Abstract
Despite improved treatment strategies for multiple myeloma (MM), patient outcomes in low- and middle-income countries remain poor, unlike high-income countries. Scarcity of specialized human resources and diagnostic, treatment, and survivorship infrastructure are some of the barriers that patients with MM, clinicians, and policymakers have to overcome in the former setting. To improve outcomes of patients with MM in Western Kenya, the Academic Model Providing Access to Healthcare (AMPATH) MM Program was set up in 2012. In this article, the program's activities, challenges, and future plans are described distilling important lessons that can be replicated in similar settings. Through the program, training on diagnosis and treatment of MM was offered to healthcare professionals from 35 peripheral health facilities across Western Kenya in 2018 and 2019. Access to antimyeloma drugs including novel agents was secured, and pharmacovigilance systems were developed. Finally, patients were supported to obtain health insurance in addition to receiving peer support through participation in support group meetings. This article provides an implementation blueprint for similar initiatives aimed at increasing access to care for patients with MM in underserved areas.
BACKGROUND
Multiple myeloma (MM) is a disorder characterized by the presence of ≥ 10% clonal bone marrow plasma cells or biopsy-proven bony or extramedullary plasmacytoma plus any myeloma-defining event (hypercalcemia, renal insufficiency, anemia, or bone lesions) or any of the biomarkers of malignancy, namely ≥ 60% clonal bone marrow plasma cells, involved to uninvolved free light chain ratio ≥ 100, or > 1 focal lesion on MRI.1 In 2018, a total of 159,985 new cases were reported globally corresponding to an age-standardized incidence rate of 2.1/100,000 persons.2 In Kenya, a lower age-standardized incidence rate of 1.3/100,000 (607 new cases) was recorded in 20183 despite research showing that MM has a higher preponderance among persons of African descent as compared with other ethnicities.4 A low diagnostic capacity is thought to be a contributing factor to this observation in Kenya and other countries of the low-middle sociodemographic index.5
CONTEXT
Key Objective
How can access to multiple myeloma (MM) care in low- and middle-income countries be improved?
Knowledge Generated
The Academic Model Providing Access to Healthcare (AMPATH) MM Program was established through a unique partnership of a Kenyan tertiary hospital and medical school with an American medical school and a leading pharmaceutical company.
The program strengthened providers' capacity to offer MM care through training, secured drug availability, and safety while assisting patients to enroll for insurance and offered peer support for psychosocial coping.
Relevance
Enhanced access to MM care, patient safety, and survivorship services are achievable in low- and middle-income countries through optimizing the abilities of the local healthcare workforce using expertise and material support from high-income countries.
There has been a steady improvement in treatment outcomes over the past 2 decades owing to the use of novel therapies with autologous stem-cell transplant. However, these benefits are yet to be seen in low- and middle-income countries (LMICs) where autologous stem-cell transplant is not available and access to new drugs is limited. The median overall survival reported from a Ghanaian tertiary hospital between 2004 and 2016 at 33 months pales in comparison with the median overall survival of 88.2 months documented in a multicenter registry study in the United States from 2009 to 2016.6,7 Inadequate access to pathologists and hemato-oncologists further aggravates the suboptimal outcomes observed in sub-Saharan African countries.8
Some of the steps taken to improve MM care in Kenya include the introduction of an oncology cover under the National Hospital Insurance Fund (NHIF) and the development of national guidelines.9,10 However, templates for successful implementation of healthcare professional (HCP) training, delivery of comprehensive treatment, drug safety, and survivorship services for MM are lacking. The Academic Model Providing Access to Healthcare (AMPATH) MM Program was established in 2012 through the support of Celgene Corporation to provide comprehensive care to patients with MM in Western Kenya. This article will discuss the program's activities, challenges, and future considerations under three themes, namely training and awareness creation, treatment and pharmacovigilance (PV), and survivorship support.
CURRENT MM CARE INFRASTRUCTURE IN KENYA
Kenya is a lower middle-income country in sub-Saharan Africa with a population of more than 47 million people11 and a developing healthcare system. Healthcare services in Kenya are provided by public, private-for-profit, and private-not-for-profit institutions with public facilities catering for more than half of outpatient visits and admissions.12 Out-of-pocket payments constituted a quarter of Kenya's total health expenditure with government and donors catering for the balance in 2015/2016.13 This presents a barrier to access to health care for a large section of the population.12 Indeed, 84% of Kenyan workers are in the informal sector, which is characterized by low irregular incomes.14 This is aggravated by the low health insurance uptake of 17% of households (2013 estimate); the National Hospital Insurance Fund (NHIF), a publicly owned social insurance fund, was subscribed to in 88% of covered households.12 For cancer care, NHIF cover provides up to six cycles of first-line chemotherapy treatment at $250 in US dollars (USD) per cycle and up to four cycles of second-line chemotherapy at $1,500 USD per cycle. Routine laboratory tests, selected imaging tests, and surgical services are also covered.9
There are only two public and a few private tertiary hospitals that can provide what is closest to comprehensive myeloma care in Kenya. These form the apex of the referral system in Kenya. A network of tertiary, secondary, and primary health facilities refers patients to the tertiary centers for appropriate diagnosis, treatment, and follow-up.
Hemato-oncologists, pathologists, and other clinicians trained in the care of MM are few and concentrated in urban centers including where the tertiary facilities are located.15 In Kenya, both lenalidomide and bortezomib, two important MM therapeutics, are registered. This however does not translate to patient access because of high costs. Other antimyeloma therapies available in Kenya include thalidomide, cyclophosphamide, melphalan, dexamethasone, and prednisolone.
Stigma and low awareness of cancer further impede access to lifesaving care.16 Besides, psychosocial support structures for cancer survivors are not well-developed in LMICs such as Kenya.17,18 Patients with MM therefore are forced to undertake a difficult journey characterized by late diagnosis, suboptimal treatment, and psychosocial distress resulting in poor outcomes.
AMPATH MM PROGRAM
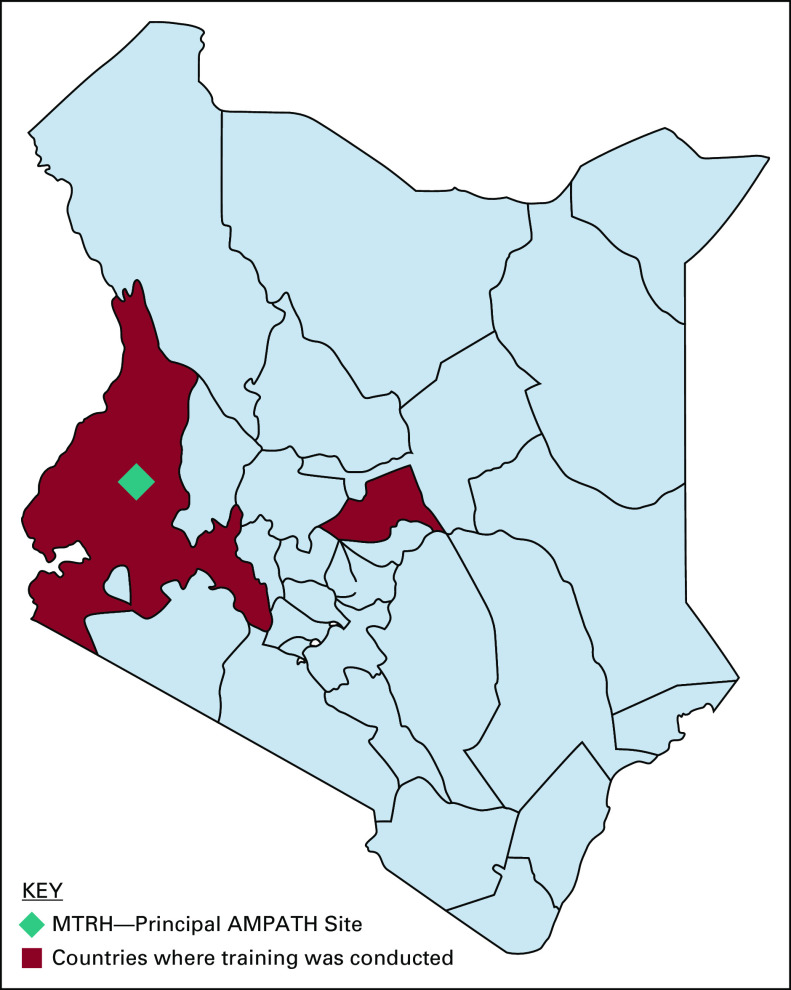
The Academic Model Providing Access to Healthcare (AMPATH) is a collaboration between Moi University School of Medicine, Moi Teaching and Referral Hospital (MTRH), the second largest national referral hospital in Kenya, and North American academic institutions led by Indiana University School of Medicine. MTRH, a principal AMPATH site, is located in Eldoret town of Uasin Gishu County and serves Western Kenya—a region of approximately 24 million residents (Fig 1).
FIG 1.
Geographic extent of training activities of the AMPATH MM Program across Western Kenya in 2018 and 2019. AMPATH, Academic Model Providing Access to Healthcare; MM, multiple myeloma; MTRH, Moi Teaching and Referral Hospital.
Initially, AMPATH focused on HIV care,19 but over the years, it has broadened its services to include primary health care and chronic disease care including diabetes, hypertension, and cancer. The latter is made possible through the AMPATH Oncology Institute (AOI). AOI has experienced periodic strategic structuring aimed at being a comprehensive cancer center.20 Various programs have been developed under the umbrella of AOI to attend to unique cancer types, and hence, the establishment of the AMPATH MM Program mandated to find, link, and retain patients with MM in care.
The program was established in 2012 in an already existing infrastructure of the AOI. MTRH was an ideal site for improving MM care because it was a referral hospital with specialized facilities (personnel and material) including clinical oncology, pathology, oncology pharmacy,21 and a health information system necessary for cancer care and was already attending to patients with MM. Faculty from Indiana University not only provided clinical care to patients with MM but also provided training to local clinicians and were key in founding the program particularly by leveraging their relationships with Celgene Corporation, a leading pharmaceutical company that specializes in developing MM therapeutics, to mobilize resources. Administrative support—human resource management, financial management, regulatory compliance, monitoring and evaluation, and procurement of supplies—was implemented through Moi University.19
At initiation, the program staff consisted of a hematologist, a pharmacist, a pharmacist assistant, and a nurse. As the number of patients grew, a pathologist, a physician assistant, and a social worker were added to the staff.
TRAINING AND AWARENESS CREATION
Before the inception of the program, only 12 patients with MM sought care at MTRH in 2012. It was felt that awareness of MM was low among local HCPs. Steps were taken to sensitize and train HCPs and medical students in Western Kenya to increase their index of suspicion for MM and expand the pool of professionals capable of diagnosing, referring, and providing definitive and supportive care using available resources. HCPs from referring primary, secondary, and tertiary facilities (public and private) in neighboring and distant counties without comprehensive cancer treatment facilities received training (Fig 1). Referring hospitals were targeted because they are closer to patients' homes and are therefore the earliest point of healthcare contact. Additionally, patients depend on these facilities for emergent healthcare needs making them important partners in MM care delivery.
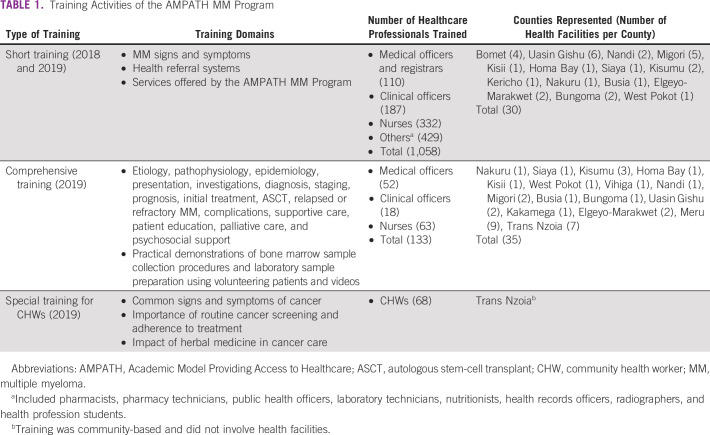
Training sessions were offered in two main formats. Short training sessions lasting between 1 and 2 hours were done during weekly continuous medical education meetings in a total of 30 peripheral health facilities in 2018 and 2019. The short training aimed to create awareness on topical aspects of MM. Following the short training, key personnel involved in MM care delivery (serving in internal medicine, renal, or orthopedic units) were then selected to attend comprehensive training at a central site. Three comprehensive training sessions of between 1 and 2 days were held at MTRH and two central sites in Meru and Trans Nzoia counties in 2019. HCP attendance of the comprehensive training sessions was 98%, 81%, and 80% of those invited for the three sites, respectively. Details of the training domains covered under short or comprehensive training, number of HCPs trained, and the geographic distribution of the peripheral health facilities they represented can be found in Table 1. All training activities were planned and executed successfully according to annual programmatic budgetary allowances.
TABLE 1.
Training Activities of the AMPATH MM Program
In 2019, a total of 68 community health workers (CHWs) from Trans Nzoia County were sensitized on common signs and symptoms of cancers, the importance of routine cancer screening, adhering to the treatment plan, and the impact of herbal medicine in cancer care. CHWs are an important part of patient support at the community level contributing to the effective linkage of patients to treatment and long-term follow-up.
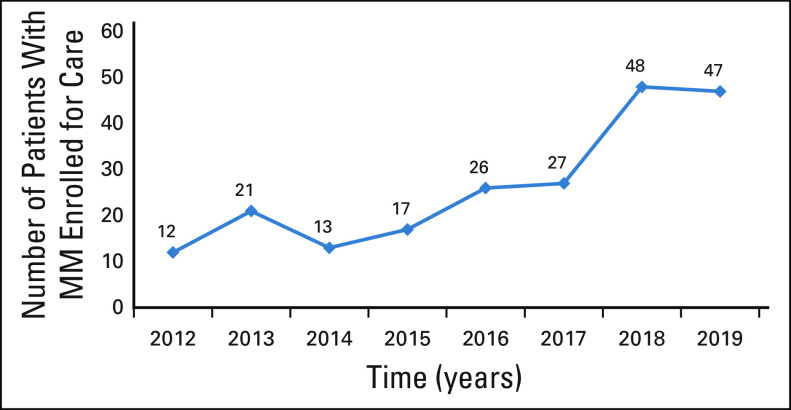
As a result of the training activities of the program, an upward trend of new patients with MM being enrolled for care at MTRH was documented (Fig 2). Improved care at the peripheral health facilities has also been observed. For instance, patients from the neighboring counties of Trans Nzoia, Kakamega, Kisumu, and Nakuru are currently able to receive weekly bortezomib injections from their respective county referral hospitals eliminating transport costs to MTRH for the same and only traveling every month for specialist assessment and to receive their refill of other oral antimyeloma drugs.
FIG 2.
The number of newly diagnosed patients with MM enrolled for care annually by the AMPATH MM Program. AMPATH, Academic Model Providing Access to Healthcare; MM, multiple myeloma.
The program plans to introduce better-structured training in which the impact of training will be evaluated to facilitate continuous improvement. To reach a wider audience, the program intends to use teleconferencing facilities in the future.
PATIENT ENROLLMENT
Patients suspected to have MM by the clinical team were supported to receive diagnostic tests majorly bone marrow examination, serum protein electrophoresis, and serum-free light chain, the latter two being acquired from commercial laboratories. Those confirmed to have symptomatic MM according to the prevailing International Myeloma Working Group (IMWG) criteria were assisted to obtain NHIF cover and have treatment initiated.22,1 The program social worker performed socioeconomic assessments ensuring that support offered was commensurate to individual patient incomes. Some patients received transport reimbursement to attend routine clinics. These activities were crucial to speeding up the diagnosis process and avoiding loss to follow-up as a result of unaffordability of care.
TREATMENT AND PV
The availability of MM chemotherapeutics was a challenge at the start of the program. Thalidomide had not been authorized to be used at MTRH by the Pharmacy and Poisons Board before 2012 because structures to safeguard its safe use had not been developed. The few patients seen at the time were prescribed for melphalan/prednisolone (MP), which they had to purchase at local community pharmacies out-of-pocket because of frequent supply interruptions at MTRH. Most community pharmacies in Western Kenya did not stock cancer chemotherapeutic agents, and patients had to pay up front before a special order was made. Costs were relatively high; for instance, melphalan per cycle would cost $60 USD, greatly affecting adherence.
In 2012, the program prioritized the acquisition of thalidomide over melphalan because of its lower propensity to cause myelosuppression.23 Lenalidomide was later availed in 2017, but because of cost implications, its use was limited to the second-line regimen or as a maintenance treatment.
To avoid embryofetal exposure to thalidomide and later lenalidomide, PV measures were put in place. A Risk Evaluation and Management Strategy (REMS) was developed with the support of Celgene Corporation. Safety pamphlets were developed and used to educate HCPs and patients on safety considerations for thalidomide/lenalidomide. This was then followed by training of HCPs at MTRH on the required safety measures when prescribing and dispensing thalidomide/lenalidomide. A nurse and a pharmacist assistant were assigned the role of offering patient education and obtaining consent before initiation of treatment and routinely before the refilling of prescriptions. Access to these agents was strictly restricted only to HCPs registered under the REMS. Patients were provided with appropriate contraception throughout thalidomide/lenalidomide treatment. Residual supplies of these drugs were retrieved from close contacts of deceased patients to avoid cases of accidental poisoning.
Adverse drug reaction monitoring was also done through phone call follow-ups and timely management offered in consultation with hemato-oncologists. These PV activities by the program enforced treatment adherence. No incidences of embryofetal malformations were reported during the lifetime of the program. Common adverse drug reactions reported can be found in a published research abstract.24 Maintaining awareness of the risks of thalidomide/lenalidomide remains a constant effort as new HCPs become part of the staff complement at MTRH and because of the human tendency to exercise less vigilance over time. Transitioning into a single electronic documentation system for REMS registration of HCPs, patients, and the medication-use process is the next step for task reduction and improved accountability.
Cyclophosphamide and zoledronic acid were made available in 2016 and later bortezomib in 2017 by the program. Because of cost reasons, bortezomib was initially reserved for relapsed MM after treatment with MP, thalidomide/dexamethasone, or thalidomide/cyclophosphamide/dexamethasone (TCD). These drugs were later absorbed into the MTRH formulary, and bortezomib was deployed as part of first-line regimens in select patients when the National Hospital Insurance Fund (NHIF) introduced a special oncology cover in 2017.9 The new oncology cover brought a huge relief as the cost of treatment was greatly reduced. Despite this, some patients who were receiving treatment were left to contend with treatment interruptions once their NHIF cover was exhausted. To cushion them, the program provided chemotherapy support necessary to achieve an acceptable intensity of treatment.
Staging using the International Staging System (ISS)25 and systematic monitoring of response to treatment according to IMWG criteria26 could not be done at MTRH as a result of high costs of β2 microglobulin, protein electrophoresis (serum and urine), immunofixation (serum and urine), and serum-free light chain tests, only obtainable from commercial laboratories during the program lifetime. Clinical recovery and occurrence of toxicity were often used to assess response to therapy. It was not uncommon for patients on MP or thalidomide/dexamethasone to receive ten or more cycles of initial treatment before toxicity set in, at which point treatment would be stopped until clinical relapse when treatment would be restarted.
After 2017, there was an increase in the use of serum M-protein to assess response; patients achieving undetectable levels of serum M-protein from initial elevated levels were considered to be in remission and were given either a treatment holiday or put on maintenance therapy if funds were available. Patients receiving bortezomib-based triple-therapy regimens were observed to achieve remission and/or clinical recovery after about four cycles, which was then adopted as the minimum threshold for initial treatment for patients on these regimens. Most patients on triple therapies were however provided with around six cycles of chemotherapy, which was optimal given the available resources.
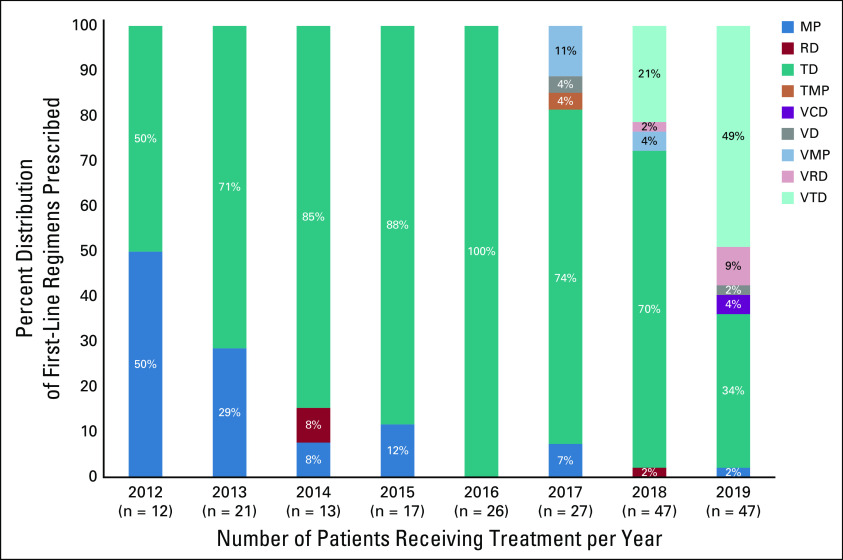
To standardize care, a combined effort from the program with the hemato-oncology, pathology, social work, and palliative care components at MTRH resulted in the development of a local protocol for MM management in March 2019. Triple therapy with a backbone of bortezomib was adopted as first-line treatment for newly diagnosed patients with MM without complications. Thanks to program initiatives (drug acquisition, PV systems, and local protocol development) and NHIF coverage, there has been a gradual increase in the use of immunomodulatory agents, proteasome inhibitors, and triple regimens as part of first-line therapies over the years (Fig 3).
FIG 3.
The trend of regimens prescribed as a first-line treatment between 2012 and 2019. MP, melphalan/prednisolone; RD, lenalidomide/dexamethasone; TD, thalidomide/dexamethasone; TMP, thalidomide/melphalan/prednisolone; VCD, bortezomib/cyclophosphamide/dexamethasone; VD, bortezomib/dexamethasone; VMP, bortezomib/melphalan/prednisolone; VRD, bortezomib/lenalidomide/dexamethasone; VTD, bortezomib/thalidomide/dexamethasone.
Sustainable provision of uninterrupted access to initial standard triple therapy and maintenance therapy for each newly diagnosed patient with MM is yet to be achieved. Moreover, options for second and subsequent lines of therapy remain limited because of the unavailability of newer-generation antimyeloma drugs such as pomalidomide, carfilzomib, and daratumumab. The program together with other stakeholders continues to search for mechanisms to minimize treatment interruptions while expanding the therapeutic arsenal for refractory or relapsed MM and toxicity-prone patients.
SURVIVORSHIP SUPPORT
Over time, the need to provide psychosocial support for patients with MM was felt, leading to the formation of a support group for patients and caregivers. Patients and caregivers received health education and psychologic counseling and were able to share their experiences through treatment during the meetings. From 2018 to 2019, four major meetings were held at MTRH where 35 to 100 participants were invited for each meeting. Attendance was encouraging, ranging from 92% to 100%. With a rising demand for support group activities and the high cost of organizing centralized meetings, schedules were developed to facilitate decentralized meetings in various regions.
Abandonment of treatment was also a common occurrence in the years before the program. The program facilitated community outreach done in the form of home visits where family health education and psychologic counseling were carried out to reestablish follow-up. A total of 10 MM patient homes were visited between 2012 and 2019. It was discovered that treatment adverse effects, lack of family support, lack of funds for transport to the hospital, and the desire to pursue alternative medicine were factors that contributed to treatment abandonment.
DISCUSSION
Training of less specialized HCPs to perform simple cancer diagnostic procedures has been advocated for as a means to increase access to diagnostic services for underserved populations in Kenya.15 This form of task-shifting has shown benefits in the diagnosis of Kaposi's sarcoma in HIV clinics across East Africa.27 Regular mentorship after initial training is however required to maintain a high level of service quality. In India, a program to improve emergency obstetric and neonatal care of staff nurses and CHWs through mentorship by nurse mentors showed that the ability to provide correct actions declined over time after initial training.28 This underscores the need for continuous education, which telehealth can effectively address. Unlike traditional training programs where the physical presence of participants is required, telehealth leverages internet technology to virtually connect specialists in academic medical centers to primary care providers in distant areas facilitating specialist skills transfer.29
The noncomprehensive coverage of cancer care by NHIF, as observed at MTRH, has been suggested to contribute to poor outcomes according to a Kenyan qualitative study.30 In an American registry study, limited access to treatment as a result of inadequate insurance coverage was found to be a contributing factor to lower survival among patients with non–Hodgkin's lymphoma.31 For Kenyan patients with MM, the current inadequate NHIF coverage, therefore, portends less-than-optimal survival outcomes. Nonetheless, having insurance coverage is better than being uncovered. In one study conducted on a cohort of patients with pediatric cancer in a Kenyan academic hospital, it was revealed that patients with insurance had greater event-free survival, lower progression, and a lower treatment abandonment rate.32 Patients with MM should therefore be encouraged to take up insurance regardless.
Successful REMS implementation in a resource-constrained setting is atypical. In LMICs, where unlimited access to cancer treatment is yet to be achieved, PV is rarely prioritized.33 In Kenya, efforts to decentralize cancer care to peripheral health facilities34 should be accompanied by REMS capacity building to ensure safety in using medications with risks of serious toxicity. In this pursuit, HCP societies can be valuable partners in the dissemination of safety information.35 On the other hand, settings with robust PV systems can achieve a reduction of the administrative burden of REMS by avoiding duplication of safety measures that are part of routine clinical practice.35
From the MTRH experience, it is worth noting that equipping public hospitals with MM diagnostics is the next logical step in facilitating quicker MM diagnoses at a lower cost. This will also open the door for implementation of guideline-recommended staging25 and response measurement,26 therefore enabling clinicians to determine the effectiveness of therapy and to avoid exposing patients with MM to toxicity from prolonged aggressive therapy.
The positive response to support group activities by participants in the AMPATH MM Program may reflect an improved quality of life. In point of fact, psychosocial support groups have been demonstrated to improve the four domains of quality of life, namely physical, psychologic, social, and spiritual among cancer survivors in a mixed qualitative/quantitative study conducted in Kenya.36 The authors, therefore, advocate for the formation of support groups in all cancer centers in LMICs where such facilities have been reported to be scarce.17,18
In conclusion, this paper has highlighted elements that contributed to the success of the AMPATH MM Program in Western Kenya. Key among these were collaboration among stakeholders and development partners, capacity building for local HCPs, prioritization of patient safety, and provision of survivorship services. Lessons derived from program implementation have been summarized as recommendations in Table 2.
TABLE 2.
Recommendations for Establishing a MM Program in a Resource-Constrained Setting

ACKNOWLEDGMENT
We are thankful to the leadership of MTRH Directorate of Hemato-Oncology, Dr Naftali Busakhala and Dr Jesse Opakas, for supporting the AMPATH MM Program. We acknowledge Dr Evangeline Njiru, Dr Beatrice Melly, Dr Nicolus Kisilu, Dr Cosmas Sang, Dr Carole Kilach, Dr Linet Kugo, Dr Paul Wasike, Mr Elvis Obura, Ms Truphena Komen, Ms Joyce Chesum, Ms Diana Flora, and Ms Yvette Oyolo for providing clinical care and organizational support. The authors also acknowledge Celgene Corporation, Indiana University, and Moi University for offering financial and administrative support to the AMPATH MM Program.
PRIOR PRESENTATION
Presented at the Virtual Kenya International Cancer Conference by the Kenya Society of Haematology and Oncology on November 21, 2020.
SUPPORT
The AMPATH Multiple Myeloma Program was supported by Celgene Corporation and Indiana University.
AUTHOR CONTRIBUTIONS
Conception and design: Mercy A. Oduor, Teresa C. Lotodo, Terry A. Vik, Kelvin M. Manyega, Fredrick C. Asirwa
Administrative support: Teresa C. Lotodo, Patrick Loehrer, Austin A. Omondi, John O. Oguda, Fredrick C. Asirwa
Financial support: Terry A. Vik
Provision of study materials or patients: Austin A. Omondi, Fredrick C. Asirwa
Collection and assembly of data: Mercy A. Oduor, Austin A. Omondi, John O. Oguda, Fredrick C. Asirwa
Data analysis and interpretation: Mercy A. Oduor, Terry A. Vik, Patrick Loehrer, John O. Oguda, Fredrick C. Asirwa
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Terry A. Vik
Research Funding: Takeda, Bristol-Myers Squibb Foundation
Kelvin M. Manyega
Research Funding: Celgene
Patrick Loehrer
Research Funding: Novartis, Lilly Foundation, Taiho Pharmaceutical
Patents, Royalties, Other Intellectual Property: US PPA/61/499,988 Gene Expression Analysis of Thymic Neoplasms Inventors: Sunil Badve, Yesim Gokmen-Polar, Patrick Loehrer
Fredrick C. Asirwa
Research Funding: Celgene, Takeda, Lilly, Bristol-Myers Squibb, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rajkumar S, Dimopoulos M, Palumbo A, et al. : International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538-e548, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Global Cancer Observatory : Kenya Fact Sheet. Lyon, France, International Agency for Research on Cancer, 2018 [Google Scholar]
- 4.Waxman A, Mink P, Devesa S, et al. : Racial disparities in incidence and outcome in multiple myeloma: A population-based study. Blood 116:5501-5506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan AJ, Allen C, Barac A, et al. : Global burden of multiple myeloma: A systematic analysis for the Global Burden of Disease Study 2016. JAMA Oncol 4:1221-1227, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acquah M, Hsing A, McGuire V, et al. : Presentation and survival of multiple myeloma patients in Ghana: A review of 169 cases. Ghana Med J 53:52-58, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ailawadhi S, Jagannath S, Lee H, et al. : Association between race and treatment patterns and survival outcomes in multiple myeloma: A Connect MM Registry analysis. Cancer 126:4332-4340, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makau-Barasa L, Greene S, Othieno-Abinya N, et al. : Improving access to cancer testing and treatment in Kenya. J Glob Oncol 4:1-8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Hospital Insurance Fund : Financial Report for the Year 2017/2018. Nairobi, Kenya, National Hospital Insurance Fund, 2018 [Google Scholar]
- 10.Ministry of Health : Kenya National Cancer Treatment Protocols. Nairobi, Kenya, Government of Kenya, 2019 [Google Scholar]
- 11.Kenya National Bureau of Statistics : 2019 Kenya Population and Housing Census. Nairobi, Kenya, Government of Kenya, 2019 [Google Scholar]
- 12.Ministry of Health : 2013 Kenya Household Health Expenditure and Utilisation Survey. Nairobi, Kenya, Government of Kenya, 2014 [Google Scholar]
- 13.Ministry of Health : Kenya National Health Accounts FY 2015/16. Nairobi, Kenya, Government of Kenya, 2019 [Google Scholar]
- 14.Andersen K. (ed): Kenya Labour Market Profile 2020. Copenhagen, Denmark, Danish Trade Union Development Agency, Analytical Unit, 2020 [Google Scholar]
- 15.Topazian H, Cira M, Dawsey S, et al. : Joining forces to overcome cancer: The Kenya cancer research and control stakeholder program. J Cancer Policy 7:36-41, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisiangani J, Baliddawa J, Marinda P, et al. : Determinants of breast cancer early detection for cues to expanded control and care: The lived experiences among women from Western Kenya. BMC Womens Health 18:81, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutebi M, Edge J: Stigma, survivorship and solutions: Addressing the challenges of living with breast cancer in low-resource areas. S Afr Med J 104:383, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Wambalaba FW, Son B, Wambalaba AE, et al. : Prevalence and capacity of cancer diagnostics and treatment: A demand and supply survey of health-care facilities in Kenya. Cancer Control 26:1-12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einterz R, Kimaiyo S, Mengech H, et al. : Responding to the HIV pandemic: The power of an academic medical partnership. Acad Med 82:812-818, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Strother RM, Asirwa FC, Busakhala NB, et al. : The evolution of comprehensive cancer care in western Kenya. J Cancer Policy 1:e25-e30, 2013 [Google Scholar]
- 21.Strother RM, Rao KV, Gregory KM, et al. : The oncology pharmacy in cancer care delivery in a resource-constrained setting in Western Kenya. J Oncol Pharm Pract 18:406-416, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Kyle R, Rajkumar S: Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23:3-9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig H, Hajek R, Tothova E, et al. : Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood 113:3435–3442, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kugo L, Oduor M, Chite F: A pharmacy-led pharmacovigilance program for multiple myeloma patients at the Moi Teaching and Referral Hospital: A five-year experience. J Oncol Pharm Pract 25, 2019. (suppl; abstr 039) [Google Scholar]
- 25.Greipp P, San Miguel J, Durie B, et al. : International Staging System for multiple myeloma. J Clin Oncol 23:3412-3420, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Paiva B, Anderson K, et al. : International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328-e346, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Laker-Oketta M, Wenger M, Semeere A, et al. : Task shifting and skin punch for the histologic diagnosis of Kaposi's sarcoma in sub-Saharan Africa: A public health solution to a public health problem. Oncology 89:60-65, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao K, Srivastava S, Warren N, et al. : Where there is no nurse: An observational study of large-scale mentoring of auxiliary nurses to improve quality of care during childbirth at primary health centres in India. BMJ Open 9:e027147, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yennurajalingam S, Amos CE, Weru J, et al. : Extension for community healthcare outcomes-palliative care in Africa program: Improving access to quality palliative care. J Glob Oncol 5:1-8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makau-Barasa LN, Greene S, Othieno-Abinya NA, et al. : A review of Kenya's cancer policies to improve access to cancer testing and treatment in the country. Health Res Policy Sys 18:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulte D, Jansen L, Brenner H: Survival disparities by insurance type for patients aged 15-64 years with non-Hodgkin lymphoma. Oncologist 20:554-561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olbara G, Martijn HA, Njuguna F, et al. : Influence of health insurance status on childhood cancer treatment outcomes in Kenya. Support Care Cancer 28:917-924, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Elshafie S, Zaghloul I, Roberti AM: Pharmacovigilance in developing countries (part I): Importance and challenges. Int J Clin Pharm 40:758-763, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Ministry of Health, Kenya : National Cancer Control Strategy 2017-2022. Nairobi, Kenya, Government of Kenya, 2017 [Google Scholar]
- 35.Frame JN, Jacobson JO, Vogel WH, et al. : Assessment of risk evaluation and mitigation strategies in oncology: Summary of the Oncology Risk Evaluation and Mitigation Strategies Workshop. J Oncol Pract 9:e24-e39, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wafula EN: Contribution of psychosocial support group in improving quality of life among female breast cancer patients in Faraja, Nairobi County-Kenya [master's thesis]. Masinde Muliro University of Science and Technology, Kakamega, Kenya, 2017 [Google Scholar]