Highlights
-
•
Theoretically, prostate cancer can spread to any part of the body.
-
•
Metastasis to axillary lymph node in a patient with normal urologic examination is rare.
-
•
It may delay the diagnosis.
-
•
A high suspicion index is essential in males presenting with symptoms suggestive of chest and abdominal-pelvic cancer.
Abbreviations: ALP, alkaline phosphatase (ALP); ALT, Alanine Aminotransferase; BP, Blood Pressure; CTscan, computerized tomography scan; DRE, digital rectal examination; H&E, Hematoxylin and Eosine; PR, Pulse Rate; PSA, Prostatic Specific Antigen; S02, oxygen saturation
Keywords: Left axillar lymphadenopathy, Rare, Case report, Metastatic prostate cancer
Abstract
Introduction and importance
Advanced prostate cancer often presents with lower urinary tract symptoms together with features of cancer on digital rectal examination. The commonest sites of metastasis include bone, liver and lungs. Metastasis to axillary lymph nodes is extremely unusual particularly as initial presentation of the disease.
Case presentation
We report an atypical case of a 40-year male patient presented with left axillary mass and normal initial urological evaluation. Histopathology and immunohistochemistry of the biopsies from the axillary mass and prostate confirmed the diagnosis of prostate adenocarcinoma. The patient declined anti-androgen monotherapy treatment and succumbed two months after establishment of the diagnosis.
Clinical discussion
Prostate cancer contributes significantly to the overall global cancer burden. Lymphatic metastasis to axillary lymph nodes is a very rare manifestation of prostate cancer and only a few cases have been reported in the literature. Therefore, clinical diagnosis of patients presenting with axillary lymphadenopathy may cause diagnostic delay. Careful physical and imaging examinations combined with pathological analysis are essential in the diagnosis of advanced prostate cancer with unusual presentation.
Conclusion
In theory, prostate cancer can cause metastatic spread to any part of the body. However, metastasis to axillary nodes has not been frequently noticed. Our report highlights the importance of considering prostate cancer among differential diagnoses in Afro-Caribbean males presenting with symptoms suggestive of chest and abdomino-pelvic cancer.
1. Introduction
Prostate cancer contributes significantly to the overall cancer burden globally. In 2018, there were estimated 1,276,106 (7.1%) new cases and the disease was responsible for 358,989 (3.8%) deaths worldwide. African-Americans have a slight higher incidence and mortality rates compared to Caucasians [1]. Clinically, prostate cancer patients usually present with obstructive uropathy and malignancy features on digital rectal examination (DRE) as well as elevated serum prostate specific antigen (PSA) [2]. However, some patients can be asymptomatic with an incidental finding on routine DRE.
Usually, prostate cancer spread locally and regionally to lymph nodes and bones [2]. It may also present as metastasis of unknown primary origin to non-regional, extra-skeletal sites rarely encountered in patients with established known prostate cancer [3]. Supra-clavicular, mediastinal, pulmonary and retro-peritoneal metastasis rarely occurs as the initial manifestation of advanced prostate cancer [4]. Pelvic and abdominal retroperitoneal lymph nodes are the most common sites of adenopathy in prostate cancer [5]. With more advanced disease, there can be involvement of peri-aortic, intra-thoracic, supra-clavicular and cervical lymph nodes; and very rarely to axillary lymph nodes [[3], [4], [5]]. Herein, we report a case of a 40-year-old male with an advanced prostate cancer presenting with left axillary lymphadenopanthy as initial presentation and a brief review of the literature. This work has been reported in line with the SCARE 2020 criteria [6].
2. Case presentation
A-40 year-old man presented to our facility with 3 month history of left axillary mass which started gradually and progressively increasing in size over time. The mass was painless and it was associated with occasional fever, slight weight loss, easy fatigability and awareness of heartbeats. The patient denied history of lower urinary tract symptoms, night sweats, coughing, and difficulty in breathing, haematuria or changes in bowel habits. He also reported occasional mild back pain and lower limbs numbness. There was no recent history of trauma to his back, aggravating or relieving factors. Past medical history revealed that the patient was on self-medication without any relief and had a positive history of prostate cancer in his family.
On examination, the patient was fully conscious. His vital signs were: BP = 106/69 mmHg, PR = 98 beats per minute, S02 = 96%, body temperature: 37.8 °C. Firm, non-tender mobile lymph node measuring about 2 × 3 cm was noted on the left axillary area. The rest of the peripheral lymph nodes were not palpable. The lower limbs had normal muscle bulkiness and tone. DRE and review of other systems were essentially unremarkable. Abdominal-pelvic malignant diseases was suspected.
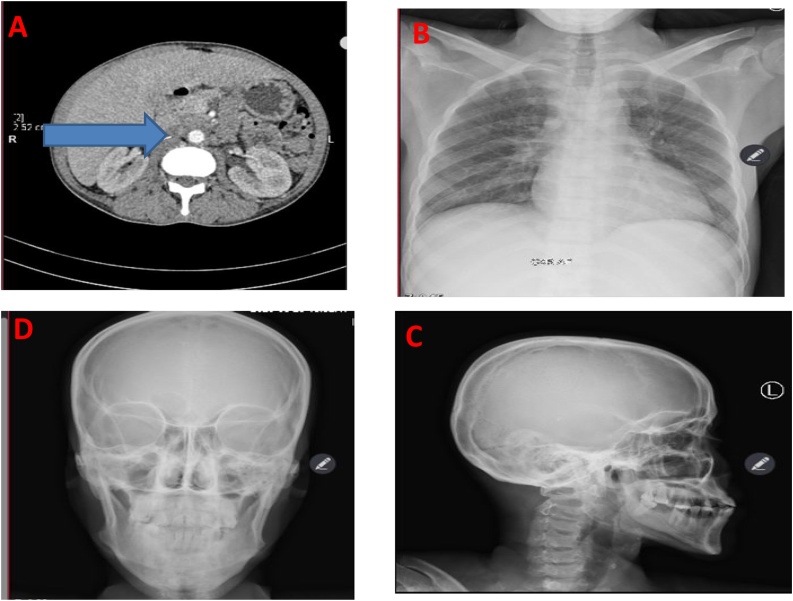
Ultrasound scan of urinary tract suggested heterogeneous prostate of 20cc; right kidney mild hydronephrosis; thick, trabeculated urinary bladder wall with residual urine volume of 44cc/116cc (38%). Computerized Tomography (CT) scan of the abdomen and pelvis revealed multiple enlarged para-aortic lymph nodes, largest measuring 2.5 × 1.6 cm; (Fig. 1A). Plain antero-posteria chest (Fig. 1B), skull (Fig. 1C&D) and pelvis x-rays were unremarkable. Full blood count suggested microcytic hypochromic anemia of 5.8 g/dl with thrombocytopenia of 96 × 10^9/L (150–500). Serum creatinine was 57 μmol/L (62–106); Aspartate Transaminase (AST) 24.77U/l (2.00–40.00), Alanine Aminotransferase (ALT) 10.51 U/l (2.00–41.00), total serum calcium 1.95 mmol/l (2.15–2.55); and alkaline phosphatase (ALP) 55.74 U/l (40–129U/L).
Fig. 1.
CT scan of the abdomen and pelvis highlighting multiple enlarged para-aortic lymph nodes (A), while the plain x-rays of chest (B) and skull (C&D) showing unremarkable findings.
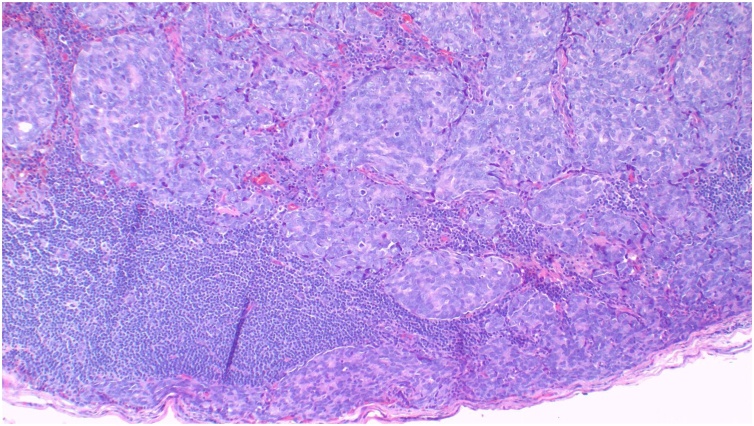
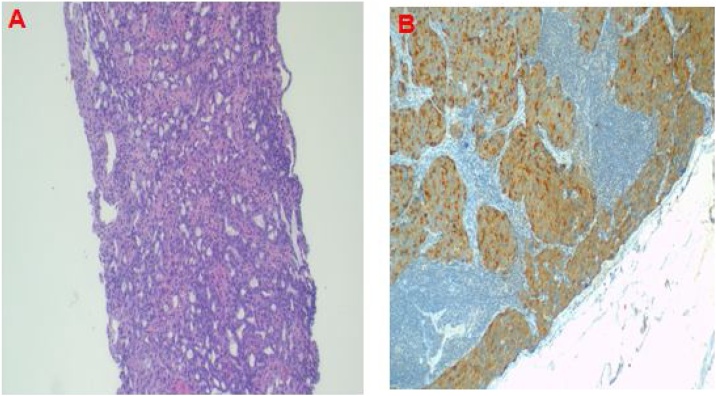
The axillary lymph note excisional biopsy was scheduled and performed by an experienced surgeon. Histopathological analysis of the specimen suggested metastatic adenocarcinoma to the lymph node, (Fig. 2). Blood PSA test was performed and revealed 86.910 ng/mL (0.001–4.000). Metastatic prostate cancer was suggested. The patient underwent prostatic trucut biopsy by a team of urological surgeons and the histopathological analysis report of the specimen concluded invasive prostatic adenocarcinoma, Gleason score (4 + 3) = 7, grade 4 (Fig. 3A). Immunohistochemistry of the axillary node biopsy with Prostatic Specific Antigen (PSA) antibody demonstrated strong tumor cell positivity (Fig. 3B). Multidisciplinary tumor board discussion proposed anti-androgen monotherapy treatment. However, the patient declined the treatment because of its association with infertility; thus he was kept on palliative care with morphine and discharged. Two months later, the patient developed sudden difficulty in breathing and succumbed while he was on the way back to the hospital. The cause of death was suspected to be disseminated prostate cancer.
Fig. 2.
Histopathology of axillary lymph node demonstrating partial effacement of the nodal architecture and replaced by an infiltrating epithelial tumour with solid cords and poorly formed glands, Haematoxylin and Eosin (H&E) 100× original magnification.
Fig. 3.
Histopathology of trucut prostate biopsy demonstrating infiltrating tumour with solid sheets and poorly formed glands, H&E 40X original magnification (A); and immunostaining of axillary node with PSA demonstrating tumour cell positivity 100× original magnification (B).
3. Discussion
Metastatic prostate cancer has a recognizable pattern of spread, most often to regional lymph nodes and the bone [7]. Reports describing atypical metastatic sites such as single or generalized lymphadenopathy with absence of other symptoms of the disease have been documented [8]. Similarly, studies on unusual presentation of prostate cancer mimicking lymphoma on radiological imaging have also been documented [9]. Furthermore, cutaneous, gastric, colonic omentum and peritoneal metastases with or without malignant ascites have been reported [10]. These can occur independently of bone metastasis and response rate to treatment is said to be similar to those with bone metastasis only [10].
Lymphatic metastasis to axillary lymph nodes is a very rare manifestation of prostate cancer [11]. Several reports of prostate cancer with non-regional supra-diaphragmatic lymphatic metastases described no cases of axillary lymph node involvement [8,12,13]. Interestingly, expression of chemokine receptors has been associated with unusual predilection of prostate cancer to lymph nodes [14]. Axillary node metastasis in the absence of classical signs and symptoms of prostate cancer can potentially lead to a considerable diagnostic and therapeutic delay because it may not be considered among differential diagnoses, [15]. Moreover, in contemporary practice many urologists do not routinely perform lymph node dissections in patients with low risk prostate cancer [2]. Therefore, despite of wide range of differential diagnoses, it is important to have a high index of suspicion for metastatic prostate cancer in young males presenting with axillary lymphadenopathy with or without signs and symptoms of prostate cancer; as it was the case in our patient.
4. Conclusion
Although metastatic prostate cancer to axillary nodes is unusual, this case is emphasizing the need for consideration of prostate cancer as an important differential diagnosis in Afro-Caribbean males presenting with symptoms suggesting chest and abdominal-pelvic malignant diseases.
Declaration of Competing Interest
All authors have declared that no competing interests exist.
Funding
The work did not receive fund from any source.
Ethical approval
There was exemption of ethical clearance.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author’s contribution
AM and BNN conceptualized and prepared the first manuscript. BNN, OJM, SK, VK, KAM and FB reviewed the patients’ medical records; planned and executed management. AM performed diagnostic investigations including histopathological and diagnostic immunochemistry analysis and prepared microscopic images. All authors read and approved the final manuscript.
Registration of research studies
Not Applicable.
Guarantor
Alex Mremi is the Guarantor of this work.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Availability of data and materials
All data used in this study are available from the corresponding author upon editorial office request.
Acknowledgements
The authors would like to thank the patient for allowing us to use the patient's information for academic purposes.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(November (6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Masood A., Iqbal N., Shohab D., Hasan A., Aimon S., Mahmood U. Clinicopathological characteristics of prostate cancer in patients presenting to a tertiary care private sector hospital. J. Coll. Phys. Surg. Pak. 2018;28(May (5)):409–411. doi: 10.29271/jcpsp.2018.05.409. [DOI] [PubMed] [Google Scholar]
- 3.Yamaga Lilian Yuri Itaya, da Cunha Marcelo Livorsi. Atypical metastases from prostate cancer detected on 68Ga-PSMA PET/CT: a case series. Int. Braz. J. Urol. 2021;47(1):205–209. doi: 10.1590/S1677-5538.IBJU.2020.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaga L.Y.I., da Cunha M.L. Atypical metastases from prostate cancer detected on 68Ga-PSMA PET/CT: a case series. Int. Braz. J. Urol. 2021;47(January-February (1)):205–209. doi: 10.1590/S1677-5538.IBJU.2020.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elabbady A., Kotb A.F. Unusual presentations of prostate cancer: a review and case reports. Arab J. Urol. 2013;11(March (1)):48–53. doi: 10.1016/j.aju.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Bubendorf L., Schöpfer A., Wagner U., Sauter G., Moch H., Willi N. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 8.Pinaquy J.B., Allard J.B., Cornelis F., Pasticier G., De Clermont H. Unusual lymph node metastases of prostate cancer detected by 18F-fluorocholine PET/CT. Clin. Nucl. Med. 2015;40(April (4)):e255–7. doi: 10.1097/RLU.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 9.Biyani C.S., Basu S., Bottomley D.M., Shah T.K. Prostatic adenocarcinoma masquerading as lymphoma and presentation with axillary-subclavian vein thrombosis. Urol. Oncol. 2003;21(January-February (1)):3–6. doi: 10.1016/s1078-1439(02)00206-5. [DOI] [PubMed] [Google Scholar]
- 10.Vinjamoori A.H., Jagannathan J.P., Shinagare A.B., Taplin M.E., Oh W.K., Van den Abbeele A.D. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am. J. Roentgenol. 2012;199(August (2)):367–372. doi: 10.2214/AJR.11.7533. [DOI] [PubMed] [Google Scholar]
- 11.Collins G.R., Lopez Y., Abreo F. Prostatic adenocarcinoma metastatic to axillary lymph node diagnosed by fine-needle aspiration biopsy. Diagn. Cytopathol. 2012;40(August (8)):751–753. doi: 10.1002/dc.21679. [DOI] [PubMed] [Google Scholar]
- 12.Cho K.R., Epstein J.I. Metastatic prostatic carcinoma to supradiaphragmatic lymph nodes. A clinicopathologic and immunohistochemical study. Am. J. Surg. Pathol. 1987;11:457–463. doi: 10.1097/00000478-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Mbwambo O.J., Mremi A., Mbwambo J.S., Bright F., Mteta A.K., Ngowi B.N. Virchow’s node as the initial presentation of metastatic prostate cancer: a case series of a common cancer in uncommon location. J. Surg. Case Rep. 2020;2020(November (11)) doi: 10.1093/jscr/rjaa476. rjaa476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heresi G.A., Wang J., Taichman R., Chirinos J.A., Regalado J.J., Lichtstein D.M. Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: report of a case, review of the literature, and analysis of chemokine receptor expression. Urol. Oncol. 2005;23(July-August (4)):261–267. doi: 10.1016/j.urolonc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Spencer J., Golding S. CT evaluation of lymph node status at presentation of prostatic carcinoma. Br. J. Radiol. 1992;65:199–201. doi: 10.1259/0007-1285-65-771-199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are available from the corresponding author upon editorial office request.