Abstract
PURPOSE
This study was designed to investigate the clinicopathologic predictors of progression-free survival (PFS) and overall survival (OS) in patients with epithelial ovarian cancer (EOC) following primary treatment in Lagos, Nigeria.
MATERIALS AND METHODS
Using data from a retrospective cohort of 126 patients who received treatment for EOC between 2010 and 2018, we identified 83 patients with a complete clinical record for subsequent data analysis. Patients' demographics and updated 2-year follow-up status were abstracted from medical records. Kaplan-Meier survival curves were compared using the log-rank test, and Cox proportional hazard models were used for multivariate analysis to identify independent predictors of survivals following treatment in EOC patients.
RESULTS
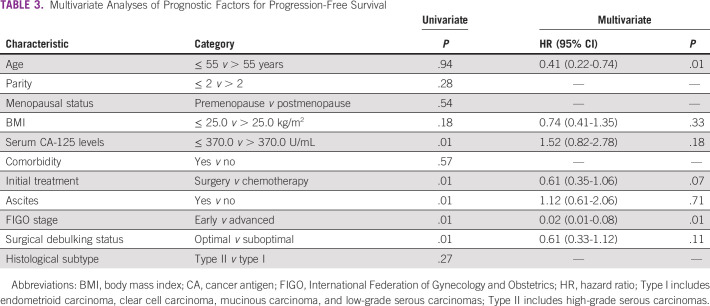
The median PFS and OS were 12 and 24 months, respectively. After adjusting for covariates in the multivariate analysis, younger age ≤ 55 years (hazard ratio [HR] = 0.40; 95% CI, 0.22 to 0.74; P = .01) and International Federation of Gynecology and Obstetrics (FIGO) stage I/II (HR = 0.02; 95% CI, 0.01 to 0.08; P = .01) were independent predictors of improved PFS, whereas being premenopausal (HR = 2.34; 95% CI, 1.16 to 4.75; P = .02) was an independent predictor of reduced OS after 2-year follow-up.
CONCLUSION
PFS could be predicted by the age and FIGO stage of the disease, whereas menopausal status was predictive of OS in patients with EOC. This knowledge should form the basis for counseling patients with ovarian cancer during their primary treatment and lend support to the importance of aggressive follow-up and monitoring for the older, premenopausal patients and those with an advanced stage of epithelial ovarian cancer. However, robust longitudinal research should be carried out to provide additional reliable insight to this information.
INTRODUCTION
Ovarian cancer (OC) is the eighth most common cancer among women worldwide and the eighth leading cause of cancer mortality. In 2018, there were almost 300,000 new patient cases and 184,799 deaths because of ovarian cancer.1 In Nigeria, it is the second most common gynecological cancer with an incidence of 30.5%.2 It is typically present in postmenopausal women with the peak incidence occurring in the early 60s.3 However, OC may also be seen in younger women, in which case it is often associated with certain genetic predispositions such as BRCA1 and BRCA2 gene mutations.4
CONTEXT
Key Objective
Are there clinicopathologic factors that predict progression-free survival (PFS) and overall survival (OS) in patients with epithelial ovarian cancer (EOC) following their primary treatment?
Knowledge Generated
The results indicated that following complete treatment of EOC with surgery and chemotherapy, patients ≤ 55 years of age and early International Federation of Gynecology and Obstetrics stage of the disease were the independent predictors of improved PFS, whereas being premenopausal was recorded as an independent predictor of poor OS after a 2-year follow-up.
Relevance
The findings from this study should form the basis for counseling patients with ovarian cancer during their primary treatment and lend support to the importance of aggressive follow-up and monitoring of the older premenopausal patients and those with an advanced stage of EOC.
Epithelial ovarian cancer (EOC) accounts for 90% of all histological types of OC5,6 with more than 70% of patients being diagnosed at the advanced stage.3 As a result of the asymptomatic nature and insidious onset of the disease, most of the cases are detected at an advanced stage.7 The standard first-line treatment at this advanced stage of the disease is optimal debulking surgery followed by adjuvant platinum-based chemotherapy4; or more recently, in women who are unfit for initial primary surgery or those in whom optimal primary debulking surgery cannot be achieved, the first-line treatment is neoadjuvant chemotherapy followed by interval debulking surgery.4,8 However, despite initial treatment and response, the recurrence rate at these advanced stages of the disease (International Federation of Gynecology and Obstetrics [FIGO], stages III-IV) may be as high as 80% usually because of chemotherapy resistance.4 There are two histological subtypes of EOC, and these include type I and II carcinomas.9 Type I carcinomas are generally slow-growing indolent neoplasms that have their precursor lesions in the ovaries,10 and these are endometrioid carcinoma, clear cell carcinoma, mucinous carcinoma, and low-grade serous carcinoma (LGSC). Type II or high-grade serous carcinomas (HGSC) are clinically aggressive neoplasms and may develop de novo from the tubal and/or ovarian surface epithelium. HGSC account for 68% of ovarian cancer and have the worst prognosis as a result of their aggressive pathologic features and usually being diagnosed at an advanced stage of the disease.9
To provide better information and personalized care to affected women, it is extremely valuable to identify the important predictors of outcome in patients with EOC in sub-Saharan Africa (SSA). Knowledge of these predictors will help to assess the efficacy of standard treatment and also the usefulness of planning and implementation of follow-up care.11 However, there is currently conflicting evidence on reliable independent predictors of survivor outcomes among patients with EOC following their complete primary treatment.12-14 A previous combined exploratory analysis of three prospectively randomized phase III multicenter trials that examined the role of surgical outcome as a prognostic factor in advanced EOC15 showed that complete tumor resection or optimal debulking is a predictor of improved progression-free survival (PFS) and overall survival (OS), whereas factors such as age, performance status, grade, FIGO stage, and histology are independent prognostic factors for OS. However, no similar study has been conducted among Black African women with EOC. This preliminary study was focused on identifying the clinicopathologic risk predictors of recurrence and death from EOC within 2 years after the primary treatment of affected women in Lagos, Nigeria. This will add to the available literature that contains studies predominantly conducted among mostly White participants in western countries of the world.
MATERIALS AND METHODS
Study Design
This was a retrospective cohort study that involved the review of case records of women with histologically confirmed EOC managed at the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria, between January 2010 and December 2018.
Eligibility Criteria
In this study, we included all patients who had a complete clinical record and relevant data for analysis and excluded patients with non-EOC and those who failed to commence treatment within 6 weeks of their presentation in LUTH. Data abstracted from patient medical records included age, parity, menopausal status, body mass index (BMI), serum cancer antigen (CA)-125 levels, coexisting morbidity (such as hypertension, diabetes mellitus, and cardiac, kidney, and liver diseases), type of primary treatment, surgical debulking status,16 presence of ascites, FIGO stage, histological subtype,9 PFS when historical information was available, and OS.
Study End Points
The study end points were to determine the clinical and pathological characteristics that predict PFS and OS in patients with EOC. PFS was determined by calculating the interval from the time of completion of primary treatment to the first evidence of progression as determined by clinical examination, elevated tumor markers (serum CA125 and/or carcinoembryonic antigen), and/or radiological studies. OS was defined as the interval from the time of completion of primary treatment until death from all causes or last follow-up since completion of treatment for patients who were still alive. The survival data were censored after a 2-year follow-up.
Statistical Analysis
Data analysis was performed using SPSS version 23.0 statistical package for Windows (IBM, Armonk, NY), and descriptive statistics were computed for all patient baseline characteristics. Characteristics of patients were described using mean and standard deviation (if normally distributed) or median and interquartile range (if skewed) for continuous variables and by frequencies and percentages for categorical variables. Kaplan-Meier estimates of PFS and OS time stratified by the various predictive factor categories were calculated and compared by employing the log-rank test statistics.17 Patients who were alive at the last follow-up or those without recurrence were censored. Multivariate Cox proportional hazard models were used to assess the association between participants’ clinicopathologic characteristics and survival outcomes while adjusting for other covariates. Backward stepwise conditional techniques were used to build the final multivariate models to include age and other variables using P < .2 to remain in the model. Associations are regarded as significant if P < .05. All P values are two-sided.
Ethical Considerations
Ethical approval for this study was obtained from the Health Research Ethics committee of the LUTH (ADM/DCST/HREC/1912) before the review of case records and data collection. Ethical principles according to the Helsinki declaration were considered during this study.
RESULTS
We recorded 126 patient cases of ovarian cancer managed in the hospital during the period under review, in which 83 were eligible for inclusion in the final analyses. Excluded from the analyses were 17 women with non-EOC, 11 who failed to undergo complete primary treatment or were lost to follow-up, three who did not commence treatment within 6 weeks of their cancer diagnosis, and 12 with insufficient clinical data.
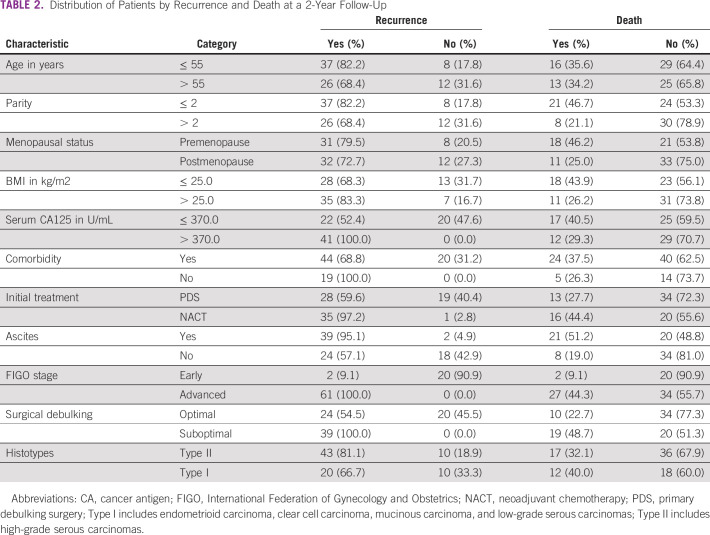
The mean age of the patients in the study group was 54.4 ± 11.5 years. Patients were predominantly in the age group 50-59 years (n = 28, 33.7%), multiparous (n = 53, 63.8%), postmenopausal (n = 44, 53.0%), and with normal body weight (n = 37, 44.6%). A larger proportion of patients had primary debulking surgery as their first upfront treatment (n = 47, 56.6%) with the majority having FIGO stage III and IV diseases (n = 61, 73.5%) and high-grade serous carcinomas (n = 53, 63.9%). Sixty-three of the patients (75.9%) had documented tumor relapse, whereas 29 (34.9%) were reported to have died at the 2-year follow-up review in this study. The median PFS and OS at 2 years were 12 months (interquartile range, 6-24 months) and 24 months (interquartile range, 23-24 months), respectively (Table 1). The distribution of patients by recurrence and death at 2-years of follow-up is shown in Table 2.
TABLE 1.
Characteristics of Patients With Epithelial Ovarian Cancer (N = 83)
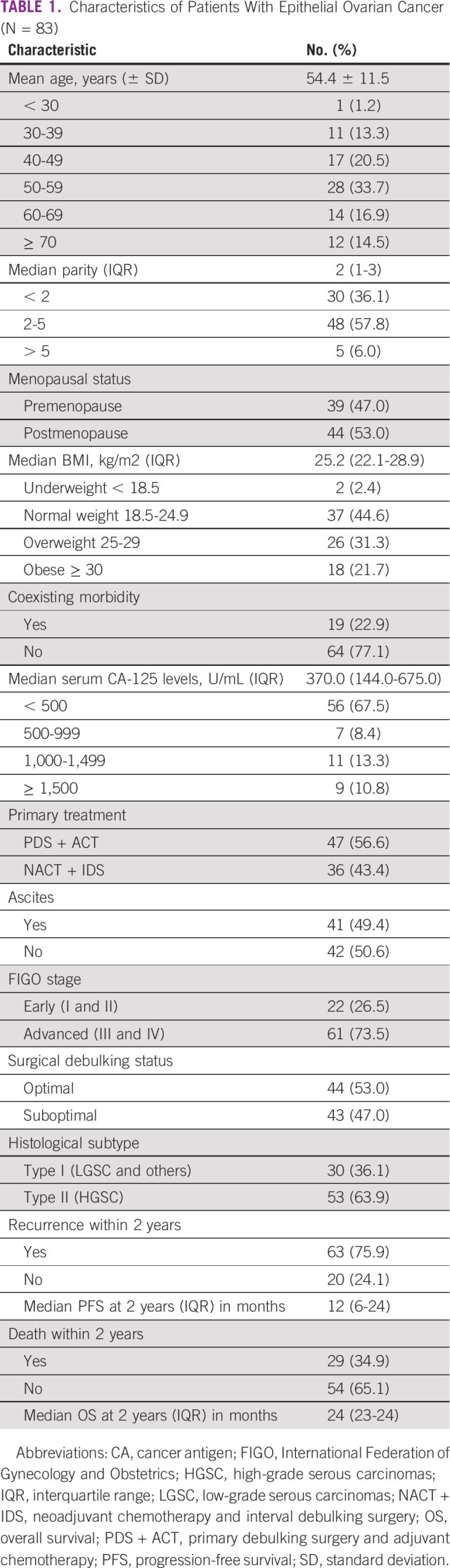
TABLE 2.
Distribution of Patients by Recurrence and Death at a 2-Year Follow-Up
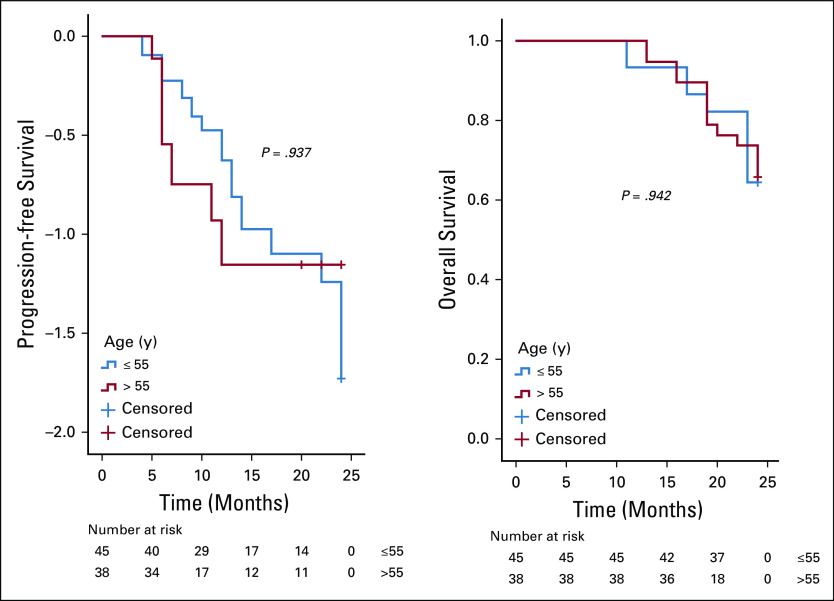
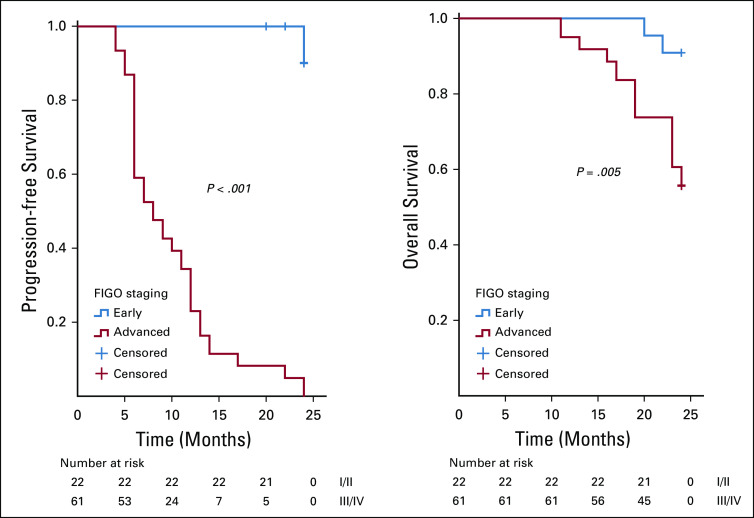
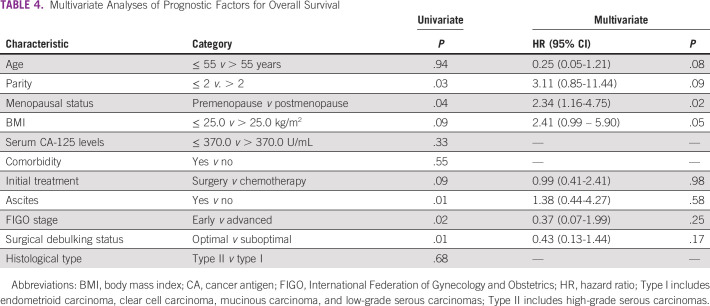
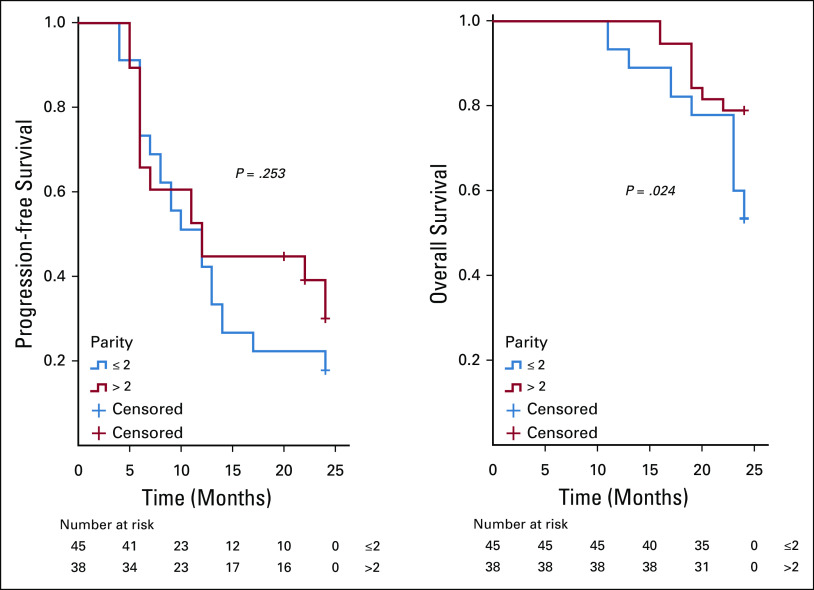
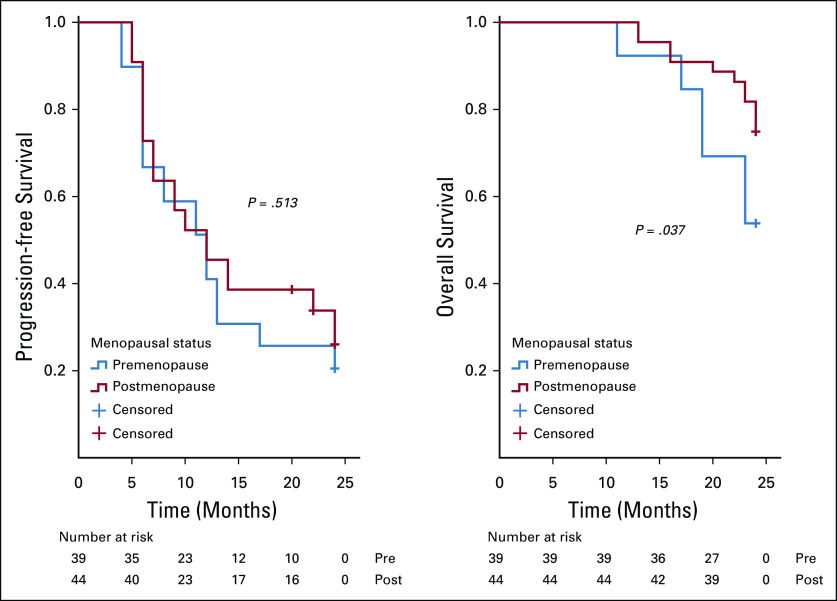
On analysis of survivals using the Kaplan-Meier estimates and log-rank statistics (Figs 1-5), it was found that age was not associated with PFS and OS, whereas both surgical debulking status and FIGO stage of the tumor were associated with survival outcomes. Parity and menopausal status were associated with OS only. After adjusting for covariates in the multivariate analysis, age ≤ 55 years (hazard ratio [HR] = 0.40; 95% CI, 0.22 to 0.74; P = .01) and FIGO stage I/II (HR = 0.02; 95% CI, 0.01 to 0.08; P = .01) were the only independent predictors of improved PFS (Table 3), whereas being premenopausal (HR = 2.34; 95% CI, 1.16 to 4.75; P = .02) was the only independent predictor of reduced OS at a 2-year follow-up (Table 4).
FIG 1.
Kaplan-Meier curve of progression-free survival (PFS) and overall survival (OS) stratified by age—age (≤ 55 v > 55 years) was not associated with PFS (P = .937) and OS (P = .942).
FIG 5.
Kaplan-Meier curve of progression-free survival (PFS) and overall survival (OS) stratified by International Federation of Gynecology and Obstetrics (FIGO) staging—FIGO stage (early v advanced) was associated with PFS (P = .001) and OS (P = .005).
TABLE 3.
Multivariate Analyses of Prognostic Factors for Progression-Free Survival
TABLE 4.
Multivariate Analyses of Prognostic Factors for Overall Survival
FIG 2.
Kaplan-Meier curve of progression-free survival (PFS) and overall survival (OS) stratified by parity—the patient’s parity (≤ 2 v > 2) was associated with OS (P = .024) but not PFS (P = .253).
FIG 3.
Kaplan-Meier curve of progression-free survival (PFS) and overall survival (OS) stratified by menopausal status—menopausal status (premenopause v postmenopause) was associated with OS (P = .037) but not PFS (P = .513).
FIG 4.
Kaplan-Meier curve of progression-free survival (PFS) and overall survival (OS) stratified by surgical debulking status—surgical debulking status (optimal v suboptimal) was associated with PFS (P = .001) and OS (P = .008). Optimal debulking is defined as when the residual disease is < 1 cm.15
DISCUSSION
This study investigated the clinicopathologic predictors of survival outcomes in patients with EOC following primary treatment in Lagos, Nigeria. We found that 63 of the patients (75.9%) had documented tumor relapse, whereas 29 (34.9%) were not alive at completion of follow-up in this study. The patient’s age and FIGO stage predicted PFS, whereas menopausal status predicted OS.
The rate of tumor recurrence recorded in this study (76.4%) is almost similar to the rate recorded in the report from a previous study conducted in the same setting in Lagos,18 whereas the high proportion of deaths recorded may be a reflection of the significantly large proportion of women (73.5%) who presented with an advanced stage disease together with its attendant poor survival outcome. Various studies outside SSA have focused on the identification of prognostic parameters for EOC, and several parameters that have been suggested to be predictive of survival in ovarian cancer include age,19 FIGO stage,20 postoperative residual tumor,11,21,22 tumor histology,23 histological grade,24 presence of ascites,25 and pretreatment serum concentrations of CA-125.26 An analysis of four prospective phase III intergroup trials in Germany that was conducted in 2016 found that patients less than age 40 years had a better PFS and OS compared with those older than 40 years.27 This is similar to the studies by Winter et al in 200719 and Chang et al in 201528 but in sharp contrast to the report from the study by Gil-Ibáñez et al29 where age was not found to be a predictor of survival in patients with ovarian cancer. Our current study showed a significant predictive effect of age on progression-free survival but not OS. This may be because age has an impact on patient ability to cope with stress related to a chronic disease state, and the altered physiology of the elderly alters the pharmacokinetics and pharmacodynamics of upfront chemotherapeutic agents used in the treatment of ovarian cancer.
According to the Gynecologic Oncology Group, an optimal surgical debulking is defined as when the residual disease is < 1 cm.16 Previous studies have shown that the ability to achieve optimal surgical debulking is the most important predictor of ovarian cancer survival,30-36 but this was not corroborated by our study. This may be due to the significant proportion of patients in this cohort (73.5%) who presented with advanced disease and because of the decreased likelihood of downgrading extensive disease by radical surgery and the poorer survival outcome of patients with high peritoneal cancer index, even after undergoing complete cytoreduction.37 We also reported that advanced FIGO stage of EOC independently predicted a reduced PFS similar to the finding by Yan et al38 and Liu et al30 but at variance with the report by Gil-Ibáñez et al.29 In contrast to our study, Liu et al30 reported in a study conducted in Tianjin, China, in 2014 that the patient’s tumor histotype is an independent prognostic factor for PFS in patients with EOC.
In contrast to our current study, Kim et al39 reported that a history of a previous parous event was associated with a significantly decreased mortality risk compared to nulliparity. This is not surprising as most parous women are older and often seek medical attention earlier compared with the younger nulliparous patients. Menopausal status is a well-known risk factor for ovarian cancer, and more than half of the patients diagnosed with ovarian cancer in our setting are postmenopausal3 as we reported in this study. There were few data regarding ovarian cancer survival in premenopausal patients; however, our study showed a reduced PFS in premenopausal patients unlike a previous study conducted by Trifanescu et al36 in 2018 where premenopausal patients with OC were shown to have a better oncologic long-term outcome. The finding of our study may be due to the relatively younger age of the premenopausal women in this cohort and the high prevalence of ovarian cancer subtypes (LGSC) associated with younger age at diagnosis that is relatively chemoresistant with its attendant high risk of relapse.40
Obesity is regarded as a major threat by increasing the incidence and mortality of different types of cancers.41 The problem is even more complex now considering the increasing incidence of obesity in most developing countries, including Nigeria, as a result of the rapid adoption of westernized lifestyles. However, at variance with data reported in the literature where increased BMI is a predictor of worst prognosis in patients with ovarian cancer,35 our study failed to show any relationship between BMI and ovarian cancer survival. In several epidemiologic studies that examined the association between serum CA-125 at diagnosis and survival from OC,42-44 there was an emphasis on the unique ability of CA-125 to independently predict OS in the setting of surgically defined disease status after primary therapy; however, our study did not find any strong association between CA-125 at diagnosis and survival in patients with EOC. This was despite our adoption of > 370 IU/mL as the cutoff in this study using stratification on the basis of the median levels of serum CA-125 recorded.
The major limitations of this study were its retrospective design that depended on effective documentation of patient history with the potential for missing data and the poor record-keeping system in our center, which resulted in the unacceptably high number of EOC patient cases with insufficient clinical data with the resultant small sample size and limited statistical power. Furthermore, the 2-year follow-up adopted may be too short for this type of study, and this may account for the relatively low OS recorded. This is also a single-center study, and thus, the findings may not be generalized to other geographical locations in Nigeria. However, this was the first study that assessed survival among women with EOC in SSA, and therefore, the preliminary data generated will form the basis for developing variables for a prediction model that will be validated in a future robust longitudinal study.
In conclusion, it is of extreme importance to identify the prognostic factors in patients with ovarian cancer to enable us to choose the most appropriate treatment strategy and to identify the risk of progression and death at follow-up. In our study, the patient age, menopausal status, FIGO clinical stage, and BMI were the only independent prognostic factors reported. The findings from this study further reiterate that once women are diagnosed with ovarian cancer, their age, the clinical stage of their disease, and their nutritional status are most likely to affect their survival. Consequently, these findings lend credence to the need for more intensive follow-up and monitoring after primary treatment for older premenopausal patients and those with an advanced stage of EOC. This can be achieved by developing a survival algorithm for triaging affected women to different approaches for immediate and long-term monitoring after completion of their primary treatment. However, a robust multicenter longitudinal study among Black African women is still required to provide additional reliable insight to this information.
ACKNOWLEDGMENT
The authors appreciate the staff of the medical record department attached to the Oncology and Pathology Studies unit of LUTH for their assistance in ensuring the retrieval of the patients’ case records.
DISCLAIMER
The views expressed in this publication are those of the authors and do not necessarily reflect those of Conquer Cancer.
SUPPORT
Supported by the Conquer Cancer International Innovation Grant under Project ID 16576.
AUTHOR CONTRIBUTIONS
Conception and design: Kehinde Sharafadeen Okunade, Adebola A. Adejimi, Ephraim O. Ohazurike, Omolola Salako,Benedetto Osunwusi, Aloy O. Ugwu, Rose I. Anorlu, Jonathan S. Berek
Financial support: Adebola A. Adejimi, Benedetto Osunwusi, Muisi A. Adenekan, Aloy O. Ugwu
Administrative support: Benedetto Osunwusi
Collection and assembly of data: Kehinde Sharafadeen Okunade, Benedetto Osunwusi, Muisi A. Adenekan, Aloy O. Ugwu, Adaiah Soibi-Harry, Olayemi Dawodu, Adeyemi A. Okunowo
Data analysis and interpretation: Kehinde Sharafadeen Okunade, Adebola A. Adejimi, Benedetto Osunwusi, Aloy O. Ugwu, Jonathan S. Berek
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Omolola Salako
Patents, Royalties, Other Intellectual Property: Oncopadi Digital Cancer Clinic
Rose I. Anorlu
Honoraria: Sanofi, Roche
Jonathan S. Berek
Leadership: Oncoquest
Research Funding: Tesaro, Karyopharm Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Yakasai IA, Ugwa EA, Otubu J: Gynecological malignancies in Aminu Kano Teaching Hospital Kano: A 3-year review. Niger J Clin Pract 16:63-66, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Okunade KS, Okunola H, Okunowo AA, et al. : A five-year review of ovarian cancer at a tertiary institution in Lagos, South-West, Nigeria. Niger J Gen Pract 14:23-27, 2016 [Google Scholar]
- 4.Matulonis UA, Sood AK, Fallowfield L, et al. : Ovarian cancer. Nat Rev Dis Primers 2:16061, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Petterson F: Annual Report on the Results of Treatment in Gynecological Cancer, FIGO, Volume 22. Stockholm, International Federation of Gynecology and Obstetrics, 1994 [Google Scholar]
- 8.Vergote I, Tropé CG, Amant F, et al. : Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943-953, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Koshiyama M, Matsumura N, Konishi I: Subtypes of ovarian cancer and ovarian cancer screening. Diagnostics (Basel) 7:12, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koshiyama M, Matsumura N, Konishi I: Recent concepts of ovarian carcinogenesis: Type I and type II. Biomed Res Int 2014:934261, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzuto I, Stavraka C, Chatterjee J, et al. : Risk of Ovarian Cancer Relapse score: A prognostic algorithm to predict relapse following treatment for advanced ovarian cancer. Int J Gynecol Cancer 25:416-422, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.du Bois A, Lück HJ, Meier W, et al. : A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 95:1320-1329, 2003 [DOI] [PubMed] [Google Scholar]
- 13.du Bois A, Weber B, Rochon J, et al. : Addition of epirubicin as a third drug to carboplatin-paclitaxel in first-line treatment of advanced ovarian cancer: A prospectively randomized gynecologic cancer intergroup trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. J Clin Oncol 24:1127-1135, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Pfisterer J, Weber B, Reuss A, et al. : Randomized phase III trial of topotecan following carboplatin and paclitaxel in first-line treatment of advanced ovarian cancer: A gynecologic cancer intergroup trial of the AGO-OVAR and GINECO. J Natl Cancer Inst 98:1036-1045, 2006 [DOI] [PubMed] [Google Scholar]
- 15.du Bois A, Reuss A, Pujade-Lauraine E, et al. : Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer 115:1234-1244, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Whitney CW, Spirtos N: Gynecologic Oncology Group Surgical Procedures Manual. Philadelphia, Gynecologic Oncology Group, 2009 [Google Scholar]
- 17.Kaplan E, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 18.Okunade KS, Adetuyi IE, Adenekan M, et al. : Risk predictors of early recurrence in women with epithelial ovarian cancer in Lagos, Nigeria. Pan Afr Med J 36:272, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winter WE III Maxwell GL Tian C et al. ; Gynecologic Oncology Group Study : Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group Study. J Clin Oncol 25:3621-3627, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Okunade KS, Dawodu O, Adenekan M, et al. : Prognostic impact of pretreatment thrombocytosis in epithelial ovarian cancer. Niger J Clin Pract 23:1141-1147, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristow RE, Tomacruz RS, Armstrong DK, et al. : Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol 20:1248-1259, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hoskins WJ, McGuire WP, Brady MF, et al. : The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 170:974-979, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Makar AP, Baekelandt M, Trope' CG, et al. : The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol 56:175-180, 1995 [DOI] [PubMed] [Google Scholar]
- 24.van Houwelingen JC, ten Bokkel Huinink WW, van der Burg ME, et al. : Predictability of the survival of patients with advanced ovarian cancer. J Clin Oncol 7:769-773, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Chi DS, Liao JB, Leon LF, et al. : Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol 82:532-537, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Schneider D, Halperin R, Halperin D, et al. : Prediction of the survival of patients with advanced ovarian cancer according to a risk model based on a scoring system. Eur J Gynaecol Oncol 19:547-552, 1998 [PubMed] [Google Scholar]
- 27.Klar M, Hasenburg A, Hasanov M, et al. : Prognostic factors in young ovarian cancer patients: An analysis of four prospective phase III intergroup trials of the AGO Study Group, GINECO and NSGO. Eur J Cancer 66:114-124, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Chang C, Chiang AJ, Wang HC, et al. : Evaluation of the time-varying effect of prognostic factors on survival in ovarian cancer. Ann Surg Oncol 22:3976-3980, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Gil-Ibáñez B, Oskay-Özcelik G, Richter R, et al. : Predictive factors in relapsed ovarian cancer for complete tumor resection. Anticancer Res 31:2583-2537, 2011 [PubMed] [Google Scholar]
- 30.Liu XH, Man YN, Wu XZ: Recurrence season impacts the survival of epithelial ovarian cancer patients. Asian Pac J Cancer Prev 15:1627-1632, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Kotsopoulos J, Rosen B, Fan I, et al. : Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol 140:42-47, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Teo MC: Update on the management and the role of intraperitoneal chemotherapy for ovarian cancer. Curr Opin Obstet Gynecol 26:3-8, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Giorda G, Gadducci A, Lucia E, et al. : Prognostic role of bowel involvement in optimally cytoreduced advanced ovarian cancer: A retrospective study. J Ovarian Res 7:72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiliotis J, Halkia E, Lianos E, et al. : Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study. Ann Surg Oncol 22:1570-1575, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Gerestein C, Eijkemans M, de Jong D, et al. : The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG 116:372-380, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Trifanescu OG, Gales LN, Trifanescu RA, et al. : Clinical prognostic factors in pre- and post-menopausal women with ovarian carcinoma. Acta Endocrinol (Buchar) 14:353-359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez A, Ngo C, Leblanc E, et al. : Surgical complexity impact on survival after complete cytoreductive surgery for advanced ovarian cancer. Ann Sur Oncol 23:2515-2521, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Yan XJ, Liang LZ, Zeng ZY, et al. : Recurrence risk factors of platinum-sensitive epithelial ovarian cancer. Ai Zheng 24:751-754, 2005 [PubMed] [Google Scholar]
- 39.Kim SJ, Rosen B, Fan I, et al. : Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer 116:964-971, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricciardi E, Baert T, Ataseven B, et al. : Low-grade serous ovarian carcinoma. Geburtshilfe Frauenheilkd 78:972-976, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopirtean C, Ciuleanu T, Cainap C, et al. : Body mass index as a prognostic factor for disease progression in patients with metastatic colorectal cancer treated with bevacizumab based systematic therapy. Acta Endo (Buc) 13:425-430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song MJ, Lee SH, Choi MR, et al. : Diagnostic value of CA 125 as a predictor of recurrence in advanced ovarian cancer. Eur J Gynaecol Oncol 34:148-151, 2013 [PubMed] [Google Scholar]
- 43.Geisler JP, Miller GA, Lee TH, et al. : Relationship of preoperative serum CA 125 to survival in epithelial ovarian cancer. J Reprod Med 41:140-142, 1996 [PubMed] [Google Scholar]
- 44.Juretzka MM, Barakat RR, Chi DS, et al. : CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecol Oncol 104:176-180, 2007 [DOI] [PubMed] [Google Scholar]