Abstract
Background:
Nothing is known about the mechanisms by which increased ceramide in the lung contributes to allergic responses and asthma severity.
Objective:
We sought to investigate the functional role of ceramide in mouse models of allergic airway disease that recapitulate the cardinal clinical features of human allergic asthma.
Methods:
Allergic airway disease was induced in mice by repeated intranasal administration of house dust mite or the fungal allergen Alternaria alternata. Processes that can be regulated by ceramide and are important for severity of allergic asthma were correlated with ceramide levels measured by mass spectrometry.
Results:
Both allergens induced massive pulmonary apoptosis and also significantly increased reactive oxygen species in the lung. Prevention of increases in lung ceramide levels mitigated allergen-induced apoptosis, reactive oxygen species, and neutrophil infiltration. In contrast, dietary supplementation of the anti-oxidant α-tocopherol decreased reactive oxygen species but had no significant effects on ceramide elevation or apoptosis, indicating that the increases in lung ceramide levels in allergen-challenged mice are not mediated by oxidative stress. Moreover, specific ceramide species were altered in bronchoalveolar lavage fluid from patients with severe asthma compared to non-asthmatics.
Conclusion:
Our data suggest that ceramide elevation after allergen challenge contributes to apoptosis, reactive oxygen species generation, and neutrophilic infiltrate that characterize the severe asthmatic phenotype. Ceramide might be the trigger of formation of Creola bodies found in the sputum of patients with severe asthma and could be a biomarker to optimize diagnosis and to monitor and improve clinical outcomes in this disease.
Keywords: asthma, ceramide, apoptosis, oxidative stress, biomarker
Graphical Abstract
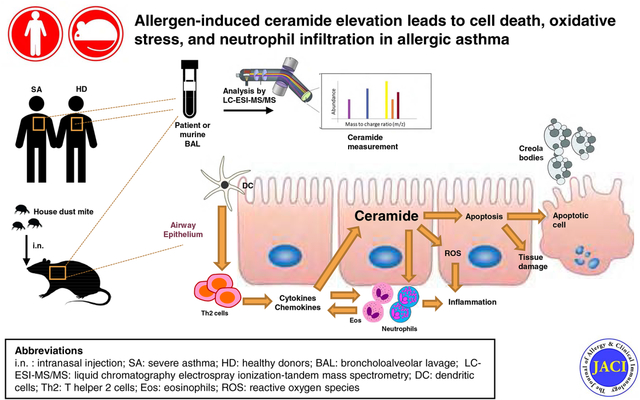
Capsule Summary
The bioactive sphingolipid metabolite ceramide is increased in lungs of allergen-challenged mice and severe asthmatic patients, promotes apoptosis, oxidative stress and neutrophil recruitment that contribute to allergic asthma and correlates with asthma severity.
INTRODUCTION
More than 25 million Americans suffer from asthma, a chronic airway disease marked by airflow obstruction, airway hyperreactivity, and pulmonary inflammation. The heterogeneous nature of asthma complicates treatment options and hampers the development of a cure. However, over the years, extensive research has advanced understanding of the initiation and progression of this disease. There is a strong genetic component to asthma and numerous genome-wide association studies (GWAS) have identified ORM (yeast)-like protein isoform 3 (ORMDL3) as a gene associated with the onset of allergic asthma, the most common type1–6. ORMDL3 is one of 3 members of the mammalian ORMDL family proteins, which are endogenous negative regulators of serine palmitoyl transferase (SPT), the rate-limiting enzyme of the de novo sphingolipid biosynthesis pathway7. This evolutionarily conserved function of ORMDL3 as a regulator of sphingolipid synthesis instigated interest into how sphingolipids may be involved in the pathology of asthma. Intriguingly, we found that although physiologically, ORMDL3 is a negative regulator of ceramide de novo biosynthesis, highly elevated pathological ORMDL3 expression, such as observed in allergic asthma, enhanced ceramide levels primarily due to increased sphingolipid degradation in the salvage pathway8. Ceramide, the central metabolite of the sphingolipid pathway9, is increased in lungs of guinea pigs by aerosol administration of ovalbumin10 and in lungs of house dust mite (HDM) challenged mice8. Moreover, inhibition of ceramide production in the lung protected HDM challenged mice from inflammation and airway hyperreactivity8. In addition, intratracheal delivery of ceramide caused lung inflammation, tissue remodeling, and airway flow obstruction11. Because ceramide is a bioactive signaling molecule involved in the regulation of several biological processes that may contribute to asthma12, 13, these results led us to investigate the functional role of ceramide in mouse models of allergic airway disease that recapitulate the cardinal clinical features of human allergic asthma. Our data suggest that ceramide elevation after allergen challenge plays a key role in airway epithelial cell apoptosis, oxidative stress, and neutrophil infiltration, processes that amplify and contribute to asthma severity in mice and humans14, 15. Moreover, specific ceramide species were altered in bronchoalveolar lavage fluid (BALF) from severely asthmatic patients compared to non-asthmatics and correlated with airway neutrophilia, suggesting that ceramide has the potential to serve as a biomarker for specific asthma endotypes and disease severity.
METHODS
Mouse models of allergic asthma
Female C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in the animal care facilities at Virginia Commonwealth University under standard temperature, humidity, and timed light conditions and provided with standard rodent chow and water ad libitum. All animal protocols and procedures were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. In an acute HDM-dependent asthma model, eight-week-old mice were challenged intranasally (i.n.) with house dust mite (15 μg HDM/25 μL saline) from Greer Laboratories (Lenoir, NC; XPB70D3A2.5) or saline on days 1 – 5 and days 8 – 12, for a total of 10 challenges. To inhibit ceramide biosynthesis, thirty minutes before HDM challenge, mice were injected intraperitoneally (i.p,) with vehicle, myriocin (0.3 mg/kg), or FB1 (0.5 mg/kg) on days 10, 11, and 12. This route of administration was selected as instilling these inhibitors directly to the lung induced massive damage and i.p. treatment with fumonisin B1 (FB1) and myriocin at these concentrations did not affect circulating lymphocytes16.
In a HDM model of allergic asthma with a strong antigen-specific IgE response, female mice (8 weeks old) were immunized by an i.p. injection of HDM (50 μg in 100 μl saline) together with 100 μl of Imject alum on day 1. On days 15, 18, and 21, mice were challenged i.n. with HDM (25 μg in 25 μl saline).
For the fungal allergen model, mice were challenged i.n. with Alternaria alternata (25 μg Alt/25 μl saline) from Greer Laboratories (XPM1D3A2.5) on days 1, 4, 7, and 10.
Tocopherol diet
C57BL/6J mice were challenged intratracheally (i.t.) with HDM (10 μg/50 μl saline) or saline 3 times per week for a total of 6 or 8 challenges. During these challenges, mice were fed either an α-tocopherol-supplemented (250 mg/kg) diet or standard rodent chow diet. 24 h following the last challenge, lung tissue was collected17.
Assessment of airway function
Mice were anesthetized with a mixture of ketamine (100 mg/kg), xylazine (10 mg/kg), and acepromazine (2 mg/kg) i.p. followed by insertion of an 18-guage cannula into the trachea and placement onto the FlexiVent apparatus (Scireq, Montreal, Canada). Ventilation was started and mice were injected i.p with the paralytic decamethonium bromide (0.5 mg). Airway hyperresponsiveness was measured in response to increasing doses of nebulized methacholine (0–100 mg/ml) as described8. Whole respiratory system resistance (R) was reported as calculated (cmH20/mL/sec) in the FlexiVent software version 8.0.4.
Mass spectrometry measurements of sphingolipids
Lipids were extracted from lung tissue and sphingolipids were quantified by liquid chromatography electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS), as previously described8.
Measurement of reactive oxygen species
Reactive oxygen species (ROS) were measured in snap-frozen lung tissue homogenized in PBS using a general oxidative stress indicator14. Briefly, lung tissue homogenates were incubated with 0.04 mM CM-H2DCFDA (ThermoFisher) for 20 min at 37° C on a thermomixer (Eppendorf) and then centrifuged at 1400 rpm for 5 min at 4° C. Aliquots of the supernatants (50 μL) were placed in wells of a 96 well black bottom plate with clear bottoms (CELLSTAR). Fluorescence was measured with a TECAN infinite M1000 Pro plate reader at an excitation wavelength of 495 nm and emission wavelength of 527 nm and normalized to protein concentration measured with the BCA Protein Assay Kit (ThermoFisher).
Clinical cohort and sample collection
Asthmatic and healthy subjects were previously recruited to the Severe Asthma Research Program (SARP1–2), an NHLBI-funded study designed to characterize molecular, cellular, and physiological phenotypes of severe and non-severe asthmatics18. Details regarding SARP1 were described previously19. Subjects 13 years of age and older with asthma and healthy control subjects were recruited and American Thoracic Society guidelines were used to categorize subjects as severe (n = 10) or non-severe asthma (n = 5) or healthy controls (n = 5)20. Control subjects were nonsmokers with no history of lung disease. BAL was performed with five 50 mL aliquots of saline, and BALF was recovered by hand suction. An aliquot of cell-free BALF supernatant (1 mL) stored at −80°C was shipped to VCU for sphingolipid analyses.
Statistical analyses
Statistical significances were determined with unpaired 2-tailed Student’s t test for comparison of 2 groups, or by ANOVA for multiple comparisons using GraphPad Prism 7.0 software (San Diego, CA). For all experiments, the normality of each group was first checked with the Shapiro-Wilk statistical test. In case of non-normally distributed data, Mann Whitney or Kruskal-Wallis tests were done. Significance defined as *P < 0.05, **P < 0.01, and ***P < 0.001. Experiments were repeated three times with similar results. For clinical correlation analyses, Pearson’s product-moment correlation (r) and the strength of the relationship (p-value) for BALF sphingolipid species and lung function and cell infiltration measurements were calculated and generated a correlation matrix plotted using Prism 7. Highly significant correlations were accepted for r>0.7 and r< −0.7 with P-values <0.05. For neutrophil populations, Spearman non-parametric correlations were determined.
BALF collection and analysis, isolation of primary lung alveolar epithelial cells, apoptosis determination with Annexin V and 7-AAD, measurement of ROS production by epithelial cells, neutrophil isolation and neutrophil attachment assays, immunocytochemistry and western blot analysis are detailed in the Methods section in this article’s Online Repository.
RESULTS
HDM challenge increases lung ceramide accompanied by increased apoptosis and ROS
To examine the role of ceramide in allergic asthma, mice were sensitized with ten intranasal injections of HDM over the duration of two weeks. This acute model stimulates airway inflammation and hypersensitivity and excessive mucus production commonly observed in asthmatic patients21, 22.Consistent with previous studies8, 23, 24, mice sensitized with HDM developed features of allergic asthma, including marked increase in BALF of eosinophils, as well as T and B cells and neutrophils to a lesser extent (Fig. 1A), and airway hyperresponsiveness (AHR) (Fig. 1B). Although a recent study suggested that HDM challenge did not affect ORMDL3 expression25, in accordance with previous reports8, 26, 27, we observed that HDM induced a robust increase in expression of ORMDL3 in lungs (Fig. 1C). As we8 and others25 reported previously, there was a significant increase in levels of multiple ceramide species with varying acyl chain lengths, particularly the predominant lung species C16:0, C24:0 and C24:1 in lungs of HDM challenged mice (Fig. 1D). However, levels of the major phosphatidylcholine 34:2 species were unaltered (Fig. 1E), suggesting that there was a specific increase in the bioactive sphingolipid metabolite ceramide.
FIG 1. House dust mite challenge increases ceramide, apoptosis, and ROS in the lung.
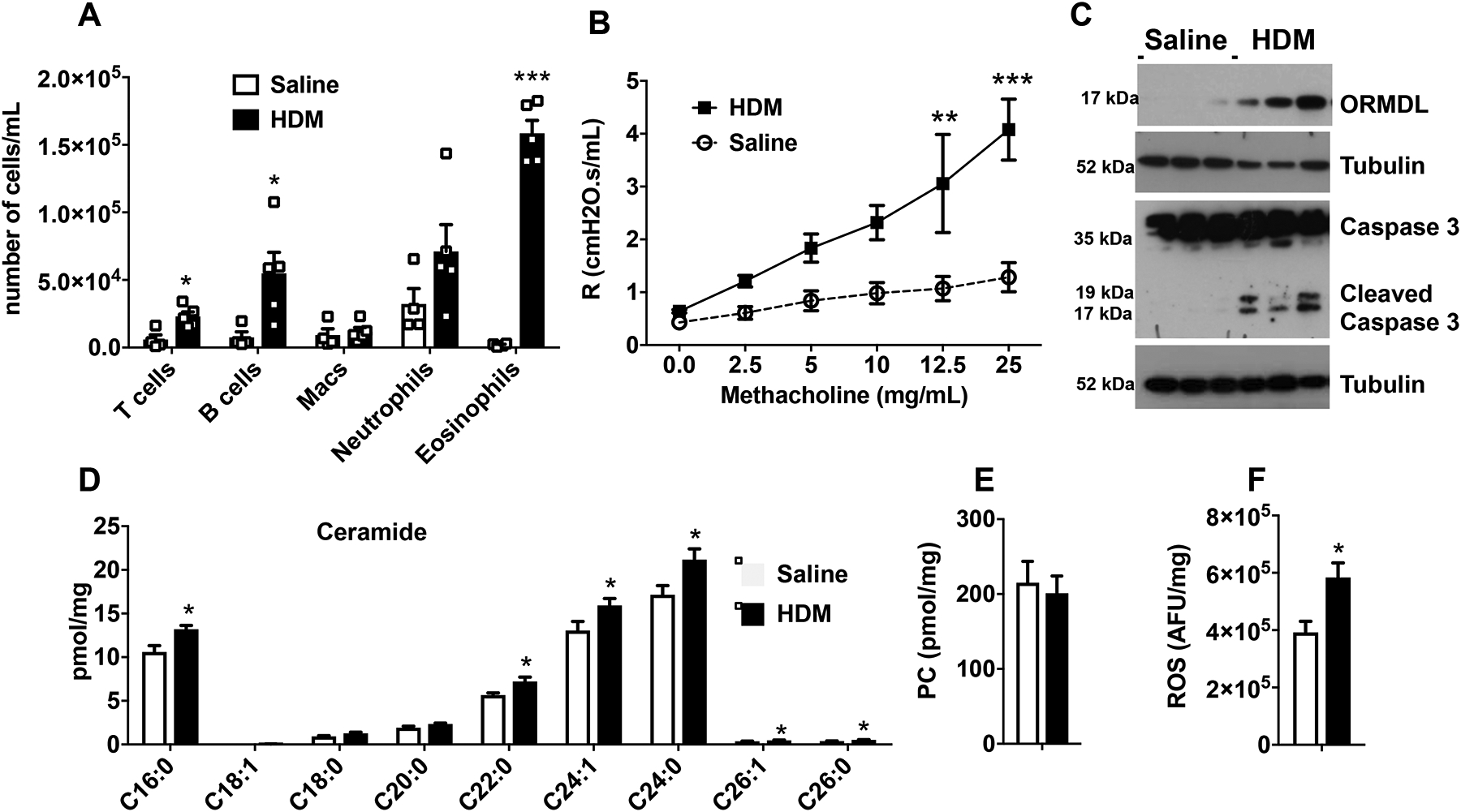
(A-D) C57Bl/6J mice were sensitized and challenged intranasally with HDM or saline and lungs examined on day 15. (A) Inflammatory cells in BALF. (B) Airway resistance in response to increasing doses of nebulized methacholine was measured with the FlexiVent system. (C) Proteins in lung lysates were separated by SDS-PAGE and immunoblotted with the indicated antibodies. Blots were stripped and re-probed with anti-tubulin to show equal loading and transfer. (D,E) Lipids were extracted and (D) ceramide species and (E) phosphatidylcholine (PC, 34:2) measured by LC-ESI-MS/MS. (F) ROS was determined with CM-H2DCFDA and fluorescence measured. Data are mean ± SEM. A: n=4 and 5 mice/group; B, C: n=3 and 3, D-F: n=4 and 4 for saline and HDM, respectively. *P < 0.05, ***P < 0.001, compared to each saline control. Similar data were obtained in 2 additional experiments.
To begin assessing the role of ceramide, we examined processes in which ceramide is known to play key roles, including apoptosis and generation of ROS9, 28 that could contribute to allergic asthma12, 13. Exposure to allergens induces extensive damage and apoptosis of airway epithelium that are related to both chronicity and severity of asthma29–31. Indeed, Creola bodies, which are clusters of apoptotic lung epithelial cells, have long been described in the sputum of asthmatic patients29, 32 Yet little is known of the initial trigger of the apoptotic process. In agreement with others14, 33, we found that HDM challenge induced activation of caspase 3, the final executionary step preceding apoptosis, as measured by increased levels of cleaved caspase 3 in immunoblots (Fig. 1C). Increased apoptosis in the lungs of HDM challenged mice was also confirmed by immunohistochemical staining with antibody against cleaved caspase 3 (Fig. E1). Since elevated ceramides can increase ROS leading to apoptosis9, 34, and it was shown that ROS and oxidative stress contribute to asthma pathology in mice and humans14, 15, we next measured ROS production. HDM exposure increased lung ROS measured fluorometrically with CM-H2DCFDA (Fig. 1F).
Consistent with elevation of lung ceramide determined by mass spectrometry (Fig. 1D), lung sections from HDM-challenged mice but not saline treated mice also displayed increased staining with a specific anti-ceramide antibody35 (Fig. 2A and Fig. E2). Lung cells with increased ceramide staining also showed increased co-staining for activated/cleaved caspase 3 (Fig. 2A), suggesting that ceramide may contribute to the increased apoptosis. Ceramide staining only partially co-localized with airway smooth muscle cells (Fig. E3). Importantly, increased ceramide and cleaved caspase-3 staining predominantly co-localized with cytokeratin 18, a marker for epithelial cells (Fig. 2B and Fig. E4), which are the first line of defense against inhaled allergens and are now recognized as important players in asthma pathogenesis36.
FIG 2. Increased ceramide staining in HDM-challenged mice co-localizes with cleaved caspase 3 in lung epithelium.
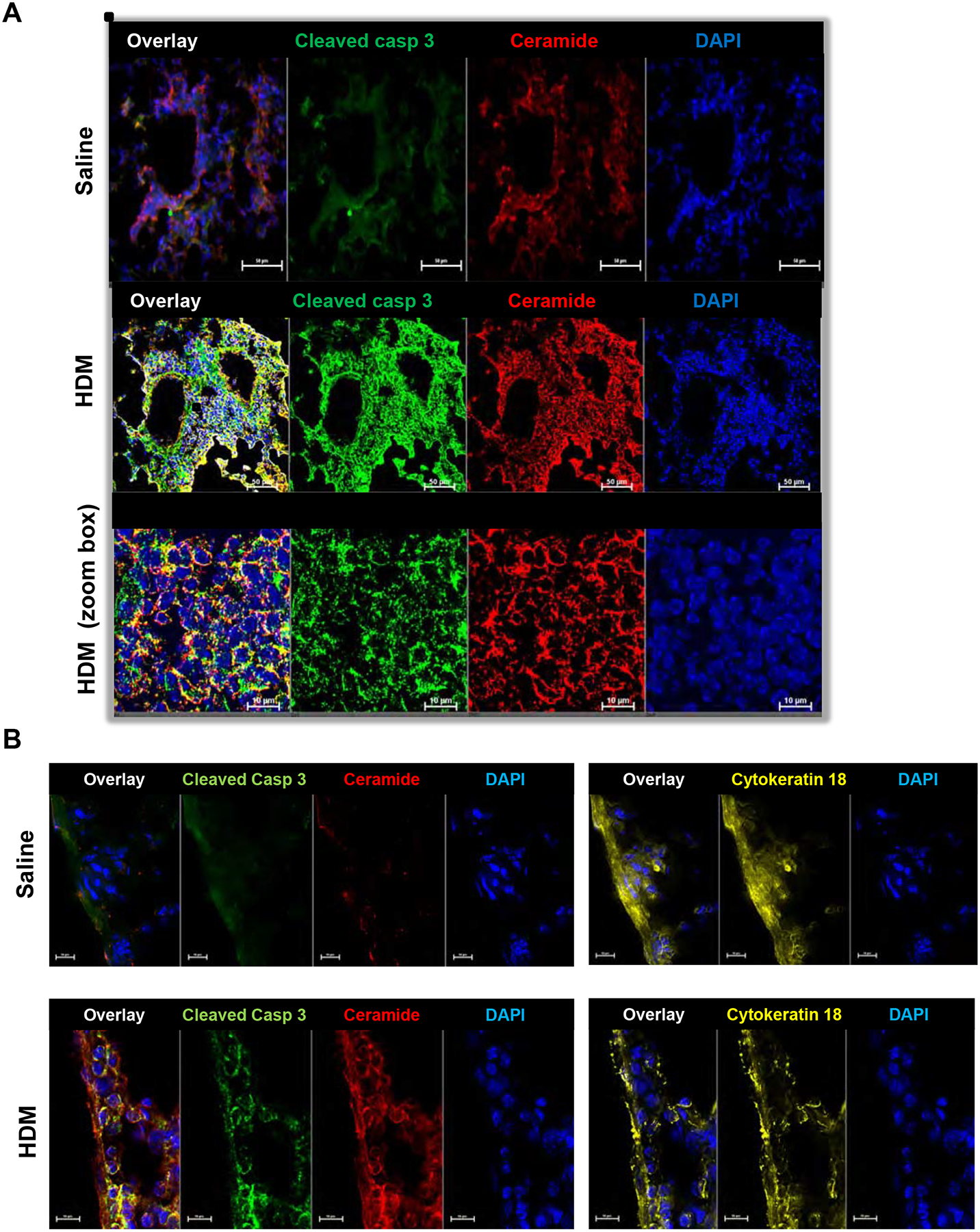
(A) Mice were challenged i.n. with HDM or saline and lungs as indicated and examined on day 15 as described in Fig 1. Lung sections were stained with anti-ceramide antibody (red), anti-cleaved caspase 3 antibody (green). Lower panels are zoom boxes indicated in middle panels. (B) Lung sections were stained with anti-ceramide antibody (red), anti-cleaved caspase 3 antibody (green) and anti-cytokeratin 18 (yellow), a marker for epithelial cells. (A,B) All sections were co-stained with DAPI to visualize nuclei (blue). Co-localization is shown in the overlay panels. Size bar: 50 or 10 μm as indicated.
Lung ceramide, apoptosis, and ROS are increased in several mouse models of allergic asthma
We next expanded our studies to examine effects of ceramide elevation, apoptosis, and ROS in other murine models of experimental asthma. In a model that induces a strong antigen-specific IgE response and mast cell hyperplasia with moderate eosinophilia37, 38, mice were sensitized i.p. with HDM in alum as an adjuvant and then challenged intranasally three times with HDM alone. Sensitization and challenges with HDM/alum induced AHR (Fig. 3A) with a significant increase in IgE and activation of mast cells (Fig. 3B and data not shown). In parallel, there was an increase in neutrophils and eosinophils in BALF (Fig. 3C). ORMDL3 expression was also increased (Fig. 3D), as well as many ceramide species (Fig. 3E). Concomitantly, caspase 3 activation (Fig. 3D) and generation of ROS (Fig. 3F) were also significantly increased.
FIG 3. Lung ceramide, apoptosis, and ROS are increased in different allergen-driven asthma models.
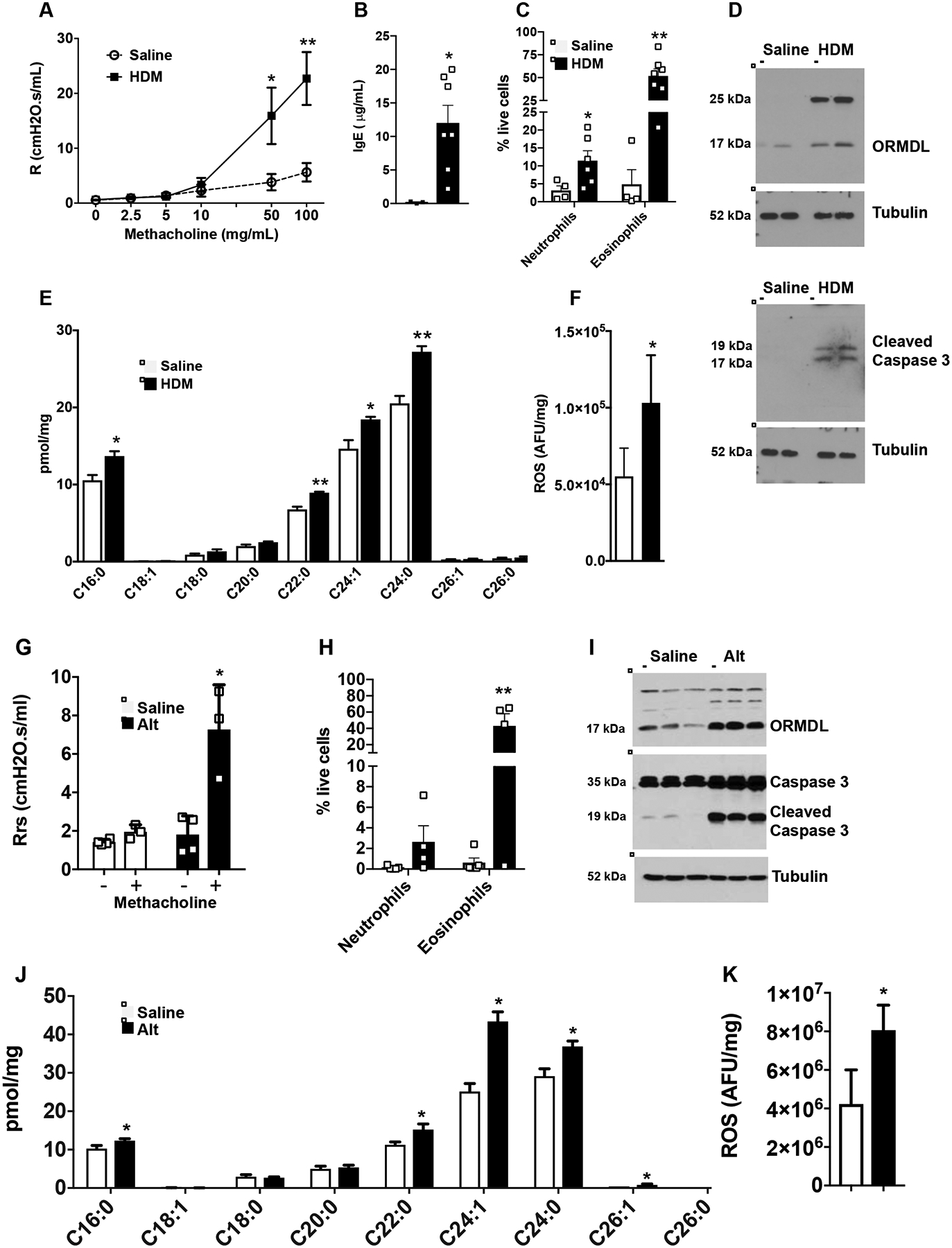
(A-F) Mice were sensitized i.p. with HDM (50 μg HDM in alum), and then challenged i.n. on days 15, 18, and 21 with HDM (25 μg in saline) or saline alone as indicated, and lungs examined on day 24. (A) Airway resistance in response to increasing doses of nebulized methacholine was measured with the FlexiVent system. (B) Eosinophils and neutrophils in BALF. (C) Serum IgE levels. (D) Proteins in lung lysates were analyzed by immunoblotting with the indicated antibodies. Blots were stripped and re-probed with anti-tubulin to show equal loading and transfer. (E) Lipids were extracted and ceramide species were measured by LC-ESI-MS/MS. (F) ROS was measured fluorometrically. A: n=10 and 7; B: n=3 and 7; C: n=4 and 6; D: n=2 and 2; E,F: n=4 and 4, respectively. *P < 0.05, **P < 0.01 compared to each saline control. (G–K) Mice were challenged i.n. with Alternaria alternata (Alt, 25 μg) or saline on days 1, 4, 7, and 10. (G) On day 11, airway resistance in response to nebulized methacholine (12 mg/mL) was measured with the FlexiVent system. (H) Eosinophils and neutrophils in BALF. (I) Western blot analysis of ORMDL, total caspase 3, and cleaved caspase 3 expression in the lung. Tubulin was used as a loading control. (J) Lung ceramide species were measured by LC-ESI-MS/MS. (K) ROS was measured fluorometrically. Data are mean ± SD. G: n=4 and 3; H: n= 5 and 4; I, n= 3 and 3; J n=3 and 4; K: n= 6 and 3 respectively. *P < 0.05, **P < 0.01 compared to each saline control.
To exclude HDM-dependent effects and to examine responses to other potent allergens, mice were exposed to the fungal extract of Alternaria alternata, another common allergen associated with allergic asthma disease39. As was reported previously25, 27, 40, Alternaria induced the hallmark features of asthma in mice, including AHR, lung and airway inflammation, eosinophilia (Fig 3G,H), as well as increased ORMDL3 (Fig. 3I). In addition to increased ORMDL3, there was also significantly increased ceramide levels, particularly C16;0, C24:0, and C24:1 species, in the lungs of sensitized mice (Fig. 3J). Concurrently, caspase 3 cleavage (Fig 3I) and ROS were also increased compared to saline treated mice (Fig. 3K). Taken together, these results demonstrate that challenge with diverse allergens increases ceramide levels that correlate with induction of apoptosis and increased levels of ROS in the lung.
Allergen induced ceramide and apoptosis is independent of ROS production
The links between ceramide, ROS, and apoptosis are complex. On the one hand, increased ROS in the lung can lead to increased ceramide production and apoptosis41, 42. On the other hand, increased ceramide can lead to ROS production and apoptosis34, 42, 43. Therefore, it was of interest to complete the elucidation of the sequence of events prevalent during allergic asthma and determine the effects of suppressing ROS production on ceramide and apoptosis. To this end, HDM-challenged mice were fed a normal diet supplemented with the anti-oxidant α-tocopherol, a vitamin E isoform that has been shown to decrease ROS in vivo and lung inflammation in response to HDM challenge17, 44. As expected, the α-tocopherol-supplemented diet significantly reduced lung ROS levels in HDM challenged mice compared to normal diet (Fig. 4A). In contrast, however, α-tocopherol supplementation did not decrease elevation of lung ceramide species induced by HDM challenge (Fig. 4B). Similarly, decreasing ROS production in these mice did not suppress apoptosis as measured by caspase 3 activation (Fig. 4C). These results suggest that allergen-induced increased ceramide and apoptosis are independent of its effects on ROS.
FIG 4. Effects of feeding a-tocopherol supplemented diet on ROS, ceramide, and apoptosis in the lung of HDM challenged mice.
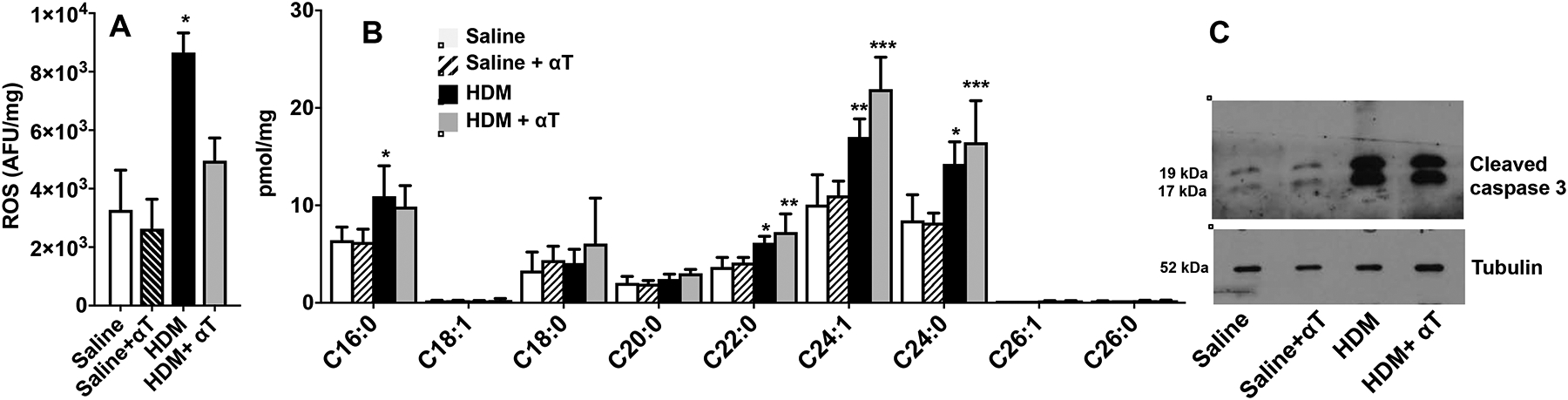
Mice fed chow diet without or with α-tocopherol (250 mg/kg) were challenged i.n. with HDM (10 μg in 50 μL saline) every other day for a total of 8 challenges. (A) ROS in lungs was determined fluorometrically by oxidation of 2,7-dichlorofluorescein. (B) Lung ceramide species were measured by LC-ESI-MS/MS. (C) Western blot analysis of cleaved caspase 3 in the lung. A,B: n=4,4,4, and 4 respectively. *P < 0.05, **P < 0.01, ***P < 0.01 compared to saline.
Allergen-induced ROS and apoptosis is ceramide-dependent
We have previously shown that treatment of HDM-challenged mice with the SPT inhibitor myriocin or fumonisin B1 (FB1), an inhibitor of ceramide synthases (CerS) (Fig. 5A), for the last 3 days of allergen challenge reduced lung ceramide levels and markedly suppressed AHR to methacholine8. Therefore, we examined whether preventing ceramide elevation affects apoptosis or ROS production. Indeed, i.p. treatment with myriocin 30 min prior to HDM challenges on days 10, 11, and 12, prevented elevation of all ceramide species in BALF (Fig. 5B), more potently than treatment with FB1. This data suggests that ceramide elevation is due to increased biosynthesis and salvage/recycling to a lesser extent. In agreement, dihydroceramides, precursors of ceramides and intermediates in its de novo biosynthesis, are also increased in lung8 and in BALF from HDM challenged mice and are decreased by these inhibitors (Fig. 5B). Acidic sphingomyelinase, which cleaves sphingomyelin to ceramide, has been implicated in bronchial asthma in mice45. However, in contrast to significant increases in ceramides and dihydroceramides, sphingomyelin levels were essentially unchanged after exposure to HDM (Fig. E5), indicating that sphingomyelin degradation is not a major contributor to ceramide elevation. Interestingly, treatment with these inhibitors not only mitigated the increase in ceramides, they markedly reduced lung epithelial cell apoptosis induced by HDM as demonstrated by reduced TUNEL staining (Fig. 5C) and reduced caspase 3 cleavage in western blots (Fig. 5D) and also completely prevented ROS formation in the lung (Fig. 5E). These results suggest that allergen-induced apoptosis and ROS production in the lung are due to elevated ceramide.
FIG 5. Inhibiting ceramide production decreases ROS and apoptosis induced by HDM.
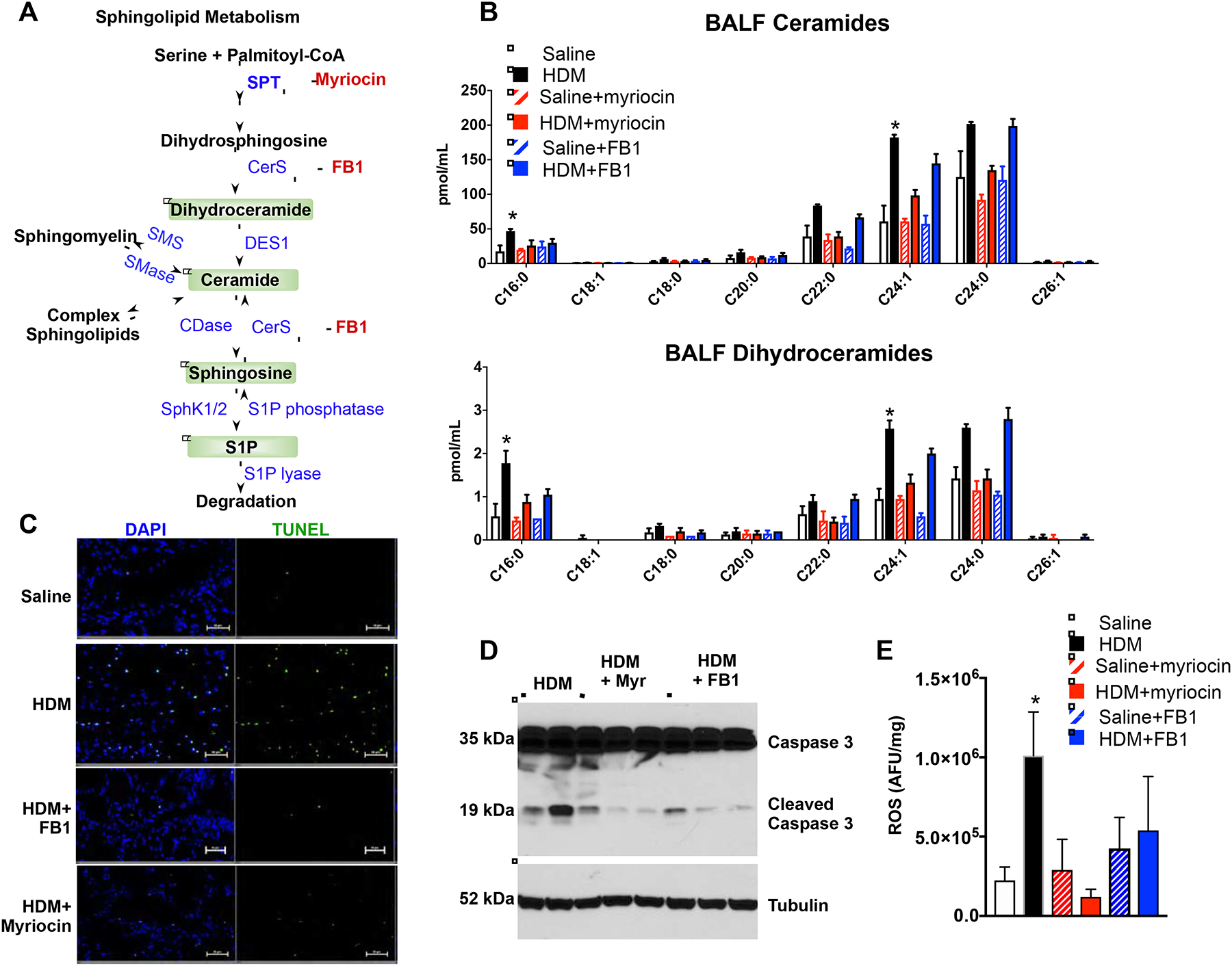
(A–C) Mice were challenged daily intranasally with HDM or saline for 5 consecutive days from days 1 to 5 and from days 8 to 12. Mice were injected i.p. with vehicle, myriocin (Myr; 0.3 mg/kg), or fumonisin B1 (FB1, 0.5 mg/kg) 30 min prior to the HDM challenges on days 10, 11, and 12 and lungs were examined on day 15. (A) Scheme of ceramide formation by de novo biosynthesis and degradation. Myriocin inhibits SPT and FB1 inhibits ceramide synthases (CerS). (B) Sphingolipids were extracted from BALF and ceramide and dihydroceramide species measured by LC-ESI-MS/MS. (C) In situ TUNEL staining of lung sections. Size bars: 50 μm. (D) Western blot analysis of cleaved caspase 3 in the lung. (E) ROS was measured fluorometrically. Data are mean ± SD. B: n=4 for saline, 4 for HDM, 2 for saline+myriocin, 4 for HDM+myriocin; 2 for saline+FB1, 4 for HDM+FB1; D: n= 2,3 and 3 respectively; E: n=3 for saline, 7 for HDM, 2 for saline+myriocin, 3 for HDM+myriocin; 2 for saline+FB1, 4 for HDM+FB1. *P < 0.05, **P < 0.01 compared to each saline control.
To elucidate the direct effect of HDM and increased ceramide on apoptosis and ROS formation, primary lung epithelial cells were treated with HDM or D-erythro-C6-ceramide, which is converted in cells to endogenous C16- and C24-ceramide46. Both HDM and C6-ceramide reduced viable cells with an increase in both early and late apoptotic cells (defined as Annexin V+) (Fig. E6A,B), as well as ROS generation, measured by fluorescence of intracellular oxidized DCF (Fig. E6C). FB1 inhibited apoptosis and ROS generation induced by HDM or C6-ceramide, whereas myriocin mitigated the effects of HDM but was less effective in C6-ceramide treated cells, consistent with the notion that generation of endogenous ceramides from short-chain ceramides requires deacylation and reacylation catalyzed by CerS46. These results suggest that, even in the absence of immune cells, HDM on its own can trigger bronchial epithelial cell apoptosis and ROS generation in a ceramide-dependent manner.
Levels of specific ceramide species associate with asthma severity in patients
Next, it was of interest to determine whether ceramide levels were also increased in asthmatic patients. BALF is the most reliable specimen to examine the fluid lining of the lower respiratory tract and is often used for physiologically relevant asthma studies47. Therefore, sphingolipid profiles were determined in BALF from healthy controls and from asthmatic patients recruited from SARP1–219 and classified by clinical activity as having no, mild, or severe disease. Relative to non-severe asthmatics, individuals with severe asthma had higher SARP Severity type scores. Spirometric measures of lung function including FEV1% and FVC% were lower in severe asthmatics than in non-severe asthmatics or healthy controls despite the usage of higher doses of inhaled corticosteroids and oral corticosteroids by severe asthmatics (Table I).
Table I.
Patient clinical characteristics and bronchiolar lavage cells
| Healthy Donors (Hd) | Non-severe Asthma (NSA) | Severe Asthma (SA) | |
|---|---|---|---|
| No. of subjects | 5 | 5 | 10 |
| Age | 30.4 (23.2–51.8) | 32 (23.7–51) | 42 (22.4–59.5) |
| % Male | 20 | 20 | 30 |
| BMI | 24.2±5.7 (19.4–33.5) | 26.3±7.5 (18.6–40.2) | 30.3±4.1 (22.8–35.1) |
| SARP Severity | 1 | 3 | 4.4±0.5** (4–5) |
| FEV1 % predicted | 112±5.6 (107–121) | 84.6±19.2 (60–106) | 54.9±21** (21–83) |
| FVC % predicted | 110±6.3 (104–119) | 83.4±20.0 (56–104) | 63.0±23.8** (36.3–101) |
| FEV1/FVC | 0.85±0 (0.78–0.88) | 0.8±0.1 (0.73–0.87) | 0.6±0.08** (0.50–0.71) |
| Max FEV1 Reversal with BD | 4.47±3.5 (0.9–6.7) | 9.7±3.0 (6.9–14.7) | 33.5±24.7 (0–47.3) |
| Oral steroids | NO | NO | YES |
| Inhaled corticosteroids | NO | YES | YES |
| High dose of inhaled corticosteroids | NO | NO | YES |
| Total cell count (millions) | 8.2±2.5 (5.6–12) | 6.1±2.8 (2.0–9.4) | 4.87±3.4 (1–11.8) |
| Alveolar Macrophages (%) | 92.3±4.9 (86.3–97.7) | 84.6±9.1 (68.7–84.2) | 80.6±11.9 (48.3–91.6) |
| Neutrophils (%) | 0.9±1.3 (0–3.2) | 3.4±3.3 (1.9–10) | 6.6±8.9 (0.8–28.3) |
| Eosinophils (%) | 0.6±0.9 (0–2.1) | 1.2±1.4 (0–3.3) | 3.5±3.7 (0–11.3) |
| Lymphocytes (%) | 6.2±4.3 (2–12.7) | 10.8±7.6 (4–19) | 9.3±5.0 (3.8–21.7) |
Values represent the mean ± SD (range). BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BD, post-bronchodilator.
P < 0.01 compared to healthy controls.
All ceramide species in BALF from severe asthmatics, except C18:1, were increased compared to healthy controls, with significant increases in C20, C26, and C26:1 ceramides (Fig. 6A). However, these increases in ceramide species were not observed in mild asthmatics. Somewhat unexpectedly, there were no significant differences in BALF levels of the bioactive sphingolipid metabolite sphingosine-1-phosphate (S1P) or the sphingoid bases, sphingosine and dihydrosphingosine, among the three groups (Fig. 6A).
FIG 6. Association between asthma severity and ceramide species that are increased in BALF.
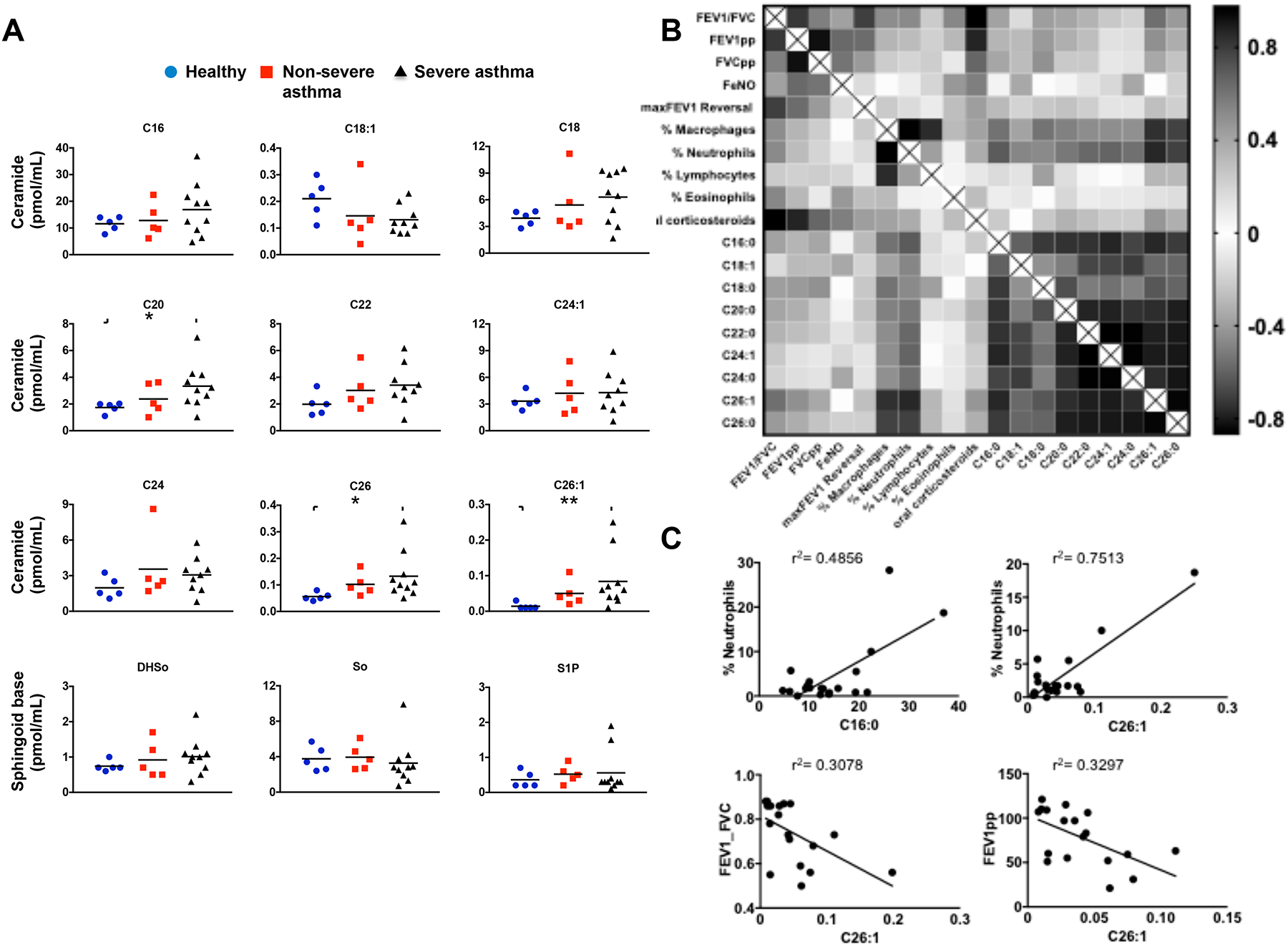
(A) Levels of ceramide species in BALF from healthy controls, non-severe asthmatics, and severe asthmatics described in Table I were measured by LC-ESI-MS/MS. * P < 0.05 compared to healthy controls. (B) Heat-map of matrix coefficient correlation of each ceramide species in the BALF and lung functions or immune cells infiltration. Visualization of the correlations between each pair of variables using product-moment coefficients. Stronger Pearson correlation coefficients (r) are represented by black color. (C) Examples of correlations between C16 or C26:1 ceramides and neutrophils, and lung functions. Goodness of fit is indicated by r2. Correlation of neutrophils with C16-ceramide (Spearman r = 0.3089 and P = 0.092) and with C26:1-ceramide (Spearman r=0.4141 and p = 0.039). Correlation of FEV1_FVC with C26:1-ceramide (Pearson r = 0.5548 and P = 0.01) and FEV1pp with C26:1-ceramide (Pearson r = 0.5742, and P =0.01).
Next, we examined whether changes of these ceramide species in the BALF correlated with lung function or immune cells infiltration. Several strong correlations were revealed by unbiased correlation matrix analysis (Fig. 6B). All ceramides with different acyl chain lengths correlated with each other. Remarkably, there were also positive correlations between ceramides, particularly C16:0, C26:1, and C26:0, with the degree of neutrophil infiltration (Fig. 6BC). Moreover, these species also correlated with airway obstruction determined by FEV1pp and FEV1/FVC (Fig. 6C). Taken together, these data suggest that specific ceramide species correlate with markers of asthma severity and airway neutrophilia, which has been associated with asthma exacerbations that are refractory to corticosteroid treatment48.
Involvement of ceramide in HDM-induced recruitment of neutrophils into the lung
We previously found that blocking ceramide elevation prevented AHR and decreased accumulation of eosinophils in HDM challenged mice8. Whereas type 2 eosinophilic inflammation is found in most asthmatics, a subset of patients has severe debilitating disease with neutrophil-predominant lung inflammation48. Because we found association in asthmatic patients between ceramide, asthma severity, and airway neutrophilia (Fig. 6B,C), it was of interest to examine the involvement of ceramides in aeroallergen-induced neutrophil recruitment into the lung. Immunofluorescence staining of lung sections with neutrophil-specific anti-Ly6G49 revealed intense staining after HDM exposure, which was greatly reduced by treatment with myriocin or FB1 (Fig. 7A). Likewise, staining for myeloperoxidase (MPO), found predominantly in neutrophil granules and a marker of their activation, was increased by HDM and suppressed by the inhibitors of ceramide formation (Fig. 7A). Furthermore, consistent with a previous report50, increasing lung ceramide by intranasal instillation of C16:0 ceramide, significantly increased numbers of neutrophils (CD11b+CD11c−SiglecF−Ly6G+) in the BALF (Fig. 7B).
FIG 7. Increased ceramide in HDM-challenged mice enhances neutrophil recruitment to the lungs.
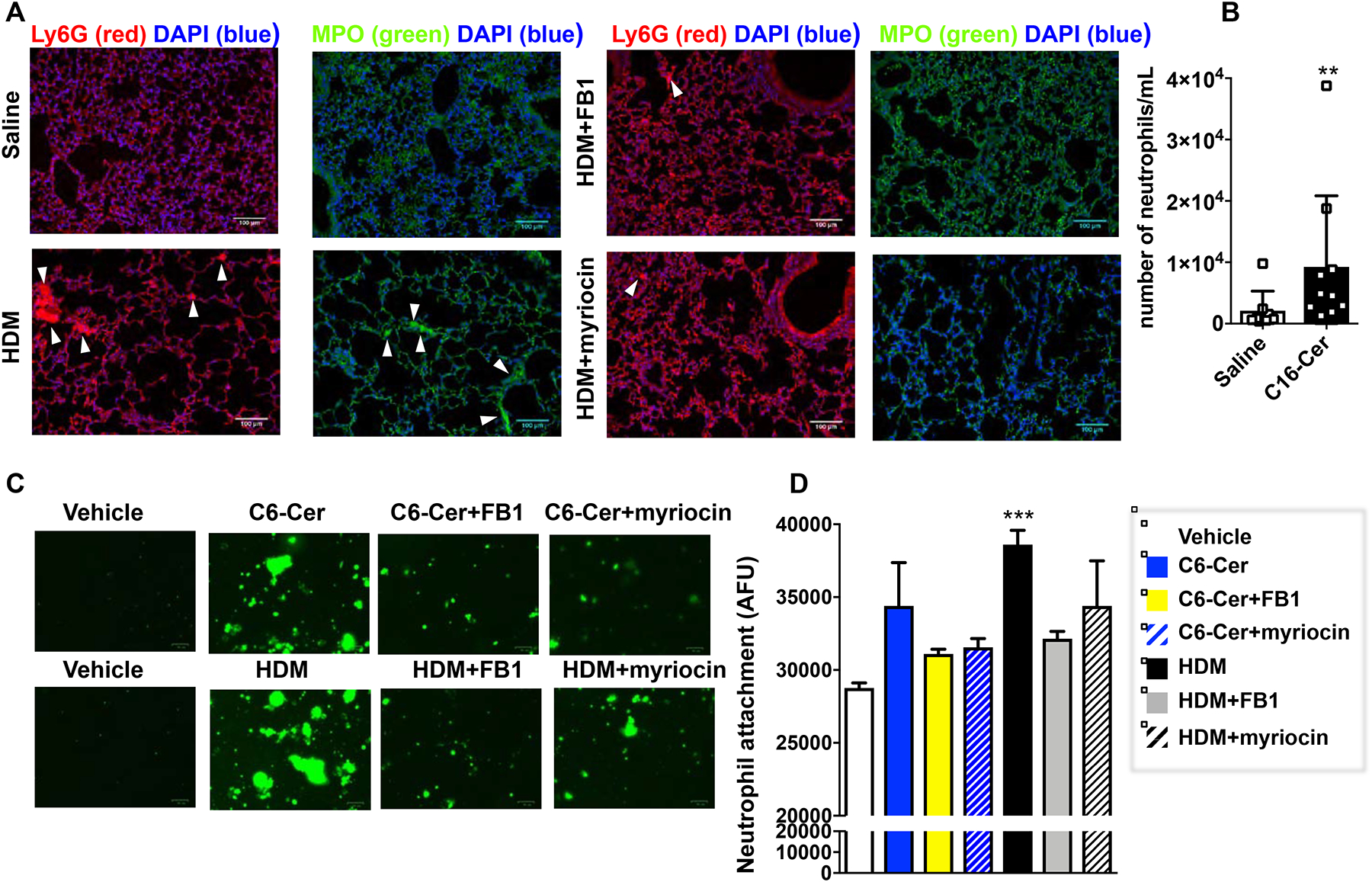
(A) Mice were challenged daily intranasally with HDM or saline for 5 consecutive days from days 1 to 5 and from days 8 to 12. Mice were injected i.p. with vehicle, myriocin (Myr; 0.3 mg/kg), or fumonisin B1 (FB1, 0.5 mg/kg) 30 min prior to the HDM challenges on days 10, 11, and 12 and lungs were examined on day 15 as described in Figure 5. Lung sections were stained with anti-Ly6G (red) or anti-MPO antibody (green) and co-stained with DAPI to visualize nuclei (blue). Size bar: 100 μm. (B,C) Primary lung epithelial cells incubated for 20 h with vehicle, D-erythro-C6-ceramide (C6-Cer, 1 μM), or HDM (100 μg/mL) in absence or presence of FB1 (1 μM) or myriocin (100 nM) as indicated. After extensive washing, purified labeled neutrophils were added for 4 h and adhesion assessed by fluorescence. (B) Following intranasal instillation of vehicle or C16:0 ceramide number of neutrophils in the BALF was determined by FACS. Data means ± SD. n = 8 and 10 mice/group, respectively. **P < 0.01. (C) Representative fluorescent images. (D) Data expressed as arbitrary fluorescence units are means ± SEM. n=5 for each group. Each sample represents an individual donor mouse. * p < 0.05 ** p < 0.01 compared to vehicle.
To further examine whether increased ceramide in the epithelium triggers neutrophil recruitment, primary lung epithelial cells were treated for 20 h with HDM or D-erythro-C6-ceramide in the absence or presence of FB1 or myriocin to increase or suppress ceramide elevation, respectively. Treatment of primary lung epithelial cells with HDM or C6-ceramide increased neutrophil adhesion to the epithelial monolayer, which was reduced by myriocin or FB1 (Fig. 7C,D). Taken together, this data support a role for ceramide elevation in allergen-initiated lung neutrophil recruitment.
Discussion
Exposure to airborne allergens such as HDM and Alternaria alternata are major causes of allergic asthma. The airway epithelium is the first line of defense of the lung and plays a key role in allergic sensitization and remodeling36, 51. These aeroallergens can also induce apoptosis and oxidative stress of the airway epithelium in mice, compromising its barrier function, increasing susceptibility to lung inflammation, leading to exacerbation of asthma36. However, the underlying mechanisms that trigger these events have not been fully elucidated. Here we demonstrated that HDM and Alternaria induced significant increases in the pro-apoptotic sphingolipid metabolite ceramide in bronchial epithelium. Prevention of ceramide elevation mitigated HDM-induced lung apoptosis, oxidative stress, and neutrophilia indicative of the critical role of ceramide in activating these processes. Importantly, pronounced reduction of AHR, eosinophils8 and neutrophils in BALF, apoptosis, and oxidative stress was observed even when inhibitors of ceramide production were only administered during the late asthmatic responses. These observations provide direct evidence for the importance of ceramide in asthma-induced pathology.
Clusters of apoptotic bronchial epithelial cells, known as Creola bodies, have long been noted in the sputum of severe asthmatics32, 52. Creola bodies were detected in sputum of 60% of pediatric asthmatic patients, which correlated with mobilization into the airway of neutrophils but not eosinophils29, 53. Consistent with data from aeroallergen challenged mice14, 54, there are several reports of apoptotic epithelial cells in endobronchial biopsies of adult patients with chronic, persistent asthma but not in biopsies from healthy volunteers29–31, 55. Based on our data, we speculate that ceramide elevation leading to caspase 3 activation, a crucial mediator of apoptosis, is the initial trigger of the apoptotic process leading to Creola body formation.
The recruitment and activation of neutrophils must be tightly regulated to balance their effector functions with their ability to damage tissues by release of proteases and ROS56. Our finding that allergen-driven airway neutrophilia was decreased by blocking ceramide elevation is important as neutrophilic inflammation in the asthmatic lung is associated with impaired lung function and more severe disease48, 56, 57. Ceramide treatment of neutrophils enhanced several pro-inflammatory pathways, including chemotaxis, phagocytosis, and neutrophil extracellular trap (NET) formation58, a process by which neutrophils externalize web-like chromatin strands containing antimicrobial peptides, proteases, and cytotoxic enzymes59. Recently it was shown that NET formation is induced in allergic lung after exposure to an aeroallergen59. Thus, it is possible that elevation of ceramide we observed in the lung and BALF may also contribute to neutrophilic inflammation in severe asthma.
Allergic asthma is associated with increases in ROS that are critical for the initiation of inflammatory responses60. ROS levels are elevated in the lavage fluid of asthmatic patients, likely produced by NADPH oxidase in immune cells infiltrating the lungs or by airway epithelial cells61. Markers of oxidative stress, including nitric oxide and 8-isoprostane, are elevated in exhaled breath condensate during asthma exacerbation and in patients with severe asthma62. Consistent with previous studies14, 63, we observed that HDM and Alternaria challenges induced lung ROS generation. Moreover, inhibitors of ceramide generation also blocked ROS production in response to these aeroallergens. However, dietary supplementation of α-tocopherol, the isoform that contributes to the anti-oxidative and anti-inflammatory effects17, 44, reduced HDM-induced ROS as expected, but had no effect on increases of ceramide or apoptosis. These data indicate that although lung oxidative stress is involved in airway inflammation64, it is not the cause for allergen-induced ceramide generation or apoptosis that leads to extensive lung damage. Moreover, the α-tocopherol supplemented diet reduced AHR and eosinophil but not neutrophil infiltration44, suggesting that an ROS-independent mechanism drives neutrophil recruitment. These results may also explain some of the inconsistent outcomes from clinical reports on the associations of vitamin E and asthma and its potential beneficial effects65, 66.
Asthma is a very heterogeneous disease with multiple phenotypes and different onset, course, and treatment responses and there is a great need for biomarkers to improve diagnosis and treatment67, 68. Interestingly, our targeted sphingolipidomic analyses showed that specific ceramide acyl chain species, including C16, the predominant species, and particularly the very long chain C20, C26, and C26:1, were increased in BALF from severe asthmatic patients but not in mild asthmatics, whereas C18:1 was reduced in both mild and severe asthmatics. Somewhat surprisingly, S1P, which has previously been implicated in allergic asthma69, 70, was not significantly increased. These results support the physiological relevance of ceramide elevation in exacerbation of allergic asthma. An untargeted metabolomics analysis of serum from healthy individuals and asthmatics also found that increased levels of ceramide positively correlated with asthma severity71. However, the physiological relevance to asthma of changes in sphingolipids in the circulation is unclear.
The strengths of this study are that it represents carefully phenotyped asthmatic patients, eliminated self-reporting bias, contains extensive information on lung function and disease severity, and corticosteroid treatment. Thus an unbiased correlation matrix analysis revealed that these ceramide species significantly correlated with lung function, and intriguingly, strongly correlated with neutrophil infiltration. Increased airway neutrophil infiltration has been associated with asthma severity and asthma exacerbations48, 57. Neutrophilia also correlates with asthma that is refractory to corticosteroids, the mainstay of asthma treatment48, 57. Here, we have identified significant associations between increased levels of specific ceramide species in BALF with neutrophilic lung inflammation, asthma severity, and resistance to corticosteroids. Hence, these ceramide species could potentially identify endotypes for corticosteroid resistant severe asthma and also be biomarkers to optimize diagnosis and to monitor and improve clinical outcomes in asthma.
Supplementary Material
Clinical Implications statement.
Our work suggests that allergen-induced ceramide elevation is the initial trigger of the apoptotic process leading to Creola body formation. Ceramide in bronchoalveolar lavage fluid has the potential to serve as a biomarker for specific asthma endotypes and disease severity.
ACKNOWLEDGMENTS
The authors thank Dr. Jeremy Allegood (Virginia Commonwealth University, Richmond, VA) for skillful sphingolipid analyses. This work was supported by NIH grant R01AI125433 (to S.S.). The fluorescence microscopy studies were supported by R01NS095215 (to E.B.). J.S. was supported by Institutional Development Award P20GM103527. The authors acknowledge the Virginia Commonwealth University Lipidomics/Metabolomics, the Flow Cytometry and the Microscopy Shared resources, which are supported in part by funding from the NIH-NCI Cancer Center Support Grant P30 CA016059.
Disclosure of potential conflict of interest: S. Spiegel received grants from the National Institutes of Health. The authors declare that they have no competing financial interests.
Abbreviations used:
- AHR
airway hyperresponsiveness
- Alt
Alternaria alternata
- BALF
bronchoalveolar lavage fluid
- CerS
ceramide synthase
- GWAS
genome-wide association studies
- FB1
fumonisin B1
- HDM
house dust mite
- LC-ESI-MS/MS
liquid chromatography-electrospray ionization-tandem mass spectrometry
- Myr
myriocin
- ORMDL3
ORM1 (yeast)-like protein 3
- ROS
reactive oxygen species
- SARP
Severe Asthma Research Program
- SPT
serine palmitoyltransferase
- S1P
sphingosine-1-phosphate
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
Sarah Spiegel, Ph.D.
Professor and Chair
Department of Biochemistry and Molecular Biology Mann T. and Sara D. Lowry Chair in Cancer Research VCU Massey Cancer Center
REFERENCES
- 1.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 2007; 448:470–3. [DOI] [PubMed] [Google Scholar]
- 2.Lluis A, Schedel M, Liu J, Illi S, Depner M, von Mutius E, et al. Asthma-associated polymorphisms in 17q21 influence cord blood ORMDL3 and GSDMA gene expression and IL-17 secretion. J. Allergy Clin. Immunol 2011; 127:1587–94. [DOI] [PubMed] [Google Scholar]
- 3.Schedel M, Michel S, Gaertner VD, Toncheva AA, Depner M, Binia A, et al. Polymorphisms related to ORMDL3 are associated with asthma susceptibility, alterations in transcriptional regulation of ORMDL3, and changes in T2 cytokine levels. J. Allergy Clin. Immunol 2015; 136:893–903. [DOI] [PubMed] [Google Scholar]
- 4.Stein MM, Thompson EE, Schoettler N, Helling BA, Magnaye KM, Stanhope C, et al. A decade of research on the 17q12–21 asthma locus: Piecing together the puzzle. J Allergy Clin Immunol 2018; 142:749–64 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmiedel BJ, Seumois G, Samaniego-Castruita D, Cayford J, Schulten V, Chavez L, et al. 17q21 asthma-risk variants switch CTCF binding and regulate IL-2 production by T cells. Nat Commun 2016; 7:13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kothari PH, Qiu W, Croteau-Chonka DC, Martinez FD, Liu AH, Lemanske RF Jr., et al. Role of local CpG DNA methylation in mediating the 17q21 asthma susceptibility gasdermin B (GSDMB)/ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3) expression quantitative trait locus. J Allergy Clin Immunol 2018; 141:2282–6 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, et al. Orm family proteins mediate sphingolipid homeostasis. Nature 2010; 463:1048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oyeniran C, Sturgill JL, Hait NC, Huang WC, Avni D, Maceyka M, et al. Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J. Allergy Clin. Immunol 2015; 136:1035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 2018; 19:175–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, et al. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. J. Pharmacol. Exp. Ther 2008; 324:548–57. [DOI] [PubMed] [Google Scholar]
- 11.Petrache I, Petrusca DN. The involvement of sphingolipids in chronic obstructive pulmonary diseases. Handb. Exp. Pharmacol 2013; 216:247–64. [DOI] [PubMed] [Google Scholar]
- 12.Sturgill JL. Sphingolipids and their enigmatic role in asthma. Adv Biol Regul 2018; 70:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James B, Milstien S, Spiegel S. ORMDL3 and allergic asthma: From physiology to pathology. J Allergy Clin Immunol 2019; 144:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan TK, Loh XY, Peh HY, Tan WN, Tan WS, Li N, et al. House dust mite-induced asthma causes oxidative damage and DNA double-strand breaks in the lungs. J Allergy Clin Immunol 2016; 138:84–96. [DOI] [PubMed] [Google Scholar]
- 15.Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-beta1 and oxidant stress in children with severe asthma: association with airflow limitation. J. Allergy Clin. Immunol 2012; 129:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson VJ, He Q, Osuchowski MF, Sharma RP. Disruption of sphingolipid homeostasis by myriocin, a mycotoxin, reduces thymic and splenic T-lymphocyte populations. Toxicology 2004; 201:67–75. [DOI] [PubMed] [Google Scholar]
- 17.Cook-Mills J, Gebretsadik T, Abdala-Valencia H, Green J, Larkin EK, Dupont WD, et al. Interaction of vitamin E isoforms on asthma and allergic airway disease. Thorax 2016; 71:954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore WC, Evans MD, Bleecker ER, Busse WW, Calhoun WJ, Castro M, et al. Safety of investigative bronchoscopy in the Severe Asthma Research Program. J Allergy Clin Immunol 2011; 128:328–36 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 2016; 4:574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–73. [DOI] [PubMed] [Google Scholar]
- 21.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol 2011; 32:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013; 38:322–35. [DOI] [PubMed] [Google Scholar]
- 23.Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 2011; 34:527–40. [DOI] [PubMed] [Google Scholar]
- 24.Cyphert-Daly JM, Yang Z, Ingram JL, Tighe RM, Que LG. Physiologic response to chronic house dust mite exposure in mice is dependent on lot characteristics. J Allergy Clin Immunol 2019; 144:1428–32 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debeuf N, Zhakupova A, Steiner R, Van Gassen S, Deswarte K, Fayazpour F, et al. The ORMDL3 asthma susceptibility gene regulates systemic ceramide levels without altering key asthma features in mice. J Allergy Clin Immunol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha SG, Ge XN, Bahaie NS, Kang BN, Rao A, Rao SP, et al. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nat. Commun 2013; 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller M, Tam AB, Cho JY, Doherty TA, Pham A, Khorram N, et al. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc. Natl. Acad. Sci. U.S.A 2012; 109:16648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature 2014; 510:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White SR. Apoptosis and the airway epithelium. J. Allergy (Cairo) 2011; 2011:9484–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Yin G, Liu J, Liu X, Zhao S. Epithelial apoptosis and loss in airways of children with asthma. J Asthma 2011; 48:358–65. [DOI] [PubMed] [Google Scholar]
- 31.Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 2013; 493:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada Y, Yoshihara S, Arisaka O. Creola bodies in wheezing infants predict the development of asthma. Pediatr. Allergy Immunol 2004; 15:159–62. [DOI] [PubMed] [Google Scholar]
- 33.Truong-Tran AQ, Ruffin RE, Foster PS, Koskinen AM, Coyle P, Philcox JC, et al. Altered zinc homeostasis and caspase-3 activity in murine allergic airway inflammation. Am J Respir Cell Mol Biol 2002; 27:286–96. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 1997; 272:11369–77. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamurthy K, Dasgupta S, Bieberich E. Development and characterization of a novel anti-ceramide antibody. J Lipid Res 2007; 48:968–75. [DOI] [PubMed] [Google Scholar]
- 36.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat. Med 2012; 18:684–92. [DOI] [PubMed] [Google Scholar]
- 37.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat. Med 2012; 18:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maltby S, Tay HL, Yang M, Foster PS. Mouse models of severe asthma: Understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation. Respirology 2017; 22:874–85. [DOI] [PubMed] [Google Scholar]
- 39.Salo PM, Arbes SJ Jr., Sever M, Jaramillo R, Cohn RD, London SJ, et al. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol 2006; 118:892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loser S, Gregory LG, Zhang Y, Schaefer K, Walker SA, Buckley J, et al. Pulmonary ORMDL3 is critical for induction of Alternaria-induced allergic airways disease. J. Allergy Clin. Immunol 2017; 139:1496–507 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castillo SS, Levy M, Thaikoottathil JV, Goldkorn T. Reactive nitrogen and oxygen species activate different sphingomyelinases to induce apoptosis in airway epithelial cells. Exp Cell Res 2007; 313:2680–6. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Yeganeh B, Ermini L, Post M. Sphingolipids as cell fate regulators in lung development and disease. Apoptosis 2015; 20:740–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Haefen C, Wieder T, Gillissen B, Starck L, Graupner V, Dorken B, et al. Ceramide induces mitochondrial activation and apoptosis via a Bax- dependent pathway in human carcinoma cells. Oncogene 2002; 21:4009–19. [DOI] [PubMed] [Google Scholar]
- 44.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol 2009; 182:4395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boll S, Ziemann S, Ohl K, Klemm P, Rieg AD, Gulbins E, et al. Acid sphingomyelinase regulates TH 2 cytokine release and bronchial asthma. Allergy 2020; 75:603–15. [DOI] [PubMed] [Google Scholar]
- 46.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, et al. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem 2002; 277:12960–9. [DOI] [PubMed] [Google Scholar]
- 47.Peters SP. Asthma phenotypes: nonallergic (intrinsic) asthma. J Allergy Clin Immunol Pract 2014; 2:650–2. [DOI] [PubMed] [Google Scholar]
- 48.Ray A, Kolls JK. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol 2017; 38:942–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 2008; 83:64–70. [DOI] [PubMed] [Google Scholar]
- 50.Kamocki K, Van Demark M, Fisher A, Rush NI, Presson RG Jr., Hubbard W, et al. RTP801 is required for ceramide-induced cell-specific death in the murine lung. Am. J. Respir. Cell Mol. Biol 2013; 48:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandhi VD, Vliagoftis H. Airway epithelium interactions with aeroallergens: role of secreted cytokines and chemokines in innate immunity. Front Immunol 2015; 6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naylor B The shedding of the mucosa of the bronchial tree in asthma. Thorax 1962; 17:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshihara S, Yamada Y, Abe T, Linden A, Arisaka O. Association of epithelial damage and signs of neutrophil mobilization in the airways during acute exacerbations of paediatric asthma. Clin Exp Immunol 2006; 144:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan X, Wang E, Xiao X, Wang J, Yang X, Yang P, et al. The role of IL-25 in the reduction of oxidative stress and the apoptosis of airway epithelial cells with specific immunotherapy in an asthma mouse model. Am J Transl Res 2017; 9:4137–48. [PMC free article] [PubMed] [Google Scholar]
- 55.Trautmann A, Schmid-Grendelmeier P, Kruger K, Crameri R, Akdis M, Akkaya A, et al. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J Allergy Clin Immunol 2002; 109:329–37. [DOI] [PubMed] [Google Scholar]
- 56.Snelgrove RJ, Patel DF, Patel T, Lloyd CM. The enigmatic role of the neutrophil in asthma: Friend, foe or indifferent? Clin Exp Allergy 2018; 48:1275–85. [DOI] [PubMed] [Google Scholar]
- 57.Panettieri RA Jr. The Role of Neutrophils in Asthma. Immunol Allergy Clin North Am 2018; 38:629–38. [DOI] [PubMed] [Google Scholar]
- 58.Corriden R, Hollands A, Olson J, Derieux J, Lopez J, Chang JT, et al. Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat Commun 2015; 6:8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnamoorthy N, Douda DN, Bruggemann TR, Ricklefs I, Duvall MG, Abdulnour RE, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu J, Li Y, Zhong W, Gao P, Hu C. Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis 2017; 9:E32–E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Um Sahiner, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma. World Allergy Organ J 2011; 4:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kharitonov SA, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 2002; 7:1–32. [DOI] [PubMed] [Google Scholar]
- 63.Uchida M, Anderson EL, Squillace DL, Patil N, Maniak PJ, Iijima K, et al. Oxidative stress serves as a key checkpoint for IL-33 release by airway epithelium. Allergy 2017; 72:1521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auerbach A, Hernandez ML. The effect of environmental oxidative stress on airway inflammation. Curr Opin Allergy Clin Immunol 2012; 12:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoskins A, Roberts JL 2nd, Milne G, Choi L, Dworski R. Natural-source d-alpha-tocopheryl acetate inhibits oxidant stress and modulates atopic asthma in humans in vivo. Allergy 2012; 67:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax 2004; 59:652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med 2012; 18:716–25. [DOI] [PubMed] [Google Scholar]
- 68.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol 2015; 135:299–310. [DOI] [PubMed] [Google Scholar]
- 69.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol 2008; 8:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price MM, Oskeritzian CA, Falanga YT, Harikumar KB, Allegood JC, Alvarez SE, et al. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. J. Allergy Clin. Immunol 2013; 131:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reinke SN, Gallart-Ayala H, Gomez C, Checa A, Fauland A, Naz S, et al. Metabolomics analysis identifies different metabotypes of asthma severity. Eur Respir J 2017; 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


