Abstract
Remodeling follows inflammatory and reparative phases of bone healing and is very pronounced in children. Unlike adults, in growing children, remodeling can restore the alignment of initially malunited fractures to a certain extent, making anatomic reduction less essential. Remodeling is not universal and ubiquitous. Animal experiments and clinical studies have proven that in a malunited fracture, the angulation corrects maximally by physeal realignment (75%) and partly by appositional remodeling of the diaphysis also known as the cortical drift (25%). Remodeling potential reduces with the increasing age of the child; lower extremities have higher remodeling potential compared to the upper extremity. Remodeling is most pronounced at the growing end of the bone and in the axis of the adjacent joint motion. Correction of a very small amount of rotational malalignment is possible, but it is clinically not relevant. Overgrowth of the bone after a fracture occurs due to hyperaemia of fracture healing. Overgrowth is the most common after paediatric femur fractures, though it is reported after fractures of the tibia and humerus as well. The orthopaedic surgeon treating children’s fractures should be familiar with regional variations of remodeling and limits of acceptance of angulation in different regions. Acceptability criteria for different bones are though well defined, but serve best as guidelines only. For the final decision-making patient’s functional capacity, parents’ willingness to wait until the completion of the remodeling process, and the experience of treating doctor should be considered concurrently. In case of the slightest doubt, a more aggressive approach should be taken to achieve a satisfactory result.
Keywords: Remodeling of fractures, Fracture healing, Limits of acceptability, Overgrowth
In growing children, remodeling can restore the alignment of initially malunited fractures to a certain extent, making anatomic reduction less essential than adults. Bone remodeling is dependent upon muscle action, joint reaction forces, and physiological stresses of body weight. Intrinsic control mechanisms like the periosteum also play a role in remodeling [1]. Knowledge of remodeling can help in managing many fractures in young children conservatively. Contrary to that, over-reliance on remodeling potential may lead to permanent deformity. Hence, a precise understanding of regional remodeling potential is crucial.
Fracture Healing in Children
Fracture healing comprises three phases: (1) inflammatory, (2) reparative, and (3) remodeling. The remodeling phase is more prolonged and pronounced in children than in adults.
Inflammatory Phase
The inflammatory phase begins immediately after the fracture with the formation of endosteal, subperiosteal, and extraperiosteal (if periosteum is torn) hematoma. Due to hematoma formation, few millimetres of bone on either side become avascular. Following the removal of this dead bone, the fracture line becomes better visible after a few days.
The vascular response initiates the cellular response. TGF-β released from the extracellular matrix of bone and platelets controls the mesenchymal precursor cells, which form osteoblasts and osteoclasts. Growth factors convert the multipotential cells into osteoprogenitor cells. Fracture bridging occurs by subperiosteal bone formation and endochondral bone formation at the endosteal areas [2].
Motion at the fracture site leads to lower oxygen tension, which leads to more cartilage formation, which is ossified later. There is gradual revascularization of the dead bone at the fracture site.
Reparative Phase
The reparative phase is highlighted by the formation of new blood vessels and the beginning of cartilage formation. Initially, neighbouring soft tissues provide vascular ingrowth to the periosteal area followed by the endosteal area. Endochondral bone is formed by calcification of the cartilage anlagen adjacent to the fracture site. Undifferentiated mesenchymal cells of the periosteum differentiate into osteoblasts and form intramembranous bone without a preceding cartilage model at the periphery.
As new bone forms under the periosteum, it is pushed away from the bone, making a collar of bone around the fracture. Primarily, this tissue is cartilaginous and fibrous and not very well ossified. It is not visible on a radiograph, but after mineralization and conversion to the bone, it becomes better visible [2].
Fracture unites clinically after the bony callus surrounds the fracture fragments and connects with the callus coming from the opposite side. At this point, the bone is clinically stable enough for the patient to begin extremity usage.
Remodeling Phase
Remodeling is the last phase of bone healing, which may last for a short time in a young child or continue throughout the growth period or even beyond the cessation of growth in an older child. Once the bone is clinically stabilized, remodeling of early soft woven bone occurs due to physiological stresses and strains. Single-cell types like osteoclasts or osteoblasts are not responsible for remodeling, but coordinated bone resorption and bone formation over large regions around the fracture are responsible for it [2].
Cartilage healing is not similar to bone healing. When the physis is injured, inflammatory and reparative phases occur, but there is no remodeling phase [2].
Remodeling of Bone in Children
Higher remodeling potential of bone in children permits treating doctor to accept suboptimal alignment with conservative treatment. The remodeling is not uniform and ubiquitous. Many factors affect bone remodeling and knowledge of which is very important for an orthopaedic surgeon treating paediatric fractures.
Remodeling can potentially correct translation, axial, and rotational deformity. It can correct shortening but not lengthening (which is occasionally iatrogenic).
Correction of Translation (side by side displacement)
It is purely a periosteal correction and is dependent on the age of the patient. In children up to the age of 10–12, complete side-to-side displacement of entire shaft width can correct practically in the entire skeleton baring some exceptions [3] (Figs. 1, 2 and 3).
Fig. 1.

Remodeling sequence of translation correction in children, a fracture with bayonet reduction, b fracture union in bayonet position, c improvement in bayonet alignment by periosteal new bone formation, d complete remodeling of fracture
Fig. 2.
a Complete translation of distal radius fracture in 3-year-old girl, b partial improvement in alignment on day of plaster removal at one month, c complete correction of translation after 2 months of injury
Fig. 3.
Full correction of complete translation of femur shaft fracture in 2-year-old girl over one and a half years
Correction of Axial Deformity (coronal and sagittal)
There are a few laws, which guide remodeling of the bone with axial deformity.
Wolff (1892) stated that new bone is produced where it is necessary mechanically (in the weight-bearing area) and is reabsorbed from areas where it is not necessary. So when fracture unites in angulation, new bone is formed in concave areas, and resorption dominates on the convex side. This process produces a certain realignment of the bone. Although it also occurs in adults, it is more remarkable in children [4]. Pioneering work by multiple authors has established that it accounts for approximately 25% of the correction and is commonly referred to as bone drift and comes from periosteum [5, 6].
According to Hueter Volkman's law (1862), after a malunited fracture, adjacent physes tend to realign perpendicular to the forces acting through them (Fig. 4). This modifies the orientation of the fracture to the bone axis [4, 7]. According to Murray, asymmetric growth of the physeal plate accounts for approximately 75% improvement in fracture angulation [5]. Wallace in a clinical study of remodeling after femoral fracture noted 74% correction at physes and only 26% at shaft [8] (Fig. 5). Freiberg also noted the larger role of epiphyseal reorientation in remodeling of distal radius fractures [9].
Fig. 4.

Remodeling of a malunited fracture in children, a normal bone, b mal-uniting fracture, c Hueter Volkman's law: asymmetrical growth of proximal and distal physis correcting bony axis (double-headed arrows) and Wolff’s law: extra callus formation on the concave side also known as cortical drift (curved arrow), d complete correction of joint alignment with persistent angulation at fracture site showing the inability of cortical drift to correct local angulation beyond certain limits
Fig. 5.
Persistent procurvatum (white arrow) after 5 years of neonatal femur fracture with good correction of mechanical axis; sufficiently proves inability of cortical drift to fully correct local angulation
After animal experiments, Murray concluded that after malunion of the fracture in the immature skeleton: (1) the overall limb and joint alignment corrects rapidly due to asymmetrical physeal growth, (2) the angulation at the fracture site rectifies slowly by the bone drift, (3) division of periosteum has a minor effect on angulation improvement, (4) under some circumstances the growth plate grows in a helix and by this mechanism torsional deformities may ensue and may rectify [5].
Factors Affecting Remodeling
Remodeling of the angular deformity is dependent on multiple factors.
Extremity—remodeling is reduced in the upper extremities compared to lower extremities, probably because the lower extremities are subjected to greater mechanical loads [7].
Skeletal age—remodeling is proportional to the remaining growth potential and is more pronounced in younger children (< 8 years) [9, 10].
Fracture site—the proximity of the fracture to a growing physis is a favourable factor for remodeling. The greater the growth potential of the physis (physes around the knee), the better will be the angulation correction [9, 10].
Degree and orientation of the deformity—if the angulation lies in the plane of movement of the adjacent joint, the more substantial will be the remodeling [9] (Fig. 6). Consequently, in the tibia and femur, malunion in the sagittal plane (procurvatum–recurvatum) shows a better remodeling than the coronal plane [10–12].
Fig. 6.
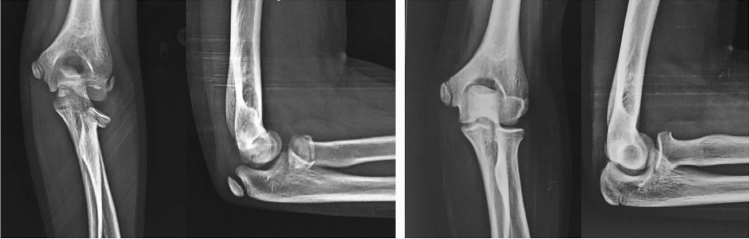
Correction of posterior angulation and translation at distal humerus as angulation was in the plane of elbow motion
Overgrowth
Overgrowth following fracture occurs as a result of hyperaemia of fracture healing. The increased vascularity spreads to the epiphyseal plate leading to growth stimulation and overgrowth [13, 14]. Neer noted overgrowth to be permanent [15]. Overgrowth has been observed after fractures of the femur, tibia, and humerus.
Femur
Shapiro followed 74 children (< 13 years old) following femoral shaft fracture until skeletal maturity and found universal femoral overgrowth. There was an average of 0.92 cm (range 0.4–2.7) femoral overgrowth, which was independent of age, fracture level, or position of fracture fragments at the time of healing. The majority of overgrowth occurred within the first 18 months of fracture. Simultaneous ipsilateral tibial overgrowth of 0.29 cm (0.1–0.5) was found in 82% of children. In 71% of children, overgrowth stopped after 3.5 years, but in 9%, it continued throughout the remaining growth period, albeit at a slower rate. They recommended that in children between 2 and 11 years, shortening of 1.5 cm may be allowed [13].
In a study of 44 children with femur fracture treated with traction Colton reported an average 8.1 mm femoral overgrowth, which was significantly greater in boys. Overgrowth was not influenced by the age, the fracture type or site, the amount of fragment overlap, or by the handedness of the patient [16]. Malkawi reported an average 8.75 mm growth acceleration in children with fracture shaft femur treated with traction, of which 6.8 mm was make-up growth and 1.95 mm was overgrowth. They noted that overriding of the fragments at the time of union stimulates growth and indicated that the greater the initial shortening, the greater the growth stimulation. Overgrowth was most significant in the 3–9 year age group. The fracture type had no effect growth stimulation [11].
Tibia
Bergmann et al. noted an average overgrowth of 5 mm in 3–10-year-old girls and 3–12-year-old boys. They also noted growth retardation in older children. Growth disturbance was not affected by the fracture type or residual angulation and lasted for 1–2 years. They recommended not to accept any shortening in girls > 10 years and boys > 12 years [17].
Shannak reported an average growth acceleration of 4.35 mm following tibia fracture, with 3.05 mm of make-up growth and 1.3 mm of overgrowth. They noted that increased shortening at the time of the reduction, comminuted fractures, fracture at the proximal and distal end, and young children had higher overgrowth [12].
Humerus
Following humerus fracture, Emmnaénus noted overgrowth in 63 of 71 children irrespective of the level of the fracture. In the majority of the children, overgrowth stopped at 18 months [14].
Lengthening After Flexible Nailing
Currently, many children from 5 years and above with femur shaft fractures are treated with flexible nailing hence it is imperative to know the amount of overgrowth following flexible nailing.
Flynn reported 5 mm lengthening/shortening in the majority of children treated for femoral fracture with flexible nailing, 6 of 58 children had 1–2 cm of inequality on early follow-up. He recommended a longer follow-up to assess the final effect of overgrowth post nailing [18]. Mazda reported lengthening in 38% of 34 patients treated with flexible nailing for femur fracture, the majority of them had < 10 mm lengthening, but 8% had lengthening of 10–15 mm [19].
Park studied femoral overgrowth in 43 children (3.6–12 years) treated with flexible intramedullary nailing with a mean follow-up of 3.5 years and found mean femoral overgrowth of 0.6 cm; 25.6% of children had an overgrowth of ≥ 1 cm [20].
Khan et al. noted limb lengthening in 15 of 29 children treated with titanium nailing in the first year, but after 3 years, only nine children were longer (average 2.7 mm); they concluded that lengthening declines at an average rate of 1.5 mm per year [21].
Rotational Malalignment
It has been proved experimentally in an animal model that rotational deformities can correct with helicoidal growth of the physeal plate [5]. In human studies, the correction of rotational malalignment by remodeling is controversial and limited to a small amount [22].
Brouwer studied 50 patients with femur shaft fractures treated conservatively after 27–32 years. They found a single case of persistent rotational malalignment without any untoward consequences. The average difference in femoral anteversion (studied by X-ray measurement) was only 5.80. There were ten patients with a rotation difference of ≥ 10°, of which five patients had reduced and five had increased anteversion compared to the unaffected side. Surprisingly, their control group also showed individual L/R differences in the rotation of 0°–15°, and angles of anteversion ranging from − 9° to + 38° (average 10.9°) [23].
David found poor remodeling of posttraumatic femoral torsional deformity in children after CT scan measurement but noted that up to 25° malrotation can be well tolerated [22]. Contrary to that, Buchholz performed MRI at 4 years follow-up in children aged 3–6 years treated with an external fixator for fracture shaft femur to study rotational deformity correction and reported full correction of rotational deformity in three of the five children [24].
Shannak et al. did not find any correction of rotational malalignment following tibial fractures [12].
Regional Variation of Remodeling and Acceptability Criteria After Closed Reduction of Fractures
Good understanding of general remodeling principles is essential, but regional variations and limits of acceptance of angulation in different regions help in quick decision-making. A ready reckoner bone-wise charts of acceptability criteria are helpful to clinicians in their daily practice (Tables 1, 2, 3, 4, 5) [25]. These charts should serve as guidelines only. Functional capacity of the patient, willingness of parents to wait till the completion of remodeling process, and the surgeon’s experience should be considered before deciding whether to depend on the remodeling capability of the specific fracture or to choose an invasive technique to obtain a satisfactory result [25].
Table 1.
Femur shaft
| Age | Varus/valgus | Sagittal |
|---|---|---|
| Birth—2 years | 30° | 30° |
| 2–5 years | 15° | 20° |
| 6–10 years | 10° | 15° |
| 11 years + | 5° | 10° |
Table 2.
Tibia shaft
| Deformity | < 8 years | > 8 years |
|---|---|---|
| Varus | 10° | 5° |
| Valgus | 5° | 5° |
| Posterior angulation | 5° | 0° |
| Anterior angulation | 10° | 5° |
| Rotation | 5° | 5° |
| Shortening | 10 mm | 5 mm |
Table 3.
Proximal humerus
| Age | Displacement |
|---|---|
| < 5 years | < 70° angulation and complete displacement |
| 5–12 years | 40–70° angulation |
| > 12 years | < 40° displacement and 50% apposition |
Table 4.
Humerus shaft
| Displacement | Angulation |
|---|---|
| Varus | 20–30° |
| Apex anterior bowing | 20° |
Table 5.
Radial shaft
| Age | Angulation | Malrotation |
|---|---|---|
| > 9 years | 15° | 45° |
| < 9 years | 10° | 30° |
Femur
Wallace studied 28 malunited fractures of the femur over 45 months and noted that an average 75% of the deformity had remodelled by three years, and remodeling was complete at 5 years. The degree of remodeling was not influenced by either the direction or the magnitude of the angulation. Younger children remodelled a little better. He concluded that in children younger than 13 years, 25° malunion in any plane will remodel, and normal alignment of the joint surfaces will be restored [8].
Malkawi from a study of children with fracture shaft femur (treated with traction) aged 2–10 years, and followed for 2–10 years recommended: (a) distraction should be avoided, (b) 15 mm of overriding may be compensated by growth stimulation, and (c) coronal plane deformity < 20° and sagittal plane deformity < 30° will culminate in a satisfactory outcome [11] (Table 1).
Tibia
Shannak in a study of tibia fracture treated conservatively in a 3–10 year age group noted that up to 10 mm shortening of may be compensated entirely or partially by growth acceleration. Varus deformity of < 15° can correct spontaneously, whereas valgus deformity and posterior angulation will persist to a certain degree and rotational deformities will not correct [12].
Similarly, Amitabh et al. studied children with tibial fracture (average age 7.2 years and average follow-up 4 years) and found anterior angular deformity to correct maximally (52.7%) followed by varus (40.9%) and valgus (23.9%), while posterior deformity had the least correction (18.5%). They noted that 12° anterior, 6° posterior, 10° varus, and 8° valgus can remodel completely [26] (Fig. 7) (Table 2).
Fig. 7.
a Comminuted tibia fracture in 10-year-old girl, b malunion in recurvatum, c failure of compete correction of recurvatum at 18 month follow-up, note normal joint orientation
Humerus
Proximal Humerus
The good prognosis of this fracture despite residual deformity following severe displacement can partly be explained by the great mobility of the shoulder joint and the extraordinary remodeling capability of the proximal humerus in children [27]. Kohler reported excellent results in children from 2 to 16 years with proximal humeral metaphyseal and epiphyseal injury treated conservatively and attributed good results to excellent remodeling in this region [28]. McBride noted that results of conservative and operative treatment were similar in this region due to excellent correction of angulation and displacement by bony remodeling [29] (Fig. 8) (Table 3).
Fig. 8.
Excellent remodelling of completely displaced proximal humerus fracture in 10-year-old boy
Shaft Humerus Fractures
O’Shaughnessy demonstrated that a majority of children with humeral shaft fractures treated nonoperatively healed with few concerns [30]. Caviglia reported that most of the humerus shaft fractures can be treated nonoperatively and malrotations or angulation beyond 15° at the mid-diaphysis or 20° close to the physis should not be accepted [31] (Fig. 9) (Table 4).
Fig. 9.
a Fracture shaft humerus in 3-year-old girl with varus deformity, b varus malunion of 27°, c excellent correction of angulation at 6 months
Proximal Radius
Arjandas noted that given the discrepancies in the literature, it is not surprising that the treatment recommendations vary greatly for proximal radial fractures. He recommended that patients with < 45° angulation should be treated with casting without manipulation and in patients with > 45° angulation, closed reduction should be attempted [32]. In children younger than 10 years, Vocke reported spontaneous correction of up to 50° angulation [33]. Steele and Graham recommended considering both the displacement and angulation in the management of radial neck fractures [34]. The overall consensus is to accept angulation up to 30° and offer a closed reduction in children with > 30° angulation [35] (Fig. 10).
Fig. 10.
a Fracture of radial neck with translation and angulation in 10-year-old male, b complete remodelling of fracture at 3-year follow-up
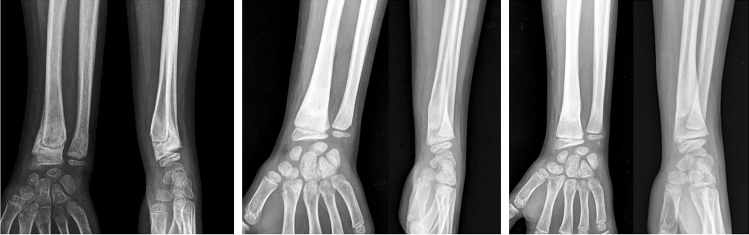
Distal Radius Fractures
Mahlmann in a study of boys younger than 14 years and girls younger than 12 years showed that angulations less than 15° and shortening of less than 1 cm will completely remodel within an average of 7.5 months. They challenged the need for a reduction under anaesthesia for this fracture [36]. Many authors have noted remodeling with higher angulations [9, 37]. Kimberly found distal radius dorsovolar malunion of 23° (15–49) and radioulnar malunion of 21° (15–33) remodelled to 8° (–2 to 21) and 10° (3–17), respectively, with remodeling speed of 2.5° (0.4–7.6) per month [37]. Crawford et al. treated children with distal radius fracture aged an average of 6.9 years by applying cast with gentle correction of angulation without anaesthesia and accepted shortening of average 5 mm ( 1–14 mm) which corrected to 00 ulnar variance in all children [38]. Plánka also treated patients with distal radius fracture without reduction and reported complete remodeling of up to 300 angulation to normal anatomic level in 86% of children younger than 12 years [39]. These results encourage more frequent use of conservative treatment on an outpatient basis in young children with distal radius fractures, avoiding the risk of anaesthesia, and saving cost.
Akar reported the highest remodeling of distal radial fractures. They noted that in children up to 10 years of age, up to 39° radial and dorsal angulation, 22° volar angulation, and complete displacement correct fully, while in older children (10–15 years), 38° dorsal angulation, 23° radial angulation, and 16° volar angulation may be accepted [40] (Fig. 11).
Fig. 11.
Malunited distal radius in 10-year-old boy with 36° of dorsal angulation showing excellent remodeling at one year follow-up
Radius and Ulna Shaft Fracture
Boeck studied remodeling after plastic deformation and noted that after 6 years of age, remodeling was less than generally accepted; in children > 6 years with cosmetically unacceptable bowing deformity and angulation of > 10°, they recommended reduction under anaesthesia [41].
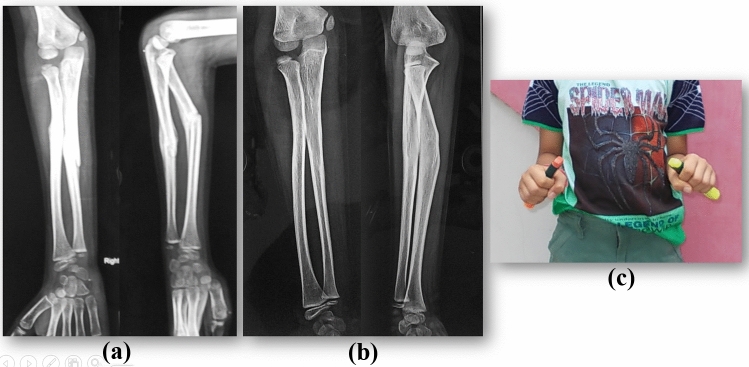
Price after studying functional results following malunion of radius and ulna shaft fractures recommended that in children < 9 years with fractures at any level, 15° of angulation, 45° of malrotation, and complete displacement is acceptable, but in children > 9 years, for proximal fractures, 10° of angulation and distal fractures, 15° angulation and 30°of malrotation can be accepted. Complete bayonet apposition was found to be acceptable for distal radius fracture if angulation was < 20° with 2 years of growth remaining [42]. Fuller found that in children < 8 years 20°, malunion remodelled completely but in children > 11 years, spontaneous correction of the malunion cannot be anticipated, although they noted some correction in boys between 8 and 10 years [43] (Fig. 12) (Table 5).
Fig. 12.
a Eight-year-old boy with malunited fracture of right radius and 30° angulation, b persistent angulation of 21° at 2 years follow-up, c restricted forearm pronation on right side
Conclusion
The remodeling potential of fractures in children is a unique phenomenon that can correct a certain amount of malalignment. It allows many paediatric fractures to be treated nonoperatively, which otherwise in an adult would necessitate operative intervention. Remodeling is the last part of the fracture healing and is not the same at all the levels of the bone. Side to side displacement (translation) of up to full shaft thickness, completely corrects in children < 10 years. This correction is periosteal in origin. After the malalignment of fractures in children, 75% of the correction comes from the realignment of growth plates (Hueter Volkman's law). Cortical drift corrects the remaining 25% with the new bone formation on the concave side, and bone resorption on the convex side (Wolff’s law); this correction is periosteal in origin. Hence, acceptance of high angulation after a diaphyseal fracture may lead to persistent deformity with a good realignment of the proximal and distal physes to the limb axis. Remodeling potential is inversely proportionate to the age and is the highest in neonates. Lower extremities have higher remodeling potential compared to the upper extremity. Remodeling is most pronounced at the growing end of the bone and along the axis of the adjacent joint motion (especially in the case of hinge joints like elbow and knee). A small amount of rotational malalignment correction is possible but it is negligible, hence rotational malalignment should not be accepted. Overgrowth of bone following femoral shaft fracture is very well known, but shortening more than 1.5 cm should not be accepted in a child with fracture shaft femur. Overgrowth following fracture of tibia is clinically insignificant and no shortening of tibial shaft fracture in children should be accepted. For the best functional results, age-wise regional acceptability criteria provide valuable insight. After accepting the angulated reduction of fracture in children, a pictorial chart showing successful remodeling of the malunited fracture is very helpful in convincing parents.
Compliance with Ethical Standards
Conflict of Interest
Author does not have any conflict of interest.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alman BA. Rockwood and Wilkins’ Fractures in Children. 8. Philadelphia: Wolters Kluwer Health; 2015. The immature skeleton; p. 27. [Google Scholar]
- 2.Jones ET. Skeletal growth and development as related to trauma. In: Green NE, editor. Skeletal Trauma in Children. 3. Philadelphia: Saunders; 2003. pp. 1–18. [Google Scholar]
- 3.von Laer L. Pediatric Fractures and Dislocations. 1. Stuttgart, New York: Thieme; 2004. Corrective mechanisms in the growing skeleton; pp. 11–16. [Google Scholar]
- 4.Gascó, J., de Pablos, J. (1997). Bone remodeling in malunited fractures in children. Is it reliable? Journal of Pediatric Orthopedics Part B. 6(2):126–32. Available from: http://journals.lww.com/01202412-199704000-00008. [DOI] [PubMed]
- 5.Murray, D.W., Wilson-MacDonald, J., Morscher, E., Rahn, B.A., Käslin, M. (1996). Bone growth and remodeling after fracture. The Journal of Bone and Joint Surgery. British Volume 78-B(1):42–50. Available from: http://online.boneandjoint.org.uk/doi/10.1302/0301-620X.78B1.0780042. [PubMed]
- 6.Karaharju EO, Ryoppy SA, Makinen RJ. Remodeling by asymmetrical epiphysial growth. An experimental study in dogs. The Journal of Bone and Joint Surgery Series B. 1976;58(1):122–126. doi: 10.1302/0301-620X.58B1.1270489. [DOI] [PubMed] [Google Scholar]
- 7.Abraham, E. (1989). Remodeling potential of long bones following angular osteotomies. Journal of Pediatric Orthopedics 9(1):37–43. Available from: http://journals.lww.com/01241398-198901000-00008 [DOI] [PubMed]
- 8.Wallace, M.E., Hoffman, E.B. (1992). Remodeling of angular deformity after femoral shaft fractures in children. The Journal of Bone and Joint Surgery. British Volume. 74(5):765–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1527131 [DOI] [PubMed]
- 9.Friberg, K.S.I. (1979). Remodeling after distal forearm fractures in children II: the final orientation of the distal and proximal epiphyseal plates of the radius. Acta Orthopaedica Scandinavica 50(6):731–9. Available from: http://www.tandfonline.com/doi/full/10.3109/17453677908991303 [DOI] [PubMed]
- 10.Stilli, S., Magnani, M., Lampasi, M., Antonioli, D., Bettuzzi, C., Donzelli, O. (2008). Remodeling and overgrowth after conservative treatment for femoral and tibial shaft fractures in children. Chirurgia degli organi di Movimento 91(1):13–9. Available from: http://link.springer.com/10.1007/s12306-007-0003-6 [DOI] [PubMed]
- 11.Malkawi, H., Shannak, A., Hadidi, S. (1986) Remodeling after femoral shaft fractures in children treated by the modified blount method. Journal of Pediatric Orthopedics 6(4):421–9. Available from: http://journals.lww.com/01241398-198607000-00006 [DOI] [PubMed]
- 12.Shannak, A.O. (1988). Tibial fractures in children. Journal of Pediatric Orthopedics 8(3):306–10. Available from: http://journals.lww.com/01241398-198805000-00010 [DOI] [PubMed]
- 13.Shapiro, F. (1981) Fractures of the femoral shaft in children: The Overgrowth Phenomenon. Acta Orthopaedica Scandinavica 52(6):649–55. Available from: http://www.tandfonline.com/doi/full/10.3109/17453678108992162 [DOI] [PubMed]
- 14.Emmnaénus, H., Hedström, Ö. (1965). Overgrowth following fracture of humerus in children. Acta Orthopaedica Scandinavica 35(1–4):51–8. Available from: http://www.tandfonline.com/doi/full/10.3109/17453676508989338 [PubMed]
- 15.Neer, CS. (1957). Treatment of fractures of the femoral shaft in children. The Journal of the American Medical Association 163(8):634. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.1957.02970430024008 [DOI] [PubMed]
- 16.Clement, D., Colton, C. (1986). Overgrowth of the femur after fracture in childhood. An increased effect in boys. The Journal of Bone and Joint Surgery. British Volume 68-B(4):534–6. Available from: http://online.boneandjoint.org.uk/doi/10.1302/0301-620X.68B4.3733825 [DOI] [PubMed]
- 17.Greiff, J., Bergmann, F. (1990). Growth disturbance following fracture of the tibia in children. Acta Orthopaedica Scandinavica 51(1–6):315–20. Available from: http://www.tandfonline.com/doi/full/10.3109/17453678008990805 [DOI] [PubMed]
- 18.Flynn, J.M., Hresko, T., Reynolds, R.A.K., Blasier, R.D., Davidson, R., Kasser, J. (2001). Titanium elastic nails for pediatric femur fractures: a multicenter study of early results with analysis of complications. Journal of Pediatric Orthopedics 21(1):4–8. Available from: http://journals.lww.com/01241398-200101000-00003 [DOI] [PubMed]
- 19.Mazda, K., Khairouni, A., Pennecot, G.F., Bensahel, H. (1997). Closed flexible intramedullary nailing of the femoral shaft fractures in children. Journal of Pediatric Orthopedics B 6(3):198–202. Available from: http://journals.lww.com/01202412-199707000-00008 [DOI] [PubMed]
- 20.Park, S.-S., Noh H, Kam M. Risk factors for overgrowth after flexible intramedullary nailing for fractures of the femoral shaft in children. Bone Joint J [Internet]. 2013 Feb;95-B(2):254–8. Available from: http://online.boneandjoint.org.uk/doi/10.1302/0301-620X.95B2.29491 [DOI] [PubMed]
- 21.Gogi N, Khan SA, Varshney MK. Limb length discrepancy following titanium elastic nailing in paediatric femoral shaft fractures. Acta Orthop Belg [Internet]. 2006 Apr;72(2):154–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16768257 [PubMed]
- 22.Davids, J.R. (1994). Rotational deformity and remodeling after fracture of the femur in children. Clinical Orthopaedics and Related Research (302):27–35. Available from: http://journals.lww.com/00003086-199405000-00006 [PubMed]
- 23.Brouwer, K.J., Molenaar, J.C., Van Linge, B. (1981). Rotational deformities after femoral shaft fractures in childhood: a retrospective study 27–32 years after the accident. Acta Orthopaedica Scandinavica 52(1):81–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7211321 [DOI] [PubMed]
- 24.Buchholz IM, Bolhuis HW, Bröker FHL, Gratama JWC, Sakkers RJB, Bouma WH. Overgrowth and correction of rotational deformity in 12 femoral shaft fractures in 3-6-year-old children treated with an external fixator. Acta Orthopaedica Scandinavica. 2002;73(2):170–174. doi: 10.1080/000164702753671759. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins, K.E. (2005). Principles of fracture remodeling in children. Injury 36(1):S3–11. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0020138304004929 [DOI] [PubMed]
- 26.Dwyer, A.J., John, B., Hora, R., Mam, M.K. (2007). Remodeling of tibial fractures in children younger than 12 years. Orthopedics 30(5):393–6. Available from: https://www.healio.com/orthopedics/journals/ortho/2007-5-30-5/%7B76fffea7-c722-4518-821b-dddda68cd03f%7D/remodeling-of-tibial-fractures-in-children-younger-than-12-years [DOI] [PubMed]
- 27.Larsen, C.F., Kiær, T., Lindequist, S. (1990). Fractures of the proximal humerus in children: Nine-year follow-up of 64 unoperated on cases. Acta Orthopaedica Scandinavica 61(3):255–7. Available from: http://www.tandfonline.com/doi/full/10.3109/17453679008993512 [DOI] [PubMed]
- 28.Kohler, R., Trillaud, J.M. (1983). Fracture and fracture separation of the proximal humerus in children: report of 136 cases. Journal of Pediatric Orthopedics 3(3):326–32. Available from: http://link.springer.com/10.1007/s12306-008-0050-7 [DOI] [PubMed]
- 29.McBride, E.D., Sisler, J. (1965) Fractures of the proximal humeral epiphysis and the juxta-epiphysial humeral shaft. Clinical Orthopaedics and Related Research 38:143–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5889085 [PubMed]
- 30.O’Shaughnessy, M.A., Parry, J.A., Liu, H., Stans, A.A., Larson, A.N., Milbrandt, T.A. (2019). Management of paediatric humeral shaft fractures and associated nerve palsy. Journal of Children's Orthopaedics 13(5):508–15. Available from: https://online.boneandjoint.org.uk/doi/10.1302/1863-2548.13.190012 [DOI] [PMC free article] [PubMed]
- 31.Caviglia, H., Garrido, C.P., Palazzi, F.F., Meana, N.V. (2005). Pediatric fractures of the humerus. Clinical Orthopaedics and Related Research (432):49–56. Available from: http://journals.lww.com/00003086-200503000-00007 [DOI] [PubMed]
- 32.Tan BHM, Mahadev A. Radial neck fractures in children. J Orthop Surg (Hong Kong). 2011;19(2):209–212. doi: 10.1177/230949901101900216. [DOI] [PubMed] [Google Scholar]
- 33.Vocke, A.K., von Laer, L. (1998). Displaced fractures of the radial neck in children. Journal of Pediatric Orthopedics 7(3):217–22. Available from: http://journals.lww.com/01202412-199807000-00007 [DOI] [PubMed]
- 34.Steele, J.A., Graham, H.K. (1992). Angulated radial neck fractures in children. A prospective study of percutaneous reduction. The Journal of Bone and Joint Surgery. British Volume 74(5):760–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1527130 [DOI] [PubMed]
- 35.Radomisli, T.E., Rosen, A.L. (1998). Controversies regarding radial neck fractures in children. Clinical Orthopaedics and Related Research 353(353):30–9. Available from: http://journals.lww.com/00003086-199808000-00005 [DOI] [PubMed]
- 36.Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. Journal of Pediatric Orthopedics Part B. 2003;12(2):109–10915. doi: 10.1097/01.bpb.0000043725.21564.7b. [DOI] [PubMed] [Google Scholar]
- 37.Jeroense, K.T.V., America, T., Witbreuk, M.M.E.H., van der Sluijs, J.A. (2015). Malunion of distal radius fractures in children. Acta Orthopaedica 86(2):233–7. Available from: http://www.tandfonline.com/doi/full/10.3109/17453674.2014.981781 [DOI] [PMC free article] [PubMed]
- 38.Crawford, S.N., Lee, L.S.K., Izuka, B.H. (2012). Closed Treatment of overriding distal radial fractures without reduction in children. The Journal of Bone and Joint Surgery American Volume 94(3):246–52. Available from: http://journals.lww.com/00004623-201202010-00008 [DOI] [PubMed]
- 39.Plánka, L., Chalupová, P., Skvaril, J., Poul, J., Gál, P. (2006). Remodeling ability of the distal radius in fracture healing in childhood. Rozhledy v Chirurgii 85(10):508–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17233178 [PubMed]
- 40.Akar, D., Köroğlu, C., Erkus, S., Turgut, A., Kalenderer, Ö. (2018). Conservative follow-up of severely displaced distal radial metaphyseal fractures in children. Cureus 9(9). Available from: https://www.cureus.com/articles/14423-conservative-follow-up-of-severely-displaced-distal-radial-metaphyseal-fractures-in-children [DOI] [PMC free article] [PubMed]
- 41.Vorlat, P., De Boeck, H. (2003). Bowing fractures of the forearm in children. Clinical Orthopaedics and Related Research 413(413):233–7. Available from: http://journals.lww.com/00003086-200308000-00026 [DOI] [PubMed]
- 42.Noonan, K.J., Price, C.T. (1998). Forearm and distal radius fractures in children. Journal of the American Academy of Orthopaedic Surgeons 6(3):146–56. Available from: http://journals.lww.com/00124635-199805000-00002 [DOI] [PubMed]
- 43.Fuller, D., McCullough, C. (1982). Malunited fractures of the forearm in children. The Journal of Bone and Joint Surgery. British Volume 64-B(3):364–7. Available from: http://online.boneandjoint.org.uk/doi/10.1302/0301-620X.64B3.7096406 [DOI] [PubMed]