Dear Editor,
The pandemic of severe-acute-respiratory-syndrome-coronavirus-2 (SARS-CoV-2) has precipitated the most extensive global vaccine development programme in history and has resulted in several vaccines receiving emergency use authorisation.1 Here, we report preliminary observations on the impact of a healthcare worker (HCW) COVID-19 vaccination programme at University Hospitals Birmingham (UHB) NHS Foundation Trust, one of the UK's largest hospital Trusts. The vast majority of vaccinated staff participating in this programme have so far received one dose of the Pfizer-BioNTech (BNT162b) vaccine.
An occupational health database of all COVID-19 positive HCWs was interrogated against an informatics search of all vaccinated HCWs. The UHB testing programme detected SARS-CoV-2 RNA from nasopharyngeal swabs using a Panther Hologic real time polymerase chain reaction platform. All HCWs are encouraged to undertake lateral flow COVID-19 tests (Innova Medical Group, Inc.); positive tests were confirmed by PCR. The Trust started its COVID-19 vaccination programme on 12/12/20, using the BNT162b vaccine. Multivariate logistic and weighted Cox regression models were used to estimate probabilitys.2
Between 28/03/20–21/03/21, UHB had 13,544 patient cases of COVID-19. Since 01/04/20, UHB have tested 32,717 staff for COVID-19, with 2721 (8.3%) HCWs testing positive. Up to 23/02/21, the Trust has delivered one dose of vaccine to 25,335 HCWs out of a possible 30,000 workforce. Between 28/01/21–21/03/21, 51% (88/174; 61 patient facing HCWs) of PCR positive UHB HCWs had been vaccinated, with 38% (66/174; 53 patient facing HCWs) having had their vaccination at least 10 days previously. Similarly, 52% percent of HCWs that reported a positive lateral flow test (57/109; 45 patient facing HCWs) had been vaccinated, with 41% (45/109; 40 patient facing HCWs) having had their vaccination >10 days previously. During this period, UHB had eight staff outbreaks with 52 HCWs being positive for COVID-19. Only three of these staff had not been vaccinated. Of all our positive HCW cases across UHB during this time period, 121 (70%) were symptomatic with mild illness. There have been no reports of vaccinated UHB HCWs requiring hospital admission for COVID-19 or COVID-19 related deaths in our vaccinated staff to date.
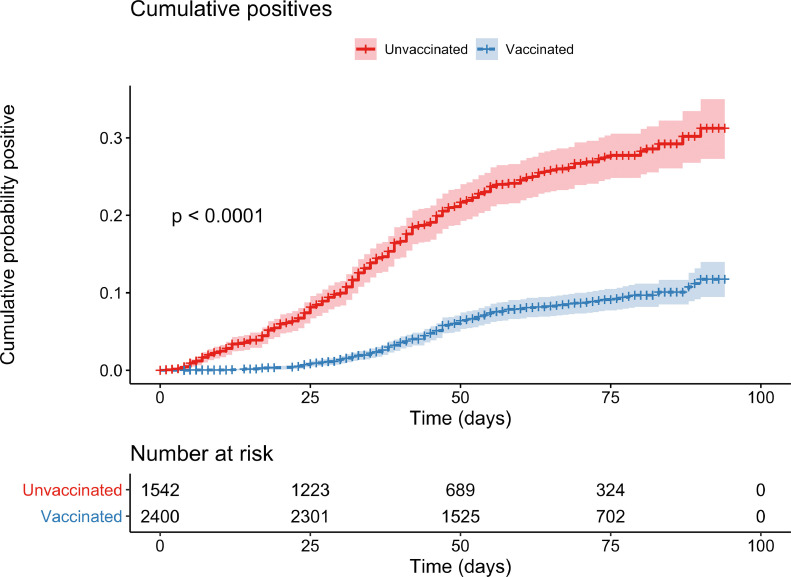
Of the 33,460 HCW positive samples; 12,701 were mapped to a HCW who was vaccinated and 5468 to a HCW who was not vaccinated. Of the vaccinated staff, there were 178 positive and 3947 negative samples; for the unvaccinated staff, those figures were 330 and 2057, respectively. A multivariate logistic regression model found that being vaccinated was associated with a decreased probability of testing positive (p = 1.40 × 10−10, odds ratio 2.35, 95% CI: 1.81–3.05). The model also found that the probability of testing positive decreases as the gap between vaccination and testing increases (p = 0.00607). A weighted cox regression demonstrated that vaccination was associated with a significantly lower hazard of testing positive during the time period in question (p < 0.0001). This model gave a generalised concordance probability of 0.24 (95% CI: 0.20, 0.28), meaning that a HCW who had been vaccinated had only a 24% probability of testing positive before an equivalent unvaccinated HCW (Fig. 1 ).
Fig. 1.
Displaying the cumulative probability, of vaccinated and non-vaccinated HCWs, testing positive for COVID-19.
Key: The Table within the Figure shows the number of vaccinated and unvaccinated HCWs observed at each time point.
Chodick et al., (2021) detailed the effectiveness of the first dose of BNT162b2 in Israel.3 They demonstrated an effectiveness of 51% of BNT162b2 vaccine against SARS-CoV-2 infection 13–24 days after immunisation with the first dose.3 This is similar to the data presented here, where 51% of HCWs testing positive for COVID-19 during a seven week period were those who had previously received one dose of BNT162b2. However, we saw a lower proportion of vaccinated positive HCWs who had the BNT162b2 (38%) >10days previously. Hunter et al., (2021) used Monte Carlo modelling to analyse the Israel data.4 They found after initial injection, case numbers increased to day 8 before declining to low levels by day 21.4 Estimating vaccine effectiveness, they concluded this was 0 at day 14 but then rose to about 90% at day 21 before levelling off.4 They concluded the cause of the initial surge in infection risk may be related to people being less cautious about maintaining protective behaviours as soon as they have the injection.4 This could also be a plausible reason why we see a significant numbers of our vaccinated HCWs being positive for SARS-CoV-2. Our data set is unique as we are looking specifically at HCWs. In our population, HCWs would be exposed to higher viral loads and infectious cases. As a result, they are more likely to get infections from SARS-CoV-2 than the general population, as per the work of Shields et al., (2020).5 It is unsurprising we see a high proportion of vaccinated HCWs testing positive. We saw the same phenomenon of vaccinated HCWs being positive in the lateral flow antigen tests. Mahese (2020) reported that the lateral flow antigen tests will identify infected individuals with the highest viral loads.6 We saw a significant proportion of our vaccinated infected HCWs positive via lateral flow, implying these HCWs could have higher viral loads. There is debate whether vaccinated individuals transmit SARS-CoV-2, however the fact a proportion of our vaccinated HCWs are positive on lateral flow tests suggestive of high viral loads, implies they will be a vector for transmission. This is further supported by the fact that 70% of our positive HCWs reported symptoms, thus increasing likelihood of transmission.
It is an important message, not just for HCW but for the wider public, that one dose of the vaccination will not prevent individuals from getting COVID-19 and potentially transmitting it. Will a second vaccination reduce HCWs having a high viral load? The clinical trial data showed the first dose of BNT162b2 having 52% efficacy (like our HCW data) and 2 doses having 95% efficacy.7 This data supports HCWs having a second dose as a priority, to prevent spread in our vulnerable settings.
We conclude that it is imperative staff remain vigilant once they are vaccinated as we are still detecting a significant number of staff acquiring COVID-19, with the potential for onwards transmission to patients and other HCWs. We have also shown that the probability of our staff getting COVID-19 is reducing with a single dose of the vaccine and this is likely to decrease further once HCWs receive their second dose.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
None.
Authors contributions
All authors have contributed to the manuscript.
Authors information
None.
Patient and public involvement
None, paper is of interest to patients and public in light of current pandemic. An observational study.
Transparency declaration
The author affirms the manuscript is an honest accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Declaration of Competing Interests
None.
Acknowledgements
We would like to thank the Infection Prevention and Control team, the Occupational Health team, the Infection Service, the Clinical Immunology Service, Informatics, the UHB vaccination hospitals hub team and the senior responsible clinicians at University Hospitals Birmingham NHS Foundation Trust.
References
- 1.Voysey M., Costa-Clemens S.A., Madhi S.A. Israel paper safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;9:99–111. doi: 10.1016/S0140-6736(20)32661-1. 397(10269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.R version 4.0.4 (2021-02-15) - The R Foundation for Statistical Computing.
- 3.Chodick G., Tene L., Patalon T., Gazit S., Ben-Tov A., Cohen D., Muhsen K. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13-24 days after immunization: real-world evidence. medRxiv preprint2021, available at https://doi.org/10.1101/2021.01.27.21250612 (last accessed 23rd March 2021)
- 4.Hunter P., Brainard J. Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of ‘real-world’ vaccination outcomes from Israel. medRxiv preprint2021, available at https://doi.org/10.1101/2021.02.01.21250957 (last accessed 23rd March 2021).
- 5.Shields A., Faustini S.E., Perez-Toledo M. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross sectional study. Thorax. 2020;75(12):1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahase E. Covid-19: pfizer vaccine efficacy was 52% after first dose and 95% after second dose. BMJ. 2020:371. doi: 10.1136/bmj.m4826. [DOI] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 VACCINE. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.