Background:
Although persistent opioid use after surgery, including cesarean birth, has been described, the risk for overdose and other serious opioid-related events (SOREs) after childbirth, specifically vaginal birth, remains unclear (1, 2).
Objective:
To assess risk for SOREs associated with postpartum opioid prescribing after childbirth, including both vaginal and cesarean births.
Methods and Findings:
We studied women aged 15 to 44 years enrolled in Tennessee Medicaid (TennCare) who were discharged after childbirth between 1 January 2007 and 20 August 2014. TennCare data were supplemented with birth certificate information and hospital discharge data from the Tennessee Hospital Discharge Data System. This study was approved by the institutional review boards of Vanderbilt University Medical Center and the Tennessee Department of Health and by the Division of TennCare.
We included women enrolled in TennCare from 180 days before birth through 41 days after hospital discharge who had no history of at least opioid prescription or opioid use disorder 180 days through 3 days before birth (baseline/antepartum period). The study exposure was the number of opioid prescriptions filled (1, 2, or ≥3) during the postpartum period (3 days before birth to 41 days after hospital discharge). Follow-up began on postdischarge day 42 and continued through the earliest of 365 days of follow-up, loss of enrollment, death, or achievement of our SORE outcome (a composite of persistent opioid use, opioid use disorder diagnosis, buprenorphine or methadone prescription fill, opioid overdose diagnosis, or opioid-related death). Persistent opioid use was defined as filling greater than a 90-day supply of opioids within a 180-day window during follow-up (excluding the postpartum exposure prescriptions), with no gaps in supply greater than 30 days.
We used adjusted Cox regression models stratified by birth type to examine the relationship between postpartum opioid prescription exposure and time to SORE, with adjustment for study covariates that could confound the relationship (Table). Multiple imputation addressed missing covariate values (<4% of total observations). We obtained separate estimates for birth type by including an interaction term in the regression model. Because a woman could contribute more than 1 birth in our analyses, we also accounted for the resulting lack of independence using the Huber-White variance estimation and calculated robust SEs for our model estimates. Analyses were performed using Stata, version 15.1 (StataCorp).
Table.
Risk for SOREs, by Number of Postpartum Prescriptions and Stratified by Delivery Type
| Outcome, by Delivery Type | No Prescription | 1 Prescription | 2 Prescriptions | ≥3 Prescriptions |
|---|---|---|---|---|
| All births, n (%) | 65 331 (31.2) | 112 951 (54.0) | 22 676 (10.8) | 8257 (4.0) |
| SORE, n | 613 | 1780 | 1182 | 1007 |
| Rate per 1000 PYs (95% CI) | 11.3 (10.5–12.3) | 19.7 (18.8–20.7) | 66.5 (62.8–70.3) | 160.0 (150.4–170.2) |
| Adjusted HR (95% CI)* | Reference | 1.4 (1.3–1.6) | 3.6 (3.2–4.0) | 7.0 (6.3–7.9) |
| SORE (excluding persistent opioid prescriptions), n | 289 | 671 | 278 | 178 |
| Rate per 1000 PYs (95% CI) | 5.3 (4.8–6.0) | 7.4 (6.9–8.0) | 15.6 (13.9–17.6) | 28.3 (24.4–32.8) |
| Adjusted HR (95% CI)* | Reference | 1.1 (0.9–1.3) | 1.6 (1.3–2.0) | 2.7 (2.1–3.4) |
| Vaginal births, n (%) | 59 392 (41.1) | 70 122 (48.5) | 11 411 (7.9) | 3763 (2.6) |
| SORE, n | 540 | 1217 | 694 | 522 |
| Rate per 1000 PYs (95% CI) | 11.0 (10.1–11.9) | 21.8 (20.6–23.1) | 78.1 (72.5–84.1) | 181.7 (167.1–197.6) |
| Adjusted HR (95% CI) | Reference | 1.5 (1.3–1.7) | 3.7 (3.3–4.2) | 7.2 (6.3–8.2) |
| SORE (excluding persistent opioid prescriptions), n | 246 | 455 | 164 | 98 |
| Rate per 1000 PYs (95% CI) | 5.0 (4.4–5.7) | 8.1 (7.4–8.9) | 18.4 (15.8–21.5) | 34.4 (28.2–41.9) |
| Adjusted HR (95% CI)* | Reference | 1.1 (0.9–1.3) | 1.8 (1.5–2.2) | 2.9 (2.3–3.8) |
| Cesarean births, n (%) | 5939 (9.2) | 42 829 (66.4) | 11 265 (17.5) | 4494 (7.0) |
| SORE, n | 73 | 563 | 488 | 485 |
| Rate per 1000 PYs (95% CI) | 15.2 (12.1–19.1) | 16.4 (15.1–17.8) | 54.8 (50.2–59.9) | 140.9 (128.9–154.0) |
| Adjusted HR (95% CI) | Reference | 1.2 (0.9–1.5) | 2.9 (2.2–3.6) | 5.9 (4.6–7.6) |
| SORE (excluding persistent opioid prescriptions), n | 43 | 216 | 114 | 80 |
| Rate per 1000 PYs (95% CI) | 8.9 (6.6–12.0) | 6.3 (5.5–7.2) | 12.8 (10.7–15.4) | 23.2 (18.7–28.9) |
| Adjusted HR (95% CI)* | Reference | 0.7 (0.5–1.0) | 1.0 (0.7–1.4) | 1.5 (1.03–2.5) |
| Sensitivity analyses (all births)† | ||||
| Including nonopioid deaths in outcome | Reference | 1.4 (1.2–1.6) | 3.5 (3.2–3.9) | 6.9 (6.2–7.7) |
| Excluding women who filled single opioid prescription during pregnancy | Reference | 1.5 (1.3–1.7) | 3.7 (3.3–4.2) | 7.6 (6.7–8.7) |
| Excluding complicated births | Reference | 1.6 (1.4–1.8) | 3.8 (3.4–4.4) | 8.0 (7.0–9.2) |
| Excluding women without enrollment for 1 y before birth | Reference | 1.5 (1.3–1.8) | 3.1 (2.6–3.7) | 6.1 (5.0–7.4) |
HR = hazard ratio; PY = person-year; SORE = serious opioid-related event.
Adjusted estimates were obtained from Cox regression models that adjusted for age, parity, income, distance to the birth hospital, race, ethnicity, tobacco use, rurality, Tennessee region, delivery year, severe maternal morbidity, perineal lacerations, bilateral tubal ligation, filling 1 opioid prescription during pregnancy, and conditions precluding nonsteroidal anti-inflammatory drug use (e.g., chronic kidney disease). Models also adjusted for mental health medications, mental health conditions, pain-related diagnoses, and procedures; these conditions were measured during both the antepartum and postpartum periods. To impute missing data for age, parity, income, and distance to the birth hospital, we used multiple imputation by chained equations and 10 imputed data sets (mi impute command in Stata). We assessed the fulfillment of the proportional hazards assumption through examination of Schoenfeld residuals and log-log plots.
Five sensitivity analyses were performed for the following scenarios: including 91 nonopioid deaths in the composite outcome, excluding 3166 women with persistent opioid use from the primary outcome, excluding women who filled a single opioid prescription during pregnancy (total n = 180 959), excluding complicated births (n = 165 640), and excluding women without enrollment for 1 year before birth (n = 103 335). The data presented are HRs and 95% CIs.
Among 209 215 births to 161 318 women (69.2% vaginal births and 30.8% cesarean births) that met inclusion criteria, 59% of vaginal births and 91% of cesarean births involved filling of 1 or more opioid prescriptions; 10.5% and 24.4%, respectively, involved filling of a second postpartum opioid prescription. We identified SOREs in 4582 women (27.2 per 1000 person-years) (persistent opioid use [69.1%], substance use disorder diagnosis [18.5%], buprenorphine or methadone prescription fill [10.1%], opioid overdose [2.1%], and opioid-related death [0.2%]).
The covariate-adjusted SORE rate increased with increasing number of postpartum opioid prescriptions (Figure). Similar patterns were demonstrated in the analysis by birth route (P for interaction = 0.173). Our findings were robust to the multiple sensitivity analyses performed (Table).
Figure.
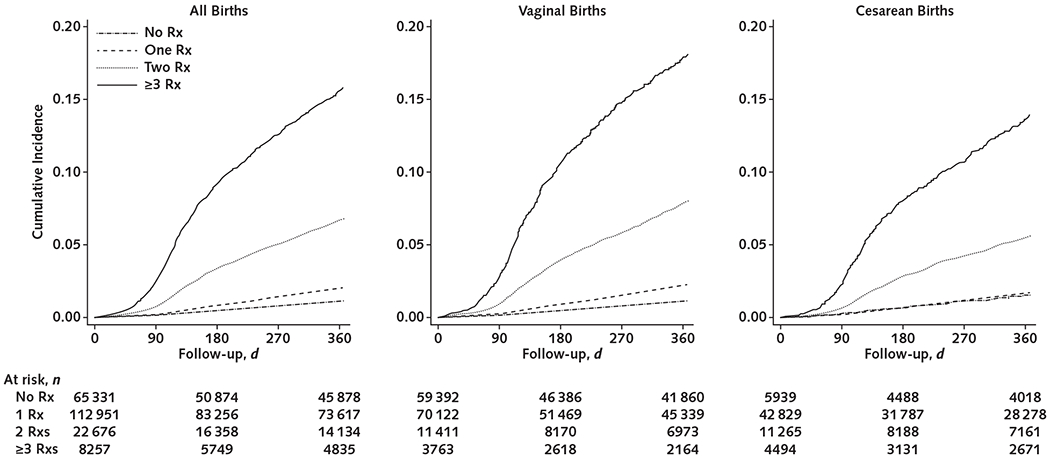
Adjusted cumulative incidence of serious opioid-related events.
Rx = prescription.
Discussion:
We found that compared with not filling prescriptions, filling opioid prescriptions in the postpartum period was strongly associated with increased risk for SOREs during the subsequent year in a prescription frequency–dependent manner. This association was consistently observed regardless of birth route. In addition, our study highlights the high frequency of vaginal births affected by opioid prescribing in our state.
Although prior literature has described persistent opioid use after cesarean birth, knowledge of risks associated with opioid prescribing after vaginal birth is limited (3). This study integrated birth certificate data with claims and hospital registry information and was designed to augment information from studies that focused exclusively on persistent opioid use. Our data indicate that opioid prescribing after vaginal birth was common in our study, involving 59% of vaginal births, and imparts a significant and equally important risk for SOREs compared with cesarean birth. Current clinical guidelines do not provide specific recommendations for opioid prescribing after childbirth (4). Design and implementation of rational opioid prescribing guidelines would be an opportunity to reduce this risk.
Our study had limitations. We utilized Medicaid prescription data to estimate opioid use from a single state heavily affected by the opioid epidemic; we lacked information on actual opioid use, including illicit use. Nevertheless, almost half of all births in the United States are covered by Medicaid (5), so studies characterizing opioid risks in this vulnerable population are important. We cannot rule out the possibility that we included some women with undisclosed opioid use disorders who stopped use during pregnancy and resumed after birth, although a sensitivity analysis restricted to women with a complete year of data before birth yielded results consistent with those of our primary analysis. Although substantially attenuated, the risk for SOREs remained strongly and consistently associated with postpartum opioid prescribing when we examined only the most severe SORE outcomes.
In summary, women receiving postpartum prescription opioids have increased risk for SOREs that is related to prescription frequency, regardless of birth route. Reducing opioid prescribing after vaginal birth and in the postpartum period in general may improve outcomes for postpartum women.
Acknowledgment:
The authors thank the Tennessee Division of TennCare of the Department of Finance and Administration and the Tennessee Department of Health for providing data for the study.
Financial Support: Dr. Osmundson was supported by grants K12HD04348317 from the National Institutes of Health and K23DA047476 from the National Institute on Drug Abuse. Dr. Min was supported by the Veterans Affairs Office of Academic Affiliations. Dr. Wiese was supported by the Vanderbilt Faculty Research Scholars program and a PhRMA Foundation Fellowship in Health Outcomes. Dr. Patrick was supported by grant K23DA038720 from the National Institute on Drug Abuse. The study was supported in part by the National Institutes of Health, National Institute on Aging, through grant R01AG043471.
Footnotes
Publisher's Disclaimer: Disclaimer: The funders of the study had no role in the design of the study, analysis or interpretation of the data, or writing of the report. The corresponding author had final responsibility for the decision to submit the manuscript for publication.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-3805.
Contributor Information
Sarah S. Osmundson, Vanderbilt University Medical Center, Nashville, Tennessee.
Jea Young Min, Vanderbilt University Medical Center, Nashville, Tennessee.
Andrew D. Wiese, Vanderbilt University Medical Center, Nashville, Tennessee.
Robert E. Hawley, Vanderbilt University Medical Center, Nashville, Tennessee.
Edward Mitchel, Vanderbilt University Medical Center, Nashville, Tennessee.
Stephen W. Patrick, Vanderbilt University Medical Center, Nashville, Tennessee.
Lauren R. Samuels, Vanderbilt University Medical Center, Nashville, Tennessee.
Marie R. Griffin, Vanderbilt University Medical Center, Nashville, Tennessee.
Carlos G. Grijalva, Vanderbilt University Medical Center and Veterans Health Administration Tennessee Valley Healthcare System, Geriatric Research Education and Clinical Center (GRECC), Nashville, Tennessee.
References
- 1.Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1–353.e18. doi: 10.1016/j.ajog.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing, and the risk of persistent opioid use after delivery [Letter]. Am J Obstet Gynecol. 2019;220:405–7. doi: 10.1016/j.ajog.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among US women. JAMA Netw Open. 2019;2:e197863. doi: 10.1001/jamanetworkopen.2019.7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35–e43. doi: 10.1097/AOG.0000000000002683 [DOI] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]


