Abstract
Diagnosing cancer earlier can enable timely treatment and optimize outcomes. Worldwide, national cancer control plans increasingly encompass early diagnosis programs for symptomatic patients, commonly comprising awareness campaigns to encourage prompt help-seeking for possible cancer symptoms and health system policies to support prompt diagnostic assessment and access to treatment. By their nature, early diagnosis programs involve complex public health interventions aiming to address unmet health needs by acting on patient, clinical, and system factors. However, there is uncertainty regarding how to optimize the design and evaluation of such interventions. We propose that decisions about early diagnosis programs should consider four interrelated components: first, the conduct of a needs assessment (based on cancer-site–specific statistics) to identify the cancers that may benefit most from early diagnosis in the target population; second, the consideration of symptom epidemiology to inform prioritization within an intervention; third, the identification of factors influencing prompt help-seeking at individual and system level to support the design and evaluation of interventions; and finally, the evaluation of factors influencing the health systems’ capacity to promptly assess patients. This conceptual framework can be used by public health researchers and policy makers to identify the greatest evidence gaps and guide the design and evaluation of local early diagnosis programs as part of broader cancer control strategies.
INTRODUCTION
Cancer control is a global health priority.1 In high-income countries (HICs), cancer incidence is increasing with other noncommunicable diseases, whereas in lower- and middle-income countries (LMICs), there is often a concordant double burden of communicable diseases.2 Furthermore, prolonged time to diagnosis and treatment is disproportionately experienced by poorer individuals, contributing to disparities in cancer outcomes within and between countries.1,3
CONTEXT
Key Objective
Prompt diagnosis and treatment of symptomatic patients can improve cancer outcomes, but this depends on many individual and health system factors. What are the key considerations that need to be taken into account when developing early diagnosis programs?
Knowledge Generated
The design, implementation, and evaluation of early diagnosis programs should be informed by the assessment of population-specific health needs; the epidemiology of symptoms of possible cancer; and the consideration of individual and health system factors influencing both timely help-seeking for new symptoms and timely investigation and treatment. Framed within the broader evidence base, case studies from the United Kingdom, Denmark, Malaysia, and Zambia are used to illustrate existing early diagnosis activities.
Relevance
This framework can help identify population-specific evidence gaps in early cancer diagnosis research and guide the design and evaluation of early diagnosis programs across the globe.
Diagnosing symptomatic cancer earlier is a feasible and cost-effective strategy4,5 that can contribute to better clinical outcomes6-9 and improve patient experience.10-12 Effective asymptomatic detection is currently only available for a few cancers, and even in countries with established population-based screening programs, the majority of patients with cancer are diagnosed following symptomatic presentation.3,13,14 Furthermore, in low-resource settings where screening programs are not available or feasible,15-17 early diagnosis (also known as clinical downstaging) strategies can support their introduction by improving clinical pathways and building diagnostic capacity.18
Early diagnosis programs consist of supporting prompt help-seeking among symptomatic individuals and/or enabling timely access to diagnosis and treatment.1 Public education campaigns aiming to raise awareness of cancer and its symptoms and signs among the general population have been conducted in both HICs and LMICs (Box 1)19,26,29-33, whereas fast-track pathways and clinical guidelines aiming to expedite the investigation, diagnosis, and treatment of symptomatic individuals have been introduced in HICs including the United Kingdom, Denmark, New Zealand, and Spain (Box 2).39,45-48
BOX 1.
Examples of Symptom Awareness Campaign Design and Evaluation in England and Malaysia
In England, national Be Clear on Cancer campaigns were developed in 2012 through the partnership of governmental agencies and Cancer Research UK, a charity. Nationwide campaigns designed to target individuals of age 50+ years have been conducted for colorectal, lung, breast, bladder, kidney, and esophagogastric cancers.19,20,25 The visual and linguistic components of the campaign were designed to engage individuals from lower socioeconomic groups and alleviate possible concerns about bothering the doctor.21 Campaign evaluations have found increasing levels of awareness and frequency of help-seeking for some symptoms and increased referrals and use of investigations for suspected cancer by primary care physicians although evidence of improvement in stage at diagnosis and survival is limited.22-25
In Malaysia, breast and colorectal cancers were the focus of Be Cancer Alert, a culturally sensitive mass media campaign led by a multisector partnership of researchers from Malaysia and the United Kingdom.26 The campaign focused on breast and colorectal cancers because of the high prevalence of these cancers in Malaysia, and the design was driven by evidence suggesting low awareness of the alarm symptoms of breast and colorectal cancer among members of the public as well as negative attitudes and beliefs regarding cancer. The campaign's logic model takes contextual factors into account, including differences in knowledge and cultural norms between the main ethnic groups in Malaysia (Malays, Chinese, and Indian) and health and media literacy.27 Recently published findings indicate improvements in symptom awareness at follow-up across ethnic and social strata although, in common with the English experience, evidence of improvements in downstream outcomes was less clear.28
BOX 2.
Fast-Track Pathways for Suspected Cancer in England and Denmark
For most common cancers, cancer survival in England (United Kingdom) and Denmark is comparatively poor to other high-income countries.34,35 This has stimulated the relative prioritization of early diagnosis as part of cancer control measures in these countries.
Fast-track (2-week-wait) referral pathways from primary care to specialist assessment were introduced in England in 1999/2000.36 In parallel, national clinical guidelines have been developed to support the pathways: certain red-flag alarm symptoms are recommended for referral based on their positive predictive value for cancer.37 Around 2.4 million symptomatic individuals are referred using this pathway annually, of which 93% occur within 14 days and around 8% are subsequently diagnosed with cancer.36,38 For individuals subsequently diagnosed with cancer (through any route), national targets for the treatment interval (time from decision to treat to first treatment) are set at a maximum of 31 days.38 In Denmark, a similar fast-track referral system was introduced in 2008 called the Cancer Patient Pathway.39
Evaluating the impact of the fast-track pathways is challenging because of the waiting time paradox, whereby individuals with more severe and acute onset symptoms are diagnosed quickly but have poorer survival compared with patients who experience longer time to diagnosis.6,7 Accounting for this phenomenon, the introduction of fast-track pathways has been associated with improvements in cancer survival.40,41 Primary care practices with higher usage of the 2-week-wait referral pathway have been associated with lower mortality in England.42 Furthermore, increasing use of the 2-week-wait pathway over time has been associated with decreasing proportions of patients diagnosed with cancer as an emergency,43 a marker of poor prognosis.44
THE NEED FOR A FRAMEWORK TO GUIDE DECISION MAKING
The timeliness of cancer diagnosis and treatment in symptomatic patients has been conceptualized as a series of intervals starting from symptom onset.12,49-51 Building on these theoretical foundations, it is important to consider how early diagnosis evidence can be translated into interventions.
Early diagnosis programs represent complex interventions; they aim to achieve changes in population-level behavior and often interdependent health system factors.52,53 The majority of early diagnosis initiatives are implemented as natural experiments,22,54,55 compounding the difficulties in measuring impact and effectiveness.
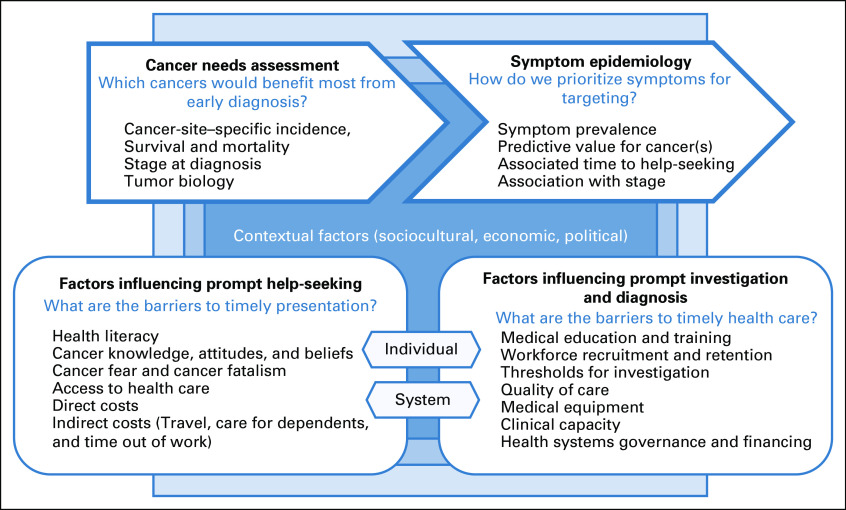
We propose a framework to aid the design and evaluation of early diagnosis programs (Fig 1). It draws on existing literature from HIC and LMIC settings, augmented by the expert opinions of the international group of authors. The framework comprises four components: first, the conduct of a needs assessment (based on cancer-site–specific statistics) to identify the cancers that may benefit most from early diagnosis in the target population; second, the consideration of symptom epidemiology to inform prioritization within an intervention; third, the identification of factors influencing prompt help-seeking at individual and system level to support the design and evaluation of interventions; and finally, the appraisal of factors influencing the health systems’ capacity to promptly assess patients.
FIG 1.
Conceptual framework for the design and evaluation of early diagnosis programs for cancer.
CANCER NEEDS ASSESSMENT
Local or contextualized epidemiological knowledge about the incidence, mortality, and survival associated with different cancers in a given setting can inform the prioritization of cancer types when designing an early diagnosis program. These considerations should ideally be based on high-quality population-based cancer registries and mortality data, although this is conditional on cancer registry infrastructure.56 Cancer-specific incidence alone may be instructive, whereas other characteristics such as the frequency and distribution of advanced stage at diagnosis among cases, site- and stage-specific survival statistics, and existing levels of awareness among the general population can further contribute to the prioritization of cancer types to be targeted by early diagnosis programs.12 In the absence of such data, as in many LMICs, data collection may be necessary to gauge capacity for early diagnosis; local- or regional-level statistics from hospital-based cancer registries or other sources could be useful. Contextual factors such as stakeholder views, sociocultural and political factors, and health system factors such as access and affordability of cancer treatment and healthcare providers will also influence how cancers are ranked by the need for early diagnosis57 and may often be stronger drivers for action particularly in settings where data capture and collection are difficult.
SYMPTOM EPIDEMIOLOGY
Early diagnosis interventions are, by their nature, centered on specific presenting symptoms of cancer. Accordingly, understanding symptom epidemiology, that is, population-level evidence regarding the nature and frequency of presenting symptoms, can help the targeting of interventions.58
Evidence on presenting symptoms may be collected retrospectively through patient recall or extracted from health records where information is captured prospectively during consultations. Self-report-based approaches provide firsthand insight, although may be associated with recall bias (because of the retrospective nature of recalling experienced symptoms) and survival bias (where patients who are the sickest are less likely to be included). In comparison, prospective health record studies allow for the study of much larger populations, but incomplete symptom elicitation and recording may introduce other biases.59 The latter may be used to derive the positive predictive value (PPV) of symptoms for cancer, which expresses the probability that following presentation with a symptom, that individual will be diagnosed with cancer.
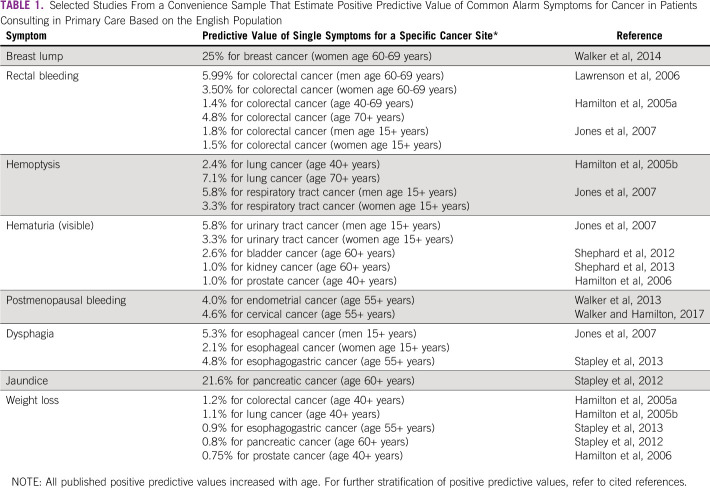
There is a large body of literature on symptom-specific PPVs for cancer based on primary care electronic health record studies in England (see Table 1 for selected examples).60-75 This evidence informed the development of an explicit PPV threshold of 3% used to prioritize symptoms for urgent investigation and fast-track 2-week-wait referral in England.36,37 Currently, there is no available evidence, and no biological reason, to suggest that the nature of cancer symptoms or their relative frequency at presentation may vary between different countries, although psychosocial and health system factors could influence how symptoms are reported.76,77 The English studies indicate that PPVs increase with age and are higher among men; other differences in demographic factors, pre-existing conditions (morbidities), and symptom prevalence could lead to variation in symptom PPVs across populations, for example, the PPV of respiratory symptoms for lung cancer in communities with high tuberculosis prevalence. As LMICs develop electronic medical records, examining country-specific symptom prevalence and PPVs will be necessary.
TABLE 1.
Selected Studies From a Convenience Sample That Estimate Positive Predictive Value of Common Alarm Symptoms for Cancer in Patients Consulting in Primary Care Based on the English Population
Nevertheless, around 50% of patients with cancer present with nonspecific or vague symptoms that are associated with much lower PPVs for cancer.78 In Denmark, patients with nonspecific symptoms (eg, unexplained weight loss, fatigue, and abdominal pain) are referred to multidisciplinary clinics where a battery of investigations (blood and urine tests and diagnostic imaging) can be conducted rapidly.39 Similarly designed clinics have been introduced in the United Kingdom to accommodate individuals who present with nonalarm symptoms.79-83
Evidence regarding pre-existing levels of symptom awareness and symptom-specific measures of diagnostic timeliness could guide the selection of symptoms in early diagnosis programs. Validated measures including the Cancer Awareness Measure,84,85 the Awareness and Beliefs about Cancer measure,86 and region-specific adaptations such as the African Women Awareness of CANcer tool designed for breast and cervical cancer87 could support such prioritization by identifying symptoms correlated with low awareness.76,88,89 Symptoms associated with the longest patient intervals (time from symptom onset to presentation) may also be particularly worthy of targeting.90
PROMPT HELP-SEEKING FOR SYMPTOMS
Psychological, social, and cultural factors influence the timeliness of help-seeking for symptoms.49,51,91,92 Individual- and group-level (eg, family or social network) barriers include cancer fear and stigma, poor health literacy, lack of trust in healthcare providers, and low expectations or perceptions regarding healthcare access or quality.3,93-98 Such barriers have been associated with demonstrably longer intervals to help-seeking for possible cancer symptoms99,100 and advanced stage at diagnosis among those diagnosed with cancer.101,102
System-wide factors also contribute to delayed presentation and inequalities in cancer outcomes. These include the proximity and accessibility of health care; the availability of infrastructure and transport services; the direct costs of seeking medical advice, diagnostic testing, and anticipated treatment; and the indirect costs of seeking help including travel and accommodation costs, care for dependents, and time out of work.103-106 Addressing the individual and system factors influencing prompt help-seeking is important for improving equitable access to cancer services, given that the above barriers are invariably more prevalent in low-resource communities.
PROMPT INVESTIGATION AND DIAGNOSIS
Many additional steps lie between prompt help-seeking for symptoms, the diagnosis of cancer, and the initiation of treatment. Postpresentational intervals in LMICs often account for a greater proportion of total time to cancer diagnosis and treatment compared with help-seeking intervals.98,107 Furthermore, focusing on early diagnosis is an important precursor to establishing cancer screening programs by increasing diagnostic capacity for all individuals with positive results.108 Early diagnosis programs have the potential to contribute to health system development as the diagnosis and treatment of cancer requires a coordinated multidisciplinary approach. The consideration of health system factors is therefore critical for early diagnosis programs.12,109,110
Timely investigation and diagnosis of cancer relies on the availability of medical equipment, imaging, pathology, and clinical laboratory services. Increasing capacity for diagnostic technologies should be preceded by the consideration of affordability and sustainability within the existing health system infrastructure for the health system and population.111 In England, the increasing volume of fast-track referrals (Box 2) has added to pressures on secondary care services such as colonoscopy provision and imaging, pointing to the need for new technologies and interventions to support risk management and clinical decision making, including new service pathways.79,112 Fecal immunochemical testing, although initially introduced in the context of bowel cancer screening, is increasingly considered as a sensitive triaging tool for patients presenting with abdominal symptoms to manage demand for colonoscopy services.113-115 Access to and availability of timely cancer treatment is also critical for early diagnosis to contribute to cancer outcomes. In Zambia, decentralized models of service provision that connect different levels of care present an effective alternative to comprehensive cancer centers, a common model of care in LMICs, while repurposing existing care pathways (Box 3).118
BOX 3.
Implementation of Primary Care–Based Breast Cancer Services in Zambia
The majority of breast cancers in Zambia are currently diagnosed at stage III/IV, and early diagnosis has been identified as a priority.116 Symptomatic individuals have to navigate multiple levels of the health system before diagnosis and treatment at the national cancer care center in Lusaka. A national assessment of health services for breast and cervical cancer identified the need for better coordination of early detection services for breast cancer.117
A breast cancer clinic was established at primary care level in a district hospital.118 Symptomatic women from surrounding primary care facilities including rural health posts, health centers, and other district hospitals are assessed at the clinic. Initially, an existing cervical cancer prevention program was used to direct eligible women for assessment and generate awareness of the new service among primary care providers. The clinic provides timely diagnostic investigation (clinical breast examination, same-visit ultrasound, and core- or fine-needle biopsy) and has surgical capacity. Patients requiring chemotherapy or radiation are referred within 2 weeks to the national cancer care center. These decentralized care pathways improved timeliness and access to diagnosis and treatment services by integrating provision with existing care pathways.118 The intervention has been accompanied by workforce development, which was identified as a barrier to cancer care delivery in Zambia.109
Recruitment and retention of a skilled health workforce including community health workers, specialists, and allied health professionals (such as radiologists, pathologists, and biomedical scientists) are also pivotal. Strengthening knowledge and awareness of cancer signs and symptoms among the healthcare workforce (particularly those working in frontline services) is of equal, if not greater, importance to raising awareness among the general population.119-123
Increasing health system capacity for timely cancer diagnosis and treatment relies on macro-level factors including health system governance and financing. Emigration of trained professionals to HICs may reduce their availability in LMICs.124 Although strengthening health systems can be challenging, creating or improving clinical pathways for the diagnosis of cancer can provide impetus and opportunities to improve healthcare delivery for other nonmalignant diseases, collinear to the universal healthcare agenda.108,124,125
DESIGNING AND EVALUATING EARLY DIAGNOSIS PROGRAMS: CHALLENGES AND OPPORTUNITIES
Early diagnosis programs comprise complex interventions, both acting on and being influenced by multiple interplaying factors.12 The conceptual framework we propose aims to make the rationale of such interventions explicit and in doing so support evidence-based strategies for earlier diagnosis.
DESIGN AND IMPLEMENTATION
In settings with fragmented or nonexistent data infrastructures, population-specific health needs assessments prior to the design and implementation of early diagnosis programs will require adaptation to available data. Nevertheless, systematically synthesizing up-to-date evidence to inform intervention design remains challenging even where routine data are readily available.55 In some instances, primary data collection may be a necessary component, using instruments such as patient questionnaires and clinical audit to identify existing barriers to early diagnosis.87,119,126
Incorporating implementation science can help identify mechanisms that enable change or lead to improved outcomes; it also helps to understand the role of contextual influencers.127,128 The use of implementation science while also embracing complexity and systems thinking can help to tackle the gap between evidence and current practice.129-132 Measuring outcomes specific to implementation can inform how best to adapt an intervention to real-life settings, determine effectiveness, and sustain and scale up efforts.127,133 The Behaviour Change Wheel128 and Normalization Process Theory134 are some of the many theories and frameworks that have been adopted to understand and assess implementation in early cancer diagnosis programs in the United Kingdom, Denmark, and Australia.135-140
MONITORING AND EVALUATION
Monitoring and evaluation are critical to the success of an early diagnosis program.12 Early diagnosis programs target changes in population-level behavior or health system improvements, which can be difficult to quantify. For example, symptom awareness campaigns may stimulate transgenerational changes in attitudes toward cancer and contribute to timely diagnosis later in the life course,141 whereas health system–wide changes may contribute to broader improvements in healthcare delivery and help streamline diagnostic pathways for cancer. Although some randomized control trials have been conducted,120,142 the majority of early diagnosis programs are natural experiments and so evaluations more often take other forms such as before and after designs and interrupted time series analysis, commonly involving mixed-methods research.22,25,27,40,135,143-145
Process and outcome indicators for monitoring early diagnosis programs have been recommended by the WHO.12 Measures should be selected based on available resources and existing infrastructure, which will influence their relative utility. For example, the proportion of patients with cancer diagnosed at early stage is often the most direct outcome indicator but is contingent on reliable and accurate cancer registration services. The patient interval (time from symptom onset to help-seeking) can be measured to evaluate possible improvements in symptom awareness among patients with cancer; here, audit methodologies or self-reported data may be more informative than health records, which can often be incomplete.146
Other metrics may be specific to certain intervention types and health system contexts. For example, a range of process and outcome measures have been examined for the evaluation of the Be Clear on Cancer campaigns in England.25 These include the cancer referral rate (the number of urgent referrals for suspected cancer made by primary care practices divided by the registered practice population) and referral scheme sensitivity (the number of cancer diagnoses resulting from an urgent referral, rather than another route to diagnosis). The primary care interval (time from first presentation in primary care to referral to secondary care) has also been highlighted as a measure of diagnostic timeliness in England, Scandinavia, and other countries50,147 but is of less relevance to jurisdictions where patients may access specialist care directly.
In conclusion, increasing cancer incidence globally compels public health agencies to instigate cancer prevention and control measures; early diagnosis programs in both HIC and LMIC settings form a critical component. Cancer-specific health needs assessment followed by the consideration of symptom epidemiology can contribute to priority setting within programs. Programs should also consider societal and health system–level factors that influence prompt help-seeking, investigation, and diagnosis of suspected cancer. The framework we propose can help to identify population-specific evidence gaps and optimize the design and evaluation of early diagnosis programs contributing to global efforts for cancer control.
ACKNOWLEDGMENT
This research arises from the CanTest Collaborative, which is funded by Cancer Research UK (C8640/A23385), of which MMK and NC are Postdoctoral Researchers, GL is Associate Director, GPR is Chair and FMW is Director. GL is supported by Cancer Research UK Clinician Advanced Scientist Fellowship (grant number: C18081/A18180). The funder has had no role in the study, writing of the report, or decision to submit the paper for publication.
DISCLAIMER
Marilys Corbex is a staff member of the World Health Organization. The author alone is responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Karla Unger-Saldaña
Honoraria: Roche
Travel, Accommodations, Expenses: Roche
Jennifer Moodley
Research Funding: Discovery Health, GlaxoSmithKline
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Minjoung Monica Koo, Karla Unger-Saldaña, Amos Deogratius Mwaka, Marilys Corbex, Jennifer Moodley, Greg P. Rubin, Georgios Lyratzopoulos
Financial support: Georgios Lyratzopoulos
Administrative support: Georgios Lyratzopoulos
Collection and assembly of data: Georgios Lyratzopoulos, Minjoung Monica Koo
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
REFERENCES
- 1.World Health Organization : WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva, 2020. https://apps.who.int/iris/handle/10665/330745
- 2.World Health Organization : Noncommunicable diseases. 2020. https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1
- 3.Balogh E, Patlak M, Nass SJ, et al. : Cancer Care in Low-Resource Areas. Washington, DC, National Academies Press, 2016. https://www.nap.edu/catalog/24743 [Google Scholar]
- 4.Ott JJ, Ullrich A, Miller AB: The importance of early symptom recognition in the context of early detection and cancer survival. Eur J Cancer 45:2743-2748, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Laudicella M, Walsh B, Burns E, et al. : Cost of care for cancer patients in England: Evidence from population-based patient-level data. Br J Cancer 114:1286-1292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tørring ML, Murchie P, Hamilton W, et al. : Evidence of advanced stage colorectal cancer with longer diagnostic intervals: A pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer 117:888-897, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal RD, Tharmanathan P, France B, et al. : Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 112:S92-S107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tørring ML, Falborg AZ, Jensen H, et al. : Advanced‐stage cancer and time to diagnosis: An International Cancer Benchmarking Partnership (ICBP) cross‐sectional study. Eur J Cancer Care (Engl) 28:e13100, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Koo MM, Swann R, McPhail S, et al. : Presenting symptoms of cancer and stage at diagnosis: Evidence from a cross-sectional, population-based study. Lancet Oncol 2045:1-7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandager M, Jensen H, Lipczak H, et al. : Cancer patients' experiences with urgent referrals to cancer patient pathways. Eur J Cancer Care (Engl) e12927, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Mendonca SC, Abel G, Saunders CL, et al. : Pre-referral general practitioner consultations and subsequent experience of cancer care: Evidence from the English Cancer Patient Experience Survey. Eur J Cancer Care (Engl) 25:478-490, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization : Guide to cancer: early diagnosis. 2017. http://apps.who.int/iris/bitstream/10665/254500/1/9789241511940-eng.pdf?ua=1
- 13.Elliss-Brookes L, McPhail S, Ives A, et al. : Routes to diagnosis for cancer—Determining the patient journey using multiple routine data sets. Br J Cancer 107:1220-1226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarma EA, Kobrin SC, Thompson MJ: A proposal to improve the early diagnosis of symptomatic cancers in the United States. Cancer Prev Res 13:715–720, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Ilbawi AM, Anderson BO: Cancer in global health: How do prevention and early detection strategies relate?. Sci Transl Med 7:278cm1, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Sankaranarayanan R, Boffetta P: Research on cancer prevention, detection and management in low- and medium-income countries. Ann Oncol 21:1935–1943, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Corbex M, Burton R, Sancho-Garnier H: Breast cancer early detection methods for low and middle income countries, a review of the evidence. Breast 21:428-434, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Devi BCR, Tang TS, Corbex M: Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: A pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol 18:1172-1176, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Public Health England : Be clear on cancer—Current campaigns. 2016. http://www.cancerresearchuk.org/health-professional/early-diagnosis-activities/be-clear-on-cancer
- 20.Cancer Research UK : About be clear on cancer. Early diagnosis Act. 2014. http://www.cancerresearchuk.org/health-professional/awareness-and-prevention/be-clear-on-cancer/about-be-clear-on-cancer
- 21.Forbes LJL, Warburton F, Richards MA, et al. : Risk factors for delay in symptomatic presentation: A survey of cancer patients. Br J Cancer 111:581-588, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ironmonger L, Ohuma E, Ormiston-Smith N, et al. : An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. Br J Cancer 112:207-216, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffat J, Bentley A, Ironmonger L, et al. : The impact of national cancer awareness campaigns for bowel and lung cancer symptoms on sociodemographic inequalities in immediate key symptom awareness and GP attendances. Br J Cancer 112:S14-S21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Public Health England : Be clear on cancer evaluation. NCRAS. 2019. http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/be_clear_on_cancer/
- 25.Lai J, Mak V, Bright CJ, et al. : Reviewing the impact of 11 national be clear on cancer public awareness campaigns, England 2012‐2016: A synthesis of published evaluation results. Int J Cancer 10.1002/ijc.33277 [epub ahead of print on September 1, 2020] [DOI] [PubMed]
- 26.Schliemann D, Donnelly M, Dahlui M, et al. : The “Be Cancer Alert Campaign”: Protocol to evaluate a mass media campaign to raise awareness about breast and colorectal cancer in Malaysia. BMC Cancer 18:881, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schliemann D, Htay MNN, Dahlui M, et al. : Impact of a mass media campaign on breast cancer symptoms awareness and screening uptake in Malaysia: Findings from a quasi-experimental study. BMJ Open 10:e036503, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schliemann D, Paramasivam D, Dahlui M, et al. : Change in public awareness of colorectal cancer symptoms following the Be Cancer Alert Campaign in the multi-ethnic population of Malaysia. BMC Cancer 20:252, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AfrOx : Early diagnosis and prevention. 2011. http://www.afrox.org/20/early-diagnosis-prevention
- 30.Cancer Australia : Campaigns and events. 2016. https://canceraustralia.gov.au/healthy-living/campaigns-events
- 31.Yaqub F: Punjab's cancer awareness campaign. Lancet Oncol 14:e92, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Puckett MC, Townsend JS, Gelb CA, et al. : Ovarian cancer knowledge in women and providers following education with inside knowledge campaign materials. J Cancer Educ 33:1285-1293, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Wide Breast Cancer : Know your lemons. 2016. https://www.worldwidebreastcancer.org/about/
- 34.Allemani C, Matsuda T, Di Carlo V, et al. : Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391:1023-1075, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold M, Rutherford MJ, Bardot A, et al. : Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol 20:1493-1505, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards M, Thorlby R, Fisher R, et al. : Unfinished business: An assessment of the national approach to improving cancer services in England 1995–2015. London. 2018.https://www.health.org.uk/publications/unfinished-business
- 37.NICE : Suspected cancer: recognition and referral. Cardiff. 2015. www.nice.org.uk/guidance/ng12 [PubMed]
- 38.NHS England : Cancer waiting times. 2020. https://www.england.nhs.uk/statistics/statistical-work-areas/cancer-waiting-times/
- 39.Vedsted P, Olesen F: A differentiated approach to referrals from general practice to support early cancer diagnosis—The Danish three-legged strategy. Br J Cancer 112:S65-S69, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen H, Tørring ML, Vedsted P: Prognostic consequences of implementing cancer patient pathways in Denmark: A comparative cohort study of symptomatic cancer patients in primary care. BMC Cancer 17:627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walters S, Benitez-Majano S, Muller P, et al. : Is England closing the international gap in cancer survival?. Br J Cancer 113:848-860, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Round T, Gildea C, Ashworth M, et al. : Association between use of urgent suspected cancer referral and mortality and stage at diagnosis: A 5-year national cohort study. Br J Gen Pract 70:e389-e398, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herbert A, Abel GA, Winters S, et al. : Cancer diagnoses after emergency GP referral or A&E attendance in England: Determinants and time trends in Routes to Diagnosis data, 2006-2015. Br J Gen Pract 69:e724-e730, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Abel GA, Hamilton W, et al. : Diagnosis of cancer as an emergency: A critical review of current evidence. Nat Rev Clin Oncol 14:45-56, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Prades J, Espinàs Ja, Font R, et al. : Implementing a cancer fast-track programme between primary and specialised care in Catalonia (Spain): A mixed methods study. Br J Cancer 105:753-759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison AJ, Foot CS: Targets and prioritization: The case of cancer in the English NHS. Qual Prim Care 20:125-129, 2012 [PubMed] [Google Scholar]
- 47.New Zealand Guidelines Group (NZGG) . 2009. Suspected Cancer in Primary Care: Guidelines for investigation, referral and reducing ethnic disparities. Wellington:New Zealand Guidelines Group. https://www.health.govt.nz/publication/suspected-cancer-primary-care-guidelines-investigation-referral-and-reducing-ethnic-disparities [PubMed] [Google Scholar]
- 48.Valentin-Lopez B, Ferrandiz-Santos J, Blasco-Amaro J-A, et al. : Assessment of a rapid referral pathway for suspected colorectal cancer in Madrid. Fam Pract 29:182-188, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Scott SE, Walter FM, Webster A, et al. : The model of pathways to treatment: Conceptualization and integration with existing theory. Br J Health Psychol 18:45-65, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Weller D, Vedsted P, Rubin G, et al. : The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br J Cancer 106:1262-1267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walter F, Webster A, Scott S, et al. : The Andersen model of total patient delay: A systematic review of its application in cancer diagnosis. J Health Serv Res Policy 17:110-118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craig P, Dieppe P, Macintyre S, et al. : Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 337:a1655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell M, Fitzpatrick R, Haines A, et al. : Framework for design and evaluation of complex interventions to improve health Framework for trials of complex interventions. Br Med J 321:694-696, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell NCN, Murray E, Darbyshire J, et al. : Designing and evaluating complex interventions to improve health care. Br Med J 334:455-459, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogilvie D, Adams J, Bauman A, et al. : Using natural experimental studies to guide public health action: Turning the evidence-based medicine paradigm on its head. J Epidemiol Community Health 74:203-208, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnelly C, Cairnduff V, Chen JJ, et al. : The completeness and timeliness of cancer registration and the implications for measuring cancer burden. Cancer Epidemiol 49:101-107, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Hamilton W, Stapley S, Campbell C, et al. : For which cancers might patients benefit most from expedited symptomatic diagnosis? Construction of a ranking order by a modified Delphi technique. BMC Cancer 15:820, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koo MM, Hamilton W, Walter FM, et al. : Symptom signatures and diagnostic timeliness in cancer patients: A review of current evidence. Neoplasia 20:165-174, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verheij RA, Curcin V, Delaney BC, et al. : Possible sources of bias in primary care electronic health record data use and reuse. J Med Internet Res 20:e185, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton W, Peters TJ, Bankhead C, et al. : Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ 339:b2998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker S, Hyde C, Hamilton W: Risk of breast cancer in symptomatic women in primary care: a case-control study using electronic records. Br J Gen Pract 64:e788-e793, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ewing M, Naredi P, Zhang C, et al. : Clinical features of patients with non-metastatic lung cancer in primary care: A case-control study. BJGP Open 2:bjgpopen18X101397, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrenson R, Logie J, Marks C: Risk of colorectal cancer in general practice patients presenting with rectal bleeding, change in bowel habit or anaemia. Eur J Cancer Care (Engl) 15:267-271, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Jones R, Latinovic R, Charlton J, et al. : Alarm symptoms in early diagnosis of cancer in primary care: Cohort study using General Practice Research Database. BMJ 334:1040, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shephard E, Neal R, Rose P, et al. : Clinical features of kidney cancer in primary care: A case-control study using primary care records. Br J Gen Pract 63:e250-e255, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collins GS, Altman DG: Identifying patients with undetected gastro-oesophageal cancer in primary care: External validation of QCancer® (Gastro-Oesophageal). Eur J Cancer 49:1040-1048, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Hamilton W, Sharp DJ, Peters TJ, et al. : Clinical features of prostate cancer before diagnosis: A population-based, case-control study. Br J Gen Pract 56:756-762, 2006 [PMC free article] [PubMed] [Google Scholar]
- 68.Hamilton W, Barrett J, Stapley S, et al. : Clinical features of metastatic cancer in primary care: A case-control study using medical records. Br J Gen Pract 65:e516-e522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamilton W, Peters TJ, Round A, et al. : What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 60:1059-1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker S, Hamilton W: Risk of cervical cancer in symptomatic women aged ≥40 in primary care: A case-control study using electronic records. Eur J Cancer Care (Engl) 63:e12706, 2017 [DOI] [PubMed] [Google Scholar]
- 71.Stapley S, Peters TJ, Neal RD, et al. : The risk of oesophago-gastric cancer in symptomatic patients in primary care: A large case-control study using electronic records. Br J Cancer 108:25-31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Astin M, Griffin T, Neal RD, et al. : The diagnostic value of symptoms for colorectal cancer in primary care: A systematic review. Br J Gen Pract 61:231-243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stapley S, Peters TJ, Neal RD, et al. : The risk of pancreatic cancer in symptomatic patients in primary care: A large case-control study using electronic records. Br J Cancer 106:1940-1944, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shephard EA, Stapley S, Neal RD, et al. : Clinical features of bladder cancer in primary care. Br J Gen Pract 62:598-604, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shephard EA, Neal RD, Rose P, et al. : Quantifying the risk of multiple myeloma from symptoms reported in primary care patients: A large case-control study using electronic records. Br J Gen Pract 65:e106-e113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forbes LJL, Simon AE, Warburton F, et al. : Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): Do they contribute to differences in cancer survival? Br J Cancer 108:292-300, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menon U Vedsted P Zalounina Falborg A et al. :. Time intervals and routes to diagnosis for lung cancer in 10 jurisdictions: cross-sectional study findings from the International Cancer Benchmarking Partnership (ICBP) BMJ Open 2019;9:e025895. doi: 10.1136/bmjopen-2018-025895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen H, Tørring ML, Olesen F, et al. : Cancer suspicion in general practice, urgent referral and time to diagnosis: A population-based GP survey and registry study. BMC Cancer 14:636, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forster AS, Renzi C, Lyratzopoulos G: Diagnosing cancer in patients with “non-alarm” symptoms: Learning from diagnostic care innovations in Denmark. Cancer Epidemiol 54:101-103, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuller E, Fitzgerald K, Hiom S, et al. : Accelerate, coordinate, evaluate programme: A new approach to cancer diagnosis. Br J Gen Pract 66:176-177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapman D, Poirier V, Vulkan D, et al. : First results from five multidisciplinary diagnostic centre (MDC) projects for non-specific but concerning symptoms, possibly indicative of cancer. Br J Cancer 123:722-729, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicholson BD, Oke J, Friedemann Smith C, et al. : The Suspected CANcer (SCAN) pathway: Protocol for evaluating a new standard of care for patients with non-specific symptoms of cancer. BMJ Open 8:1-8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sewell B, Jones M, Gray H, et al. : Rapid cancer diagnosis for patients with vague symptoms: A cost-effectiveness study. Br J Gen Pract 70:e186-e192, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stubbings S, Robb K, Waller J, et al. : Development of a measurement tool to assess public awareness of cancer. Br J Cancer 101:S13-S17, 2009. (suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robb K, Stubbings S, Ramirez AJ, et al. : Public awareness of cancer in Britain: A population-based survey of adults. Br J Cancer 101:S18–S23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon AE, Forbes LJL, Boniface D, et al. : An international measure of awareness and beliefs about cancer: Development and testing of the ABC. BMJ Open 2:e001758, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moodley J, Scott SE, Mwaka AD, et al. : Development and validation of the African Women Awareness of CANcer (AWACAN) tool for breast and cervical cancer. PLoS One 14:e0220545, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su TT, Goh JY, Tan J, et al. : Level of colorectal cancer awareness: A cross sectional exploratory study among multi-ethnic rural population in Malaysia. BMC Cancer 13:376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rendle KA, Sarma EA, Quaife SL, et al. : Cancer symptom recognition and anticipated delays in seeking care among U.S. adults. Am J Prev Med 57:e1-e9, 2019 [DOI] [PubMed] [Google Scholar]
- 90.Koo MM, von Wagner C, Abel GA, et al. : The nature and frequency of abdominal symptoms in cancer patients and their associations with time to help-seeking: Evidence from a national audit of cancer diagnosis. J Public Health (Bangkok) 40:e388-e395, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitaker KL, Scott SE, Wardle J: Applying symptom appraisal models to understand sociodemographic differences in responses to possible cancer symptoms: A research agenda. Br J Cancer 112:S27-S34, 2015. (suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCutchan GM, Wood F, Edwards A, et al. : Influences of cancer symptom knowledge, beliefs and barriers on cancer symptom presentation in relation to socioeconomic deprivation: A systematic review. BMC Cancer 15:1000, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Humphrys E, Burt J, Rubin G, et al. : The influence of health literacy on the timely diagnosis of symptomatic cancer: A systematic review. Eur J Cancer Care (Engl) 28:e12920, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mwaka AD, Okello ES, Wabinga H: Perceptions and beliefs of lay people from northern Uganda regarding surgery for diagnosis and treatment of cervical cancer. Psychooncology 27:1965-1970, 2018 [DOI] [PubMed] [Google Scholar]
- 95.MacArtney J, Malmström M, Overgaard Nielsen T, et al. : Patients' initial steps to cancer diagnosis in Denmark, England and Sweden: What can a qualitative, cross-country comparison of narrative interviews tell us about potentially modifiable factors? BMJ Open 7:e018210, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Unger-Saldaña K, Infante-Castañeda CB: Breast cancer delay: A grounded model of help-seeking behaviour. Soc Sci Med 72:1096-1104, 2011 [DOI] [PubMed] [Google Scholar]
- 97.Moodley J, Cairncross L, Naiker T, et al. : From symptom discovery to treatment—Women's pathways to breast cancer care: a cross-sectional study. BMC Cancer 18:312, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foerster M, McKenzie F, Zietsman A, et al. : Dissecting the journey to breast cancer diagnosis in sub‐Saharan Africa: Findings from the multicountry ABC‐DO cohort study. Int J Cancer 10.1002/ijc.33209 [epub ahead of print on July 14, 2020] [DOI] [PMC free article] [PubMed]
- 99.Smith LK, Pope C, Botha JL: Patients' help-seeking experiences and delay in cancer presentation: A qualitative synthesis. Lancet 366:825-831, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Mullins MA, Peres LC, Alberg AJ, et al. : Perceived discrimination, trust in physicians, and prolonged symptom duration before ovarian cancer diagnosis in the African American Cancer Epidemiology Study. Cancer 125:4442-4451, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lyratzopoulos G, Liu MP-H, Abel GA, et al. : The Association between fatalistic beliefs and late stage at diagnosis of lung and colorectal cancer. Cancer Epidemiol Biomarkers Prev 24:720-726, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anakwenze C, Bhatia R, Rate W, et al. : Factors related to advanced stage of cancer presentation in Botswana. J Glob Oncol 1-9, 2018. http://ascopubs.org/doi/10.1200/JGO.18.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shahid S, Teng T-HK, Bessarab D, et al. : Factors contributing to delayed diagnosis of cancer among Aboriginal people in Australia: A qualitative study: Table 1. BMJ Open 6:e010909, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Youl P, Aitken J, Turrell G, et al. : The impact of rurality and disadvantage on the diagnostic interval for breast cancer in a large population-based study of 3202 women in Queensland, Australia. Int J Environ Res Public Health 13:1156, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dianatinasab M, Fararouei M, Mohammadianpanah M, et al. : Impact of social and clinical factors on diagnostic delay of breast cancer. Medicine (Baltimore) 95:e4704, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berraho M, Obtel M, Bendahhou K, et al. : Sociodemographic factors and delay in the diagnosis of cervical cancer in Morocco. Pan Afr Med J 12:1-8, 2012 [PMC free article] [PubMed] [Google Scholar]
- 107.Unger-Saldaña K: Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol 5:465, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ginsburg O, Yip C, Brooks A, et al. : Breast cancer early detection: A phased approach to implementation. Cancer 126:2379-2393, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horton S, Camacho Rodriguez R, Anderson BO, et al. : Health system strengthening: Integration of breast cancer care for improved outcomes. Cancer 126:2353-2364, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rubin G, Berendsen A, Crawford SM, et al. : The expanding role of primary care in cancer control. Lancet Oncol 16:1231-1272, 2015 [DOI] [PubMed] [Google Scholar]
- 111.World Health Organization : WHO list of priority medical devices for cancer management. 2017. https://apps.who.int/iris/bitstream/handle/10665/255262/9789241565462-eng.pdf?sequence=1 [DOI] [PubMed]
- 112.Rubin G, Walter F, Emery J, et al. : Reimagining the diagnostic pathway for gastrointestinal cancer. Nat Rev Gastroenterol Hepatol 15:181-188, 2018 [DOI] [PubMed] [Google Scholar]
- 113.van Melle M, Yep Manzano SI, Wilson H, et al. : Faecal immunochemical test to triage patients with abdominal symptoms for suspected colorectal cancer in primary care: Review of international use and guidelines. Fam Pract, 2020. https://academic.oup.com/fampra/advance-article/doi/10.1093/fampra/cmaa043/5831482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Westwood M, Lang S, Armstrong N, et al. : Faecal immunochemical tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: A systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med 15:189, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicholson BD, James T, Paddon M, et al. : Faecal immunochemical testing for adults with symptoms of colorectal cancer attending English primary care: A retrospective cohort study of 14 487 consecutive test requests. Aliment Pharmacol Ther 1-11, 2020. http://doi.wiley.com/10.1111/apt.15969 [DOI] [PubMed] [Google Scholar]
- 116.McKenzie F, Zietsman A, Galukande M, et al. : Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer—Disparities in outcomes (ABC-DO) study. Int J Cancer 142:1568-1579, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chibwesha C, Pinder LF, Musonda A, et al. : A comprehensive assessment of breast and cervical cancer control infrastructure in Zambia. J Cancer Policy 13:24-29, 2017 [Google Scholar]
- 118.Songiso M, Pinder LF, Munalula J, et al. : Minimizing delays in the breast cancer pathway by integrating breast specialty care services at the primary health care level in Zambia. JCO Glob Oncol 859-865, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Unger-Saldaña K, Fitch-Picos K, Villarreal-Garza C: Breast cancer diagnostic delays among young Mexican women are associated with a lack of suspicion by health care providers at first presentation. J Glob Oncol 5:1-12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pace LE, Dusengimana JMV, Shulman LN, et al. : Cluster randomized trial to facilitate breast cancer early diagnosis in a rural district of Rwanda. J Glob Oncol 1-13, 2019. http://ascopubs.org/doi/10.1200/JGO.19.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Unger-Saldaña K, Miranda A, Zarco-Espinosa G, et al. : Health system delay and its effect on clinical stage of breast cancer: Multicenter study. Cancer 121:2198-2206, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mwaka AD, Okello ES, Wabinga H, et al. : Symptomatic presentation with cervical cancer in Uganda: A qualitative study assessing the pathways to diagnosis in a low-income country. BMC Womens Health 15:15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sardi A, Orozco-Urdaneta M, Velez-Mejia C, et al. : Overcoming barriers in the implementation of programs for breast and cervical cancers in Cali, Colombia: A pilot model. J Glob Oncol 5:1-9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morgan GW, Foster K, Healy B, et al. : Improving health and cancer services in low-resource countries to attain the sustainable development goals target 3.4 for noncommunicable diseases. J Glob Oncol 1-11, 2018. http://ascopubs.org/doi/10.1200/JGO.18.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Atun R, Cavalli F: The global fight against cancer: Challenges and opportunities. Lancet 391:412-413, 2018 [DOI] [PubMed] [Google Scholar]
- 126.Swann R, McPhail S, Witt J, et al. : Diagnosing cancer in primary care: Results from the National Cancer Diagnosis Audit. Br J Gen Pract 68:e63-e72, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Proctor E, Silmere H, Raghavan R, et al. : Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Heal Ment Heal Serv Res 38:65-76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Michie S, van Stralen MM, West R: The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci 6:42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chambers D, Vinson C, Norton W: Advancing the Science of Implementation Across the Cancer Continuum (ed 1). Oxford University Press, 2018. http://www.oxfordmedicine.com/view/10.1093/med/9780190647421.001.0001/med-9780190647421 [Google Scholar]
- 130.Peters DH, Adam T, Alonge O, et al. : Republished research: Implementation research: What it is and how to do it. Br J Sports Med 48:731-736, 2014 [DOI] [PubMed] [Google Scholar]
- 131.Sivaram S, Sanchez MA, Rimer BK, et al. : Implementation science in cancer prevention and control: A framework for research and programs in low- and middle-income countries. Cancer Epidemiol Biomarkers Prev 23:2273-2284, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rositch AF, Unger‐Saldaña K, DeBoer RJ, et al. : The role of dissemination and implementation science in global breast cancer control programs: Frameworks, methods, and examples. Cancer 126:2394-2404, 2020 [DOI] [PubMed] [Google Scholar]
- 133.Stirman SW, Miller CJ, Toder K, et al. : Development of a framework and coding system for modifications and adaptations of evidence-based interventions. Implement Sci 8:65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.May C, Finch T: Implementing, embedding, and integrating practices: An outline of normalization process theory. Sociology 43:535-554, 2009 [Google Scholar]
- 135.McCutchan G, Smits S, Ironmonger L, et al. : Evaluation of a national lung cancer symptom awareness campaign in Wales. Br J Cancer 122:491-497, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smits S, McCutchan G, Wood F, et al. : Development of a behavior change intervention to encourage timely cancer symptom presentation among people living in deprived communities using the behavior change wheel. Ann Behav Med, 2016. http://link.springer.com/10.1007/s12160-016-9849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Toftegaard BS, Bro F, Vedsted P: A geographical cluster randomised stepped wedge study of continuing medical education and cancer diagnosis in general practice. Implement Sci 9:159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dikomitis L, Green T, Macleod U: Embedding electronic decision-support tools for suspected cancer in primary care: A qualitative study of GPs' experiences. Prim Health Care Res Dev 16:548-555, 2015 [DOI] [PubMed] [Google Scholar]
- 139.Chiang PPC, Glance D, Walker J, et al. : Implementing a QCancer risk tool into general practice consultations: An exploratory study using simulated consultations with Australian general practitioners. Br J Cancer 112:S77-S83, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Green T, Martins T, Hamilton W, et al. : Exploring GPs' experiences of using diagnostic tools for cancer: A qualitative study in primary care. Fam Pract 32:101-105, 2015 [DOI] [PubMed] [Google Scholar]
- 141.Rosendal M, Jarbøl DE, Pedersen AF, et al. : Multiple perspectives on symptom interpretation in primary care research. BMC Fam Pract 14:167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Emery JD, Gray V, Walter FM, et al. : The improving rural cancer outcomes trial: A cluster-randomised controlled trial of a complex intervention to reduce time to diagnosis in rural cancer patients in Western Australia. Br J Cancer 117:1459-1469, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dahl TL, Vedsted P, Jensen H: The effect of standardised cancer pathways on Danish cancer patients' dissatisfaction with waiting time. Dan Med J 64, 2017 [PubMed] [Google Scholar]
- 144.Rubin G, Gildea C, Wild S, et al. : Assessing the impact of an English national initiative for early cancer diagnosis in primary care. Br J Cancer 112:S57-S64, 2015. (suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Toftegaard BS, Bro F, Falborg AZ, et al. : Impact of a continuing medical education meeting on the use and timing of urgent cancer referrals among general practitioners—A before-after study. BMC Fam Pract 18:44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smith SM, Whitaker KL, Cardy AH, et al. : Validation of self-reported help-seeking, and measurement of the patient interval, for cancer symptoms: An observational study to inform methodological challenges in symptomatic presentation research. Fam Pract 1-7, 2019. https://academic.oup.com/fampra/advance-article/doi/10.1093/fampra/cmz047/5569995 [DOI] [PubMed] [Google Scholar]
- 147.Lyratzopoulos G, Saunders CL, Abel GA, et al. : The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br J Cancer 112:S35-S40, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]