Summary
BMI1-expressing cancer stem cells (CSCs) play a key role in the development, progression, therapy resistance, recurrence, and metastasis of head and neck squamous cell carcinoma (HNSCC). Here, we present a chemically-induced HNSCC mouse model, genetically and pathologically similar to human HNSCC. This protocol describes how to use genetic lineage tracing based on the Cre-loxP recombination strategy, which allows us to study the regulation and targeting of BMI1+ CSCs in primary tumors and lymph node metastases.
For complete details on the use and execution of this protocol, please refer to Chen et al. (2017) and Jia et al. (2020)
Subject areas: Cancer, Microscopy, Model Organisms, Stem Cells
Graphical abstract
Highlights
-
•
A protocol for inducing squamous cell carcinoma (SCC) in mice
-
•
In vivo lineage tracing of BMI1+ cancer stem cells in primary tumors
-
•
In vivo lineage tracing of BMI1+ cancer stem cells in metastatic lymph nodes
-
•
Immunostaining and immunohistochemistry for pathology analysis
BMI1-expressing cancer stem cells (CSCs) play a key role in the development, progression, therapy resistance, recurrence, and metastasis of head and neck squamous cell carcinoma (HNSCC). Here, we present a chemically-induced HNSCC mouse model, genetically and pathologically similar to human HNSCC. This protocol describes how to use genetic lineage tracing based on the Cre-loxP recombination strategy, which allows us to study the regulation and targeting of BMI1+ CSCs in primary tumors and lymph node metastases.
Before you begin
Animal breeding
Bmi1CreER (JAX:010531) ( Madisen et al., 2010) and R26tdTomato (JAX:007908) (Sangiorgi and Capecchi, 2008) transgenic mouse strains are obtained from The Jackson Laboratory and crossed to yield Bmi1CreER; R26tdTomato double-positive mice that are then backcrossed for at least 5 generations to C57BL/6J mice. C57BL/6J congenic offspring mice are used for tumor induction. To ensure successful crossbreeding, genomic DNA is isolated from tail clips and genotyped following the instructions provided by The Jackson Laboratory website. All animals are housed under specific pathogen-free (SPF) conditions. All procedures are performed based on the UCLA Animal Research Committee-approved protocols.
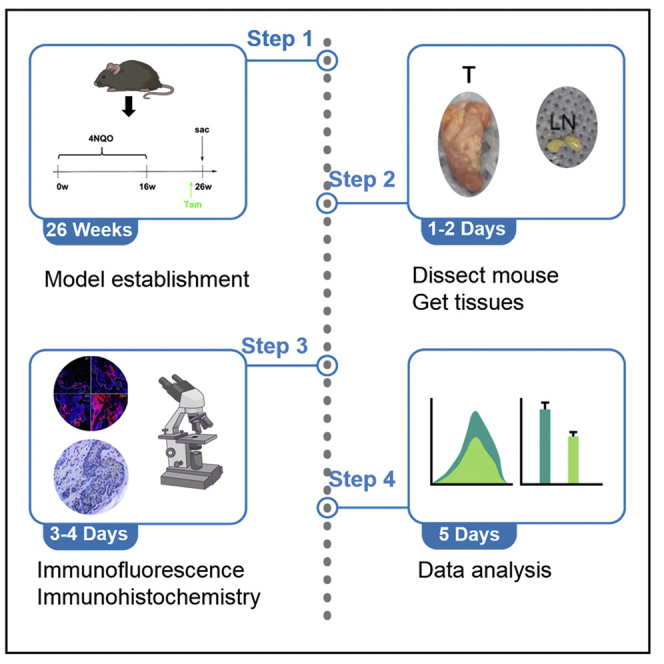
Induction of mouse HNSCC using carcinogen
Timing: 24–26 weeks
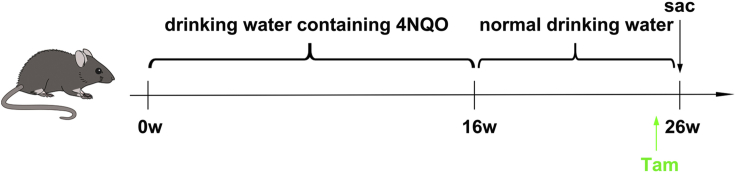
First, to make a stock of carcinogen, 4-Nitroquinoline N-oxide (4-NQO, Santa Cruz, sc-256815) is dissolved in propylene glycol (Sigma-Aldrich, W294025) at 5 mg/mL and stored for no longer than 3 months at 4°C. Both female and male mice are used for HNSCC induction. When mice reach the age of 6 weeks, dilute the 4-NQO stock solution in the feeding water for 100 times and feed the mice with 4NQO water for 16 weeks. The drinking water was changed every two weeks. After that, feed the mice with normal drinking water for another 8-10 weeks (Figure 1). Of note, we originally used 4-NQO from Sigma-Aldrich (N8141). Accidently, we find that mice tolerate 4-NQO from Santa Cruz better.
Figure 1.
Experimental design for tracing Bmi1+ cells in mouse HNSCC induced by 4NQO
Lineage tracing
Timing 2–4 h
Tamoxifen (Sigma-Aldrich, T5648) is dissolved in mineral oil (Sigma-Aldrich, M5904) at 20 mg/mL on a nutator for 2–4 h at 37°C. After fully dissolved, tamoxifen solution is aliquoted in 1.5 mL Eppendorf tubes, covered with foil, and stored for a week at −20°C. For in vivo labeling of BMI1+ CSCs, Bmi1CreER; R26tdTomato mice are injected with tamoxifen intraperitoneally at a dose of 225 mg per 1 kg body weight 24 hrs before sacrificing mice (Figure 1).
CRITICAL: 4-NQO stock solution should be prepared in light-shielded water bottles and kept for no more than 3 months at −20°C. For 4NQO waste, place it in a suitable, labeled container for waste disposal.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-pan-Cytokeratin | Santa Cruz | Cat#sc-8018, RRID# AB_627396 |
| Rabbit polyclonal anti-Keratin 5 | BioLegend | Cat#905501, RRID#AB_2565050 |
| Chemicals, peptides, and recombinant proteins | ||
| 4-Nitroquinoline N-oxide (4NQO) | Santa Cruz | sc-256815 |
| Tamoxifen | Sigma-Aldrich | Cat#T5648 |
| EdU | Sigma-Aldrich | Cat#T511285 |
| Tissue-Tek OCT Compound | Sakura | Cat#25608-930 |
| Fisherbrand Superfrost Plus Microscope Slides | Thermo Fisher Scientific | Cat#12-550-15 |
| DAPI | Sigma-Aldrich | Cat#D9542 |
| Vectashield with DAPI | Vector Laboratories | Cat#H-1200-10 |
| Permount Mounting Medium | Fisher Scientific | Cat#SP15-100 |
| SlowFade Antifade Reagents | Thermo Fisher Scientific | Cat#S36937 |
| Critical commercial assays | ||
| Click-iT EdU Alexa Fluor 488 Imaging Kit | Thermo Fisher Scientific | Cat#C10337 |
| DAKO EnVision+ Kits | QIAGEN | Cat#74004 |
| H&E Staining Kit | Abcam | Cat#Ab245880 |
| Experimental models: Organisms/strains | ||
| Mouse: Bmi1Cre-ER | Jackson Laboratory JAX:010531 | |
| Mouse: R26tdTomato | Jackson Laboratory JAX:007908 | |
| Software and algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij |
| CellSens software | Olympus | http://www.olympus-lifescience.com/en/software/cellsens/ |
| Other | ||
| Bone scissors | Thermo Fisher Scientific | Cat#08-990 |
| Sharp dissection scissors | Thermo Fisher Scientific | Cat#08-935 |
| Forceps | Thermo Fisher Scientific | Cat#12-000-127 |
| Phospho-buffered saline | Thermo Fisher Scientific | Cat#10-010-023 |
| 4% Paraformaldehyde | Santa Cruz | Cat#sc-281692 |
| Hemostatic cotton | Thermo Fisher Scientific | Cat#NC1641241 |
| Pap pen | Thermo Fisher Scientific | Cat#23-769-533 |
| Coplin jar | Thermo Fisher Scientific | Cat#19-4 |
Materials and equipment
Prepare an 25 cm × 25 cm foam board wrapped with aluminum foil to serve as a dissection board, six 25 G sterile needles, 1 mL syringe, 5 mg/mL EdU in PBS, 70% ethanol spray, a bone scissor (#08-990, Fisher Scientific), a sharp dissection scissor (#08-935, Fisher Scientific), two forceps (#12-000-127, Fisher Scientific), a marker pen, phospho-buffered saline (PBS), 4% paraformaldehyde fix solution (PFA), paper towels, hemostatic cotton (#NC1641241, Fisher Scientific), two 15 mL falcon tubes, a 15-cm ruler, a sharps container, 30% sucrose solution in PBS, a disposable embedding mold, a balance, a pap pen (#23-769-533, Fisher Scientific), and a Leica EZ4 dissection microscope.
CRITICAL: Mark any chemicals or reagents that are harmful or toxic with a brief explanation of the hazard, and state clearly how to take precautions when handling these agents.
PFA is moderately toxic by inhaling and skin contact, therefore carefully handle it in the chemical hood.
Alternatives: Mention any known alternative materials or equipment in this format.
Step-by-step method details
Tissues harvesting
Timing: 2–4 h
-
1.
To label BMI1+ CSCs, Bmi1CreER; R26tdTomato mice are injected with tamoxifen intraperitoneally at a dose of 225 mg per 1 kg body weight 24 h before sacrificing the mice. For in vivo lineage tracing of BMI1+ CSCs and their progenies, mice are injected with tamoxifen intraperitoneally at a dose of 225 mg per 1 kg body weight 2–6 weeks before ending the experiments.
-
2.
To label proliferating tumor cells, mice bearing HNSCC are first injected intraperitoneally with EdU at a dose of 25 μg/g body weight after reaching the experimental endpoint.
-
3.
90 min after EdU injection, mice are then euthanized using CO2 asphyxiation.
-
4.
Use the needles to pin the mouse limbs to the dissection board to secure the body. Spray the body with 70% ethanol.
-
5.
Use forceps to grab the skin and cut a long incision through the midline from the chin to the upper chest region of the mouse using scissors.
-
6.
Gently insert scissors under the skin to separate the skin from the underlying tissues.
-
7.
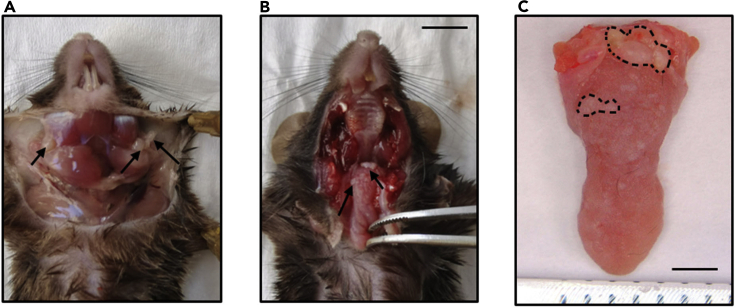
Once the skin is fully detached, cut and open the skin laterally at the chest level and pin the skin with needles to expose the cervical lymph nodes (Figure 2A).
-
8.
Carefully excise the lymph nodes with sharp scissors under the Leica EZ4 Stereo Microscope (Use hemostatic cotton to swab the blood that might hinder the identification of the lymph nodes).
-
9.
Place the lymph nodes into a 15 mL falcon tube filled with 10 mL 4% PFA at 22°C–25°C.
-
10.
After collecting all the lymph nodes, cut the joints on both sides of the jaws with a bone scissor. Open the jaws as wide as possible to expose the tongue tissues (Figure 2B). Cut the whole tongue using a sharp scissor.
-
11.
Take photos of the tongue using the Leica EZ4 Stereo Microscope with a ruler (Figure 2C).
-
12.
Put the tongue in the 4% PFA tube mentioned above (The tissue can be kept in 4% PFA up to 72 h before over-fixation).
-
13.
After 24 h, remove PFA and wash the tongue and lymph nodes three times with PBS for 30 min at 22°C–25°C. Cut the tongue into halves sagittally along the mid-line of the HNSCC lesion. Keep half of the lesion for paraffin section and the other half for cryosection.
Figure 2.
Dissection and photographing of mouse HNSCC
(A) Dissecting cervical lymph nodes.
(B and C) Tumor lesions on the tongue.
Scale bar for (A and B), 500 μm; Scale bar for (C), 200 μm.
Cryo-section and immunostaining
Timing: 1–2 days
-
14.
Discard PBS and pour in 10 mL of 30% sucrose solution in PBS into the 15 mL tube. Keep the tissue at 4°C for 48 h until the tissue sinks in the solution.
-
15.
Embed the tissue in pre-chilled optimal cutting temperature (OCT) compound in an appropriate embedding mold. Freeze on dry ice and store tissue at −80°C until sectioning.
-
16.
Cut the cytosections at a thickness of 8–10 μm using a Leica cryostat and collect the sections with Superfrost Slides (Fisher Scientific Cat#12-550-15).
-
17.
Let the slides dry for 5 min at 22°C–25°C.
-
18.
Fix in 4% PFA for 10 min in a humidified chamber, 200 μL/sample, and spread with a new pipette tip (operate in the chemical hood).
-
19.
Wash the slides in PBS for 5 min three times.
-
20.
Make blocking buffer (5% Normal Donkey Serum, 1% bovine serum albumin, 0.2% Triton X-100 in 1 × PBS).
-
21.
Trace around the sections with a pap pen and add 200–400 μL block buffer per slide.
-
22.
Block in a humidified chamber for 30 min at 22°C–25°C.
-
23.
Decant block buffer and add anti-pan-cytokeratin antibodies (1:500, Santa Cruz, Cat#sc-8018) or anti-keratin 5 (1:500; BioLegend, Cat#905501) diluted in block buffer to each slide (200 μL per slide) and cover with a plastic coverslip. Because some hematopoietic cells in lymph nodes express BMI1, the sections of cervical lymph nodes need to be stained with anti-pan-cytokeratin antibodies to confirm that BMI1+ CSCs are metastatic tumor cells.
-
24.
Incubate at 40C in a humidified chamber.
-
25.
The next day, lift off the coverslip by dipping in PBS until coverslip slips off the slide on its own
-
26.
Place slide into a Coplin jar with 1 × PBS/0.1% Triton x-100 (PBS-T). Wash for 5 min.
-
27.
Repeat this wash 2 more times.
-
28.
Dilute secondary antibodies in PBS-T 1:200 and add 200–300 μL per slide (no coverslip) and incubate at 22°C–25°C in humidified chamber for 45 min.
-
29.
Wash in PBS-T (in a Coplin jar) 3 times (5 min per wash) and then 2 times in PBS (no Triton)
-
30.
Dip slide in ddH20 and then add 1–2 drops of Vectashield with DAPI (#H-1200-10, Vector Laboratories) and carefully place glass coverslip over while avoiding trapping bubbles under the slide.
-
31.
Fix slide with nail polish around the coverslip.
-
32.
Acquire the images with a fluorescent microscopy running CellSens software (Olympus http://www.olympus-lifescience.com/en/software/cellsens/).
Paraffin section and H&E staining
Timing: 2–3 days
-
33.
Transfer tissue to 70% ethanol for fixation for 14–16 h at 22°C–25°C.
-
34.
Transfer tissue to 80% ethanol for 30 min at 22°C–25°C.
-
35.
Transfer tissue to 95% ethanol for 30 min at 22°C–25°C.
-
36.
Transfer tissue to 100% ethanol for 30 min at 22°C–25°C. Repeat this step twice.
-
37.
Transfer tissue to Histoclear II for 30 min at 22°C–25°C. Repeat this step twice.
-
38.
Incubate tissue in melted paraffin in 56°C–58°C for 30 min.
-
39.
After incubation, transfer tissue into a labeled cassette – you can leave this indefinitely until ready to embed into molds.
-
40.
Embed and label accordingly.
-
41.
Cut the paraffin section at a thickness of 5 μm using a Leica RM2235 manual microtome and collect the sections with Superfrost Slides (Fisher Scientific Cat#12-550-15).
-
42.
Deparaffinize the sections in Histoclear II for 3 min at 22°C–25°C. Repeat twice.
-
43.
Rehydrate the sections into 100% ethanol solution for 1 min at 22°C–25°C.
-
44.
Transfer the sections into 95% ethanol solution for 1 min at 22°C–25°C.
-
45.
Transfer the sections into 80% ethanol solution for 1 min at 22°C–25°C.
-
46.
Transfer the sections into 70% ethanol solution for 1 min at 22°C–25°C.
-
47.
Transfer the sections into deionized H2O for 5 min at 22°C–25°C.
-
48.
Perform H&E staining using a H&E Staining Kit (Abcam, ab245880) according to the manufacturer’s guidance.
-
49.
Mount the slides with Permount Mounting Medium (#SP15-100, Fisher Scientific) and a cover slide.
-
50.
Air dry the slides in the chemical hood for 14–16 h.
-
51.
Acquire the images with a microscopy running CellSens software (Olympus http://www.olympus-lifescience.com/en/software/cellsens/).
Expected outcomes
Formation of HNSCC
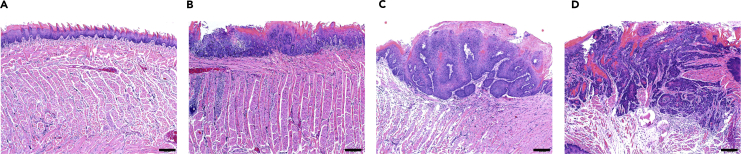
After drinking 4-NQO-contained water for 16 weeks and then drinking normal water for 8–10 weeks, lesions can be observed in the tongue of mice. Most of the lesions are SCC invading the tongue muscle with cervical lymph node metastasis, but some of these lesions may be papilloma (Figures 3A–3D). Of note, to determine cervical lymph node metastasis, it is important to stain the section with anti-pan-cytokeratin antibodies because it is very difficult to distinguish metastatic tumor cells from lymphocytes by H&E staining.
Figure 3.
Histology of normal tongue epithelium and HNSCC
(A) normal tongue epithelium (grade 0).
(B) epithelial dysplasia confined to tongue epidermis (grade 1).
(C) distinct invasion, unclearness of basement membrane, diffuse infiltration into the superficial portion of the muscle layer (grade 2).
(D) loss of the basement membrane, extensive invasion into deep muscle layer (grade 3).
Scale bars, 200 μm.
Presence of tomato+ cells
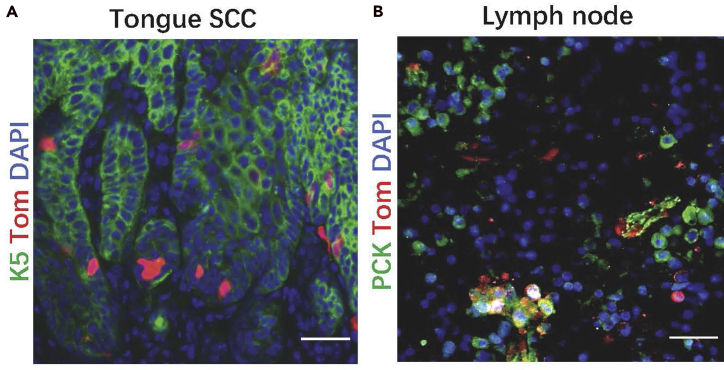
1 day after the injection of tamoxifen, Tomato+ cells can be detected in the primary HNSCC which can be readily detected in the invasive front (Figure 4A) and metastatic lymph nodes (Figure 4B). The colonies formed by labeled cells at the invasive front are expanded progressively over time.
Figure 4.
Tomato+ CSCs in the invasive front of primary and metastatic HNSCC
(A) Tomato+ CSCs in the invasive front of HNSCC.
(B) Metastatic Tomato+ CSCs in cervical lymph nodes.
Scale bar for (A and B), 50 μm. Figure 4A reprinted with permission from Chen et al., 2017.
Metastatic tumor cells in the cervical lymph nodes
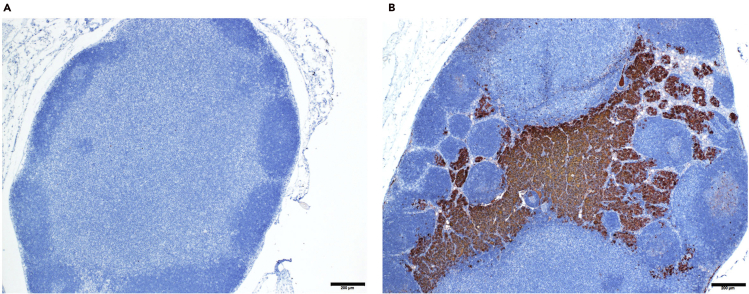
After IHC staining of anti-pan cytokeratin antibodies, the sections of lymph nodes with metastatic HNSCC cells would show positive brown signals (Figures 5A and 5B).
Figure 5.
Representative PCK staining of non-metastatic and metastatic lymph nodes
(A) Non-metastatic lymph nodes with negative PCK staining.
(B) Metastatic lymph nodes with positive PCK staining.
Scale bars, 200 μm.
Quantification and statistical analysis
-
1.Quantification of BMI1+ CSCs
- At least three sections from each HNSCC lesions were analyzed. Tomato+ tumor cells from 3–5 fields are counted manually in each section. The percentage of Tomato+ cells is calculated by dividing those cells with tumor cells and then averaged from the sections.
-
2.Tumor number count and tumor grade quantification
- Cut 15–20 sections for each sample, stain the first and last sections with H&E, and count SCC numbers under a microscope. The depth of the tumor invasion in the tongue is determined in the H&E stained sections according to the following criteria: showing signs of normal appearance (grade 0, Figure 3A); epithelial dysplasia confined to tongue epidermis (grade 1, Figure 3B); distinct invasion, unclearness of the basement membrane, drop and diffuse infiltration into the superficial portion of the muscle layer (grade 2, Figure 3C); loss of the basement membrane, extensive invasion into deep muscle layer (grade 3, Figure 3D).
-
3.Lymph node metastasis quantification
- Immunostain the sections of cervical lymph nodes with anti-pan-cytokeratin antibodies and count the lymph nodes with positive cytokeratin staining signals as metastatic lymph nodes. The percentage of lymph nodes with metastasis is calculated and compared (Figures 5A and 5B).
-
4.Statistics methods
- All statistical analysis is achieved using SPSS software. For comparison of tumor numbers in control and knockout mice, the differences are assessed using two-way ANOVA. For HNSCC invasion grades, the Cochran-Armitage test is performed. For a percentage of mice with positive lymph node metastasis, Fisher’s exact test is performed.
Limitations
Several limitations may exist for this mouse model. It takes a relatively long time to successfully induce HNSCC. Precautions need to be taken to handle the carcinogen of 4-NQO that can damage human skin upon contact. Human HNSCC consists of HPV- and HPV+ tumors, while this mouse model is only relevant to HPV- HNSCC.
Troubleshooting
Problem 1
Mouse is dead before developing HNSCC (“Induction of mouse HNSCC using carcinogen” section).
Potential solution
Reduce 4-NQO induction dose and time. Use 4-NQO from a different vendor.
Problem 2
Mouse does not develop HNSCC (“Induction of mouse HNSCC using carcinogen” section).
Potential solution
Increase the concentration of 4-NQO in the drinking water and feeding times. Use 4-NQO from a different vendor.
Problem 3
No obvious lineage tracing (step 1).
Potential solution
It could be due to inefficient Cre enzyme activation. Increase the dosage or number of TAM for injection.
Problem 4
Few lymph nodes collected (steps 4–9)
Potential solution
Dissect the mice carefully to avoid blood flood into the neck region and use hemostatic cotton to get rid of the blood.
Problem 5
Low Tomato fluorescent signal (steps 14–32).
Potential solution
When the Tomato fluorescent signal is too low, try to use an antibody against Tomato to detect the signal.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Cun-Yu Wang (cwang@dentistry.ucla.edu).
Materials availability
All materials described in this protocol are commercially available.
Data and code availability
This study did not generate/analyze new datasets/code.
Acknowledgments
The protocol was developed at UCLA. C.Y.W. was supported by NIH/NIDCR grants R01DE15964 and R01DE029173.
Author contributions
Experimental design and conception were done by D.C., W.Z., L.J., C.W., and C.Y.W. Animal breeding and carcinogen induction of transgenic mice, sample harvesting, IHC staining, image processing, and quantification and statistical analysis were performed by D.C., W.Z., L.J., and C.Y.W. D.C. and C.Y.W. wrote the manuscript with input from all other authors.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Demeng Chen, Email: chendm29@mail.sysu.edu.cn.
Cun-Yu Wang, Email: cwang@dentistry.ucla.edu.
References
- Chen D., Wu M., Li Y., Chang I., Yuan Q., Ekimyan-Salvo M., Deng P., Yu B., Yu Y., Dong J. Targeting BMI1+ cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell Stem Cell. 2017;20:621–634. doi: 10.1016/j.stem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Zhang W., Wang C.Y. BMI1 inhibition eliminates residual cancer stem cells after PD1 blockade and activates antitumor immunity to prevent metastasis and relapse. Cell Stem Cell. 2020;27:238–253. doi: 10.1016/j.stem.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E., Capecchi M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze new datasets/code.