Abstract
Background
In England, the reopening of universities in September 2020 coincided with a rapid increase in SARS-CoV-2 infection rates in university aged young adults. This study aimed to estimate SARS-CoV-2 antibody prevalence in students attending universities that had experienced a COVID-19 outbreak after reopening for the autumn term in September 2020.
Methods
A cross-sectional serosurvey was conducted during 02–11 December 2020 in students aged ≤ 25 years across five universities in England. Blood samples for SARS-CoV-2 antibody testing were obtained using a self-sampling kit and analysed using the Abbott SARS-CoV-2 N antibody and/or an in-house receptor binding domain (RBD) assay.
Findings
SARS-CoV-2 seroprevalence in 2,905 university students was 17.8% (95%CI, 16.5–19.3), ranging between 7.6%-29.7% across the five universities. Seropositivity was associated with being younger likely to represent first year undergraduates (aOR 3.2, 95% CI 2.0–4.9), living in halls of residence (aOR 2.1, 95% CI 1.7–2.7) and sharing a kitchen with an increasing number of students (shared with 4–7 individuals, aOR 1.43, 95%CI 1.12–1.82; shared with 8 or more individuals, aOR 1.53, 95% CI 1.04–2.24). Seropositivity was 49% in students living in halls of residence that reported high SARS-CoV-2 infection rates (>8%) during the autumn term.
Interpretation
Despite large numbers of cases and outbreaks in universities, less than one in five students (17.8%) overall had SARS-CoV-2 antibodies at the end of the autumn term in England. In university halls of residence affected by a COVID-19 outbreak, however, nearly half the resident students became infected and developed SARS-CoV-2 antibodies.
Keywords: SARS-CoV-2 infection, COVID-19, Universities, Transmission, Students
Background
Young adults have the highest rates of SARS-CoV-2 infection but rarely develop severe COVID-19, require hospitalisation, intensive care admission, or die of the infection. Consequently, university students are expected to have mild transient illness if infected with SARS-CoV-2. Given the close proximity of many university students living in high-density residential housing and their extensive connected social networks compared to the general population, the potential for rapid spread of SARS-CoV-2 in university settings is of concern. In England, this concern was reaffirmed by the large numbers of PCR-confirmed cases1 and outbreaks2 in young adults when universities reopened in September 2020. PCR-testing was widely available for students when the universities reopened but only recommended for individuals with characteristic symptoms of COVID-19 (fever, new onset of cough, loss of smell or taste) and would, therefore, miss asymptomatic, atypical and mildly symptomatic infections. Testing also require the students to attend community testing centres when they are symptomatic. In contrast to PCR testing which only provide a point estimate of symptomatic disease prevalence, serum antibodies provide a more accurate estimate of prior infection and extent of transmission during outbreaks in institutional settings, as has been demonstrated previously in care homes, for example.3
In England and elsewhere, large number of outbreaks have been reported in universities and, because of public health concerns about asymptomatic infections fuelling these outbreaks and potentially contributing to wider community transmission, Public Health England (PHE) initiated a rapid serological evaluation of SARS-CoV-2 antibodies in universities across England that had experienced a COVID-19 outbreak to assess the extent of infection and transmission, the scale of outbreaks and risk factors for SARS-CoV-2 infection among university students. The findings of this serosurveillance will provide valuable information on the potential risk of future infections and outbreaks and implications for outbreak management and control in university settings.
Methods
Study design
PHE conducted a cross-sectional serosurvey during 2–11 December 2020 to estimate the prevalence of SARS-CoV-2 antibodies in students attending the following five universities across England: Leeds Beckett University, Newcastle University, University of Manchester, Oxford Brookes University and Reading University. The serosurvey was initiated rapidly after reports of large outbreaks in English universities, at a time when universities were implementing mass SARS-CoV-2 rapid testing programmes, just before the end of the autumn term (September to December 2020). All participating universities had reported an outbreak of COVID-19 to PHE between September and November 2020 (Supplementary Table 1).
Participants
University students aged 25 years or under who were enrolled with the university during the 2020/2021 academic year were invited to participate by email from the university or recruited by a PHE representative on-site on the day of mass lateral flow device (LFD) testing, along with invitation posters around the campus (Supplementary Table 1). Students were eligible to participate irrespective of whether they had prior confirmed COVID-19 or COVID-19 related symptoms.
Data sources
Participants provided online consent and completed a short online questionnaire. Information was collected on demographics, COVID-19 related illness or symptoms; accommodation type; whether they were aware of confirmed cases within their accommodation; and their participation in COVID-19 vaccine or other COVID-19 trials. Additional data were requested from universities regarding number of COVID-19 cases during the autumn term and occupancy of specific halls of residence within the university, which were verified through university and private accommodation provider websites.
Community seroprevalence estimates were obtained from the NHS Blood and Transplant (NHSBT) serological surveillance coordinated by PHE, which provides age-stratified seroprevalence across different geographic regions by testing samples from healthy adult blood donors using the Euroimmun Spike based assay.
Laboratory testing
Following online consent and questionnaire completion, participating students provided a blood sampling using the TASSO-SST OnDemand device to collect about 400 μL of capillary blood.4 The students were provided a TASSO kit containing the Tasso device, instructions, an alcohol swab and a plaster. The TASSO-SST OnDemand device attaches to the skin on the upper arm with a light adhesive. When the button is pressed, a vacuum forms and a lancet pricks the surface of the skin. The vacuum draws blood out of the capillaries and into a serum separator tube attached to the bottom of the Tasso Button. The blood sample is collected within 5 min and the device is removed from the arm. The blood-filled tube is then capped and sent to PHE for SARS-CoV-2 antibody testing by post (using the packaging and pre-paid envelope provided) or through courier collection from designated collection points at each university site.
At PHE, the samples were centrifuged, and sera tested for SARS-CoV-2 antibodies using the Abbott Architect SARS-CoV-2 IgG (nucleoprotein assay). The Abbot is highly specific (99.9%, 95% CI: 99.4–100; cut 0.8) and sensitive (92.7%,95% CI: 90.2–94.8), especially within the first three months after infection.5 Sera with insufficient volume for the Abbott assay were tested using a PHE in house receptor binding domain (RBD) assay (specificity 98.1%, 95% CI 97.3–98.8%; sensitivity 89.8%, 95%CI, 86.0–92.9).6 Results of the antibody testing were reported back to individual participants.
Statistical analyses
Data are mainly descriptive and presented as numbers and percentages. Tests for association with SARS-CoV-2 antibody positivity were performed using a mixed-effects logistic regression model, which allows for differences across the universities with the aim of making results generalisable to the student population. A multivariable model to explore demographic factors was fitted adjusting for sex, age group, ethnicity and accommodation type. Being unwell with COVID-19 symptoms and having a confirmed COVID-19 case in their accommodation were assessed in a mixed-effects regression models that accounted for university as a random effect.
Data for healthy blood donors aged 17–24 years, by region in England during Week 46 -Week 51 from the NHSBT serological collection were used to compare university seropositivity estimates to corresponding regional seropositivity estimates.
Sub-group analyses were conducted on those living in halls of residence, where data were available on resident occupancy and capacity of the hall, with the aim of exploring the effects of demographic factors and accommodation characteristics on SARS-CoV-2 antibody seropositivity. Both univariable and multivariable hierarchical mixed effects models were fitted, including individual hall of residence nested within the university. Factors explored were sex, age group, ethnicity, hall size and sharing facilities. Analyses were conducted using Stata v.15.0 (Statacorp, Tx).
Ethics approval
The study protocol was approved by the PHE Research Ethics and Governance Group – NR0245.
Funding
This study was funded by Public Health England. The authors had sole responsibility for the study design, data collection, data analysis, data interpretation, and writing of the report. The authors are all employed by PHE, the study funder, which is a public body — an executive agency of the Department of Health and Social Care.
Results
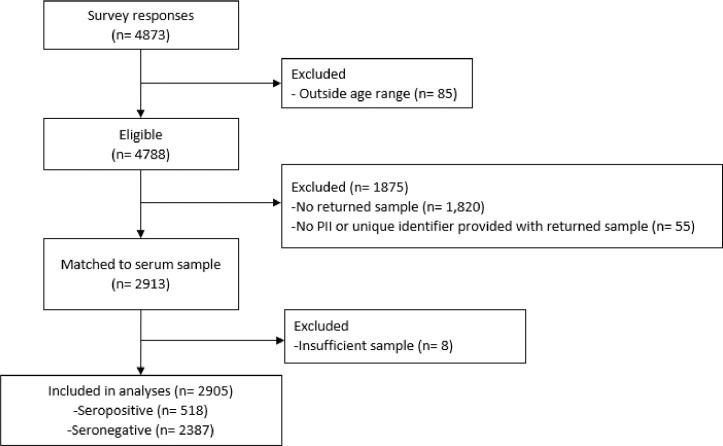
In total, 4873 students completed the online questionnaire but 85 were excluded because they were outside the specified age-range. Of the remaining 4788 participants, 2913 returned a blood sample with participant identifiable information (Fig. 1 ). Eight (0.3%) samples had insufficient volume for testing using either assay. Thus, 2905 were included in the final analysis, including 2702 (93%) with sufficient sample for testing with the Abbott assay and 203 (7%) with insufficient serum for the Abbott assay were tested using the RBD assay.
Fig. 1.
. Flow diagram for study participants. The number of study participants (n) are provided. Reasons for exclusion are given.
Participants
Of the 2905 participants, 2565 (88.3%) were white and 1818 were female (62.6%). The median age of participants was 20 (IQR, 19–21) years. In total, 872 participants (30.0%) lived in halls of residence and 387/872 (44.4%) reported a confirmed COVID-19 case in their accommodation. At the same time, 2033 (70.0%) lived in other residential settings and 479 (23.6%) reported a confirmed COVID-19 case in their accommodation (Table 1 ). The characteristics of study participants in individual university are summarised in Supplementary Table 2.
Table 1.
Characteristics of study participants – overall and stratified by valid and matched antibody test result.
| Characteristics |
Total n (%) |
Seropositive n (%) |
|---|---|---|
| Overall | 2905 (100) | 518 (17.8) |
| Gender | ||
| Male | 1074 (37) | 203 (18.9) |
| Female | 1818 (62.6) | 313 (17.2) |
| Prefer not to say | 13 (0.5) | 2 (15.4) |
| Age group | ||
| 17–19 | 892 (30.7) | 261 (29.3) |
| 20–22 | 1739 (59.9) | 231 (13.3) |
| 23–25 | 274 (9.4) | 26 (9.5) |
| Ethnicity | ||
| White | 2565 (88.3) | 462 (18) |
| Black | 36 (1.2) | 6 (16.7) |
| Asian | 157 (5.4) | 23 (14.6) |
| Mixed | 122 (4.2) | 23 (18.9) |
| Other | 25 (0.9) | 4 (16) |
| Medical condition (self-report) | ||
| Yes | 418 (14.4) | 62 (14.8) |
| No | 2459 (84.6) | 453 (18.4) |
| Unknown | 28 (1) | 3 (10.7) |
| Living in halls of residence | ||
| Halls of residence | 872 (30.0) | 252 (28.9) |
| Non- Halls of residence | 2033 (70.0) | 266 (13.1) |
| Accommodation type | ||
| Halls of residence | 872 (30.0) | 252 (28.9) |
| Off-campus housing | 1922 (66.2) | 250 (13) |
| Family Home | 110 (3.8) | 15 (13.6) |
| Other | 1 (0.03) | 1 (100) |
| Symptoms at any time since 1st Jan 2020 | ||
| Symptomatic | 1006 (34.6) | 305 (30.3) |
| No symptoms | 1589 (54.7) | 130 (8.2) |
| Not known | 310 (10.7) | 36 (11.6) |
| Symptomatic after starting university (of those symptomatic) | 553 (55) | 244 (44.1) |
| Previous PCR positive (self-report) | ||
| Previous PCR positive | 241 (8.3) | 199 (82.6) |
| Reported no previous PCR positive | 2664 (91.7) | 319 (12.0) |
| Previous PCR positive Sept – Nov 2020 | 216 (7.4) | 183 (84.7) |
| Confirmed case in accommodation | ||
| Confirmed case | 866 (29.8) | 310 (35.8) |
| No confirmed case | 1874 (64.5) | 186 (9.9) |
| Not Known | 165 (5.7) | 22 (13.3) |
| University | ||
| University A | 500 (17.2) | 80 (16) |
| University B | 445 (15.3) | 132 (29.7) |
| University C | 784 (27.1) | 143 (18.2) |
| University D | 546 (18.8) | 115 (21.1) |
| University E | 630 (21.7) | 48 (7.6) |
The characteristics of students who completed the online questionnaire but didn't provide a blood sample with participant identifiable information (n = 1875) were generally similar to those who did return a sample (median age 21 (IQR 20–22) years, 1229 (65.6%) were female, 1613 (86.0%) were white and 512 (27.3%) individuals lived in halls of residence).
Seroprevalence of SARS-CoV-2 antibodies in university and community settings
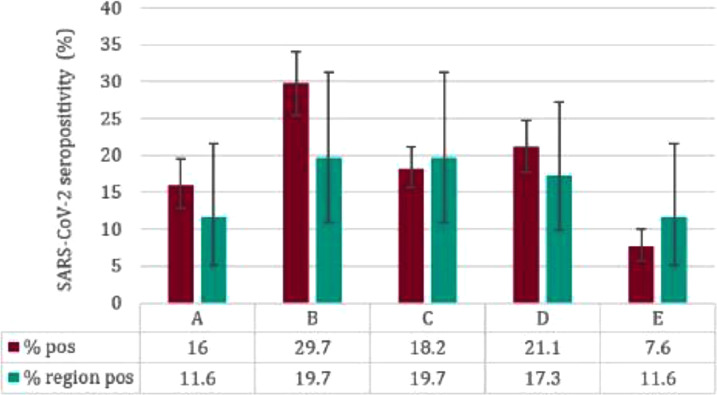
In total, 518/ 2905 participants (17.8%; 95%CI, 16.5–19.3%) were seropositive for SARS-CoV-2 IgG. This compares with 13.7% (95% CI 11.1–16.9%), amongst healthy blood donors aged 17–24 years in England during calendar Weeks 46 to 51. Seropositivity varied across the five universities, ranging from 7.6% to 29.7% (Table 1). Seropositivity in University A, B and D was higher compared to their respective regional community blood donor seroprevalence, but this was not statistically significant (Fisher's Exact p > 0.05) (Fig. 2 , Supplementary Table 3).
Fig. 2.
. University seropositivity by regional community blood donor seroprevalence estimates.
Previous COVID-19 history
Overall, 1006 (34.6%) participants reported COVID-19 related symptoms since 01 January 2020, including 553 (55% of those with COVID-19 symptoms; 19.0% of all participants) with symptom onset after starting university in the autumn term. Seropositivity was higher in participants reporting COVID-19 symptoms after starting university (244/553; 44.1% seropositive, 95% CI 39.9–48.4) compared to those who experienced symptoms prior to university (89/453; 19.7% seropositive, 95% CI 16.1–23.6). Among the 1589 participants who reported no COVID-19 related symptoms, 130 (8.2%) were seropositive.
Additionally, of 241 participants who reported a positive SARS-CoV-2 PCR test, 216 reported that the PCR test had been performed test during the autumn term (between September and November 2020), and 84.7% (183/216) were seropositive (Supplementary Table 4).
Factors associated with seropositivity in university students
In the univariate analysis, students aged 17–19 years had 4.1 times (95% CI, 2.7–6.4) greater odds of being seropositive than 23–25-year-olds, while those living in halls of residence had 2.9 times (95% CI, 2.4–3.5) greater odds of testing seropositive than those living in other accommodation types. In shared accommodation settings, having a confirmed case within the accommodation setting was significantly associated with seropositivity (OR 4.5, 95%CI 3.7–5.6). These associations remained independently significant in the multivariable logistic regression model (Table 2 ).
Table 2.
Potential risk factors for antibody positivity for students.
| Variable | Seropositive n/N (%) |
Univariable regression OR (95% CI) |
P value | Multivariable regressiona OR (95% CI) |
Adjusted P value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 203/1074 (18.9) | Ref | Ref | ||
| Female | 313/1818 (17.2) | 0.87(0.71–1.06) | 0.16 | 0.83(0.68–1.02) | 0.08 |
| Prefer not to say | 2/13 (15.4) | 0.63(0.14–2.89) | 0.55 | 0.71(0.15–3.36) | 0.67 |
| Age group | |||||
| 23–25 | 26/274 (9.5) | Ref | Ref | ||
| 20–22 | 231/1739 (13.3) | 1.59(1.03–2.45) | 0.04 | 1.67(1.08–2.57) | 0.02 |
| 17–19 | 261/892 (29.3) | 4.11(2.66–6.35) | <0.01 | 3.16(2.02–4.93) | <0.01 |
| Ethnicity | |||||
| White | 462/2565 (18) | Ref | Ref | ||
| Black | 6/36 (16.7) | 1.05(0.42–2.6) | 0.92 | 0.88(0.35–2.24) | 0.79 |
| Asian | 23/157 (14.6) | 0.82(0.51–1.29) | 0.39 | 0.7(0.44–1.12) | 0.14 |
| Mixed | 23/122 (18.9) | 1.09(0.68–1.75) | 0.73 | 1.02(0.63–1.67) | 0.93 |
| Other | 4/25 (16) | 0.93(0.31–2.78) | 0.90 | 0.73(0.24–2.27) | 0.59 |
| Living in halls of residence | |||||
| Non- Halls of residence | 266/2033 (13.1) | Ref | Ref | ||
| Halls of residence b | 252/872 (28.9) | 2.89(2.36–3.54) | <0.01 | 2.14(1.7–2.68) | <0.01 |
| Accommodation type | |||||
| Off-campus housing | 250/1922 (13) | Ref | Ref | ||
| Halls of residence | 252/872 (28.9) | 2.87(2.33–3.52) | <0.01 | 2.13(1.69–2.68) | <0.01 |
| Family Home | 15/110 (13.6) | 0.83(0.47–1.48) | 0.53 | 0.88(0.49–1.58) | 0.66 |
| Other | 1/1 (100) | – | – | – | – |
| Sharing facilities | |||||
| Sharing bedroom | |||||
| No | 493/2686 (18.4) | Ref | Ref | ||
| Yes | 20/195 (10.3) | 0.5(0.31–0.8) | <0.01 | 0.73(0.45–1.19) | <0.01 |
| Unknown | 5/24 (20.8) | – | – | – | – |
| Sharing bathroom | |||||
| No | 214/747 (28.6) | Ref | Ref | ||
| Yes | 304/2158 (14.1) | 0.43(0.35–0.53) | <0.01 | 0.73(0.57–0.95) | <0.01 |
| No. of individuals student shares kitchen with | |||||
| 0–3 | 119/949 (12.5) | Ref | Ref | ||
| 4–7 | 332/1657 (20) | 1.92(1.53–2.42) | <0.01 | 1.43(1.12–1.82) | 0.03 |
| 8 or more | 65/290 (22.4) | 2.99(2.09–4.27) | <0.01 | 1.53(1.04–2.24) | <0.01 |
| History of COVID-19 related symptoms | |||||
| Symptoms at any time since 1st Jan 2020 | |||||
| No symptoms | 130/1589 (8.2) | Ref | Ref | <0.01 | |
| Symptomatic | 305/1006 (30.3) | 4.51(3.62–5.61) | <0.01 | 4.3(3.43–5.38) | <0.01 |
| Not known | 36/310 (11.6) | 1.25(0.85–1.84) | 0.26 | 1.05(0.71–1.57) | 0.80 |
| Symptomatic before or after starting university | |||||
| Before | 89/453 (19.6) | Ref | Ref | ||
| After | 244/553 (44.1) | 3.15(2.35–4.21) | <0.01 | 2.57(1.89–3.49) | <0.01 |
| Confirmed case in accommodation | |||||
| No confirmed case | 186/1874 (9.9) | Ref | Ref | ||
| Confirmed case | 310/866 (35.8) | 4.52(3.67–5.58) | <0.01 | 3.57(2.86–4.44) | <0.01 |
| Not Known | 22/165 (13.3) | 1.22(0.72–2.06) | 0.45 | 0.95(0.58–1.56) | 0.83 |
Adjusted for sex, age, ethnicity, living in halls of residence.
Adjusted for sex, age, ethnicity
OR = odds ratio; CI = confidence interval
Univariable and Multivariable models included university as a random effect.
When assessing the sharing of facilities in the different accommodation types, the odds of seropositivity increased with the number of individuals sharing a kitchen (sharing with 4 to 7 individuals, OR 1.92, 95%CI 1.53–2.42; sharing with 8 or more individuals OR 2.99, 95% CI 2.09–4.27, compared to sharing with 0–3 individuals). Those sharing a bathroom, however, had lower odds of seropositivity compared to those who didn't share a bathroom (OR 0.4, 95 CI% 0.4–0.5). In the multivariable logistic regression model, these associations also remained independently significant (Table 2).
Halls of residence – subgroup analyses
Subgroup analysis of those who lived in halls of residence (n = 697) found that living in a hall with larger numbers of residents was associated with higher seropositivity, although this association was not statistically significant (Table 3 ). A similar pattern was also seen with students sharing a kitchen, with the odds of seropositivity increasing with a greater number of individuals sharing a kitchen. This association was, however, only statistically significant in the multivariable model. Sharing a bedroom increased the odds of being seropositive (OR 3.8, 95% CI 1.1–13.1) but sharing a bathroom lowered the risk (OR 0.6, 95% CI 0.3–0.9).
Table 3.
Potential risk factors for students living in halls of residence.
| Variable |
Seropositive n/N (%) |
Univariable regression OR (95% CI) |
P value |
Multivariable regressiona OR (95% CI) |
Adjusted P value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 92/273 (33.7) | ||||
| Female | 128/421 (30.4) | 0.74(0.51–1.08) | 0.12 | 0.63(0.43–0.93) | 0.02 |
| Prefer not to say | 1/3 (33.3) | 1.83(0.12–28.97) | 0.67 | 2.58(0.12–55.72) | 0.54 |
| Age group | |||||
| 23–25 years | 176/443 (39.7) | Ref | Ref | ||
| 20–22 years | 41/218 (18.8) | 2.22(0.68–7.17) | 0.18 | 2.06(0.63–6.72) | 0.23 |
| 17–19 years | 4/36 (11.1) | 3.52(1.13–11.02) | 0.03 | 3.1(0.97–9.91) | 0.06 |
| Ethnicity | |||||
| White | 195/596 (32.7) | Ref | Ref | ||
| Black | 3/10 (30) | 2.24(0.46–11.01) | 0.32 | 2.65(0.53–13.28) | 0.24 |
| Asian | 13/48 (27.1) | 1.29(0.6–2.78) | 0.51 | 1.19(0.54–2.58) | 0.67 |
| Mixed | 9/33 (27.3) | 0.71(0.29–1.72) | 0.45 | 0.79(0.33–1.93) | 0.61 |
| Other | 1/10 (10.0) | 0.17(0.02–1.46) | 0.11 | 0.18(0.02–1.58) | 0.12 |
| Hall size | |||||
| Small (<300) | 42/196 (21.4) | Ref | Ref | ||
| Medium (300–699) | 103/331 (31.1) | 1.46(0.76–2.78) | 0.25 | 1.46(0.75–2.86) | 0.27 |
| Large (700+) | 76/170 (44.7) | 2.04(0.97–4.3) | 0.06 | 2.06(0.99–4.29) | 0.05 |
| Sharing facilities | |||||
| No. of individuals student shares kitchen with | |||||
| 0–3 | 26/83 (31.3) | Ref | Ref | ||
| 4 to 7 | 145/413 (35.1) | 1.5(0.82–2.73) | 0.19 | 1.57(0.84–2.93) | 0.16 |
| 8+ | 50/201 (24.9) | 1.9(0.9–4.01) | 0.10 | 2.35(1.1–5.01) | 0.03 |
| Share bathroom | |||||
| No | 163/435 (37.5) | Ref | Ref | ||
| Yes | 58/262 (22.1) | 0.55(0.32–0.93) | 0.03 | 0.43(0.24–0.77) | <0.01 |
| Share bedroom | |||||
| No | 214/677 (31.6) | Ref | Ref | ||
| Yes | 7/20 (35.0) | 3.84(1.13–13.06) | 0.03 | 7.65(2.15–27.25) | <0.01 |
| Case rate in halls of residence (self-report) | |||||
| <8% | 50/184 (27.2) | Ref | Ref | ||
| 8–20% | 101/206 (49) | 2.28(1.13–4.56) | 0.02 | 2.54(1.53–4.23) | <0.01 |
| 20%+ | 35/71 (49.3) | 2.81(1.1–7.16) | 0.03 | 2.25(1.16–4.35) | 0.02 |
| Unknown | 35/236 (14.8) | – | – | – | – |
| Confirmed case in accommodation | |||||
| No confirmed case | 52/297 (17.5) | Ref | Ref | ||
| Confirmed case | 156/323 (48.3) | 3.02(1.96–4.65) | <0.01 | 2.75(1.77–4.27) | <0.01 |
| Not known | 13/77 (16.9) | 0.79(0.38–1.62) | 0.51 | 0.74(0.35–1.57) | 0.43 |
All univariable estimates include individual hall of residence nested within University accounted for using a hierarchical mixed effects model
bAll multivariable estimates are adjusted for sex, age group, ethnicity, hall size, kitchen sharing, shared bathroom, shared bedroom, with individual hall of residence nested within University accounted for using a hierarchical mixed effects model
OR = odds ratio; CI = confidence interval.
For individuals who lived in halls of residence with available information on self-reported COVID-19 cases during the autumn term (n = 461), those who lived in halls of residence with 8–20% (OR 2.3, 95% CI 1.1–4.6) or >20% (OR 2.8, 95% CI 1.1–7.2) self-reported case rate had significantly higher odds of being seropositive compared to students living in halls of residence with <8% self-reported case rate. These associations remained statistically significant in the multivariable model.
Within halls of residence, students who reported a confirmed case in their accommodation setting had greater odds of testing positive for antibodies compared to those who didn't report a confirmed case in their accommodation (OR 3.02, 95% CI 1.96–4.65). This association remained in the multivariable model but was attenuated (OR 2.75, 95% CI 1.77–4.27).
Discussion
This cross-sectional UK study of SARS-CoV-2 antibody seroprevalence among the university student population found that 17.8% of university students had evidence of prior infection with SARS-CoV-2 by December 2020. This is higher than the seroprevalence reported by Office for National Statistics study of individuals over 16 years of age from England in December 2020 (12.1%, 95% CI: 11.6%−12.7%).7
We observed a large variation in seropositivity rates between universities, ranging from 7.6% to 29.7%. When compared with blood donors aged 17–24 years in England during Weeks 46 to 51 (the only available data source in England with seroprevalence reported by region and age group), three of five universities had higher seroprevalence compared to their respective regional blood donor prevalence, although these differences were not statistically significant. Factors that may contribute to the observed variation between universities include differences in student populations including proportions living in university halls of residence, history of confirmed COVID-19 infection or COVID-19 related symptoms, as well as the size and duration of outbreaks during the autumn term. Seropositivity is also likely to be different in campus compared to city-integrated universities in terms of increased contact between the students and mixing with the general population, respectively.
Seropositivity was significantly associated with younger students, especially first year undergraduates, those living in halls of residence and those who shared a kitchen with a greater number of fellow students. Intriguingly, those who shared a bathroom had lower odds of antibody positivity compared to those who didn't share a bathroom even after adjusting for accommodation type. Sharing a kitchen may be an important factor for transmission due to the higher degree of close proximity interactions and potential sharing of utensils within the setting. A recent case study published by the Office of National Statistics also found that the risk of SARS-CoV-2 infection in two universities was greater in residential settings such as halls of residence, with little evidence of virus spread during face-to-face teaching in classrooms and lecture theatres.8
Data on SARS-CoV-2 antibody prevalence amongst the university population are limited. A seroprevalence study in a Los Angeles university student population in May 2020 found antibody positivity rates of only 4.0%, similar to the community seroprevalence at the time. The low seroprevalence is likely explained by the stay at home order with closure of the physical university campus in March 2020.9 When compared with other educational settings such as primary schools at the end of the autumn term 2020 in England (10.4%; 95% CI 8.8–12.3), including 8.7% (31/358; 95% CI 6.2–12.1) of students and 11.2% (96/858; 95% CI 9.2–13.5) of staff), antibody seroprevalence among university students was higher.10
We used SARS-CoV-2 antibodies to assess prior infection, which captures asymptomatic, symptomatic and mild, transient infections even if the individuals did not have a confirmatory PCR test. Serology also provides a cumulative measure of infection rates within an institutional setting, thus providing a more accurate representation of the true burden and spread of infection. For this study, we primarily used the Abbott assay which detects N-antibodies within 7–14 days infection, has a high sensitivity and specificity and detects antibodies earlier than other antibody assays, particularly those that measure SARS-CoV-2 spike protein antibodies which take longer to develop after infection5. The very high seropositivity rates among students living in halls of residence with high self-reported case rates (>8%) during the autumn term highlights the ability of the virus to spread rapidly in closed settings, as has been demonstrated in care homes,3 prisons,11 and cruise ships.12 Public health interventions should target early identification, testing, confirmation and isolation of suspected cases, potentially through rapid mass testing,13 and implementation of infection control measures, to interrupt transmission and control the spread of the virus in such settings.
At the same time, however, less than one in five university students overall had SARS-CoV-2 antibodies, indicating that the majority of this population remains susceptible to future SARS-coV-2 infections and outbreaks when they return to campus.
The strength of this study was the large number of participants recruited rapidly in five universities across England. The demographics of the recruited cohort is similar to the UK higher education student population in 2018/19, reported to be 57% female and 76% of white ethnicity14. Furthermore, once recruited, we achieved a high rate of return of blood samples, including 93% that had sufficient volume for antibody testing using a commercial assay, highlighting the willingness of students to participate in the study and demonstrating the acceptability of the Tasso device as a blood collection device for serosurveys.
The main limitation of the study is the convenience sampling strategy adopted. Due to the urgency of this work close to the end of the academic term, the study was open to all students who were 25 or under but the characteristics of those who took part – and, therefore, risk factors such as household contacts– may be different to those who did not take part. Furthermore, many students had already returned to their family home prior to the beginning of our study and, therefore, the sample may not be entirely representative of the wider student population. The recruitment model adopted by University C and D could also introduce bias as only students that were booked for mass lateral flow device (LFD) testing were targeted. Mass LFD testing programmes, for example, excluded students who were confirmed PCR positive in the last 90 days. It is not known how generalizable these findings may be to other university populations as the size and configuration of universities across the country are variable, influencing contact patterns and thus potential spread of infection.
Our findings in December 2020, however, do indicate that a large proportion of the student population remain susceptible to infection despite large outbreaks reported in each of these participating universities during the autumn term. We have also identified an important public health need to support universities with early identification and isolation of suspected cases and rapid implementation of infection control measures to interrupt the spread the virus and prevent large outbreaks in halls of residence. With the recent emergence of highly transmissible SARS-CoV-2 variants of concern,15 too, ongoing surveillance including serosurveillance will play a critical role in monitoring infection and transmission of SARS-CoV-2 in educational settings, including universities, when they re-open.
Declarations of Competing Interest
None.
Data sharing
Applications for relevant anonymised data should be submitted to the Public Health England Office for Data Release.
CRediT authorship contribution statement
Amoolya Vusirikala: Conceptualization, Methodology, Writing - original draft, Formal analysis, Project administration, Writing - review & editing. Heather Whitaker: Writing - original draft, Formal analysis, Project administration, Writing - review & editing. Samuel Jones: Project administration, Writing - review & editing. Elise Tessier: Project administration, Writing - review & editing. Ray Borrow: Project administration, Writing - review & editing. Ezra Linley: Project administration, Writing - review & editing. Katja Hoschler: Methodology, Project administration, Writing - review & editing. Frances Baawuah: Conceptualization, Project administration, Writing - review & editing. Shazaad Ahmad: Project administration, Writing - review & editing. Nick Andrews: Project administration, Writing - review & editing. Mary Ramsay: Conceptualization, Project administration, Writing - review & editing. Shamez N Ladhani: Conceptualization, Project administration, Writing - review & editing. Kevin E Brown: Conceptualization, Methodology, Writing - original draft, Project administration, Writing - review & editing. Gayatri Amirthalingam: Conceptualization, Methodology, Writing - original draft, Project administration, Writing - review & editing.
Acknowledgments
Public Health England (PHE) UNICOVID team members and staff within Clinical Services Unit, PHE Colindale for laboratory support.
We would like to thank all the universities that participated in this study and their staff who made this study possible. We would also like thank the Newcastle City Council Public Health Team.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.04.028.
Appendix. Supplementary materials
References
- 1.Coronavirus cases by local authority: epidemiological data. Available at: https://www.gov.uk/government/publications/coronavirus-cases-by-local-authority-epidemiological-data- 17-december-2020. Accessed: 10 January 2021.
- 2.Public Health England (PHE). National flu and COVID-19 surveillance reports. Available at: https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports. Accessed: 10 January 2021
- 3.Ladhani S.N., Jeffery-Smith A., Patel M., Janarthanan R., Fok J., Crawley-Boevey E., et al. EClinicalMedicine: High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: prospective cohort study, England. 2020;28:100597. [DOI] [PMC free article] [PubMed]
- 4.Tasso, Inc. Available at: https://www.tassoinc.com/tasso-sst. Accessed: 05 January 2021
- 5.Public Health England (PHE). Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARSCoV-2 antibodies Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/890566/Evaluation_of_Abbott_SARS_CoV_2_IgG_PHE.pdf Accessed: 20 January 2021
- 6.Jeffery-Smith A., Dun-Campbell K., Janarthanan R., Fok J., Crawley-Boevey E., Vusirikala A., et al. Lancet Regional Health- Europe: Infection and transmission of SARS-CoV-2 in London care homes reporting no cases or outbreaks of COVID-19: prospective observational cohort study, England 2020. 2021:100038. [DOI] [PMC free article] [PubMed]
- 7.Office for National Statistics (ONS). Coronavirus (COVID-19) Infection Survey: antibody data for the UK, January 2021. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsinthecommunityinengland/antibodydatafortheukjanuary2021 Accessed: 20 January 2021
- 8.Office for National Statistics (ONS). How has coronavirus (COVID-19) spread among students in England? Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/educationandchildcare/articles/howhascoronaviruscovid19spreadamongstudentsinengland/2020-12-21 Accessed: 20 January 2021
- 9.Tilley K., Ayvazyan V., Martinez L., Nanda N., Kawaguchi E.S., O'Gorman M., et al. Lancet Regional Health- Europe: A cross-sectional study examining the seroprevalence of severe acute respiratory syndrome coronavirus 2 antibodies in a university student population. 2020;67(6):763–8. [DOI] [PMC free article] [PubMed]
- 10.Ladhani, S., Prospective active national surveillance of preschools and primary schools for SARS-CoV-2 infection and transmission in England, June 2020 (January 11, 2021). Available at SSRN: https://ssrn.com/abstract=3764198.
- 11.Hagan, L.M., Williams S.P., Spaulding A.C., Toblin R.L., Figlenski J., Ocampo J., et al. Mass testing for SARS-CoV-2 in 16 prisons and jails—six jurisdictions, United States, April–May 2020. Morb Mortal Wkly Rep 2020 Aug 21;69(33):1139. [DOI] [PMC free article] [PubMed]
- 12.Moriarty L.F., Plucinski M.M., Marston B.J., Kurbatova E.V., Knust B., Murray E.L., et al. Public health responses to COVID-19 outbreaks on cruise ships—worldwide, February–March 2020. Morb Mortal Wkly Rep. 2020 Mar 27;69(12):347–52. [DOI] [PMC free article] [PubMed]
- 13.Larremore D.B., Wilder B., Lester E., Shehata S., Burke J.M., Hay J.A., et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021 Jan 1;7(1):eabd5393. [DOI] [PMC free article] [PubMed]
- 14.Higher Education Statistics Agency (HESA). Higher Education Student Statistics; UK 2018/19. Available at: https://www.hesa.ac.uk/news/16-01-2020/sb255-higher-education-student-statistics/numbers. Accessed: 11 January 2021
- 15.Public Health England (PHE). Investigation of novel SARS-COV-2 variant, Variant of Concern 202012/01. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959438/Technical_Briefing_VOC_SH_NJL2_SH2.pdf. Accessed: 15 January 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.