Abstract
Purpose
The aims of this study were to investigate if/how the presence of lymphedema affects the sensation of the upper limb and to assess whether complex decongestive physiotherapy (CDP) has a favorable impact on sensory testing.
Methods
A total of 27 patients with unilateral stage 2 breast cancer–related lymphedema (BCRL) were included in the study. Bilateral circumferential measurements were taken with a tape measure at different levels. Based on these measurements, limb volumes were determined by summing segment volumes derived from the truncated cone formula. Circumferential measurements and ultrasonographic evaluations (epidermis, dermis, and subcutaneous fat thicknesses) were performed at 10 cm distal to the elbow crease. The Semmes–Weinstein monofilament (SWM), static and moving two-point discrimination, pressure pain threshold (PPT), and tactile localization tests were also applied at the same site. After an initial evaluation, all patients underwent CDP phase 1 program. All the evaluations were repeated at the end of the treatment period.
Results
Before CDP, affected sides had significantly higher values than the unaffected sides in terms of SWM (p < 0.001), static (p = 0.002) and moving (p = 0.011) two-point discrimination, PPT (p = 0.001), and tactile localization (p < 0.001) values. After CDP, SWM (p = 0.002), static (p = 0.009) and moving (p = 0.024) two-point discrimination, PPT (p = 0.014), and tactile localization (p < 0.001) values decreased significantly on the affected sides.
Conclusion
BCRL seems to reduce light touch, static and moving two-point discrimination, PPT, and tactile localization sensations, whereas CDP seems to improve these sensory perceptions in women with BCRL. Ultrasonographic measurements also appear to be promising for prompt and convenient follow-up in the management of BCRL.
Trial registration
Clinical Trial Registration Number: NCT04296929 (date of registration: March 5, 2020)
Keywords: Lymphedema, Semmes–Weinstein, Two-point discrimination, Pressure pain threshold, Tactile localization, Ultrasound
Introduction
With the therapeutic advances, the survival rates as well as certain long-term complications have increased among breast cancer survivors. For instance, breast cancer–related lymphedema (BCRL) is commonplace in musculoskeletal practice and has the potential to cause significant disability during daily activities [1]. Therefore, in addition to lymphedema, breast cancer patients might also suffer other conditions after surgery, radiotherapy, or chemotherapy, such as neuropathy up to 30%, myofascial pain up to 44%, fatigue up to 94%, 20–40% upper body problems, and 18–54% functional limitations [2–8], which contribute to patients’ particular complaints, such as arm pain, numbness, or feeling of heaviness [9, 10]. Women with lymphedema more frequently report pain and demonstrate bilateral impairments in shoulder range of motion, greater restrictions in upper limb activities and upper limb strength, and sensory disturbances compared to women without lymphedema [9, 11]. While there are several studies in the literature on physical, functional, and emotional problems in women with BCRL, the effects of lymphedema on sensory parameters have not been explored. Accordingly, the purpose of this study was twofold. First, we aimed to investigate if/how the presence of lymphedema (affected/unaffected sides) affects the ultrasonographic measurements of soft tissue layers and skin sensation. Secondly, we aimed to assess whether complex decongestive physiotherapy (CDP) has a favorable impact (pre- and post-treatment) on limb volume/circumference and, thus, soft tissue ultrasound measurements and sensory parameters. To this end, aside from circumferential measurements and sensory evaluations (light touch, static and moving two-point discrimination, pressure pain threshold, and tactile localization sensation perceptions), we also included ultrasonographic (i.e., morphological) evaluations of different layers of skin and subcutaneous tissues in this study.
Materials and methods
Subjects
Between November 2019 and November 2020, women with unilateral stage 2 BCRL who had completed active breast cancer treatments (i.e., surgery, radiotherapy, and chemotherapy) at least 12 months before were recruited for the study if they were at least 18 years old and literate. Stage 2 lymphedema was determined based on the findings of 2 cm or more difference in limb circumference measurement at any level [12], stiffness of the skin, pitting edema with strong pressure, no reduction in edema with elevation, and fibrotic changes on ultrasound [13]. They were excluded in the presence of any of the following: current recurrence of breast cancer, bilateral involvement, smoking, diabetes mellitus, pre-existing neuromusculoskeletal conditions, edema due to other reasons (e.g., primary lymphedema, heart diseases), contraindications for CDP (e.g., cardiac edema, active infection), analgesic use in the last 24 h, and previous treatment for lymphedema in the last 12 months.
The study protocol was approved by the local ethics committee (date: 28.11.2019, No: KA – 19,120) of our university, and all subjects gave written informed consent based on the principles set out in the Helsinki Declaration.
Assessment and outcome measures
This study utilized a prospective before-and-after study design. A sociodemographic and clinical questionnaire was used to collect information on age, height, weight, education level, smoking habit, and health status (including medications and comorbidities). Circumferential measurements, ultrasonographic evaluations, and sensory tests were applied bilaterally before CDP and after 3 weeks of CDP.
Circumferential measurements and volumetric calculations
A flexible tape was used to measure the circumferences at the wrist (ulnar styloid level) and at each 5-cm segment until the axilla. Measurements were taken horizontally using a slight pressure. Volume was calculated bilaterally from circumferential values using the truncated cone formula which yielded excellent inter- and intra-observer reproducibility (0.97 and 0.98, respectively) in comparison to water displacement [14]. Considering the difference between the dominant and non-dominant arm volume, a 3.3% volume correction was performed in women with dominant arm involvement [15]. The severity of lymphedema was determined by the difference between the calculated volumes of the affected and unaffected arms (moderate lymphedema: 250–500 ml difference; severe lymphedema: > 500 ml difference) [16]. In addition, bilateral circumferential measurements were made at 10 cm distal to the elbow crease (the level at which ultrasonographic measurements were made) [17]. Intra- and inter-rater reliability levels were found to be excellent for circumferential measurements (intraclass correlation coefficient (ICC): 0.998 and 0.997, respectively) in patients with BCRL. Standard errors of measurements (SEM) were 0.13 cm (0.5%) for intra-rater assessment and 0.17 cm (0.7%) for inter-rater assessment of the forearm [18].
Ultrasonographic measurements
Ultrasound (US) imaging was performed using a 5–12-MHz linear probe (Logiq P5; GE Medical Systems, Milwaukee, WI, USA), and all the US measurements were performed by a single physiatrist (LÖ) with more than 20 years of expertise in musculoskeletal US. During the procedure, the participants were seated with their forearms supinated and extended on a pillow. At 10 cm distal to the elbow crease, along the line parallel to the arm axis from the midpoint of the medial and lateral epicondyles, the measurements were performed bilaterally [17]. Epidermis, dermis, and subcutaneous fat thicknesses were measured using plenty of gel and with minimum probe compression using the automatic calculation feature of the US device. Intra- and inter-rater ICC values were found to be excellent (0.95 and 0.99, respectively) for US measurements of the forearm in patients with BCRL [19].
Sensory testing
Before and after the 3 weeks of CDP, bilateral sensory tests (tactile sensitivity, static and moving two-point discrimination, pressure pain threshold, and tactile localization) were administered. During the sensory tests, the participants were seated with their forearms supinated and extended on the examination table. They were informed in detail about the tests before the assessments. The patients were asked to close eyes and turn the head towards the opposite side of the testing arm. On the last day of the 3 weeks of CDP, for each patient, the compression bandages were removed and, before the sensory measurements, the patients were asked to wash their arms, lie down, and rest for 30 min in order to reduce the effect of compression on sensory perception. As one of the most objective sensory tests [20, 21], the Semmes–Weinstein monofilaments (SWMs) (North Coast Medical, Morgan Hill, CA, USA) were used to assess the tactile sensitivity of the upper extremities—on volar region of the forearm, 10 cm distal to the midpoint of the medial and lateral epicondyles. The tests were started with the smallest diameter monofilament and progressed successively with larger diameter monofilaments until the correct responses were obtained [20, 21]. Each monofilament was touched three times with 2-s intervals, and the patients were asked to say “yes” when they felt the monofilament on their skin.
Static two-point discrimination test was performed with an esthesiometer, on the volar region of the forearm (between the regions 8–12 cm distal to the midpoint of the medial and lateral epicondyles). The test was started with a 5-mm gap between the tips of the esthesiometer. For the two-point discrimination, the tip was tapped randomly in the test area, either single or double. When the skin was touched at two points, care was taken to apply simultaneous and equal pressure. A 2-s break was given between each application. When seven out of 10 responses of the patient were correct, the answer was considered correct. In case of incorrect answers, the test was continued until the correct answer was reached by increasing the distance between the two ends of the esthesiometer by 5 mm. The lowest range of value that the patient reported correctly was recorded in mm [22, 23]. Similarly, moving two-point discrimination test was also performed with an esthesiometer in the volar region of the forearm 8–12 cm distal to the midpoint of the medial and lateral humeral epicondyles. The tips of the esthesiometer—placed as one or two points—were moved from proximal to distal. If seven of the 10 responses given by the patient were correct, the test was considered correct. If the answer was wrong, the gap between the tips of the esthesiometer was increased by 5 mm until the right answer was obtained [24].
Pressure pain threshold (PPT) measurement was assessed with an algometer (JTech Algometer Commander) on both upper extremities in the volar region of the forearm 10 cm distal to the midpoint of the medial and lateral humeral epicondyles. With the 1 cm2 head of the algometer, pressure was applied vertically and the patient was asked to say “yes” when he/she felt uncomfortable with the pressure. The measurement was repeated three times, and the arithmetic mean of the three trials was recorded as the PPT value [25].
The tactile localization test was performed in the volar region of the forearm 8–12 cm distal to the midpoint of the medial and lateral humeral epicondyles, using a pencil and ruler. The patients’ eyes were closed, and the body surface was touched with a pencil. The patient was asked to open his eyes and show the point that was touched. The distance between the point actually touched and the point indicated by the patient was measured with a ruler in cm and recorded. A total of three measurements were performed, and the arithmetic mean of the three trials was recorded [26, 27].
Complex decongestive physiotherapy
After the initial evaluation, all patients underwent CDP phase 1 program which comprised skin care, manual lymphatic drainage (MLD), compression bandages, and exercises for BCRL as recommended by the International Society of Lymphology consensus [13]. CDP was applied by a qualified physiotherapist five sessions per week, for 3 weeks (a total of 15 sessions, 60 min per session). MLD was applied using the Földi method and was followed by daily multi-layered short stretch bandaging worn for 23 h a day (excluding the 1-h treatment session). Abdominal breathing exercises and remedial exercises were performed with the bandages on. The participants were advised to avoid skin damages (e.g., insect bite, cut, and burn) and to protect their skin during daily life activities (e.g., using a thimble when sewing) [28].
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences software, version 21.0 (IBM SPSS Statistics; IBM Corporation, Armonk, NY, USA). Normal distribution of the variables was tested by the Kolmogorov–Smirnov/Shapiro–Wilk test. Descriptive statistics are presented with mean and standard deviation for normally distributed numerical variables. Median and interquartile ranges were used for non-normally distributed numerical variables while numbers and percentages were used for categorical variables. The Wilcoxon test was used to compare the affected vs. unaffected sides and pre- vs. and post-treatment values. The Mann–Whitney U test was used to compare data pertaining to dominant vs. non-dominant upper limb involvement. Correlation analyses between skin thickness measurements (epidermis, dermis, and subcutaneous fat) and sensory tests (SWM, static and moving two-point discrimination, PPT, and tactile localization) were performed using Spearman’s correlation coefficients. Statistical significance was set at p < 0.05. Power analysis was done using the G*Power program. The post hoc power analysis (N = 27) of the Wilcoxon test for SWM values between the pre- and post-treatment results of the affected sides achieved a power of 98% with a significance level of 0.002.
Results
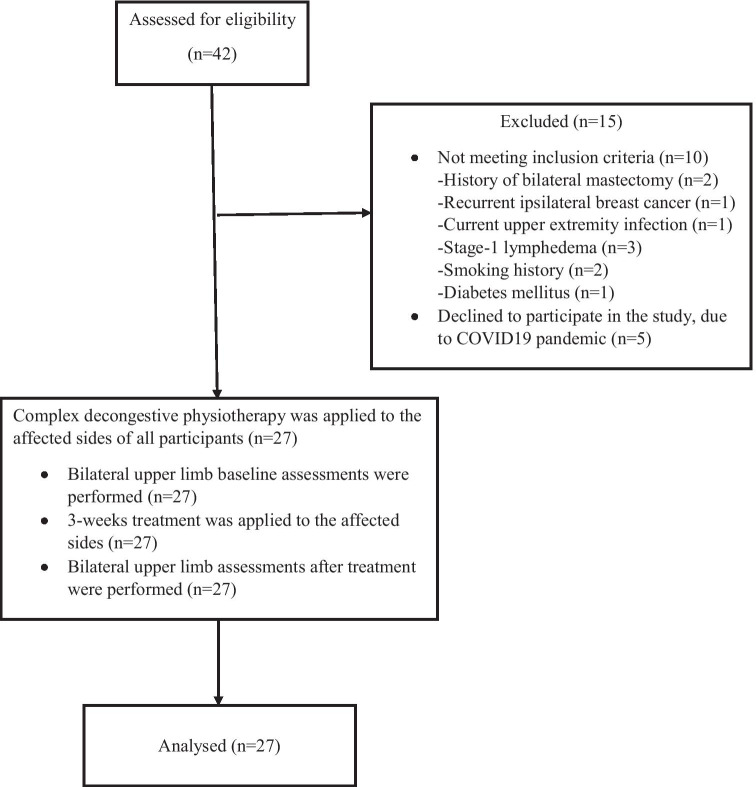
A total of 42 subjects applied for lymphedema treatment. Twenty-seven subjects (mean age 59.1 ± 8.56 years; body mass index 30.6 ± 4.42 kg/m2; mean lymphedema duration 49.8 ± 35.1 months) met the inclusion criteria and completed all the study procedures. Figure 1 shows a flow diagram of the study subjects. Clinical characteristics of the subjects are given in Table 1. Before the CDP, all the volumetric, circumferential, and ultrasonographic measurements were found to be higher (p < 0.05) in the affected limbs compared to those in the unaffected limbs (Table 2). After the CDP, while volumetric, circumferential, and ultrasonographic measurements (except epidermis) decreased significantly on the affected sides (p < 0.001), they remained the same on the unaffected sides (p > 0.05) (Table 3).
Fig. 1.
Flow diagram of the subjects
Table 1.
Sociodemographic and clinical characteristics of the study subjects
| Age (years) | 59.1 ± 8.56 |
|---|---|
| BMI (kg/m2) | 30.6 ± 4.42 |
| Education (years) | 10.2 ± 3.8 |
| Cancer diagnosis onset (months) | 84.7 ± 38.9 |
| Lymphedema duration (months) | 49.8 ± 35.1 |
| Lymphedema severity (moderate/severe) | 9/18 |
| Affected side (R/L) | 11/16 |
| Dominant/non-dominant involvement | |
| Dominant side, n (%) | 11 (40.7) |
| Non-dominant side, n (%) | 16 (59.3) |
| Radiotherapy (yes), n (%) | 25 (92.6) |
| Radiotherapy duration (n) | 25 (25–28) |
| Chemotherapy (yes), n (%) | 24 (88.9) |
| Chemotherapy (number of cycles) | 6 (4–6) |
| Type of operation | |
| Lumpectomy + ALND | 6 |
| Radical mastectomy + ALND | 5 |
| Modified radical mastectomy + ALND | 11 |
| Breast conserving surgery + ALND | 5 |
| Removed lymph nodes (n) | 21.4 ± 7.6 |
Data are presented as mean ± standard deviation or number (%) or median (interquartile range)
BMI body mass index, ALND axillary lymph node dissection
Table 2.
Pre-treatment values of the calculated volume, forearm circumference, and soft tissue measurements
| Affected side (N = 27) | Unaffected side (N = 27) | p | |
|---|---|---|---|
| Total limb volume (cm3) | 2964.2 ± 323.5 | 2398.4 ± 182.6 | < 0.001* |
| Circumference on forearm (cm) | 28.4 ± 3.1 | 23.3 ± 2.3 | < 0.001* |
| Soft tissue thickness measures on forearm | |||
| Epidermis (mm) | 0.041 ± 0.013 | 0.034 ± 0.008 | 0.023* |
| Dermis (mm) | 0.24 ± 0.05 | 0.1 ± 0.022 | < 0.001* |
| Subcutaneous fat (mm) | 1.44 ± 0.4 | 1.01 ± 0.28 | < 0.001* |
Circumference and soft tissue measurements pertain to a site 10 cm distal to the elbow, and data are presented as mean ± standard deviation
*Statistically significant difference between the affected and unaffected sides
Table 3.
Pre- and post-treatment values of the calculated volume, forearm circumference, and soft tissue measurements
| Pre-treatment (N = 27) | Post-treatment (N = 27) | p | |
|---|---|---|---|
| Affected side | |||
| Total limb volume (cm3) | 2964.2 ± 323.5 | 2661.5 ± 249.9 | < 0.001* |
| Circumference on forearm (cm) | 28.4 ± 3.1 | 25.8 ± 2.6 | < 0.001* |
| Soft tissue thickness on forearm | |||
| Epidermis (mm) | 0.041 ± 0.013 | 0.04 ± 0.009 | 0.496 |
| Dermis (mm) | 0.24 ± 0.05 | 0.18 ± 0.41 | < 0.001* |
| Subcutaneous fat (mm) | 1.44 ± 0.4 | 1.22 ± 0.36 | < 0.001* |
| Unaffected side | |||
| Total limb volume (cm3) | 2398.4 ± 182.6 | 2407.1 ± 200.7 | 0.11 |
| Circumference on forearm (cm) | 23.3 ± 2.3 | 23.5 ± 2.3 | 0.884 |
| Soft tissue thickness on forearm | |||
| Epidermis (mm) | 0.034 ± 0.008 | 0.036 ± 0.006 | 0.134 |
| Dermis (mm) | 0.1 ± 0.022 | 0.11 ± 0.023 | 0.449 |
| Subcutaneous fat (mm) | 1.01 ± 0.28 | 1.00 ± 0.3 | 0.586 |
Circumference and soft tissue measurements pertain to a site 10 cm distal to the elbow, and data are presented as mean ± standard deviation
*Statistically significant difference between the pre- and post-treatment values
Before the CDP, affected sides had significantly higher values for SWM (p < 0.001), static (p = 0.002) and moving (p = 0.011) two-point discrimination, PPT (p = 0.001), and tactile localization (p < 0.001) scores (Table 4). After the CDP, SWM (p = 0.002), static (p = 0.009) and moving (p = 0.024) two-point discrimination, PPT (p = 0.014), and tactile localization (p < 0.001) values were decreased significantly on the affected sides. Sensory test results remained the same on the unaffected sides after the CDP (p > 0.05) (Table 5).
Table 4.
Pre-treatment sensory assessments
| Affected side (N = 27) | Unaffected side (N = 27) | p | |
|---|---|---|---|
| Semmes–Weinstein monofilament | 3.22 (2.44–3.61) | 2.44 (2.36–3.22) | < 0.001* |
| Static 2-point discrimination (cm) | 3.5 (2.5–4.5) | 3.0 (2.5–3.0) | 0.002* |
| Moving 2-point discrimination (cm) | 2.5 (2.0–3.5) | 2.5 (2.0–2.5) | 0.011* |
| Pressure pain threshold (kPa) | 9.6 ± 1.4 | 8.5 ± 1.2 | 0.001* |
| Tactile localization (cm) | 1.84 ± 0.52 | 1.45 ± 1.37 | < 0.001* |
Data are presented as mean ± standard deviation or median (interquartile range)
*Statistically significant difference between the affected and unaffected sides
Table 5.
Sensory parameters before and after complex decongestive physiotherapy
| Pre-treatment (N = 27) | Post-treatment (N = 27) | p | |
|---|---|---|---|
| Affected side | |||
| Semmes–Weinstein monofilaments | 3.22 (2.44–3.61) | 2.83 (2.44–3.22) | 0.002* |
| Static 2-point discrimination (cm) | 3.5 (2.5–4.5) | 3.5 (3.0–3.5) | 0.009* |
| Moving 2-point discrimination (cm) | 2.5 (2.0–3.5) | 2.5 (2.3–3.0) | 0.024* |
| Pressure pain threshold (kPa) | 9.6 ± 1.4 | 8.7 ± 1.2 | 0.014* |
| Tactile localization (cm) | 1.84 ± 0.52 | 1.76 ± 1.32 | < 0.001* |
| Unaffected side | |||
| Semmes–Weinstein monofilaments | 2.44 (2.36–3.22) | 2.44 (2.36–2.83) | 0.149 |
| Static 2-point discrimination (cm) | 3.0 (2.5–3.0) | 3.0 (2.5–3.0) | 0.132 |
| Moving 2-point discrimination (cm) | 2.5 (2.0–2.5) | 2.0 (2.0–2.5) | 0.366 |
| Pressure pain threshold (kPa) | 8.5 ± 1.2 | 8.3 ± 1.6 | 0.101 |
| Tactile Localization (cm) | 1.45 ± 1.37 | 1.16 ± 0.41 | 0.301 |
Data are presented as mean ± standard deviation or median (interquartile range)
*Statistically significant difference between the affected and unaffected sides
When the participants were grouped according to the involvement of their dominant vs. non-dominant upper limbs, there was no statistically significant difference in terms of SWM, static and moving two-point discrimination, PPT, and tactile localization values (p > 0.05). Correlation analyses showed no significant relationships between skin thickness measurements (epidermis, dermis, and subcutaneous fat) and sensory tests (SWM, static and moving two-point discrimination, PPT, and tactile localization) (all p > 0.05).
Discussion
According to the results of this study, ipsilateral upper extremity sensory perception decreased in women with BCRL and CDP improved the light touch, static and moving two-point discrimination, PPT, and tactile localization sensory perceptions. To the best of the authors’ knowledge, this is the first study to explore the effects of CDP on sensory evaluations of lymphedematous extremity and the relationship between ultrasonographic skin thickness measurements and sensory perceptions in women with BCRL.
Limited studies in the literature mentioned the changes in sensory functions after breast cancer treatments. Smoot et al. [9] evaluated light touch using SWM and vibration with a biothesiometer in breast cancer patients with or without lymphedema whereby the severity of lymphedema was mostly mild. It was determined that light touch (but not vibration) sensation was significantly decreased in patients with lymphedema as compared to those without. Using SWM, Civelek [29] found no difference between the affected and unaffected sides of the cases with mild lymphedema after breast cancer treatment. In contrast/addition to these aforementioned reports, several sensory tests (i.e., light touch, static and moving two-point discrimination, PPT, and tactile localization) were applied in our study and the patient population comprised patients with moderate to severe lymphedema.
Concerning the possible mechanisms, it was reported that light touch sensation decreased as the mechanical properties of the skin (e.g., hardness, epidermal thickness, and stretch response) increased [30]. It is thought that the decrease in sensory perception of SWM, static and moving two-point discrimination, PPT, and tactile localization, together with epidermis, dermis, and subcutaneous fat thicknesses, might be related to the decrease in the ability to activate afferent conduction. Increased soft tissue thickness can cause a greater distinction between the mechanoreceptors and the external stimuli and has a significant effect on afferent firing at the perceptual threshold. In addition, the transmission ability of the stimulus force applied to the skin to activate the mechanoreceptors may be affected by skin hardness and thickness [30]. In stage 2 lymphedema, the skin is hard and the godet test is also positive with strong pressure. Moreover, in stage 2 lymphedema, the extremity is enlarged and nerve endings possibly become responsible for a wider area. Therefore, this could be another reason for the decrease in sensation. Additionally, peripheral nerve entrapments associated with lymphedema or fibroblast infiltration might also be contributory as regards the eventual sensory disturbance [31].
Stimulating flow through lymphatic vessels and activating the collateral circulation, CDP causes increased fluid and protein emission, softens fibrotic tissues, and improves histological changes associated with lymphedema. Currently, it is the most effective and the gold standard conservative treatment for volume reduction in lymphedema [16, 32]. Auriol et al. [33] reported that skin elasticity increased and soft tissue thickness decreased after CDP. Keser and Esmer [34] examined the acute effects after one session of MLD and found that the PPT and pressure pain tolerance increased in healthy subjects. In our study, not only MLD but also all components of CDP were applied for 3 weeks. Additionally, to eliminate the effects of compression on sensation, evaluations were made 30 min after the bandages were removed. Of note, it was observed that the PPT perception decreased as lymphedema subsided; in other words, the sensory perceptions improved. However, the effect of each component of CDP on sensation is unknown. CDP softens the skin hardness and decreases soft tissue thickness in lymphedema patients [35]. Edema reduction and possible/favorable changes in the skin histology might have contributed to the improvement in the sensory parameters. Moreover, components of CDP provide a high level of sensory inputs to the patients [36, 37] and they might have positively contributed as well.
This study has some major limitations. First, sensory evaluations were acquired from a single region. Herein, since more than one sensory perception was preferred to be assessed in detail and as a high concentration of the subject is a prerequisite during the assessments, we have taken a single region to avoid distraction and inconvenience. Second, due to the current COVID-19 pandemic, both the number of patients who applied for lymphedema treatment and that of those who volunteered to participate in our study decreased significantly. Likewise, due to the insufficient number of patients, subgroup analysis either as regards different breast cancer treatments (e.g., surgical approaches, chemotherapy, and radiotherapy) which may also cause sensory deficits [38] or as regards the presence/absence of lymphedema was considered to be inappropriate. Therefore, further studies with larger sample sizes are warranted.
Conclusion
To conclude, in light of this study findings, we imply that BCRL reduces sensory perceptions of light touch, static and moving two-point discrimination, PPT, and tactile localization. Being aware that BCRL is commonplace in daily clinical practice and that sensory disturbances can expose these patients to injuries, it is noteworthy that relevant patients be treated with CDP not only for edema reduction but also to improve their sensory functions. There is definitely a need for future randomized controlled studies investigating sensory functions in breast cancer patients with or without lymphedema, also taking into account different cancer therapies they receive. Moreover, further studies with additional sensory evaluations at multiple sites and perhaps coupled with specific electrophysiological correlates are definitely awaited.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Emine Baran, Levent Özçakar, Serap Özgül, Sercan Aksoy, and Türkan Akbayrak. The first draft of the manuscript was written by Emine Baran, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
N/A
Code availability
N/A
Declarations
Ethics approval and consent to participate
The Hacettepe University Ethical Committee approved the present study (date: 28.11.2019, No: KA – 19120). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emine Baran, Email: eminekbaran@gmail.com.
Levent Özçakar, Email: lozcakar@yahoo.com.
Serap Özgül, Email: serapky@yahoo.com.
Sercan Aksoy, Email: saksoy07@yahoo.com.
Türkan Akbayrak, Email: takbayrak@yahoo.com.
References
- 1.Penn IW, Chang YC, Chuang E, Chen CM, Chung CF, Kuo CY, Chuang TY. Risk factors and prediction model for persistent breast-cancer-related lymphedema: a 5-year cohort study. Support Care Cancer. 2019;27(3):991–1000. doi: 10.1007/s00520-018-4388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses’ Health Study. Cancer. 2000;89(11):2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::AID-CNCR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16(3):197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98(8):521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, Schmitz KH. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(S8):2237–2249. doi: 10.1002/cncr.27467. [DOI] [PubMed] [Google Scholar]
- 6.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(S8):2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 7.Lacomba MT, Del Moral OM, Zazo JLC, Gerwin RD, Goñí ÁZ. Incidence of myofascial pain syndrome in breast cancer surgery: a prospective study. Clin J Pain. 2010;26(4):320–325. doi: 10.1097/AJP.0b013e3181c4904a. [DOI] [PubMed] [Google Scholar]
- 8.Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes JM, Lunet N. Neurological complications of breast cancer: a prospective cohort study. The Breast. 2015;24(5):582–587. doi: 10.1016/j.breast.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Smoot B, Wong J, Cooper B, Wanek L, Topp K, Byl N, Dodd M. Upper extremity impairments in women with or without lymphedema following breast cancer treatment. J Cancer Surviv. 2010;4(2):167–178. doi: 10.1007/s11764-010-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orhan C, Üzelpasaci E, Baran E, Nakip G, Özgül S, Aksoy S, Akbayrak T. The reliability and validity of the Turkish version of the Lymphedema Life Impact Scale in patients with breast cancer–related lymphedema. Cancer Nurs. 2020;43(5):375–383. doi: 10.1097/NCC.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 11.Gursen C, Dylke ES, Moloney N, Meeus M, De Vrieze T, Devoogdt N, De Groef A (2021) Self-reported signs and symptoms of secondary upper limb lymphoedema related to breast cancer treatment: systematic review. Eur J Cancer Care e13440. 10.1111/ecc.13440 [DOI] [PubMed]
- 12.Armer J, Stewart B. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43(3):118. [PMC free article] [PubMed] [Google Scholar]
- 13.Executive Committee of the International Society of Lymphology The diagnosis and treatment of peripheral lymphedema: 2020 consensus document of the International Society of Lymphology. Lymphology. 2020;53:3–19. [PubMed] [Google Scholar]
- 14.Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82(12):1201–1212. doi: 10.1093/ptj/82.12.1201. [DOI] [PubMed] [Google Scholar]
- 15.Sagen Å, Kåresen R, Risberg MA. The reliability of a simplified water displacement instrument: a method for measuring arm volume. Arch Phys Med Rehabil. 2005;86(1):86–89. doi: 10.1016/j.apmr.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Kligman L, Wong RK, Johnston M, Laetsch NS. The treatment of lymphedema related to breast cancer: a systematic review and evidence summary. Support Care Cancer. 2004;12(6):421–431. doi: 10.1007/s00520-004-0627-0. [DOI] [PubMed] [Google Scholar]
- 17.Ozcan DS, Oken O, Aras MD, Koseoglu B. Is ultrasonography a useful method to evaluate the effectiveness of complex decongestive therapy in breast cancer-related lymphedema? Lymphology. 2017;50(2):84–94. [PubMed] [Google Scholar]
- 18.Chen YW, Tsai HJ, Hung HC, Tsauo JY. reliability study of measurements for lymphedema in breast cancer patients. Am J Phys Med Rehabil. 2008;87(1):33–38. doi: 10.1097/PHM.0b013e31815b6199. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Lee CH, Heo SJ, Moon MH. The clinical usefulness of lymphedema measurement technique using ultrasound. Lymphat Res Biol. 2021 doi: 10.1089/lrb.2019.0070. [DOI] [PubMed] [Google Scholar]
- 20.Moberg E. Objective methods for determining the functional value of sensibility in the hand. J Bone Joint Surg Br. 1958;40(3):454–476. doi: 10.1302/0301-620X.40B3.454. [DOI] [PubMed] [Google Scholar]
- 21.Tracey EH, Greene AJ, Doty RL. Optimizing reliability and sensitivity of Semmes-Weinstein monofilaments for establishing point tactile thresholds. Physiol Behav. 2012;105(4):982–986. doi: 10.1016/j.physbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Moberg E. Two-point discrimination test. A valuable part of hand surgical rehabilitation, eg in tetraplegia. Scand J Rehabil Med. 1990;22(3):127–134. [PubMed] [Google Scholar]
- 23.Nolan MF. Two-point discrimination assessment in the upper limb in young adult men and women. Phys Ther. 1982;62(7):965–969. doi: 10.1093/ptj/62.7.965. [DOI] [PubMed] [Google Scholar]
- 24.Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474–481. doi: 10.1016/S0363-5023(78)80143-9. [DOI] [PubMed] [Google Scholar]
- 25.Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36(5):642–646. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- 26.Skirven TM, Osterman AL, Fedorczyk J, Amadio PC, Felder S, Shin EK (2020) Rehabilitation of the hand and upper extremity: E-book. Elsevier Health Sciences
- 27.Halnan C, Wright GH. Tactile localization. Brain. 1960;83:677–700. doi: 10.1093/brain/83.4.677. [DOI] [PubMed] [Google Scholar]
- 28.Vignes S, Porcher R, Arrault M, Dupuy A. Long-term management of breast cancer-related lymphedema after intensive decongestive physiotherapy. Breast Cancer Res Treat. 2007;101(3):285–290. doi: 10.1007/s10549-006-9297-6. [DOI] [PubMed] [Google Scholar]
- 29.Civelek G. Effects of breast cancer related lymphedema on hand muscle strength, hand functions and sensory loss of hand. Cukurova Med J. 2016;41(2):208–216. doi: 10.17826/cutf.200040. [DOI] [Google Scholar]
- 30.Strzalkowski ND, Triano JJ, Lam CK, Templeton CA, Bent LR. Thresholds of skin sensitivity are partially influenced by mechanical properties of the skin on the foot sole. Physiol Rep. 2015;3(6):e12425. doi: 10.14814/phy2.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganel A, Engel J, Sela M, Brooks M. Nerve entrapments associated with postmastectomy lymphedema. Cancer. 1979;44(6):2254–2259. doi: 10.1002/1097-0142(197912)44:6<2254::AID-CNCR2820440638>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Bakar Y, Berdici B, Şahin N, Pala ÖO. Lymphedema after breast cancer and its treatment. J Breast Health. 2014;10:6–14. doi: 10.5152/tjbh.2014.1651. [DOI] [Google Scholar]
- 33.Auriol F, Vaillant L, Pelucio-Lopes C, Machet L, Diridollou S, Berson M, et al. Study of cutaneous extensibility in lymphoedema of the lower limbs. Br J Dermatol. 1994;131(2):265–269. doi: 10.1111/j.1365-2133.1994.tb08503.x. [DOI] [PubMed] [Google Scholar]
- 34.Keser I, Esmer M. Does manual lymphatic drainage have any effect on pain threshold and tolerance of different body parts? Lymphat Res Biol. 2019;17(6):651–654. doi: 10.1089/lrb.2019.0005. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Shin BW, Jeong HJ, Kim GC, Kim DK, Sim YJ. Ultrasonographic evaluation of therapeutic effects of complex decongestive therapy in breast cancer-related lymphedema. Ann Rehabil Med. 2013;37(5):683. doi: 10.5535/arm.2013.37.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomide L, Matheus J, Candido dos Reis F. Morbidity after breast cancer treatment and physiotherapeutic performance. Int J Clin Pract. 2007;61(6):972–982. doi: 10.1111/j.1742-1241.2006.01152.x. [DOI] [PubMed] [Google Scholar]
- 37.Da Luz SCT, Da Silva Honório GJ. The role of physiotherapy in female breast cancer. In: Brandão S, Da Roza T, Ramos I, Mascarenhas T, editors. Women’s health and biomechanics: where medicine and engineering meet. Cham: Springer International Publishing; 2018. pp. 71–82. [Google Scholar]
- 38.Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(S8):2250–2260. doi: 10.1002/cncr.27463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A
N/A