Fig. 2. Structure of AuxSen and critical steps in the engineering process.
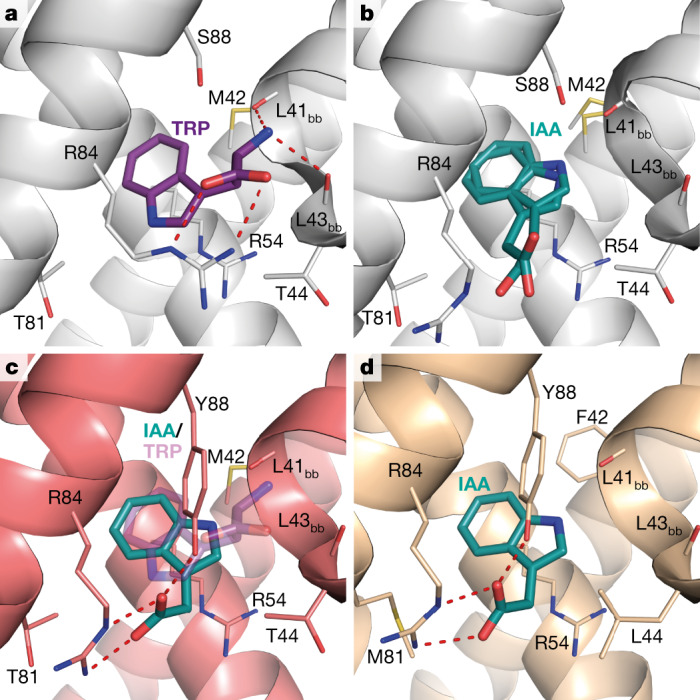
a, b, Structure of TrpR bound to the native ligand TRP (purple, Protein Data Bank (PDB) ID: 1ZT9) (a) and to the design-target IAA (green) (b). IAA is rotated by 180° in the binding pocket compared with TRP. Owing to a lack of stabilization when binding to TrpR, IAA shows conformational freedom; two alternative conformations are shown. c, The mutation S88Y sterically precludes the positioning of TRP (transparent purple) in the binding site while favouring the binding of IAA. d, Structure of the final AuxSen variant (TrpR(M42F/T44L/T81M/N87G/S88Y)) bound to IAA. The ligand is firmly packed in the enhanced hydrophobic pocket of TrpR and is anchored to R84 as well as Y88, resulting in a high affinity of AuxSen for IAA. All structures are superimposed on the Cα of residues 20–60 of both chains. Red dashed lines show polar interactions between ligand and side-chain atoms. The subscript ‘bb’ labels residues that have interactions of backbone atoms with the ligand.
