Abstract
Despite adherence to treatment, individuals living with HIV have an increased risk for developing cognitive impairments, referred to as HIV-associated neurological disorders (HAND). Due to continued growth in the HIV population, particularly amongst the aging cohort, the neurobiological mechanisms of HAND are increasingly relevant. Similar to other viral proteins (e.g. Tat, Gp120, Vpr), the Negative Factor (Nef) is associated with numerous adverse effects in the CNS as well as cognitive impairments. In particular, emerging data indicate the consequences of Nef may be facilitated by the modulation of cellular autophagy as well as its inclusion into extracellular vesicles (EVs). The present review examines evidence for the molecular mechanisms by which Nef might contribute to neuronal dysfunction underlying HAND, with a specific focus on autophagy and EVs. Based on the these data, we propose an integrated model by which Nef may contribute to underlying neuronal dysfunction in HAND and highlight potentially novel therapeutic targets for HAND.
Keywords: HAND, HIV-1 Nef, Autophagy, Exosome
1. Introduction:
Human immunodeficiency virus (HIV) infection and its associated neurological consequences remain a consistent public health concern. Recent reports suggested that an additional 1.7 million people were infected with the virus in 2018, bringing the total number of people living with HIV to 37.9 million worldwide (UN AIDS 2019). Despite improved patient outcomes due to enhanced diagnostic testing and targeted therapies, there remains no effective vaccine for HIV, or a permanent cure for HIV-infected individuals (Davenport et al. 2019). Moreover, HIV continues to be a leading cause of morbidity across the globe (World Health Organization 2018). However, the implementation of combined anti-retroviral therapy (cART) has transformed this once fatal disease into a chronic, manageable condition (Deeks et al. 2013; Saylor et al. 2016). Following its introduction in the mid-1990s, cART has substantially increased the life expectancy of the HIV population, which is now approaching that of the general population (Fauci and Marston 2015; Trickey et al. 2017).
Although current treatments can control HIV infection, infected individuals maintain increased risks for numerous chronic comorbidities, including HIV-Associated Neurological Disorder (HAND) (Sengupta and Siliciano 2018). Amongst HIV infected individuals, HAND is estimated to afflict up to half of patients, even if cognitive deficits are not self-reported or viral loads are suppressed to undetectable levels (Heaton et al. 2010; Simioni et al. 2009). Broadly, these detriments can reflect impairments in several cognitive domains, including executive functioning (e.g. impulsivity, problem solving), memory (e.g. immediate retrieval, working memory) and attention (e.g. sustained attention, strategy shifting) (Clifford and Ances 2013). According to recently adopted criteria, HAND can further be delineated into asymptomatic neurocognitive impairment, mild neurocognitive disorder or dementia, depending on the severity of impairment and subsequent impact on daily functioning (Antinori et al. 2007). Thus, it is such neurocognitive impairments that remain a persistent challenge amongst HIV infected individuals, even those who adhere to therapeutic regimens and maintain long term viral suppression.
In the cART era, the prevelance and clinical presentaiton of HAND has shifted. Specifically, findings from the comprehensive CHARTER (the CNS HIV antiretroviral therapy effects research) cohort indicated an 80% reduction in the most severe forms of cognitive impairment since cART implementation, although milder cognitive deficits were found to persist at comparable rates. While only 2% of virally suppressed individuals are estimated to display severe deficits (i.e. compared to 10–15% pre-CART), more than half (52%) maintain detectable cognitive impairments upon neuropsychological assessment (McArthur et al. 2010). Subsequent reports disputed these findings and suggested the rates of HAND might be substantially lower, in part due to inconsistent testing criteria (Gisslén et al. 2011; McDonnell et al. 2014). Despite active debate surrounding the prevalence of HAND, it is evident that HIV infected individuals in the cART era can maintain significantly greater risk for the development and progression of neurocognitive deficits compared to the general population (Grant et al. 2014; Schouten et al. 2016). Furthermore, such likelihoods remain elevated even if alternative neuropsychological criteria used to asses HIV infected persons (group et al. 2016; Su et al. 2015)
Several key variables render HIV+ individuals at increased risk for HAND, including advancing age, drug use and co-infections (Table 1). Aging is particularly relevant in the cART era, where decreased mortality rates and increased life expectancies have resulted in approximately half of HIV+ individuals reaching at least 50 years of age (Goodkin et al. 2001). In turn, this cohort is consistently found to be at a significantly greater risk for developing HAND (Chiesi et al. 1996; Janssen et al. 1992; L. Fazeli et al. 2014; Valcour et al. 2004). Notably, this age-associated risk for HAND could be due to long term adherence to antiretroviral treatment itself; for instance, cART induces metabolic changes (e.g. central obesity, dyslipidemia, and insulin resistance) which are themselves considered risk factors for dementia (Behrens et al. 1999; Falutz 2007; Narciso et al. 2001; Whitmer et al. 2008). In addition, illicit drug use, including methamphetamine and cocaine, is known to be significantly higher amongst HIV patients who develop HAND, compared to those who do not use illegal substances (Ayuso-Mateos et al. 2000; Byrd et al. 2011). However, it remains unclear if this association is mediated by pre-existing substance use disorders, which themselves can result in cART interruption and increase risk for HAND (Kamal et al. 2017; Meyer et al. 2013). Along with aging and drugs of abuse, several studies have also shown that HIV patients coinfected with other viruses or bacteria have increased risks for developing cognitive impairments (Cherner et al. 2005; Hestad et al. 2019; Marra et al. 2013; Ryan et al. 2004).
Table1.
Factors contributing the pathogenesis of HIV associated neurological disorders
| Host Factors | Age |
|
| Neuroinflammation |
|
|
| HAART |
|
|
| Substance Abuse |
|
|
| Co-morbid conditions |
|
|
| Metabolism |
|
|
| Viral Factors | HIV-1 Genes and proteins | |
| Tat |
|
|
| GP120 |
|
|
| VPR |
|
|
| NEF |
|
|
Although HAND’s prevalence has shifted and risk factors have been identified, the continued growth of the HIV population and ensuing development of neurological impairments has increased focus on the neurobiological underpinnings of HAND. For instance, the implementation of a varieity of neurimaging techniques (e.g. functional magnetic resonance imaging (fMRI), positron emission tomography (PET)) has elucidated structural and functional alterations in specific brain regions (e.g. frontal cortex, hippocampus) that are associated with HAND (Ances and Hammoud 2014; Sanford et al. 2017). However, evidence has also begun to eluciate the molecular mechanisms that might contribute to neuronal dysfunction underlying HAND, including the aberrant consequences of HIV viral proteins.
2. HIV viral proteins in the development of HAND
Although HIV-1 was initially thought to target peripheral immune cells (T-lymphocytes, monocytes/macrophages, dendritic cells), subsequent findings illustrated that CNS immune cells are also susceptible to infection (e.g. macrophages, microglia and astrocytes) (Gartner et al. 1986; Koenig et al. 1986; Pope et al. 1994; Weissman et al. 1995). While the exact mechanisms of infiltration remain debated, the virus is thought to cross the blood-brain-barrier (BBB) within weeks of systemic infection through infiltrating monocytes or infected CD4+ T lymphocytes (Spudich and Gonzalez-Scarano 2012; Valcour et al. 2012). Once in the CNS, microglia and perivascular macrophages serve as persistent and latent viral reservoirs, provided that they are the only resident cells in brain parenchyma that have been shown to support productive HIV-1 infection (Chen et al. 2017; Crowe et al. 2003; Williams et al. 2001). Conversely, while limited evidence suggests neurons and other non-neuronal cells (e.g. astrocytes) may also be infected, it remains unclear if such cell types can support constitutive viral replication (Bissel and Wiley 2004).
Several mechanisms are suggested to contribute to HIV-associated neuronal dysfunction. A chonrically dysregualted neuroinflammatory profile may be a casual factor, provided that HIV elevates levels of various pro-inflammatory cytokines and chemokines in the CNS, including tumor necrosis factor alpha (TNFα), interleukin 1β, 6 and 8 (IL-1β, IL-6, IL-8) and interferon α (IFNα) (Kaul et al. 2001; Tyor et al. 1992). Indeed, compared to uninfected controls and non-demented counterparts, HIV infected individuals suffering from cognitive dysfunction display significantly elevated levels of IFNα in cerebrospinal fluid (CSF) (Rho et al. 1995). Modified microglial functions may also contribute to HAND. Post-mortem tissue analyses indicate that microglia densities are positively correlated with the degree of cognitive dysfunction or impairment in HIV infected individuals. (Anthony et al. 2005; Ghorpade et al. 2005; Glass et al. 1995; Huang et al. 2011). Moreover, amongst virally suppressed individuals who remain cognitively sound, impaired executive performance is nonetheless associated with greater microglia activation across multiple cortical regions, whereas such an association is absent among uninfected controls (Garvey et al. 2014). In addition, abnormal astrocytic functioning may contribute to neurological dysfunction in HIV, given that the percentage of astrocytes harboring the virus is known to significantly correlate with increasing severity of cognitive deficits (Churchill et al. 2009). Consistent with the adverse effects of infected microglia, infected astrocytes can also compromise BBB integrity by altering gap junctions, and disrupt glutamate homeostasis via attenuated expression of amino acid transporters (Eugenin et al. 2011).
While some evidence indicates that HIV-associated neurocognitive impairments are caused by an altered neuroimmune profile or aberrant glial functioning, others suggest the adverse effects of specific viral proteins may ultimately contribute to HAND (Ellis et al. 2007; Nath 2002). The HIV genome is itself composed of nine genes that encode fifteen viral proteins (Frankel and Young 1998). Gag, pol, and env code for structural proteins (MA, CA, NC), enzymes (Pro, RT, IN, RNase H), and envelope proteins (gp120, gp41), while the remaining genes code for regulatory (Tat, Rev) and accessory proteins (Vif, Vpr, Vpu, and Nef). In addition to viral replication, these proteins maintain a plethora of roles, including the regulation of host-cell gene expression, metabolic modifications, and alterations in intracellular signaling cascades (Frankel and Young 1998; Swanson and Malim 2008; Wyatt and Sodroski 1998).
As suggested by initial investigations of Gp120, the neurobiological mechanisms underlying HAND may be due the adverse consequences of HIV viral proteins (Barnes 1987). For instance, Gp120 induces the production of proinflammatory cytokines, such as IL-1B, TNFA, and IL6, that subsequently elevates rates of apoptosis in both neuronal and non-neuronal cells (Cheung et al. 2008; Li et al. 2005; Yeung et al. 1995). Interestingly, such deteriorated neuronal variability could be inhibited by the application of anti-oxidant enzymes, suggesting Gp120’s induction of ROS might also contribute to compromised neuronal functioning in HAND (Louboutin et al. 2012; Reddy et al. 2012). Gp120 has also been shown to disrupt ion regulation and glutamate homeostasis amongst neurons and glia, which can subsequently contribute to contextual memory impairments in vivo (Fernandes et al. 2007; Holden et al. 1999; Wang et al. 2004).
Along with Gp120, the HIV-1 viral protein R (Vpr) may also contribute to neuronal dysfunction underlying HAND. Indeed, Vpr is neurotoxic across a variety of primary cell models, including human hippocampal neurons as well as rat cortical and striatal neurons (Huang et al. 2000; Patel et al. 2000; Piller et al. 1998; Sabbah and Roques 2005). Such compromised viability could be due to direct consequences in neuron’s themselves, given that Vpr can impair the functional capacitates of neural mitochondria while also inhibiting axonal stability (Kitayama et al. 2008; Wang et al. 2017). Conversely, the consequences of this viral protein may be equally aversive across all CNS cell types; for instance, it can disrupt calcium regulation and induce pro-inflammatory cytokine expression in both neuronal and non-neuronal cells alike (Mamik et al. 2017; Na et al. 2011; Rom et al. 2009). Moreover, in vivo expression of Vpr in the rodent brain can result in significantly decreased performance across long-term, spatial memory tasks as well as short-term, working memory tasks (Torres and Noel 2014).
Along with Gp120 and Vpr, the Trans-activator of transcription (Tat) may play a role in facilitating HAND, especially given its persistent detection in the CNS despite viral suppression (Johnson et al. 2013). Indeed, Tat compromises a variety of homeostatic processes in the brain, including neuronal viability, synapse formation and neuroinflammatory profiles (Kim et al. 2008; Nath et al. 1999; Sabatier et al. 1991). In particular, recent in vitro evidence demonstrates Tat’s capacity to not only induce and enhance aberrant protein aggregation, but also its ability to do so in concert with pathologically relevant polypeptides, such as Amyloid β (Aβ) (Fan and He 2016; Hategan et al. 2017; Rempel and Pulliam 2005). Moreover, a substantial body of evidence from preclinical models has illustrated both the exogenous application or endogenous expression of Tat in the CNS results numerous cognitive impairments across a variety of behavioral paradigms (Carey et al. 2012; Fitting et al. 2008; Fitting et al. 2018; Harricharan et al. 2015; Jacobs et al. 2019; Nookala et al. 2018; Zhao et al. 2020). Together, these data further suggest the abnormal persistence of HIV viral proteins in the CNS may contribute to neuronal dysfunction underlying HAND.
In addition to the previously characterized proteins, limited yet accumulating data also suggest a prominent role for the Nef protein in facilitating HIV-associated neurological deficits. Therefore, its association with the development of neurological impairments in HIV, as well as its potential pathogenic mechanisms in the CNS, warrant due consideration.
3. HIV Nef
The Negative Factor (Nef) protein is a multifunctional, 27– 34-kDa polypeptide that has historically been understudied in the context of HAND (Carroll and Brew 2017; Rao et al. 2014). Its gene is located at the 3’-end of HIV-1, HIV-2, and SIV, partially overlapping the 3’ long terminal repeat (LTR) (Laprevotte et al. 2001). A combination of X-ray crystallography and nuclear magnetic resonance has characterized this viral protein’s three-dimensional structure (Arold et al. 1997; Lee et al. 1996). Specifically, it is composed of a folded core (residues 55–65 and 84–203), with flexible N-terminal (residues 1–54) and C-terminal (residues 204–206) domains, as well as a central flexible loop (residues149–179) within the folded core (Geyer and Peterlin 2001). In addition, its myristoylated N-terminus allows for its association with the cytosolic face of cellular membranes and is required for Nef’s cellular interactions (Fackler et al. 1997; Geyer et al. 1999). Such structural features may account for Nef’s abundant interactions with host cell proteins (Figure 1).
Figure 1: Structural motifs of HIV-1 Nef.
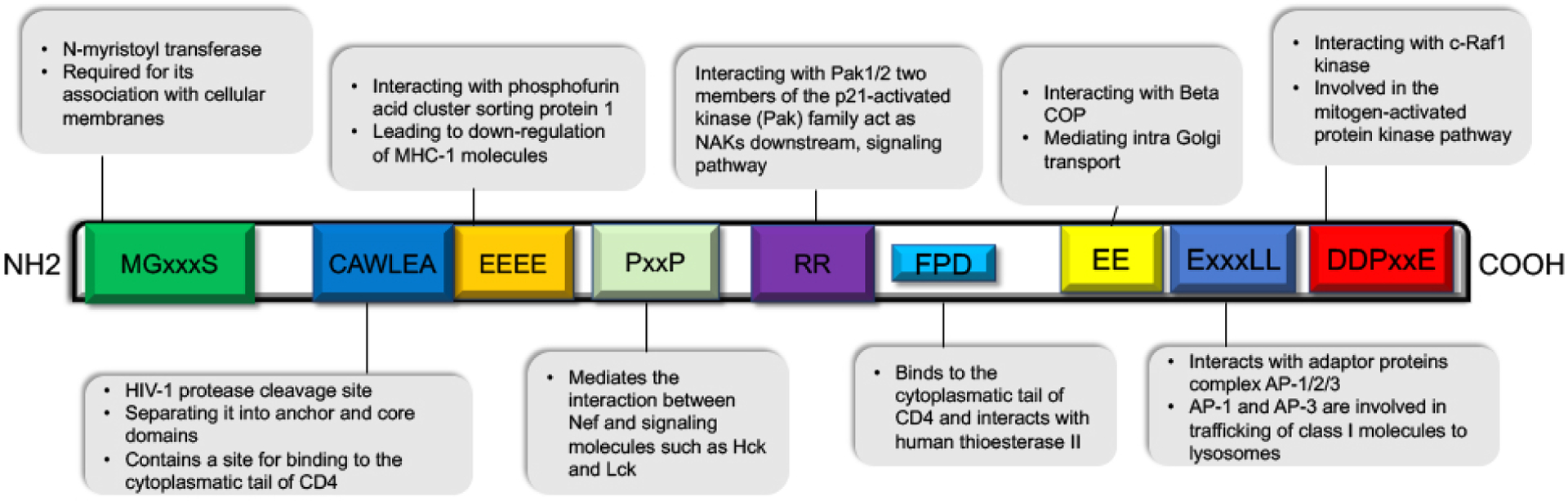
Conserved structural domains of HIV-1 Nef and their interacting proteins are depicted. Nef modification motifs: N-myristoylation site (MGxxx) and HIV-1 protease cleavage site (CAWLEA). Signaling motifs: Proline rich motif (PxxP), RR and DDPxxE. Internalization and trafficking motifs: EEEE, FPD, EE, ExxxLL, and DD.
As its name implies, Nef was initially considered an inhibitor of viral genome transcription, but studies have since shown that Nef is essential for the maintenance of high viral loads, and promotes disease progression to AIDS (Gorry et al. 2007; Hanna et al. 1998; Thompson et al. 2003). For example, HIV-1 particles produced in the presence of Nef are ten times more infectious than particles produced in its absence (Delassus et al. 1991; Kestler et al. 1991). Conversely, HIV strains that lack a functional Nef protein result in delayed disease progression; here, strains with deletions in the Nef gene, due to truncated 3’-LTRs, result in normal CD4 counts and lower viral loads up 14 years post-infection, even in the absence of cART treatment (Deacon et al. 1995; Learmont et al. 1999).
In addition to its roles in HIV pathogenesis, evidence also indicates a potential role for Nef in the HAND development. This postulation was spurred by initial post-mortem analyses of brain tissue from HIV infected individuals; while only half of all patients maintained detectable Nef-positive cells, this rate increased to ~85% amongst those individuals who met behavioral criteria for dementia (Ranki et al. 1995). Along with altered concentrations of Nef within the CNS, HIV individuals displaying cognitive deficits maintain specific structural subtypes of Nef, compared to patients who remain cognitively stable. Following structural bioinformatics, computational modeling and proteomic analyses, findings suggest the Nef protein in the brains of individuals suffering from HAND maintain distinct structural alignments that potentially alter its conformational transitions and subsequent binding potentials (Lamers et al. 2011). Moreover, the Nef structural signatures associated with HAND, including modifications to SRC homology 3 (SH3), apetela 2 (AP-2) and cytokine bindings domains, were found to be specific to the CNS, further suggesting a role for Nef in contributing to HIV associated neurological dysfunction (Lamers et al. 2018).
Nef may promote HAND through a number of complimentary mechanisms in the brain. Amongst neuronal and non-neuronal cell types, exposure to the Nef protein results in significantly decreased metabolic activity and increased rates of cell death (Trillo-Pazos et al. 2000). In vivo expression of Nef not only recapitulates such neuronal loss, but impairs locomotor activity and triggers a robust neuroinflammatory response, including an upregulation of interferon-gamma-inducible protein 10 (IP-10); furthermore, such increased expression of IP-10 has also been detected in the brains of HIV individuals suffering from severe cognitive deficits (van Marle et al. 2004). Along with neuronal loss, Nef may contribute to cognitive impairments by inducing the recruitment of peripheral immune cells into the CNS (Koedel et al. 1999). Specifically, transplantation of Nef-expressing glia into the rat brain results in increased recruitment of peripheral macrophages, as well as neuronal apoptosis and a neuroinflammatory profile; in turn, these animals maintain significantly decreased performance on a variety of cognitive measures, including spatial and non-spatial memory tasks (Chompre et al. 2013; Mordelet et al. 2004). Such Nef-induced recruitment of peripheral immune cells and ensuing behavioral impairments may in part be due to Nef’s capacity to increase expression of CCL2 in the brain, a chemoattractant for circulating monocytes (Chompre et al. 2019; Lehmann et al. 2019) . Along with these mechanisms, emerging data suggest that Nef may facilitate neurological complications through two other pathogenic processes, cellular autophagy and extracellular vesicles.
4. Nef and Autophagy
Autophagy is a dynamic, self-digestive process that captures, isolates and degrades intracellular materials, such as damaged organelles (e.g. mitochondria), intracellular microbes and potentially toxic protein aggregates, in order to maintain a homeostatic cytoplasmic environment and ensure efficient protein quality control (PQC) (Kaur and Debnath 2015). This pathway is characterized by the formation of autophagosomes that sequester damaged cytoplasmic substrates, and ensuing fusion with lysosomes that leads to the degradation of targeted materials (Glick et al. 2010; Reggiori et al. 2012). Although it can serve as an innate self-defense mechanism to control viral spread, particularly follow initial infection, abnormal cellular autophagy is often correlates with HIV pathogenesis (Chiramel et al. 2013; Killian 2012; Lennemann and Coyne 2015).
Investigations have demonstrated such dysregulated autophagy may be caused by HIV-1 proteins themselves. For instance, following exposure to recombinant Tat, primary rodent hippocampal neurons display dose and time dependent decreases in levels of LC3, a protein which is otherwise responsible for initiating autophagosome formation; furthermore, such Tat-induced variation in autophagy is accompanied by elevated neurodegeneration in vivo, particularly in the CA3 region of the hippocampus (Fields et al. 2015; Hui et al. 2012). Similarly disrupted autophagic mechanisms are found in response to Gp120, while data from primary neuronal culture and transgenic models suggest this viral protein’s effects on autophagy may be due to the modulation of mTOR-dependent signaling cascades (Fields et al. 2013; Liu et al. 2019b).
Similar to other HIV-1 viral proteins, Nef can significantly disrupt cellular autophagy (Kyei et al. 2009). Here, Nef’s binding to beclin-1 (BECN1) and ensuing inhibition of autophagosome formation silence autophagy to the levels that are equivalent to uninfected cells (Campbell et al. 2015). The sequestration of BECN1 and inhibition of autophagy by Nef has been replicated in a variety of cell lines and primary cultures, while recent evidence also indicates this effect could be due to Nef’s indirect yet further blockade of BECN1 by enhancing its binding to Bcl2, as well as Nef’s capacity to prevent LC3 lipidation (Castro-Gonzalez et al. 2020). In turn, Nef-dependent inhibition of autophagy can significantly increase cellular susceptibility to apoptosis (Gupta et al. 2017).
The variation in autophagy within the CNS due to Nef may play a crucial role in HAND development, although further investigations employing neuronal cell models are necessary to validate this postulation. One study examined these claims directly utilizing primary human astrocytes expressing the Nef protein following adenoviral transduction. As indicated by the accumulation of ATG8 and p62, Nef emulated the autophagic blockade induced by bafilomycin treatment. Along with morphological alterations indicative of apoptosis, co-transduction with the tandem LC3 vector illustrated that Nef inhibited autophagosome fusion with lysosomes (Saribas et al. 2015).
5. Nef and Extracellular Vesicles
Extracellular vesicles (EVs), are membranous compartments that enable the direct exchange of materials between cells, including nucleic acids, lipids and proteins (Tetta et al. 2013). These vesicles comprise a heterogeneous population of membrane vesicles including microvesicles and exosomes with size variation between 50 nm and 500 nm (Raposo and Stoorvogel 2013b). Exosomes originate from the invagination of endosomal membranes that bud inwardly as intra-luminal vesicles (ILVs) during the biogenesis of multivesicular bodies (MVBs); in turn, MVBs can then fuse with lysosomes and be degraded, or fuse with the plasma membrane and release their ILVs as exosomes (Colombo et al. 2014). By endocytosis or fusion with the plasma membrane, exosomes subsequently release their content into recipient cells (Raposo and Stoorvogel 2013a).
Although they can transfer materials that enhance or maintain physiological homeostasis, a growing body of evidence suggests that exosomes may also facilitate pathogenic processes by transferring deleterious materials (e.g. viral proteins, aggregated proteins, miRNAs, cytokines etc.) (Arakelyan et al. 2017; Konadu et al. 2015; Levy 2017). Indeed, numerous viral proteins can be detected in excreted exosomes from HIV infected individuals, including Nef, Vpr, Gag, Pol and Tat (Anyanwu et al. 2018). As indicated by Gp-120 containing exosomes, such inclusion of viral proteins can double the rate of infection in naive tissue (Arakelyan et al. 2017). Likewise, exosomes from a variety of cell types (primary mouse astrocytes, CD4+ T cells, 293T, U373) can incorporate Tat; in turn, exposure to such Tat-containing exosomes impairs neural cell viability and blunts neurite elongation (Rahimian and He 2016a; Rahimian and He 2016b). Thus, Nef-containing exosomes may also induce similar dysregulations in the CNS and contribute to neuronal dysfunction underlying HAND.
Focus on Nef-containing exosomes was initiated due to reports of extracellular Nef and Nef-containing microvesicles in the circulation of HIV infected individuals, as well evidence that such soluble protein species reliably induce apoptosis in target cells (Fujii et al. 1996; James et al. 2004; Raymond et al. 2011). Subsequent investigations confirmed Nef’s unique myristoylated N-terminus that enables its incorporation into exosomes to a degree that exceeds other viral proteins (i.e. Tat) (Lenassi et al. 2010). Moreover, Nef-containing exosomes are found across various cell models (Jurkat, SupT1, TZM-bl, HeLa, CIITA, Primary T-Cells) and remain functional following fusion with target cells, where they are capable of transactivating HIV LTRs and reducing viability by up to 70% (Campbell et al. 2008; Lenassi et al. 2010). Interestingly, recent in vitro and in vivo data suggest the Nef released by exosomes into recipient cells may impair functioning by augmenting pro-inflammatory signaling (i.e. TNFα, IL-6) as well as compromising a cell’s lipidome (i.e. lipid raft rearrangements, altered cholesterol efflux) (Mukhamedova et al. 2019). Furthermore, inclusion of biologically functional Nef into exosomes appears to be a conserved process, as it has been demonstrated in both non-human primate models as well as primary human astrocytes (McNamara et al. 2018; Pužar Dominkuš et al. 2017).
Emerging evidence provided by the investigations employing neural systems indicate the adverse effects of Nef-containing EVs in the CNS (Table.2). Neural cell lines exposed to plasma derived Nef-EVs from patients with HAD displayed several abnormalities, including enhanced APP expression as well as elevated production and section of toxic amyloid beta (e.g. Aβ1–42); furthermore, compared to exosomes from cognitively sound individuals, exposure to exosomes from individuals diagnosed with HAND similarly induced increased levels of toxic Aβ (Khan et al. 2016). Provided their capacity to serve as latent viral reservoirs, it is notable that microglial derived Nef-exosomes may be particularly potent, as these vesicles can significantly increase BBB permeability and potentiate pro-inflammatory signaling in surrounding CNS macrophages (Raymond et al. 2016). In addition, Nef-containing EVs secreted by Nef transduced primary human astrocytes induce elevated oxidative stress following reuptake by proximal primary neurons (Saribas et al. 2018). Moreover, such Nef-exosomes can also impair functional efficiencies in neurons, as indicated by blunted action potential frequency and decreased spiking activity (Saribas et al. 2018).
Table 2.
In vitro and ex vivo studies for Nef-EVs effects in the CNS
| Model | Nef-EVs Donor Cells | Nef-EVs Recipient cells | Observed effects of Nef-EVs | Reference |
|---|---|---|---|---|
| In vitro cell culture | Nef expressing primary human fetal astrocytes | Primary human fetal neurons | Oxidative stress and neuronal electrophysiological impairment | (Sami Saribas, Cicalese et al. 2018) |
| Nef expressing microglia (CHME5) | In vitro Blood-Brain- Barrier model | Reduced the expression of tight junction protein ZO-1 | (Raymond, Diaz et al. 2016) | |
| Nef expressing HEK 293T cells | In vitro Blood-Brain- Barrier model | Reduced transendothelial electrical resistance (TEER) and increased permeability of the BBB | (Raymond, Diaz et al. 2016) | |
| Nef expressing HEK 293T cells | Microglia (CHME5) | Induces microglial cytokine/ chemokine secretion | (Raymond, Diaz et al. 2016) | |
| Ex vivo | Plasma derived Nef-EVs from patients with HAD | Neuroblastoma cell line (SH-SY5Y) | Enhanced APP expression and Increased expression and secretion of Aβ and Aβ peptides | (Khan, Lang et al. 2016) |
In addition to the adverse effects of Nef on recipient cells, due to its strong association with EVs, Nef-EVs are also considered as a potential drug and antigen delivery tool. Recently, the exosomes carrying mutant Nef (Nef mut) has gained attention as a potential CTL vaccine candidate (Di Bonito et al. 2017; Ferrantelli et al. 2019; Ferrantelli et al. 2018). Nef mut lacks adverse effects of Nef associated with HIV pathogenesis, has high levels of incorporation into EVs, and acts an exosome anchoring domain that can fuse with heterologous protein and loaded with an antigen of interest. (Lattanzi and Federico 2012; Manfredi et al. 2016).
Conclusions
Human immunodeficiency virus (HIV) infection and its neurological comorbidities remain a major health concern worldwide, despite advances in treatment. HAND effect the majority of patients living with HIV, while advanced aged, illicit drug use, and co-infection increase the risk for such cognitive deficits. Within the CNS, a variety of cell types (i.e. microglia, perivascular macrophages, astrocytes) are susceptible to HIV-1 infection and subsequently release viral particles, such as viral proteins. In turn, HIV infiltration of the CNS is associated with several dysregulated processes that are thought to contribute to HAND, including an altered neuroinflammatory profile and aberrant functioning of non-neuronal cells. As exemplified by Gp120, Vpr and Tat, such dysregulated mechanisms may ultimately be a result of viral proteins themselves. In particular, recent evidence suggests Nef is a crucial modulator of neuronal functioning and may contribute to HAND, even in patients receiving cART, by altering cellular autophagy as well as its inclusion into EVs.
Although its functions within the CNS require further elucidation, we propose a mechanistic model by which Nef may contribute to underlying neuronal dysfunction in HAND (Figure 2). Specifically, we highlight Nef’s capacity to inhibit autophagy across multiple cell types, which impairs the degradation of autophagosomes that contains damaged organelles and viral proteins, such as Nef itself. In addition, Nef is readily incorporated into, and translocated by EVs which can subsequently release functional Nef protein in adjacent cells including neurons. Moreover, given that autophagosomes can fuse with MVBs (i.e. amphisomes) and release their content as EVs, it is possible that Nef induced autophagy dysregulation may potentiate the effects of Nef-containing EVs and vice versa (Sami Saribas et al. 2018). Along with its other aberrant consequences (i.e. metabolic alterations, neuroinflammatory modulation, recruitment of peripheral immune cells etc.), Nef’s impairment of autophagy and inclusion into EVs may lead to its uptake by neurons and ultimately compromise neuronal functioning and contribute to cognitive impairments amongst HIV infected individuals.
Figure 2: Potential molecular crosstalk between autophagy and endosome biogenesis leading to Nef release from HIV infected glial cells.
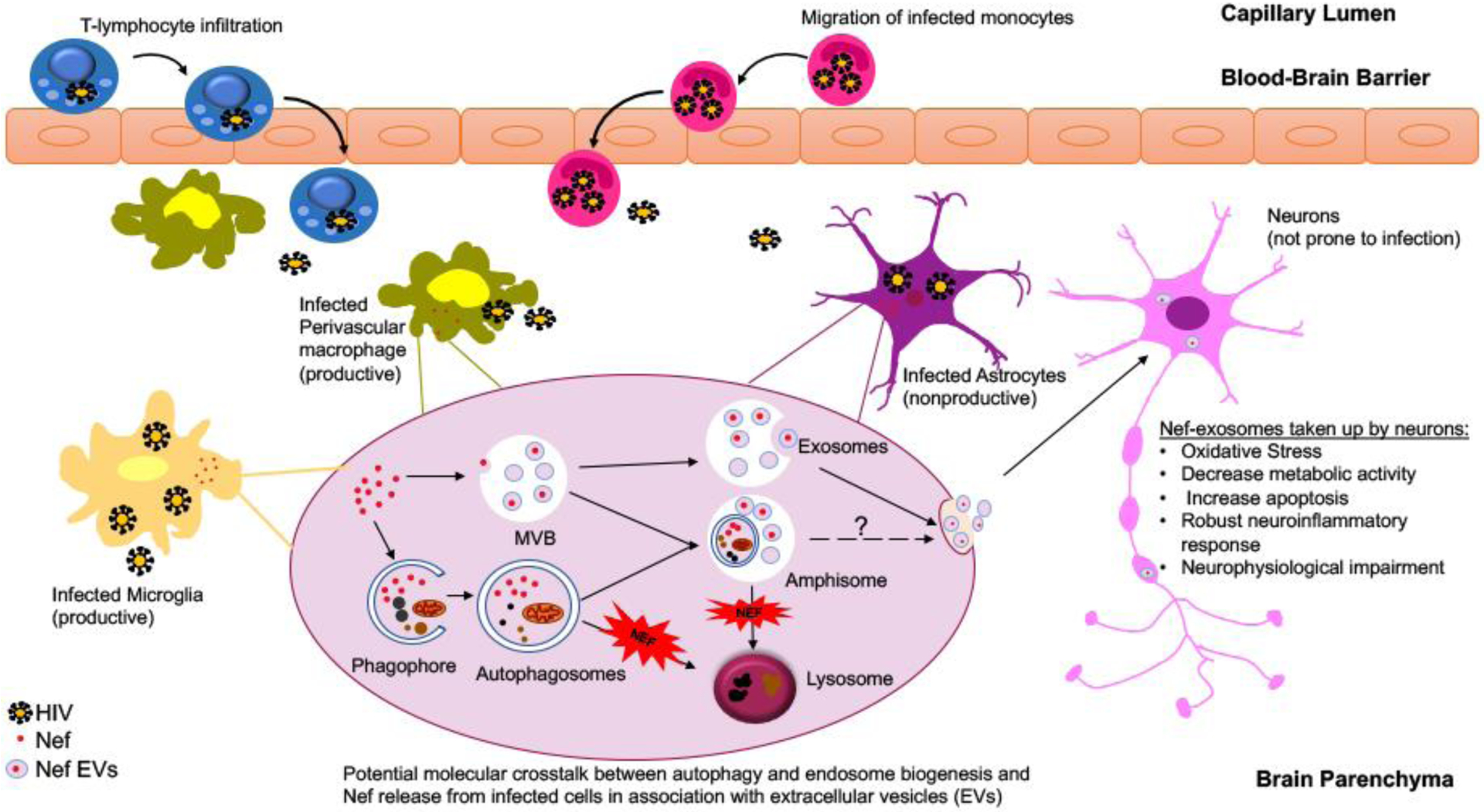
Several models were put forth to explain how cell-associated virus can be responsible for HIV infection of the brain. Infected T-lymphocytes and monocyte-derived macrophages can migrate into the brain from the peripheral circulation and seed the virus in the brain. While perivascular macrophages and microglia are considered as the viral reservoirs supporting the productive viral infection in the brain, HIV establishes infection in astrocytes by abortive infection leading to viral replication and release of viral proteins such as Nef. Limited yet accumulating data suggest a prominent role for Nef release from infected cells through two pathogenic processes, cellular autophagy and extracellular vesicle biogenesis and potentially by utilizing an establishing crosstalk between these two pathways. Within the HIV infected cells, Nef blocks autophagy by inhibiting the fusion of autophagosome with lysosomes. Autophagosomes may fuse with multivesicular bodies (MVBs) to produce organelles termed amphisomes, which can subsequently either fuse with lysosomes for content degradation or fuse the plasma membrane and secrete their content in the extracellular matrix. Released Nef exosomes can be picked up by the neurons leading to neuronal toxicity and impairment.
Given the available evidence of Nef-specific mechanisms in HAND, several therapeutic approaches could prove beneficial and warrant further investigation. Pharmacological enhancement of neuronal autophagy or lysosomal degradation is one such approach, provided that impaired autophagy increases the secretion of Nef-containing exosomes that subsequently hinder neuronal functioning (Saribas et al. 2018). Indeed, the modulation of autophagy is increasingly recognized as a potent therapeutic strategy for a variety of neurodegenerative pathologies (Liu and Li 2019; Liu et al. 2019a).
In addition, the targeted inhibition of Nef on the surface of exosomes can inhibit exosomal reuptake at recipient cells, suggesting the inhibition of extracellular yet membrane-bound Nef (i.e. via monoclonal antibodies or nanoparticle antagonists) may help preserve efficient neuronal functioning (Khan et al. 2016). Such neurotropic immunotherapies warrant particular consideration, provided their increasing application in Alzheimer Disease. In light of rapid advancing gene editing techniques, it may also prove beneficial to target Nef transcripts directly, as functional Nef mRNA can be translocated between cells via exosomes, potentially exacerbating the pathogenic contributions of Nef in HAND (Abudayyeh et al. 2019; Khan et al. 2016).
Acknowledgments
This work was supported, in part, by grants awarded by the NIH to IKS. SSY and MD are supported by Interdisciplinary and Translational Research Training in NeuroAIDS (T32MH079785).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References:
- Abudayyeh OO et al. (2019) A cytosine deaminase for programmable single-base RNA editing Science 365:382–386 doi: 10.1126/science.aax7063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIDS U (2019) Global HIV & Aids statistics - 2019 fact sheet.
- Ances BM, Hammoud DA (2014) Neuroimaging of HIV-associated neurocognitive disorders (HAND) Curr Opin HIV AIDS 9:545–551 doi: 10.1097/COH.0000000000000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony I, Ramage S, Carnie F, Simmonds P, Bell J (2005) Influence of HAART on HIV-related CNS disease and neuroinflammation Journal of Neuropathology & Experimental Neurology 64:529–536 [DOI] [PubMed] [Google Scholar]
- Antinori A et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders Neurology 69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyanwu SI et al. (2018) Detection of HIV-1 and Human Proteins in Urinary Extracellular Vesicles from HIV Advances in virology 2018 [DOI] [PMC free article] [PubMed]
- Arakelyan A, Fitzgerald W, Zicari S, Vanpouille C, Margolis L (2017) Extracellular Vesicles Carry HIV Env and Facilitate Hiv Infection of Human Lymphoid Tissue Scientific Reports 7:1695 doi: 10.1038/s41598-017-01739-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arold S, Franken P, Strub M-P, Hoh F, Benichou S, Benarous R, Dumas C (1997) The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling Structure 5:1361–1372 doi: 10.1016/S0969-2126(97)00286-4 [DOI] [PubMed] [Google Scholar]
- Ayuso-Mateos JL, Pereda M, Gómez del Barrio A, Echevarria S, Fariñas MC, García-Palomo D (2000) Slowed Reaction Time in HIV-1-Seropositive Intravenous Drug Users without AIDS Eur Neurol 44:72–78 doi: 10.1159/000008200 [DOI] [PubMed] [Google Scholar]
- Barnes D (1987) Solo actions of AIDS virus coat Science 237:971–973 doi: 10.1126/science.3039663 [DOI] [PubMed] [Google Scholar]
- Behrens G et al. (1999) Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors: AIDS 13:F63–F70 doi: 10.1097/00002030-199907090-00001 [DOI] [PubMed] [Google Scholar]
- Bissel SJ, Wiley CA (2004) Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations Brain Pathol 14:97–108 doi: 10.1111/j.1750-3639.2004.tb00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA et al. (2011) Neurocognitive Impact of Substance Use in HIV Infection: JAIDS Journal of Acquired Immune Deficiency Syndromes 58:154–162 doi: 10.1097/QAI.0b013e318229ba41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Rawat P, Bruckman RS, Spector SA (2015) Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration PLoS pathogens 11:e1005018 doi: 10.1371/journal.ppat.1005018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TD, Khan M, Huang MB, Bond VC, Powell MD (2008) HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions Ethn Dis 18:S2-14-19 [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP (2012) Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse Behavioural brain research 229:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A, Brew B (2017) HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment F1000Res 6:312 doi: 10.12688/f1000research.10651.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Gonzalez S et al. (2020) HIV-1 Nef counteracts autophagy restriction by enhancing the association between BECN1 and its inhibitor BCL2 in a PRKN-dependent manner Autophagy:1–25 doi: 10.1080/15548627.2020.1725401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NC, Partridge AT, Sell C, Torres C, Martín-García J (2017) Fate of microglia during HIV-1 infection: From activation to senescence?: Microglial Dysfunction During HIV-1 Infection Glia 65:431–446 doi: 10.1002/glia.23081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M et al. (2005) Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine Neurology 64:1343–1347 doi: 10.1212/01.WNL.0000158328.26897.0D [DOI] [PubMed] [Google Scholar]
- Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG (2008) Signaling Mechanism of HIV-1 gp120 and Virion-Induced IL-1β Release in Primary Human Macrophages The Journal of Immunology 180:6675–6684 doi: 10.4049/jimmunol.180.10.6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesi A et al. (1996) Epidemiology of AIDS Dementia Complex in Europe JAIDS Journal of Acquired Immune Deficiency Syndromes 11:39–44 [DOI] [PubMed] [Google Scholar]
- Chiramel A, Brady N, Bartenschlager R (2013) Divergent Roles of Autophagy in Virus Infection Cells 2:83–104 doi: 10.3390/cells2010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chompre G, Cruz E, Maldonado L, Rivera-Amill V, Porter JT, Noel RJ (2013) Astrocytic expression of HIV-1 Nef impairs spatial and recognition memory Neurobiology of Disease 49:128–136 doi: 10.1016/j.nbd.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chompre G, Martinez-Orengo N, Cruz M, Porter JT, Noel RJ (2019) TGFβRI antagonist inhibits HIV-1 Nef-induced CC chemokine family ligand 2 (CCL2) in the brain and prevents spatial learning impairment Journal of Neuroinflammation 16:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR (2009) Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia Ann Neurol 66:253–258 doi: 10.1002/ana.21697 [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder The Lancet Infectious Diseases 13:976–986 doi: 10.1016/S1473-3099(13)70269-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C (2014) Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. In: Schekman R, Lehmann R (eds) Annual Review of Cell and Developmental Biology, Vol 30, vol 30. Annual Review of Cell and Developmental Biology. Annual Reviews, Palo Alto, pp 255–289. doi: 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Crowe S, Zhu T, Muller WA (2003) The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection Journal of Leukocyte Biology 74:635–641 doi: 10.1189/jlb.0503204 [DOI] [PubMed] [Google Scholar]
- Davenport MP, Khoury DS, Cromer D, Lewin SR, Kelleher AD, Kent SJ (2019) Functional cure of HIV: the scale of the challenge Nat Rev Immunol 19:45–54 doi: 10.1038/s41577-018-0085-4 [DOI] [PubMed] [Google Scholar]
- Deacon NJ et al. (1995) Genomic Structure of an Attenuated Quasi Species of HIV-1 from a Blood Transfusion Donor and Recipients Science 270:988–991 doi: 10.1126/science.270.5238.988 [DOI] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Havlir DV (2013) The end of AIDS: HIV infection as a chronic disease The Lancet 382:1525–1533 doi: 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delassus S, Cheynier R, Wain-Hobson S (1991) Evolution of human immunodeficiency virus type 1 nef and long terminal repeat sequences over 4 years in vivo and in vitro Journal of Virology 65:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bonito P et al. (2017) Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation Int J Nanomedicine 12:4579–4591 doi: 10.2147/ijn.S131309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair Nature Reviews Neuroscience 8:33–44 [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW (2011) Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism The Journal of neuroscience : the official journal of the Society for Neuroscience 31:9456–9465 doi: 10.1523/JNEUROSCI.1460-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT et al. (1997) Association of Human Immunodeficiency Virus Nef Protein with Actin is Myristoylation Dependent and Influences its Subcellular Localization Eur J Biochem 247:843–851 doi: 10.1111/j.1432-1033.1997.00843.x [DOI] [PubMed] [Google Scholar]
- Falutz J (2007) Therapy Insight: body-shape changes and metabolic complications associated with HIV and highly active antiretroviral therapy Nat Rev Endocrinol 3:651–661 doi: 10.1038/ncpendmet0587 [DOI] [PubMed] [Google Scholar]
- Fan Y, He JJ (2016) HIV-1 Tat induces unfolded protein response and endoplasmic reticulum stress in astrocytes and causes neurotoxicity through glial fibrillary acidic protein (GFAP) activation and aggregation J Biol Chem 291:22819–22829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Marston HD (2015) Ending the HIV–AIDS Pandemic — Follow the Science N Engl J Med 373:2197–2199 doi: 10.1056/NEJMp1502020 [DOI] [PubMed] [Google Scholar]
- Fernandes SP, Edwards TM, Ng KT, Robinson SR (2007) HIV-1 protein gp120 rapidly impairs memory in chicks by interrupting the glutamate–glutamine cycle Neurobiology of Learning and Memory 87:1–8 doi: 10.1016/j.nlm.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Ferrantelli F et al. (2019) The Intracellular Delivery Of Anti-HPV16 E7 scFvs Through Engineered Extracellular Vesicles Inhibits The Proliferation Of HPV-Infected Cells Int J Nanomedicine 14:8755–8768 doi: 10.2147/ijn.S209366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrantelli F, Manfredi F, Chiozzini C, Anticoli S, Olivetta E, Arenaccio C, Federico M (2018) DNA Vectors Generating Engineered Exosomes Potential CTL Vaccine Candidates Against AIDS, Hepatitis B, and Tumors Mol Biotechnol 60:773–782 doi: 10.1007/s12033-018-0114-3 [DOI] [PubMed] [Google Scholar]
- Fields J et al. (2015) HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders The Journal of neuroscience : the official journal of the Society for Neuroscience 35:1921–1938 doi: 10.1523/JNEUROSCI.3207-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J et al. (2013) Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer Journal of NeuroVirology 19:89–101 doi: 10.1007/s13365-012-0145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF (2008) Neonatal intrahippocampal injection of the HIV-1 proteins gp120 and Tat: differential effects on behavior and the relationship to stereological hippocampal measures Brain Research 1232:139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, McLaurin KA, Booze RM, Mactutus CF (2018) Dose-dependent neurocognitive deficits following postnatal day 10 HIV-1 viral protein exposure: Relationship to hippocampal anatomy parameters International Journal of Developmental Neuroscience 65:66–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Young JAT (1998) HIV-1: Fifteen Proteins and an RNA Annu Rev Biochem 67:1–25 doi: 10.1146/annurev.biochem.67.1.1 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Otake K, Tashiro M, Adachi A (1996) Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells FEBS Lett 393:93–96 doi: 10.1016/0014-5793(96)00859-9 [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz D, Kaplan M, Gallo R, Popovic M (1986) The role of mononuclear phagocytes in HTLV-III/LAV infection Science 233:215–219 doi: 10.1126/science.3014648 [DOI] [PubMed] [Google Scholar]
- Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, Winston A (2014) Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART: AIDS 28:67–72 doi: 10.1097/01.aids.0000432467.54003.f7 [DOI] [PubMed] [Google Scholar]
- Geyer M, Munte CE, Schorr J, Kellner R, Kalbitzer HR (1999) Structure of the anchor-domain of myristoylated and non-myristoylated HIV-1 Nef protein 1 1Edited by A. R. Fersht Journal of Molecular Biology 289:123–138 doi: 10.1006/jmbi.1999.2740 [DOI] [PubMed] [Google Scholar]
- Geyer M, Peterlin BM (2001) Domain assembly, surface accessibility and sequence conservation in full length HIV-1 Nef FEBS Letters 496:91–95 doi: 10.1016/S0014-5793(01)02394-8 [DOI] [PubMed] [Google Scholar]
- Ghorpade A et al. (2005) Neuroinflammatory responses from microglia recovered from HIV-1-infected and seronegative subjects Journal of Neuroimmunology 163:145–156 doi: 10.1016/j.jneuroim.2005.01.022 [DOI] [PubMed] [Google Scholar]
- Gisslén M, Price RW, Nilsson S (2011) The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 11:356 doi: 10.1186/1471-2334-11-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 38:755–762 [DOI] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms The Journal of pathology 221:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin K et al. (2001) Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality Journal of Clinical Epidemiology 54:S35–S43 doi: 10.1016/S0895-4356(01)00445-0 [DOI] [PubMed] [Google Scholar]
- Gorry PR et al. (2007) Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo Retrovirology 4:66 doi: 10.1186/1742-4690-4-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I et al. (2014) Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline Neurology 82:2055–2062 doi: 10.1212/WNL.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- group obotPs et al. (2016) Defining cognitive impairment in people-living-with-HIV: the POPPY study BMC Infect Dis 16:617 doi: 10.1186/s12879-016-1970-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta MK et al. (2017) HIV-1 Nef-induced cardiotoxicity through dysregulation of autophagy Sci Rep 7:8572 doi: 10.1038/s41598-017-08736-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P (1998) Nef Harbors a Major Determinant of Pathogenicity for an AIDS-like Disease Induced by HIV-1 in Transgenic Mice Cell 95:163–175 doi: 10.1016/S0092-8674(00)81748-1 [DOI] [PubMed] [Google Scholar]
- Harricharan R, Thaver V, Russell VA, Daniels WM (2015) Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats Behavioral and Brain Functions 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hategan A et al. (2017) HIV Tat protein and amyloid-β peptide form multifibrillar structures that cause neurotoxicity Nat Struct Mol Biol 24:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study Neurology 75:2087–2096 doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Chinyama J, Anitha MJ, Ngoma MS, McCutchan JA, Franklin DR, Heaton RK (2019) Cognitive Impairment in Zambians With HIV Infection and Pulmonary Tuberculosis: JAIDS Journal of Acquired Immune Deficiency Syndromes 80:110–117 doi: 10.1097/QAI.0000000000001880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden CP, Haughey NJ, Nath A, Geiger JD (1999) Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes Neuroscience 91:1369–1378 doi: 10.1016/S0306-4522(98)00714-3 [DOI] [PubMed] [Google Scholar]
- Huang M-B, Weeks O, Zhao L-J, Saltarelli M, Bond VC (2000) Effects of extracellular human immunodeficiency virus type 1 vpr protein in primary rat cortical cell cultures Journal of neurovirology 6:202–220 [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao L, Jia B, Wu L, Li Y, Curthoys N, Zheng JC (2011) Glutaminase Dysregulation in HIV-1-Infected Human Microglia Mediates Neurotoxicity: Relevant to HIV-1-Associated Neurocognitive Disorders Journal of Neuroscience 31:15195–15204 doi: 10.1523/JNEUROSCI.2051-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Haughey NJ, Geiger JD (2012) Role of endolysosomes in HIV-1 Tat-induced neurotoxicity ASN Neuro 4:243–252 doi: 10.1042/AN20120017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs IR et al. (2019) Inhibitory Control Deficits Associated with Upregulation of CB 1 R in the HIV-1 Tat Transgenic Mouse Model of Hand Journal of Neuroimmune Pharmacology 14:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC (2004) Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors J Virol 78:3099–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK (1992) Epidemiology of human immunodeficiency virus encephalopathy in the United States Neurology 42:1472–1472 doi: 10.1212/WNL.42.8.1472 [DOI] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A (2013) Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein Proceedings of the National Academy of Sciences 110:13588–13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal S et al. (2017) The Presence of Human Immunodeficiency Virus-Associated Neurocognitive Disorders Is Associated With a Lower Adherence to Combined Antiretroviral Treatment Open Forum Infectious Diseases 4 doi: 10.1093/ofid/ofx070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia Nature 410:988–994 doi: 10.1038/35073667 [DOI] [PubMed] [Google Scholar]
- Kestler HW, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC (1991) Importance of the nef gene for maintenance of high virus loads and for development of AIDS Cell 65:651–662 doi: 10.1016/0092-8674(91)90097-i [DOI] [PubMed] [Google Scholar]
- Khan MB, Lang MJ, Huang M-B, Raymond A, Bond VC, Shiramizu B, Powell MD (2016) Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Aβ1–42 secretion in SH-SY5Y neural cells Journal of NeuroVirology 22:179–190 doi: 10.1007/s13365-015-0383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian M (2012) Dual role of autophagy in HIV-1 replication and pathogenesis AIDS Res Ther 9:16 doi: 10.1186/1742-6405-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA (2008) Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death Journal of Neuroscience 28:12604–12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama H, Miura Y, Ando Y, Hoshino S, Ishizaka Y, Koyanagi Y (2008) Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction Journal of Virology 82:2528–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U et al. (1999) HIV type 1 Nef protein is a viral factor for leukocyte recruitment into the central nervous system The Journal of Immunology 163:1237–1245 [PubMed] [Google Scholar]
- Koenig S et al. (1986) Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy Science 233:1089–1093 doi: 10.1126/science.3016903 [DOI] [PubMed] [Google Scholar]
- Konadu KA et al. (2015) Association of Cytokines With Exosomes in the Plasma of HIV-1-Seropositive Individuals J Infect Dis 211:1712–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei GB et al. (2009) Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages The Journal of Cell Biology 186:255–268 doi: 10.1083/jcb.200903070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli P L, Crowe M, A. Ross L, Wadley V, Ball K, E. Vance D (2014) Cognitive Functioning In Adults Aging With HIV: A Cross-Sectional Analysis Of Cognitive Subtypes And Influential Factors JCRHAP 1:54–68 doi: 10.14302/issn.2324-7339.jcrhap-13-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL et al. (2018) Brain-specific HIV Nef identified in multiple patients with neurological disease Journal of neurovirology 24:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Poon AF, McGrath MS (2011) HIV-1 nef protein structures associated with brain infection and dementia pathogenesis PloS one 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprevotte I, Pupin M, Coward E, Didier G, Terzian C, Devauchelle C, Hénaut A (2001) HIV-1 and HIV-2 LTR Nucleotide Sequences: Assessment of the Alignment by N-block Presentation, “Retroviral Signatures” of Overrepeated Oligonucleotides, and a Probable Important Role of Scrambled Stepwise Duplications/Deletions in Molecular Evolution Molecular Biology and Evolution 18:1231–1245 doi: 10.1093/oxfordjournals.molbev.a003909 [DOI] [PubMed] [Google Scholar]
- Lattanzi L, Federico M (2012) A strategy of antigen incorporation into exosomes: comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles Vaccine 30:7229–7237 doi: 10.1016/j.vaccine.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Learmont JC et al. (1999) Immunologic and Virologic Status after 14 to 18 Years of Infection with an Attenuated Strain of HIV-1 — A Report from the Sydney Blood Bank Cohort N Engl J Med 340:1715–1722 doi: 10.1056/NEJM199906033402203 [DOI] [PubMed] [Google Scholar]
- Lee C-H, Saksela K, Mirza UA, Chait BT, Kuriyan J (1996) Crystal Structure of the Conserved Core of HIV-1 Nef Complexed with a Src Family SH3 Domain Cell 85:931–942 doi: 10.1016/S0092-8674(00)81276-3 [DOI] [PubMed] [Google Scholar]
- Lehmann MH, Lehmann JM, Erfle V (2019) Nef-induced CCL2 expression contributes to HIV/SIV brain invasion and neuronal dysfunction Front Immunol 10:2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenassi M et al. (2010) HIV Nef is Secreted in Exosomes and Triggers Apoptosis in Bystander CD4+ T Cells Traffic 11:110–122 doi: 10.1111/j.1600-0854.2009.01006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennemann NJ, Coyne CB (2015) Catch Me If You Can: The Link between Autophagy and Viruses PLOS Pathogens 11:e1004685 doi: 10.1371/journal.ppat.1004685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E (2017) Exosomes in the Diseased Brain: First Insights from In vivo Studies Frontiers in Neuroscience 11 doi: 10.3389/fnins.2017.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Galey D, Mattson MP, Nath A (2005) Molecular and cellular mechanisms of neuronal cell death in HIV dementia Neurotox Res 8:119–134 doi: 10.1007/bf03033824 [DOI] [PubMed] [Google Scholar]
- Liu J, Li L (2019) Targeting Autophagy for the Treatment of Alzheimer’s Disease: Challenges and Opportunities Frontiers in molecular neuroscience 12 doi: 10.3389/fnmol.2019.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu W, Yang H (2019a) Balancing Apoptosis and Autophagy for Parkinson’s Disease Therapy: Targeting BCL-2 ACS Chem Neurosci 10:792–802 doi: 10.1021/acschemneuro.8b00356 [DOI] [PubMed] [Google Scholar]
- Liu S et al. (2019b) The Dual Role of HIV-1 gp120 V3 Loop-Induced Autophagy in the Survival and Apoptosis of the Primary Rat Hippocampal Neurons Neurochem Res 44:1636–1652 doi: 10.1007/s11064-019-02788-3 [DOI] [PubMed] [Google Scholar]
- Louboutin J-P, Agrawal L, Reyes BaS, van Bockstaele EJ, Strayer DS (2012) Gene delivery of antioxidant enzymes inhibits human immunodeficiency virus type 1 gp120-induced expression of caspases Neuroscience 214:68–77 doi: 10.1016/j.neuroscience.2012.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamik MK, Hui E, Branton WG, McKenzie BA, Chisholm J, Cohen EA, Power C (2017) HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation Journal of Neuroimmune Pharmacology 12:233–248 [DOI] [PubMed] [Google Scholar]
- Manfredi F, Di Bonito P, Arenaccio C, Anticoli S, Federico M (2016) Incorporation of Heterologous Proteins in Engineered Exosomes Methods Mol Biol 1448:249–260 doi: 10.1007/978-1-4939-3753-0_18 [DOI] [PubMed] [Google Scholar]
- Marra CM et al. (2013) Neurocognitive impairment in HIV-infected individuals with previous syphilis Int J STD AIDS 24:351–355 doi: 10.1177/0956462412472827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A (2010) HIV-associated neurocognitive disorders: ‘mind the gap’ Ann Neurol:NA-NA doi: 10.1002/ana.22053 [DOI] [PubMed] [Google Scholar]
- McDonnell J et al. (2014) Minimal Cognitive Impairment in UK HIV-Positive Men Who Have Sex With Men: Effect of Case Definitions and Comparison With the General Population and HIV-Negative Men JAIDS Journal of Acquired Immune Deficiency Syndromes 67:120–127 doi: 10.1097/QAI.0000000000000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RP et al. (2018) Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses mBio 9:e02344–02317 doi: 10.1128/mBio.02344-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JP, Althoff AL, Altice FL (2013) Optimizing care for HIV-infected people who use drugs: evidence-based approaches to overcoming healthcare disparities Clin Infect Dis 57:1309–1317 doi: 10.1093/cid/cit427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordelet E et al. (2004) Histopathological and cognitive defects induced by Nef in the brain FASEB J 18:1851–1861 doi: 10.1096/fj.04-2308com [DOI] [PubMed] [Google Scholar]
- Mukhamedova N et al. (2019) Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells PLOS Pathogens 15:e1007907 doi: 10.1371/journal.ppat.1007907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na H et al. (2011) Interactions between human immunodeficiency virus (HIV)-1 Vpr expression and innate immunity influence neurovirulence Retrovirology 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narciso P et al. (2001) Metabolic and Morphologic Disorders in Patients Treated with Highly Active Antiretroviral Therapy since Primary HIV Infection Annals of the New York Academy of Sciences 946:214–222 doi: 10.1111/j.1749-6632.2001.tb03914.x [DOI] [PubMed] [Google Scholar]
- Nath A (2002) Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia The Journal of infectious diseases 186:S193–S198 [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO (1999) Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes A hit and run phenomenon J Biol Chem 274:17098–17102 [DOI] [PubMed] [Google Scholar]
- Nookala AR, Schwartz DC, Chaudhari NS, Glazyrin A, Stephens EB, Berman NE, Kumar A (2018) Methamphetamine augment HIV-1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors Brain, Behavior, and Immunity 71:37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH (2018) The top 10 causes of death.
- Patel CA, Mukhtar M, Pomerantz RJ (2000) Human Immunodeficiency Virus Type 1 Vpr Induces Apoptosis in Human Neuronal Cells Journal of Virology 74:9717–9726 doi: 10.1128/JVI.74.20.9717-9726.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller SC, Jans P, Gage PW, Jans DA (1998) Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: Implications for AIDS pathology Proceedings of the National Academy of Sciences 95:4595–4600 doi: 10.1073/pnas.95.8.4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M et al. (1994) Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1 Cell 78:389–398 doi: 10.1016/0092-8674(94)90418-9 [DOI] [PubMed] [Google Scholar]
- Pužar Dominkuš P, Ferdin J, Plemenitaš A, Peterlin BM, Lenassi M (2017) Nef is secreted in exosomes from Nef.GFP-expressing and HIV-1-infected human astrocytes J Neurovirol 23:713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian P, He JJ (2016a) Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein Journal of neurovirology 22:774–788 doi: 10.1007/s13365-016-0451-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian P, He JJ (2016b) HIV-1 Tat-shortened neurite outgrowth through regulation of microRNA-132 and its target gene expression Journal of neuroinflammation 13:247–247 doi: 10.1186/s12974-016-0716-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki A et al. (1995) Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia AIDS 9:1001–1008 doi: 10.1097/00002030-199509000-00004 [DOI] [PubMed] [Google Scholar]
- Rao VR, Ruiz AP, Prasad VR (2014) Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND) AIDS Res Ther 11:13 doi: 10.1186/1742-6405-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W (2013a) Extracellular vesicles: Exosomes, microvesicles, and friends J Cell Biol 200:373–383 doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W (2013b) Extracellular vesicles: exosomes, microvesicles, and friends J Cell Biol 200:373–383 doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD, Campbell-Sims TC, Khan M, Lang M, Huang MB, Bond VC, Powell MD (2011) HIV Type 1 Nef is released from infected cells in CD45(+) microvesicles and is present in the plasma of HIV-infected individuals AIDS Res Hum Retroviruses 27:167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD et al. (2016) Microglia-derived HIV Nef+ exosome impairment of the blood–brain barrier is treatable by nanomedicine-based delivery of Nef peptides Journal of NeuroVirology 22:129–139 doi: 10.1007/s13365-015-0397-0 [DOI] [PubMed] [Google Scholar]
- Reddy PVB, Agudelo M, Atluri VSR, Nair MP (2012) Inhibition of Nuclear Factor Erythroid 2-Related Factor 2 Exacerbates HIV-1 gp120-Induced Oxidative and Inflammatory Response: Role in HIV Associated Neurocognitive Disorder Neurochem Res 37:1697–1706 doi: 10.1007/s11064-012-0779-0 [DOI] [PubMed] [Google Scholar]
- Reggiori F, Komatsu M, Finley K, Simonsen A (2012) Autophagy: More Than a Nonselective Pathway International Journal of Cell Biology 2012:1–18 doi: 10.1155/2012/219625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L (2005) HIV-1 Tat inhibits neprilysin and elevates amyloid β AIDS 19:127–135 [DOI] [PubMed] [Google Scholar]
- Rho MB, Wesselingh S, Glass JD, Mcarthur JC, Choi S, Griffin J, Tyor WR (1995) A Potential Role for Interferon-α in the Pathogenesis of HIV-Associated Dementia Brain, Behavior, and Immunity 9:366–377 doi: 10.1006/brbi.1995.1034 [DOI] [PubMed] [Google Scholar]
- Rom I, Deshmane SL, Mukerjee R, Khalili K, Amini S, Sawaya BE (2009) HIV-1 Vpr deregulates calcium secretion in neural cells Brain Research 1275:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P, Bank tMHB (2004) Neuropsychiatric impact of hepatitis C on advanced HIV Neurology 62:957–962 doi: 10.1212/01.WNL.0000115177.74976.6C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier JM et al. (1991) Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1 Journal of Virology 65:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah EN, Roques BP (2005) Critical implication of the (70–96) domain of human immunodeficiency virus type 1 Vpr protien in apoptosis of primary rat cortical and straital neurons Journal of neurovirology 11:489–502 [DOI] [PubMed] [Google Scholar]
- Sami Saribas A, Cicalese S, Ahooyi TM, Khalili K, Amini S, Sariyer IK (2018) HIV-1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef-mediated neurotoxicity Cell Death & Disease 8:e2542–e2542 doi: 10.1038/cddis.2016.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances BM, Collins DL (2017) Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era: JAIDS Journal of Acquired Immune Deficiency Syndromes 74:563–570 doi: 10.1097/QAI.0000000000001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saribas AS, Khalili K, Sariyer IK (2015) Dysregulation of autophagy by HIV-1 Nef in human astrocytes Cell Cycle 14:2899–2904 doi: 10.1080/15384101.2015.1069927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D et al. (2016) HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment Nature Reviews Neurology 12:234–248 doi: 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J et al. (2016) Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy: AIDS 30:1027–1038 doi: 10.1097/QAD.0000000000001017 [DOI] [PubMed] [Google Scholar]
- Sengupta S, Siliciano RF (2018) Targeting the Latent Reservoir for HIV-1 Immunity 48:872–895 doi: 10.1016/j.immuni.2018.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S et al. (2009) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia: AIDS:1 doi: 10.1097/QAD.0b013e3283354a7b [DOI] [PubMed] [Google Scholar]
- Spudich S, Gonzalez-Scarano F (2012) HIV-1-Related Central Nervous System Disease: Current Issues in Pathogenesis, Diagnosis, and Treatment Cold Spring Harbor Perspectives in Medicine 2:a007120–a007120 doi: 10.1101/cshperspect.a007120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T et al. (2015) Multivariate normative comparison, a novel method for more reliably detecting cognitive impairment in HIV infection: AIDS:1 doi: 10.1097/QAD.0000000000000573 [DOI] [PubMed] [Google Scholar]
- Swanson CM, Malim MH (2008) SnapShot: HIV-1 proteins Cell 133:742–742. e741 [DOI] [PubMed] [Google Scholar]
- Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G (2013) Extracellular vesicles as an emerging mechanism of cell-to-cell communication Endocrine 44:11–19 doi: 10.1007/s12020-012-9839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KA et al. (2003) Decreased Neurotropism of nef Long Terminal Repeat ( nef/LTR)-Deleted Simian Immunodeficiency Virus Journal of Neurovirology 9:442–451 doi: 10.1080/13550280390218715 [DOI] [PubMed] [Google Scholar]
- Torres L, Noel RJ (2014) Astrocytic expression of HIV-1 viral protein R in the hippocampus causes chromatolysis, synaptic loss and memory impairment Journal of neuroinflammation 11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey A et al. (2017) Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies The Lancet HIV 4:e349–e356 doi: 10.1016/S2352-3018(17)30066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP (2000) Recombinant nef HIV-IIIB protein is toxic to human neurons in culture Brain Research 864:315–326 doi: 10.1016/s0006-8993(00)02213-7 [DOI] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE (1992) Cytokine expression in the brain during the acquired immunodeficiency syndrome Ann Neurol 31:349–360 doi: 10.1002/ana.410310402 [DOI] [PubMed] [Google Scholar]
- Valcour V et al. (2012) Central Nervous System Viral Invasion and Inflammation During Acute HIV Infection The Journal of Infectious Diseases 206:275–282 doi: 10.1093/infdis/jis326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V et al. (2004) Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort Journal of Neuroimmunology 157:197–202 doi: 10.1016/j.jneuroim.2004.08.029 [DOI] [PubMed] [Google Scholar]
- van Marle G et al. (2004) Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10 Virology 329:302–318 doi: 10.1016/j.virol.2004.08.024 [DOI] [PubMed] [Google Scholar]
- Wang Y, Santerre M, Tempera I, Martin K, Mukerjee R, Sawaya BE (2017) HIV-1 Vpr disrupts mitochondria axonal transport and accelerates neuronal aging Neuropharmacology 117:364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z et al. (2004) Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: Potential role in neuropathogenesis Journal of Neurovirology 10:25–32 doi: 10.1080/753312749 [DOI] [PubMed] [Google Scholar]
- Weissman D, Li Y, Orenstein JM, Fauci AS (1995) Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells J Immunol 155:4111–4117 [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K (2008) Central obesity and increased risk of dementia more than three decades later Neurology 71:1057–1064 doi: 10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- Williams KC et al. (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS J Exp Med 193:905–915 doi: 10.1084/jem.193.8.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R, Sodroski J (1998) The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens Science 280:1884–1888 [DOI] [PubMed] [Google Scholar]
- Yeung MC, Pulliam L, Lau AS (1995) The HIV envelope protein gp120 is toxic to human brain-cell cultures through the induction of interleukin-6 and tumor necrosis factor-α: AIDS 9:137–144 doi: 10.1097/00002030-199509020-00004 [DOI] [PubMed] [Google Scholar]
- Zhao X, Fan Y, Vann PH, Wong JM, Sumien N, He JJ (2020) Long-term HIV-1 Tat Expression in the Brain Led to Neurobehavioral, Pathological, and Epigenetic Changes Reminiscent of Accelerated Aging Aging and disease:0 [DOI] [PMC free article] [PubMed]


