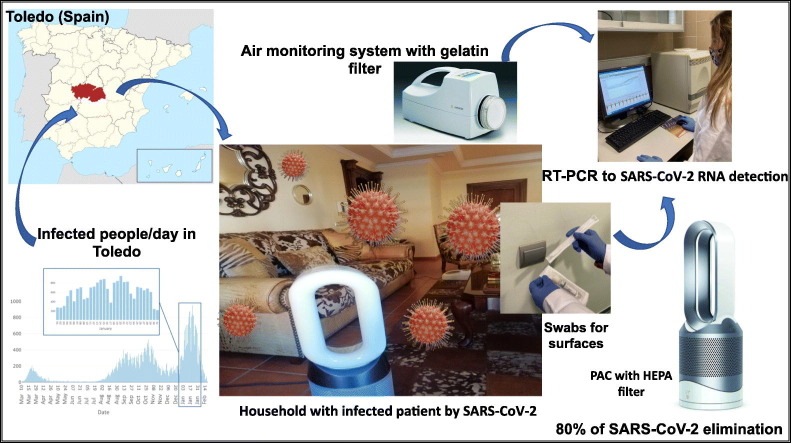
Abstract
The transmission of SARS-CoV-2 virus through aerosols has become an outstanding issue, where plenty of spread aspects are being analyzed. Portable Air Cleaners (PAC) with high-efficiency particulate air (HEPA) filters have been discussed as an adjunctive means for indoor environments coronavirus decontamination. This study evaluates, first, the air and surfaces SARS-COV-2 RNA contamination due to positive patients in households, and second, the efficiency of a PAC with HEPA filter to eliminate virus. A total of 29 air and surface samples were collected inside 9 households, by using an air portable collector with gelatin filters and swabs. SARS-CoV-2 RNA detection was performed using real-time reverse transcription polymerase chain reaction (RT-PCR). Overall, all the air samples collected before using PAC and 75% of swab samples were positive for SARS-CoV-2. After the PAC usage, all samples except one were negative, displaying a 80% device effectiveness. Portable HEPA cleaners usage allowed the removal of SARS CoV-2 and, therefore, they could be recommended for places with inadequate ventilation, considering the limitations and functionality of the device.
Keywords: Viral particles, Portable Air Cleaners, Airborne SARS-CoV-2, Surfaces
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a newly emerging virus that was first reported in Wuhan (China) in December 2019. SARS-CoV-2 is a positive-sense single-stranded RNA virus, contagious in humans, that has caused an unprecedented public health emergency worldwide (WHO, 2021a). Most people infected by SARS-CoV-2, develop only mild (40%) or moderate (40%) disease, but approximately 15% of them suffer from severe disease requiring oxygen support, and 5% have critical disease with complications such as respiratory failure, acute respiratory distress syndrome, sepsis and septic shock, thromboembolism, and/or multiorgan failure, including acute kidney injury and cardiac injury (TNCPERET, 2020).
The first epidemiological and virological studies suggested that transmission occurred mainly by person-to-person contact, through respiratory droplets, also called flügge droplets (larger drops emitted by coughing, sneezing, or talking), or by contact with contaminated objects and surfaces (Burke et al., 2020; Lauer et al., 2020; Liu et al., 2020; Ong et al., 2020; Somsen et al., 2020). Subsequently, it was shown that, in closed and poorly ventilated spaces, the predominant route of infection was through aerosols (particles smaller than 1 mm of diameter) containing viable viral particles, generated by infected people (Prather et al., 2020; Qian et al., 2020; van Doremalen et al., 2020; Arslana et al., 2020). For this reason, the WHO (2021b) issued recommendations to prevent contagion, including the use of face masks, physical distancing, avoiding crowds and an adequate ventilation of indoor spaces. Regarding this last recommendation, both natural and mechanical methods can be used in order to get indoor ventilation. Various air purifying/cleaning technologies can exist as part of a building's central heating, ventilation, and air conditioning (HVAC) system. When it is not possible to achieve an optimal ventilation rate in a specific indoor environment, the use of Portable Air Cleaners (PACs) carrying high-efficiency particulate air (HEPA) filters could be considered. For a PAC to be effective at removing viruses from indoor air, the unit should have the ability to remove small airborne particles, in the range of 0.1 to 1 μm of diameter, although larger droplets may also carry viruses (EPA, 2019).
No direct scientific evidence exists demonstrating the effectiveness of PACs in reducing COVID-19 transmission, and the precise role of HEPA filtration, in preventing infectious diseases is unclear (ECDC, 2020). However, it has been demonstrated that PACs with HEPA filters reduce aerosol concentration and accelerate their removal in rooms with low ventilation rates (Zhao et al., 2020a; Ren et al., 2021). Moreover, the Centers for Disease Control and Prevention (CDC) suggested the use of portable HEPA purifiers as an adjunctive infection control strategy for SARS-CoV-1, the causative agent of the 2003 SARS outbreak (CDC, 2005). Therefore, these devices could also be considered as a complementary airborne SARS-COV-2 elimination procedure (Christopherson et al., 2020; Zhao et al., 2020b), in addition to an overall improvement indoor air quality contributor.
In order to clarify these aspects, both the optimization of a method to detect the presence of COVID-19 RNA in indoor air, and a subsequent study to determine the effectiveness of a PAC with HEPA filter were carried out. For the first of the objectives, indoor air of households inhabited by a Covid-19 positive patient was sampled by using a MD8 Airport (Sartorius) collector with gelatin filters. In parallel, sampling of different surfaces in the same rooms was carried out by using sterile pre-moistened swabs with a specific virus preservation solution. Analysis of viral RNA from both types of samples was carried out by real-time RT-PCR and once optimized this method, the effectiveness of a PAC with HEPA filter was assessed by viral RNA presence detection before and after cleaner usage.
2. Material and methods
2.1. Sampling
We conducted the present study in January 2021, during the third wave pandemic peak over Spain (https://cnecovid.isciii.es/). Sampling was carried out in several households, located in different towns in Toledo province (region in the center of Spain). All households had natural ventilation, but they were inadequately ventilated, due to heating was on and windows and doors closure. In some of them, one COVID-19 positive PCR test patient was confined, and others were inhabited by healthy people, which were considered as negative controls. A total number of 13 swabs and 16 air samples were collected and analyzed (Table 1 ).
Table 1.
Summary of patients and sampling information.
| Room | Age | Air sampling | Surface sampling | Symptoms in sampling day | Severity of the illnessa | Diagnosis by PCR test | Daysb | Use of PAC | Room size (m2) | T (°C) | R.H. (%) | PM2.5c (μg/m3) | PM10c (μg/m3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 28 | Yes | No | Headache, fatigue, loss of smell and taste | Severe | Yes | 5 | Yes | 15 | 21 | 60 | 5 | 6 |
| B | 21 | Yes | Yes | Sore throat, fatigue | Mild | Yes | 3 | Yes | 16 | 23 | 48 | 5 | 5 |
| C | 60 | Yes | No | No symptoms | Mild | Yes | 5 | Yes | 60 | 22 | 50 | 6 | 7 |
| D | 54 | Yes | No | Cough, fever, fatigue | Very severe | Yes | 2 | Yes | 13 | 22 | 47 | 5 | 6 |
| E | 35 | Yes | No | Cough, fever, fatigue | Mild | Yes | 3 | Yes | 27 | 22 | 58 | 6 | 6 |
| F | 75 | Yes | Yes | Control = No symptoms | No | Yes | 22 | 23 | 44 | 6 | 7 | ||
| G | 44 | Yes | Yes | Control = No symptoms | No | Yes | 17 | 21 | 48 | 5 | 5 | ||
| H | 46 | Yes | No | Fever, fatigue | Mild | Yes | 5 | No | 22 | ||||
| I | 48 | Yes | No | Headache, fatigue, loss of smell and taste | Mild | Yes | 47 | No | 16 |
Medical diagnostic.
Number of days since the date of clinical diagnosis to the sampling day.
PM concentrations after the PAC usage.
The air samples were collected by using an MD8 Airport Portable Air collector (Sartorius AG, Gottingen Germany) with gelatin membrane filters (80 mm of diameter). Each aspiration cycle with a 50 L/min flow, lasted 20 min; being 1 m3 the total sampled volume. For sampling, the collector was positioned 1.5 m above the floor and 2 m far from the room occupant, who was not wearing a mask, in order to recover the ambient aerosols instead of the direct emitted drops. During sampling only one person was at the room whose dimensions are detailed in Table 1.
The same day and after the first sampling, one PAC with HEPA filter (Dyson, Pure Hot+Cool™) was placed in the center of the room, ensuring unobstructed airflow. This device automatically measured temperature, relative humidity (R.H.) and particles in suspension (PM2.5 and PM10) reporting about them in real time. Manufacturer information indicates that the HEPA filter captures 99.95% of particles down to 0.1 μm. Gases, such as NO2 and volatile organic compounds, are captured by an active carbon filter. Then, it projects purified air with Air Multiplier ™ technology, with a clean air delivery rate of 290 L/s, oscillating up to 360° to project purified air throughout the room. According to manufacturer's recommendations, its effectiveness is optimal in spaces of up to 27 m2 (82 m3). The air samples for real-time RT-PCR analysis were taken again when PM2.5 and PM10 concentrations were between 5 and 7 μg/m3, as indicated by the PAC, and never below 1 h of operating. These concentrations were lower than the levels established by Spanish legislation for PM2.5 (<20 μg/m3) (RD102/2011) or the value recommended by the WHO (2015) (10 μg/m3).
In addition, sampling of potentially contaminated surfaces and objects existing in the room, such as TV's remote control, doorknob, mobile phone, different switches and furniture, was carried out by using sterile pre-moistened swabs with a specific virus preservation solution (Citoswab, UK).
Both, the gelatin filters and the swabs, were transported refrigerated to the laboratory and stored frozen at −80 °C until processing.
2.2. Laboratory procedures
Once in the laboratory, the gelatin filters were dissolved in 4 mL of diethyl pyrocarbonate-water, centrifuged at 3000 ×g for 5 min and incubated at 37 °C for 10 min to dissolve the gelatin, following the manufacturer's instructions. Viral RNA was extracted from 150 μL of the solution obtained from gelatin filters and, in the case of the swab, from the same quantity of the virus preservation solution, by using the NucleoSpin RNA Virus kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. Quality control of this kit, as informed by the manufacturer, included testing of the columns with rRNA and MS2 phage RNA, as well as absence of RNases and yield and efficiency of purification, at both by real-time RT-PCR. RNA concentration and purity were determined with a NanoDrop spectrophotometer (Biotek, Synergy HT, USA) by calculating the ratio of the optical densities obtained at the wavelengths of 260/280 and 260/230 nm, respectively. Complementary DNA (cDNA) was synthesized from 1 μg of DNase-treated RNA and detection of SARS-CoV-2 RNA was carried out by real-time RT-PCR targeting the Nucleocapsid (N), the Spike (S) and the RNA-dependent RNA polymerase gene (RdRP), using the primers described in Table 2 . The positive control for real-time RT-PCR was an in vitro transcribed RNA derived from the strain BetaCoV_Wuhan_WIV04_2019 (EPI_ISL_402124), kindly given by Gortazar's group (Fernández de Mera et al., 2020). RNAse free water was used as a negative control. Real time RT-PCR was performed by using an ABI PRISM 7500 Fast Sequence Detection System instrument and software (Applied Biosystem, Foster City, CA). The real-time RT-PCR was performed under thermal cycling conditions consisting of an initial 1 min denaturation at 50 °C and 10 min of further denaturation at 95 °C, followed by 50 cycles of 15 s of denaturation at 95 °C and 60 s of annealing/extension at 60 °C. The result was considered positive when the Cycle threshold (Ct) value was ≤41 (Fernández de Mera et al., 2020). SYBR-Green One-Step real time PCR Master Mix (Thermo Fisher) was used in the assays, which, as informed by the manufacturer, presents high sensitivity, with a limit of detection as low as 2 copies of target; high reproducibility and high specificity, minimizing primer-dimer formation and nonspecific amplification. Primers were supplied by Sigma-Merck.
Table 2.
List of primers used for real time RT-PCR and amplified fragment sizes in base pairs (Park et al., 2020).
| Target gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Size (pb) |
|---|---|---|---|
| RdPR | AGAATAGAGCTCGCACCGTA | CTCCTCTAGTGGCGGCTATT | 101 |
| S | GCTGGTGCTGCAGCTTATTA | AGGGTCAAGTGCACAGTCTA | 107 |
| N | CAATGCTGCAATCGTGCTAC | GTTGCGACTACGTGATGAGG | 117 |
3. Results and discussion
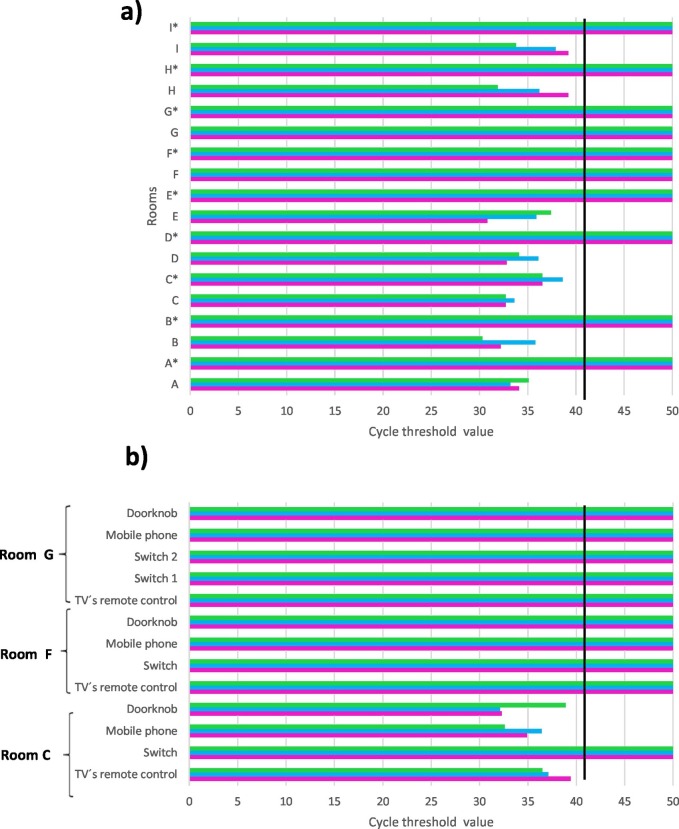
The RNA extraction procedure was successful for all the samples, being the values for RNA concentration between 84.1 and 101.2 ng/μL. Results for SARS-CoV-2 obtained from real-time RT-PCR analysis of air samples, both before and after using of PAC with HEPA filter, are shown in Fig. 1 a. Ct values ranged from 30.8 to 39.2 when RdPR primers were used, from 33.2 to 38.6 with S primers and from 30.3 to 37.4 with N primers. Therefore, all the air samples, collected by using the MD8 Airport Portable Air Collector (Sartorius) and gelatin filters previously to use the PAC with HEPA filter (Dyson), from households inhabited by COVID patients, were positive for SARS-CoV-2. The same procedure for air sampling had been tested with good results in COVID-19 isolation ward of hospitals in Italy and China (Razzini et al., 2020; Chen et al., 2020), but in our case the air volume sampled was smaller, only 1 m3 compared to the 2 or 3 m3 sampled by the other authors.
Fig. 1.
Real-time RT-PCR results for the three pairs of primers ( RdPR,
RdPR,  S and
S and  N). a) Air samplings in the rooms; *corresponds to values from samples taken after using the PAC. b) Surface samplings. Black line indicates values of Ct ≤ 41, which means positive real-time RT-PCR (Fernández de Mera et al., 2020).
N). a) Air samplings in the rooms; *corresponds to values from samples taken after using the PAC. b) Surface samplings. Black line indicates values of Ct ≤ 41, which means positive real-time RT-PCR (Fernández de Mera et al., 2020).
When the samples taken from the surfaces of households inhabited by COVID patients were analyzed, all except one, were positive for SARS-CoV-2 with Ct values ranging between 32.1 and 39.4 for the three pairs of primers used (Fig. 1b).
On the other hand, both air and surface samples taken in households without any infected people (rooms F and G in Table 1) were negative for SARS-CoV-2, confirming the method's validity (Fig. 1a and b). These results support the idea of an important spread of SARS-CoV-2 through aerosols and point out the need of find systems to eliminate the virus in this medium.
For this purpose, we checked the effectiveness of the PAC with HEPA filter (Dyson), which captures particles down to 0.1 μm, to remove airborne SARS-CoV-2. Table 1 details PM2.5, PM10, temperature and R.H. values in the air, measured after using of the PAC at each of the rooms sampled. When air samples taken after using the cleaner were analyzed for SARS-CoV-2, all except one were negative, displaying an effectiveness of 80%. It is worth highlighting that the PAC was not effective in the room C, with a size larger than the recommended by the device (27 m2), although the operating time was longer than in other rooms (>2 h). However, in the room of the patient E, whose size was in the recommended limit, the concentration of particles in the air reduced after 2 h of cleaning, and the result from real-time RT-PCR analysis was negative. These facts show the importance of following the manufacturer's recommendations, using as many devices as necessary, according to their filtration capacity and the size of the room.
In summary, to our knowledge, this is the first study reporting the analyses of the presence of SARS-CoV-2 in the air of households inhabited by COVID patients. The method used for the detection of the viral RNA, showed good results, confirming its validity, even with low sampled air volume. In addition, we have studied the effectiveness of a PAC with HEPA filter to remove airborne SARS-CoV-2, finding also good results when the room size sampled follows to the manufacturer's recommendations. In this sense, the PACs may be especially useful in places where infected and healthy people live together and the complete isolation of the sick people is difficult, compromising the health of the rest of the inhabitants of the household.
The use of these devices in schools, offices, and commercial buildings, to complement existing HVAC systems, should be considered, particularly in areas where adequate ventilation is difficult to achieve. Therefore, PACs utilization for SARS-CoV-2 elimination may be useful as an adjunctive infection control measure, but it should be undertaken having in mind functionality and limitations of both PACs and HEPA filters. In this sense, it is important to know that factors such as the accumulation of particles in the filter, its possible clogging, or the age of the device, can affect the effectiveness of the PAC. Because of it, and when used with this objective, it will be advisable to replace or clean the filters with a higher frequency than that for ordinary use (Zhao et al., 2020b).
Another important aspect to be considered is to ensure the correct handling and disposal of HEPA filters after their lifetime. Accumulation of viruses on filters could represent a real risk of environmental contamination, so that whenever air purifiers are used, filters should be collected and disposed as medical waste or disinfected thoroughly to prevent secondary contamination (Zhao et al., 2020b).
4. Conclusions
This study has reported interesting findings about presence of SARS-CoV-2 in households inhabited by COVID patients and about the real effectiveness of a PAC with HEPA filter to terminate it. It is very important to consider the room size recommendations usage where PACs are going to be installed in order to get the proposed objective.
Authors are aware of the limitations of this preliminary study, because of the small sample size and only one PAC testing, but the results have been highly promising. For this reason, a further study should analyze a higher number of samples from different origins, such as health-care settings, schools, etc. Such additional research should consider new and assorted mitigation strategies, such as PACs based on different types of filtration systems (HEPA filters, positively charged polyvinylidene fluoride (PVDF) nanofiber filter to electrostatic capture of particle, etc.), HVAC with efficient filtration systems, or the decontamination of air using UVC and/or ozone. Carrying out of such larger scale studies is crucial to end with the current negative consequences of the world pandemic.
CRediT authorship contribution statement
M.R, S.S. and A. R. collected the samples. M.R. did the analyses. All authors processed data and drafted the manuscript and contributed to discussion of the results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Authors want to thank to Dyson the loan of the PACs and to Sartorius for free filters. We strongly thank to E. Sánchez for checking and editing the manuscript.
Editor: Pavlos Kassomenos
References
- Arslana M., Xu B., El-Din M.G. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743:140709. doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R.M., Midgley C.M., Dratch A., Fenstersheib M., Haupt T., Holshue M., et al. Active monitoring of persons exposed to patients with confirmed COVID-19 – United States, January–February 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(9):245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Centers for Disease Control and Prevention. III. Infection control in healthcare facilities: public health guidance for community-level preparedness and response to severe acute respiratory syndrome (SARS) 2005. https://www.cdc.gov/sars/guidance/iinfection/healthcare.html
- Chen G.M., Ji J.J., Jiang S., Xiao Y., Qi Z., Ren L., et al. Detecting environmental contamination of acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in isolation wards and fever clinics. Biomed. Environ. Sci. 2020;33(12):943–947. doi: 10.3967/bes2020.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson D.A., Yao W.C., Lu M., Vijayakumar R., Sedaghat A.R. High-efficiency particulate air filters in the era of COVID-19: function and efficacy otolaryngology. Head Neck Surg. 2020;163(6):1153–1155. doi: 10.1177/0194599820941838. [DOI] [PubMed] [Google Scholar]
- ECDC . European Centre for Disease Prevention and Control; Stockholm: 2020. European Centre for Disease Prevention and Control. Heating, Ventilation, and Air-conditioning Systems in the Context of COVID-19: First Update. (Accessed 23 Feb 2021) [Google Scholar]
- EPA . United States Environmental Protection Agency; 2019; Washington, DC: 2019. United States Environmental Protection Agency. Air Cleaners, HVAC Filters, and Coronavirus (COVID-19)https://www.epa.gov/coronavirus/air-cleaners-hvac-filters-and-coronavirus-covid-19 (Accessed 22 Feb 2021) [Google Scholar]
- Fernández de Mera I., Rodríguez del Río F.J., de la Fuente J., Pérez Sancho M., Hervás D., Moreno I., Domínguez M., Domínguez L., Gortázar C. Detection of environmental SARS-CoV-2 RNA in a high prevalence setting in Spain. Transbound. Emerg. Dis. 2020;1-6 doi: 10.1111/tbed.13817. [DOI] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Won J., Choi B.Y., Lee C.J. Optimization of primer sets and detection protocols for SARS-CoV-2 of coronavirus disease 2019 (COVID-19) using PCR and real-time PCR. Exp. Mol. Med. 2020;52:963–977. doi: 10.1038/s12276-020-0452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather K.A., Marr L.C., Schooley R.T., McDiarmid M.A., Wilson M.E., Milton D.K. Airborne transmission of SARS-CoV-2. Science. 2020;370(6514):303–304. doi: 10.1126/science.abf0521. [DOI] [PubMed] [Google Scholar]
- Qian H., Miao T., Liu L., Zheng X., Luo D., Li Y. Indoor transmission of SARS-CoV-2. Indoor Air. 2020 doi: 10.1111/ina.12766. [DOI] [PubMed] [Google Scholar]
- Razzini K., Castrica M., Menchett L., Maggi L., Negroni L., Orfeoa N.V., et al. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19ward of a hospital in Milan, Italy. Sci. Total Environ. 2020;742:140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RD 102/2011 Relativo a la mejora de la calidad del aire. Ministerio de la Presidencia BOE-A-2011-1645. https://www.boe.es/eli/es/rd/2011/01/28/102/con (Accessed 23 Feb 2021)
- Ren Y.H., Marzouk Q., Richard T., Pembroke K., Martone P., Venner T., et al. Effects of mechanical ventilation and portable air cleaner on aerosol removal from dental treatment rooms. J. Dent. 2021;105 doi: 10.1016/j.jdent.2020.103576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen G.A., van Rijn C., Kooij S., Bem R.A., Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir. Med. 2020;8(7):658–659. doi: 10.1016/S2213-2600(20)30245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TNCPERET The novel coronavirus pneumonia emergency response epidemiology team. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 Novel Coronavirus diseases (COVID-19) -China 2020. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D., Holbrook M., Gamble A., Williamson B., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. 2015. https://apps.who.int/iris/bitstream/handle/10665/69477 (Accessed 23 Feb 2021) [PubMed]
- WHO . 2021. World Health Organization 2021. Clinical Management of COVID-19. WHO/2019-nCoV/clinical/2021.1. [Google Scholar]
- WHO 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (Accessed 22 Feb 2021)
- Zhao B., An N., Chen C. Using an air purifier as a supplementary protective measure in dental clinics during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Liu Y., Chen C. Air purifiers: a supplementary measure to remove airborne SARS-CoV-2. Build. Environ. 2020;177:106918. doi: 10.1016/j.buildenv.2020.106918. [DOI] [PMC free article] [PubMed] [Google Scholar]