Abstract
Wastewater-based surveillance for SARS-CoV-2 has been used for the early warning of transmission or objective trending of the population-level disease prevalence. Here, we describe a new use-case of conducting targeted wastewater surveillance to complement clinical testing for case identification in a small community at risk of COVID-19 transmission. On 2 July 2020, a cluster of COVID-19 cases in two unrelated households residing on different floors in the same stack of an apartment building was reported in Singapore. After cases were conveyed to healthcare facilities and six healthy household contacts were quarantined in their respective apartments, wastewater surveillance was implemented for the entire residential block. SARS-CoV-2 was subsequently detected in wastewaters in an increasing frequency and concentration, despite the absence of confirmed COVID-19 cases, suggesting the presence of fresh case/s in the building. Phone interviews of six residents in quarantine revealed that no one was symptomatic (fever/respiratory illness). However, when nasopharyngeal swabs from six quarantined residents were tested by PCR tests, one was positive for SARS-CoV-2. The positive case reported episodes of diarrhea and the case's stool sample was also positive for SARS-CoV-2, explaining the SARS-CoV-2 spikes observed in wastewaters. After the case was conveyed to a healthcare facility, wastewaters continued to yield positive signals for five days, though with a decreasing intensity. This was attributed to the return of recovered cases, who had continued to shed the virus. Our findings demonstrate the utility of wastewater surveillance as a non-intrusive tool to monitor high-risk COVID-19 premises, which is able to trigger individual tests for case detection, highlighting a new use-case for wastewater testing.
Keywords: Wastewater-based epidemiology, COVID-19, Non-intrusive COVID-19 surveillance, COVID-19 case detection
Graphical abstract
1. Introduction
Wastewater-based testing for SARS-CoV-2 has been shown to be a useful COVID-19 surveillance tool (Daughton, 2020). At wastewater treatment plants, it could provide early warning of transmission or objective trending of the population-level disease prevalence (Thompson et al., 2020). In countries such as the Netherlands (Medema et al., 2020), Spain (Randazzo et al., 2020), France (Wurtzer et al., 2020) and the United States (Nemudryi et al., 2020), the detection of SARS-CoV-2 RNA at wastewater treatment plants preceded symptomatic cases. Wastewater surveillance has also been used as an additional indicator to provide prevalence trends of SARS-CoV-2 in the sampled communities (National Institute for Public Health and the Environment, 2020; Peccia et al., 2020). More recently, surveillance at high-density living premises types, such as student hostels, has also been carried out (Chelvan, 2021; Peiser, 2020).
In Singapore, more than 58,000 COVID-19 cases have been reported from January to December 2020, mainly in community clusters and migrant worker dormitories. Similar to other countries, active wastewater monitoring is also being conducted in Singapore since February 2020 at water reclamation plants for wide area surveillance, and at high density living premises types, such as worker dormitories to provide timely assessment of the COVID-19 situation (Mohan, 2020; National Environment Agency, 2020).
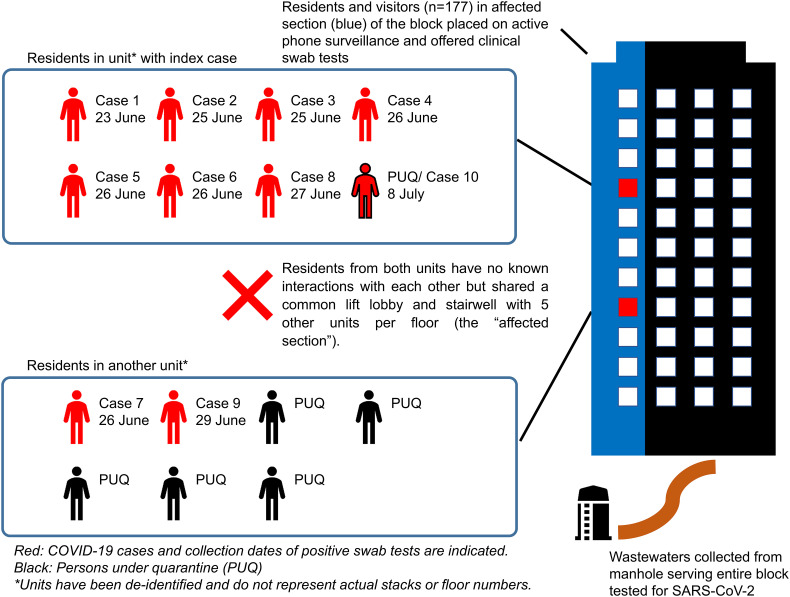
On 2 July 2020, the Ministry of Health in Singapore reported a cluster of COVID-19 cases in two unrelated households on different floors of the same apartment building (Goh, 2020). Six apartment units on each floor of this building share lifts, lift lobbies and stairwell. The first case (index case) was reported on 23 Jun 2020, followed by eight additional cases identified through active clinical surveillance. Six of these were among seven family members who reside with the index case. Two remaining cases were among seven residents in another unit neighboring the index case (Fig. 1 ). Residents of the two affected units had no known interactions.
Fig. 1.
Schematic representation of the cluster of cases in the residential building. The cluster comprised of nine cases residing in two units located on different floors of the same apartment building. Residents from both units had no known interactions, but shared common lift lobby and stairwell with five other units in each floor (the “affected section”).
All cases were conveyed to healthcare facilities for clinical management and isolation, and household members in the two affected units were placed under home quarantine for 14 days. Active clinical surveillance that comprised a one-time offer of PCR testing and phone interview for symptoms was conducted among other residents and visitors of the affected building. To complement clinical surveillance, wastewater surveillance was implemented from 4 to 20 July 2020 for continuous monitoring of the building.
Here, we describe a new use-case of wastewater surveillance that facilitated the detection of a new COVID-19 case prior to onset of symptoms in the affected apartment building, demonstrating the utility of wastewater surveillance as a non-intrusive tool to monitor high-risk COVID-19 premises.
2. Materials and methods
2.1. Clinical surveillance
Persons under quarantine (PUQs) who resided in the two affected units (one and five PUQs, respectively) were placed on active phone surveillance. Nasopharyngeal swab PCR tests were conducted at the start and end of their quarantine period. Another 152 residents and 25 visitors of the affected section of the building were offered PCR tests and were placed on active phone surveillance and monitored for fever or respiratory symptoms until 12 July 2020. All epidemiological investigations and outbreak containment measures were implemented under the Infectious Diseases Act (Singapore Statutes Online, 2003).
2.2. Wastewater sampling
Wastewater samples were collected from a manhole serving the residential building using an autosampler (Aquacell P2 Multiform, Aquamatic, United Kingdom). Hourly wastewater composites were collected during the diurnal sewage peaks (0500–1500 h and 1700–2200 h) from 4 to 20 July 2020. Each hourly composite sample collected (n = 159) included four 200 mL wastewater samples drawn from the manhole every 15 min. Nevertheless, samples could not be collected due to low sewage flow during certain hours. Details of sample collection and the list of samples successfully collected are provided in the Supplementary Data (Tables S1 and S2). All samples were transported daily to the laboratory on ice at 4–8 °C.
2.3. Virus concentration and RNA extraction
An aliquot of 45 mL of each hourly composite sample was concentrated using an adapted polyethylene glycol (PEG) precipitation method (Ahmed et al., 2020; World Health Organization, 2003). Briefly, each sample aliquot was clarified through centrifugation at 4000 ×g for 30 min at 4 °C. The supernatant was added to 3.6 g of PEG (8% w/v, polyethylene glycol 8000, Sigma Aldrich) and 0.875 g of sodium chloride (Sigma Aldrich). The supernatant-PEG mixture was incubated overnight at 4 °C on a rotating shaker at 200 rpm and was subsequently centrifuged at 4000 ×g for 180 min at 4 °C. The supernatant was removed, and the virus pellet was suspended in 300 μL of phosphate buffered saline.
This adapted method had a SARS-CoV-2 recovery rate of 59.5 ± 10.4% and was chosen because of its higher recovery rate in our laboratory settings when compared with other reported virus concentration methods (Ahmed et al., 2020). The recovery rate for the virus concentration method was obtained using SARS-CoV-2 positive wastewater samples with known SARS-CoV-2 virus concentration, where virus concentrations of the samples had been previously determined.
The recovery rate was obtained using the following formula, and tests were conducted in duplicate:
Virus RNA was extracted from 200 μL of the suspended virus pellet using the KingFisher Flex System and MagMAX Viral Pathogen II Kit (ThermoFisher, USA) according to the manufacturer's recommendations.
2.4. Molecular testing
SARS-CoV-2 ORF1ab gene (World Health Organization, 2020) and the PMMoV faecal indicator (Gu et al., 2018) were detected in RNA extracted from wastewater samples by using quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR) assays described elsewhere (Niu et al., 2020; Zhang et al., 2006). PMMoV, which is widespread and abundant in human faeces (Kitajima et al., 2018; Rosario et al., 2009), was primarily used as an indicator of human faecal contamination in wastewater samples and as an indirect indicator of PCR inhibition. A negative PMMoV result was assumed to indicate either the absence of faecal matter in tested samples or inhibition of PCR assay due to various inhibitors present in wastewater.
The original assays were optimized to perform a one-step RT-PCR, by using Luna Universal Probe One-Step RT-qPCR Kit (NEB, USA). Details on assay optimisation are provided in the Supplementary Data. Each PCR reaction was carried out in a 20 μL final reaction volume containing 1× Luna Universal Probe One-Step Reaction Mix, 1× Luna WarmStart RT Enzyme Mix, either 0.5 μM (ORF1ab) or 0.9 μM (PMMoV) of each primer, either 0.25 μM (ORF1ab) or 0.2 μM (PMMoV) of the probe and 2.5 μL of RNA template. The reaction protocol consisted of reverse transcription for 10 min at 55 °C, initial denaturation at 95 °C for 1 min, and 45 amplification cycles of denaturation at 95 °C for 10 s and extension at 55 °C for 30 s. The copy numbers of each target were calculated from quantification cycle (Cq) values by using standard curves generated from serial dilutions of known copy numbers of a SARS-CoV-2 RNA synthetic control (complete genome based, Twist Synthetic SARS-CoV-2 RNA Control 2, MN908947.3; Twist Bioscience, USA) and PMMoV RNA synthetic control (110 bp; Integrated DNA Technologies, Singapore). Both standard curves were generated by using triplicates of 5-log serial dilutions. The thresholds of detection were determined based on the dynamic range of amplification for ORF1ab and PMMoV targets. The lowest level of detection for ORF1ab was 10 copies of synthetic SARS-CoV-2 RNA per reaction at an average Cq of 39.36. The respective value for PMMoV target was 150 copies of synthetic RNA per reaction at an average Cq of 39.96. Therefore, evidence of amplification at Cq <40 was considered as a “positive signal” for each target. All tests were performed in triplicates and an average concentration is reported.
3. Results
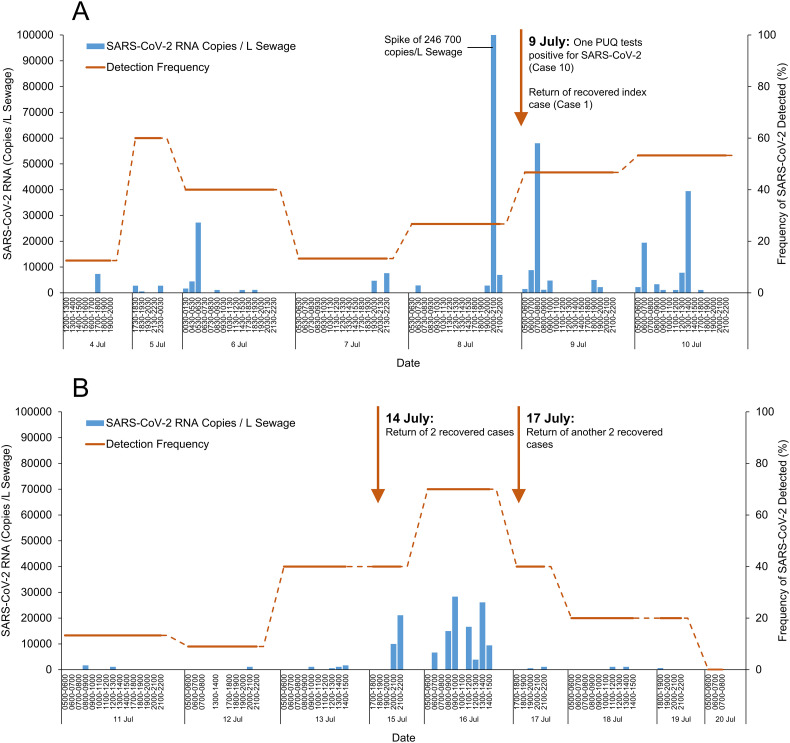
The one-time swab tests were done on 110 residents and 13 visitors of the affected building from 1 to 3 July 2020. All tests yielded negative SARS-CoV-2 results. However, SARS-CoV-2 was detected in one of eight wastewater samples collected on the first day of deployment on 4 July 2020 (7000 RNA copies/L sewage) (Fig. 2A ), suggesting active virus shedding in the building. SARS-CoV-2 was detected in wastewaters daily from 4 to 9 July 2020, with frequent spikes ranging from 600 to 246,700 RNA copies/L sewage (Fig. 2A).
Fig. 2.
SARS-CoV-2 virus RNA in wastewater samples collected from the apartment building. Panel A – SARS-CoV-2 RNA levels from 4 to 10 July 2020; frequent spikes in virus RNA levels corroborated the new case reported on 9 July 2020. Panel B – SARS-CoV-2 RNA levels from 11 to 20 July 2020; detection of SARS-CoV-2 RNA levels during 15–16 July 2020 and subsequent decline in virus RNA signals corroborated the return of two recovered cases on 14 July 2020. Return of another two recovered cases on 17 July 2020 did not result in spikes of SARS-CoV-2 RNA levels.
Despite positive wastewater test results, none of the persons under quarantine (n = 6) in the two affected apartment units reported COVID-19 symptoms. Nasopharyngeal swab PCR tests were carried out on all persons under quarantine on 8 July 2020, and one was positive for SARS-CoV-2 (Cq value 22.64, 22.16). The new case (Case 10) was subsequently conveyed to a healthcare facility. Case 10 reported gastrointestinal symptoms, with episodes of diarrhea since 7 July 2020. The stools of case 10 were positive for SARS-CoV-2 on 11 July 2020.
The continuous screening of wastewater facilitated the identification of a COVID-19 case in the affected building. Additionally, the diarrheal episodes and positive stool of this new case explained the spikes of virus RNA levels observed from 4 to 9 July 2020. Notably, SARS-CoV-2 RNA was detected in wastewaters more than three days before the case recalled diarrheal, fever and respiratory symptoms, suggesting that viral shedding in faecal material could have started in the pre-symptomatic period.
The index case (Case 1) returned from the healthcare facility on 8 July 2020 after clinical recovery. Therefore, wastewater signals detected on 9 July 2020 could be either due to viral shedding from the newly identified case (Case 10) or from the returned index case. However, SARS-CoV-2 levels declined after the newly identified case was conveyed to a healthcare facility (1100–39,400 to 600–1700 RNA copies/L sewage from 10 to 13 July 2020) (Fig. 2B), suggesting that subsequent viral shedding was from the recovered index case, rather than from another additional new case/s. This contrasts to the frequent spikes in virus RNA levels observed from 4 to 9 July 2020, before the new case was identified.
Interestingly, SARS-CoV-2 levels increased again during 15–16 July 2020 (3900–28,300 RNA copies/L sewage) and was attributed to two recovered cases who returned on 14 July 2020 (Fig. 2B). SARS-CoV-2 levels were not detected from the evening of 19 July 2020, corroborating the viral shedding pattern of recovered cases and the cessation of active virus transmission in the building.
4. Discussion
This report describes a new use-case for wastewater surveillance, in which monitoring of a relatively small community at high risk of COVID-19 provided actionable data that informed operational decisions. Positive and intensifying SARS-CoV-2 signals in wastewater triggered intrusive individual tests for case identification, while negative or weakening signals assured the gradual cessation of transmission. In contrast to the widely reported, regional-based surveillance at wastewater treatment plants implemented for situational assessment (La Rosa et al., 2020; Prado et al., 2021), this targeted approach of wastewater surveillance complemented the cost- and logistics- intensive individual testing to facilitate early identification of a COVID-19 case.
Detection of virus RNA in wastewaters before the onset of symptoms in the present study supports the notion that faecal material can be positive for SARS-CoV-2, even when cases are asymptomatic (Ahmad et al., 2020; Tang et al., 2020). It is now known that SARS-CoV-2 shedding can persist longer in stool samples than in nasopharyngeal swabs (Gupta et al., 2020). Taken together, this suggests that stool samples have a longer detection window for SARS-CoV-2 than other commonly-tested biological samples, underscoring the utility of wastewater testing as a COVID-19 surveillance strategy.
The present study also revealed the importance of longitudinal wastewater surveillance for the continuous monitoring of SARS-CoV-2 in the community. Single time point detection of SARS-CoV-2 in wastewaters provided limited information on whether a positive signal originates due to viral shedding by newly-emerging cases or from a recovered case. In contrast, continued monitoring and trend analyses could reveal whether positive signals occur due to viral shedding either by a new or a recovered case. Our findings also emphasized that the contribution of residual viral shedding from recovered cases must be accounted for when modelling wastewater surveillance-based estimates of COVID-19 prevalence in affected communities.
4.1. Limitations
It was a challenge to discern whether viral signals were due to new or recovered cases, although the increasing or decreasing trends of viral RNA levels could be ascribed to new and recovered cases respectively. Another limitation was that wastewater samples could not be collected in every hour for each sampling day due to low sewage flow. Although 24-h daily composites have been recommended for other surveillance studies (Centres for Disease Control and Prevention, 2020), hourly composites provided improved sensitivity for case detection presumably because samples were not diluted by negative samples collected at different hours. However, the hourly approach requires a large number of wastewater samples to be tested, and could thus be a limitation for laboratories with limited resources.
Despite demonstrating a new use-case for wastewater surveillance, the approach described in the present study might be resource-intensive and/or limited by the access to suitable sampling sites to capture the targeted population.
5. Conclusions
-
•
This study highlights a new use-case for non-intrusive wastewater testing at high-risk sites that facilitates case identification with minimal inconvenience to the community.
-
•
The study demonstrated that viral shedding by even a single case could be detected in wastewater from a small community.
-
•
The findings also emphasized limitations when interpreting single time point wastewater test results, as positive signals might be either from active infections or recovered cases. Longitudinal monitoring of wastewater could thus be appropriate in wastewater-based COVID-19 surveillance programmes.
CRediT authorship contribution statement
Judith Chui Ching Wong: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Joanna Tan: Formal analysis, Investigation, Writing – original draft. Ying Xian Lim: Formal analysis, Investigation, Writing – original draft. Sathish Arivalan: Methodology, Investigation. Hapuarachchige Chanditha Hapuarachchi: Methodology, Investigation, Writing – review & editing. Diyar Mailepessov: Investigation. Jane Griffiths: Investigation. Praveena Jayarajah: Investigation. Setoh Yin Xiang: Investigation. Wei Ping Tien: Investigation. Swee Ling Low: Investigation. Carmen Koo: Investigation. Surya Pavan Yenamandra: Investigation. Marcella Kong: Investigation. Vernon Jian Ming Lee: Supervision, Writing – review & editing. Ng Lee Ching: Supervision, Conceptualization, Methodology, Formal analysis, Writing – review & editing.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
Acknowledgements
The authors would like to thank Ms. Quek Hui Leng for her administrative support as well as Janelle Thompson, Stefan Wuerst, Karina Gin and Eric Alm for their useful discussions.
Funding/support
This study was internally funded by the National Environment Agency - Singapore.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.147419.
Appendix A. Supplementary data
Supplementary material
References
- Ahmad I., Flanagan R., Staller K. Increased internet search interest for GI symptoms may predict COVID-19 cases in U.S. hotspots. Flinical Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centres for Disease Control and Prevention Developing a Wastewater Surveillance Sampling Strategy. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance/developing-a-wastewater-surveillance-sampling-strategy.html Retrieved 1 April 2021 from.
- Chelvan V.P. Channel News Asia; 2021. 437 NUS UTown Hostel Residents Swabbed for COVID-19 After Viral Material Found in Wastewater Sample.https://www.channelnewsasia.com/news/singapore/nus-utown-hostel-residents-swabbed-covid-19-moh-14473672 Retrieved 1 April 2021 from. [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T. The Straits Times; 2020. 9 Covid-19 Cases in Blk 111 Tampines St 11, Residents of 58 Households and Their Visitors Offered Testing.https://www.straitstimes.com/singapore/health/coronavirus-9-confirmed-covid-19-cases-detected-in-blk-111-tampines-st-11-residents [Google Scholar]
- Gu X., Tay Q.X.M., Te S.H., Saeidi N., Goh S.G., Kushmaro A., Thompson J.R., Gin K.Y. Geospatial distribution of viromes in tropical freshwater ecosystems. Water Res. 2018;137:220–232. doi: 10.1016/j.watres.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Parker J., Smits S., Underwood J., Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Color. Dis. 2020;22(6):611–620. doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Sassi H.P., Torrey J.R. Pepper mild mottle virus as a water quality indicator. Clean Water. 2018;1(19) doi: 10.1038/s41545-018-0019-5. [DOI] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mohan M. Channel News Asia; 2020. From Manhole to Sampling Bottle: How Wastewater Helps Indicate Presence of COVID-19 in Foreign Worker Dormitories.https://www.channelnewsasia.com/news/singapore/foreign-worker-dormitories-sampling-testing-covid19-wastewater-12953408 Retrieved 1 April 2021 from. [Google Scholar]
- National Environment Agency NEA Leads Scientific Team in Wastewater Surveillance Trials for Assessment Of COVID-19 Transmission. 2020. https://www.nea.gov.sg/media/news/news/index/nea-leads-scientific-team-in-wastewater-surveillance-trials-for-assessment-of-covid-19-transmission Retrieved 1 April 2021 from.
- National Institute for Public Health and the Environment Sewage Research: Decline of Novel Coronavirus in the Netherlands. 2020. https://www.rivm.nl/en/news/sewage-research-decline-of-novel-coronavirus-in-netherlands Retrieved 1 April 2021 from.
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu P., Lu R., Zhao L., Wang H., Huang B., Ye F., Wang W., Tan W. Vol. 2. China CDC Weekly; 2020. Three Novel Real-time RT-PCR Assays for Detection of COVID-19 Virus; pp. 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser J. The University of Arizona says it caught a dorm’s covid-19 outbreak before it started. Its secret weapon: poop. Wash. Post. 2020 https://www.washingtonpost.com/nation/2020/08/28/arizona-coronavirus-wastewater-testing/ Retrieved 1 April 2021 from. [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Resende P.C., Motta F.C., Eppinghaus A.L.F., Chagas do Vale V.H., Braz R.M.S., de Andrade J.D.S.R., Maranhão A.G., Miagostovich M.P. Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res. 2021;191 doi: 10.1016/j.watres.2021.116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario K., Symonds E.M., Sinigalliano C., Stewart J., Breitbart M. Pepper mild mottle virus as an indicator of fecal pollution. Appl. Environ. Microbiol. 2009;75(22):7261–7267. doi: 10.1128/AEM.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singapore Statutes Online Infectious Diseases Act, Chapter 137. 2003. https://sso.agc.gov.sg/Act/IDA1976 Retrieved 1 April 2021 from.
- Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N., Wu W.J., Yuan C., Yu M.L., Li P., Yan J.B. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26(6):1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal V.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184 doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO/V&B/03.03; 2003. Guidelines for Environmental Surveillance of Poliovirus Circulation.http://archives.who.int/vaccines-documents/DocsPDF03/www737.pdf [Google Scholar]
- World Health Organization Coronavirus Disease (COVID-19) Technical Guidance: Laboratory Testing for 2019-nCoV in Humans. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance Retrieved 9 April from.
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Euro Surveill. 2020;25(50) doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Breitbart M., Lee W.H., Run J.Q., Wei C.L., Soh S.W., Hibberd M.L., Liu E.T., Rohwer F., Ruan Y. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 2006;4(1) doi: 10.1371/journal.pbio.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material