Abstract
Pabinafusp alfa is a fusion protein comprising a humanized anti-human transferrin receptor (TfR) antibody and human iduronate-2-sulfatase. It was developed as a novel modality to target central nervous system-related symptoms observed in patients with mucopolysaccharidosis type II (MPS II, also known as Hunter syndrome). As the fusion protein contains an entire IgG1 molecule that binds TfR, there may be specific safety concerns, such as unexpected cellular toxicity due to its effector functions or its ability to inhibit iron metabolism, in addition to general safety concerns. Here, we present the comprehensive results of a nonclinical safety assessment of pabinafusp alfa. Pabinafusp alfa did not exhibit effector functions, as assessed by antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity studies in TfR-expressing hematopoietic cells. Repeat-dose toxicity studies in cynomolgus monkeys showed that pabinafusp alfa did not induce any significant toxicological changes at doses up to 30 mg/kg/week upon intravenous administration for up to 26 weeks. Interaction of transferrin with TfR was not inhibited by pabinafusp alfa, suggesting that the effect of pabinafusp alfa on the physiological iron transport system is minimal, which was confirmed by toxicity studies in cynomolgus monkeys. These findings suggest that pabinafusp alfa is expected to be safe for long-term use in individuals with MPS II.
Keywords: Mucopolysaccharidosis type II, Anti-transferrin receptor antibody, Toxicity, Effector function, Antibody-dependent cellular cytotoxicity, Complement-dependent cytotoxicity
Abbreviations: ADA, anti-drug antibody; ADCC, antibody-dependent cellular cytotoxicity; BBB, blood-brain barrier; CDC, complement-dependent cytotoxicity; CSF, cerebrospinal fluid; CNS, central nervous system; ERT, enzyme-replacement therapy; Fc, fragment crystalizable; FOB, functional observational battery; GAG, glycosaminoglycan; Hb, hemoglobin; Ht, hematocrit; IDS, iduronate-2-sulfatase; mAb, monoclonal antibody; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MPS II, mucopolysaccharidosis type II; NOAEL, no observed adverse effect level; pAb, polyclonal antibody; QWBA, quantitative whole-body autoradioluminography; RBC, red blood cell; Ret, reticulocyte; Tf, transferrin; TfR, transferrin receptor; TK, toxicokinetics
1. Introduction
Mucopolysaccharidosis type II (MPS II, also known as Hunter syndrome) is a rare X-linked recessive lysosomal storage disease caused by disease-associated variants in the gene encoding iduronate-2-sulfatase (IDS) [1,2]. Patients with MPS II suffer from various systemic manifestations such as hepatosplenomegaly, cardiac and respiratory distress, and bone and joint malformation. Patients with severe MPS II also progressively develop neurocognitive impairment, resulting in severe mental retardation within the first decade of their lives [[2], [3], [4]]. Symptoms of MPS II occur primarily because of the pathological accumulation of IDS substrate glycosaminoglycans (GAGs)—namely heparan sulfate and dermatan sulfate—throughout the body. An enzyme-replacement therapy (ERT) with recombinant human IDS has been developed and is currently the only approved treatment for MPS II [[5], [6], [7], [8], [9]]. Although conventional intravenous ERT relieves most somatic symptoms by reducing GAG deposition in peripheral tissues [[5], [6], [7], [8], [9]], it fails to redress the neurocognitive impairment because it is unable to cross the blood-brain barrier (BBB) [5,6].
We developed a BBB-penetrable IDS-antibody fusion protein, pabinafusp alfa (investigational code name, JR-141), comprising human IDS fused to an anti-human transferrin receptor (hTfR) antibody. Intravenously administered pabinafusp alfa has been shown to cross the BBB and enter the central nervous system (CNS) of hTfR knock-in/Ids-knock-out mice, an animal model of MPS II, and of cynomolgus monkeys [10]. Pabinafusp alfa decreases the amount of GAGs deposited in both peripheral and CNS tissues, thus preventing neurobehavioral abnormalities in MPS II mice [11]. Clinical studies have also shown that pabinafusp alfa reduces GAG levels in the serum and cerebrospinal fluid (CSF) and has beneficial effects on somatic and neurocognitive manifestations [[12], [13], [14]].
Antibody-based drugs have been utilized for the treatment of a wide variety of diseases, including cancer, inflammation, and autoimmunity, as they have high target specificity [15]; for example, in cancer therapy, antibodies recognize and kill the target cells via complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) [16,17]. However, in the case of pabinafusp alfa, the anti-hTfR antibody is used to facilitate BBB penetration by binding the TfR expressed on capillary endothelial cells in the brain and subsequent receptor-mediated transcytosis [10]. This also enhances cellular uptake of the fused enzyme through TfR, in addition to the mannose-6-phosphate receptor, throughout the body. Thus, cytotoxicity mediated by the antibody, if present, would be an unfavorable side effect of pabinafusp alfa. Moreover, the TfR-binding property of the drug raises concerns about its potential to alter iron metabolism when administered chronically.
Here, we describe the results of comprehensive nonclinical safety assessments of pabinafusp alfa. Four-week repeat-dose toxicity studies in sexually mature and juvenile monkeys and a 26-week repeat-dose study with dosage of pabinafusp alfa of up to 30 mg/kg/week were conducted. In addition, we performed in vitro assays to evaluate the effector functions (CDC and ADCC) of pabinafusp alfa and to elucidate its influence on transferrin (Tf) and TfR binding.
2. Materials and methods
2.1. Test substances
Pabinafusp alfa (JR-141), a recombinant fusion protein comprising a humanized anti-hTfR antibody and hIDS, was produced as previously described [10].
2.2. Cells
CCRF-CEM (human T lymphoblast cell line) and K562 (human myelogenous leukemia cell line) were obtained from the Japanese Collection of Research Bioresearch Cell Bank. HEL92.1.7 (human erythroblast cell line) was obtained from the American Type Culture Collection. The cells were stored under liquid nitrogen before use.
2.3. Animal experiments
All the toxicity studies were approved by the Institutional Animal Care and Use Committee (Approval No.: IACUC250-155/250-183/250-166/250-129) and were performed at the Drug Safety Research Laboratories, Shin Nippon Biomedical Laboratories, Ltd., which has been accredited by AAALAC International, in accordance with the animal welfare bylaws of the company. The cynomolgus monkeys (Macaca fascicularis) used were all purpose-bred for research. Detailed protocols of the animal experiments are described below.
2.4. CDC and ADCC assays
The test samples used were pabinafusp alfa and humanized anti-hTfR monoclonal antibody (mAb), the parent antibody for pabinafusp alfa. For the CDC assay, target cells (CCRF-CEM) were cultured, and 5 × 104 cells (50 μL/well) were added to a 96-well clear-bottom plate. The test sample solutions (25 μL) were added to the appropriate wells. Next, 25 μL of diluted normal human serum complement (Quidel, San Diego, CA) was added to each well at a final human serum concentration of 10%, and the cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 4 h. Cytotoxicity was detected using a CytoTox-Fluor™ Cytotoxicity Assay Kit (Promega, Madison, WI) according to the manufacturer's instructions. Fluorescence (excitation at 485 nm and emission at 520 nm) was measured using a fluorescence microplate reader (Molecular Devices, San Jose, CA).
For ADCC measurement, the ADCC Reporter Bioassay Core kit (Promega) was used according to the manufacturer's instructions. Briefly, the target cells (CCRF-CEM, K562, or HEL92.1.7) were cultured, and 1 × 104 cells (25 μL/well) were added to a 96-well white plate. The test sample solutions (25 μL) were added to the appropriate wells. Next, 25 μL of a separately prepared effector cell suspension (Jurkat cells stably expressing the V158 variant FcγRIIIa receptor and an NFAT response element driving expression of firefly luciferase, provided in the kit) was added to each well and incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 6 h. Cytotoxicity was determined based on the chemiluminescence intensity using the GloMax® Discover System (Promega) according to the manufacturer's instructions.
2.5. Tf-TfR interaction
Tf-TfR interactions in the presence or absence of the test samples were analyzed based on surface plasmon resonance using Biacore T200 (GE Healthcare, Buckinghamshire, UK). The test samples analyzed were pabinafusp alfa, anti-hTfR mAb, and anti-hTfR polyclonal antibody (anti-hTfR pAb; R&D Systems, Minneapolis, MN). The samples were diluted with running buffer (HBS-P+; GE Healthcare) to a final concentration of 100 nmol/L (capture solutions). The extracellular domain of hTfR (a recombinant protein produced in-house using Chinese hamster ovary cells) was bound to each test sample for saturation prior to the addition of human holo-Tf (R&D Systems). The final concentration of hTf was 200 nmol/L. A Series S Sensor Chip NTA (GE Healthcare) was docked on the Biacore T200 and equilibrated with the running buffer, and measurements were made under standard conditions. The relative binding ratio was quantified by calculating the ratio of the Tf-TfR binding response (resonance unit) in the presence of test samples to the Tf-TfR binding response without test samples but with human IgG1 (Sigma-Aldrich, St. Louis, MO).
2.6. Safety evaluation in cynomolgus monkeys
Toxicity studies were conducted under GLP compliance and related International Council for Harmonisation guidelines. Cynomolgus monkeys were selected as the animal species for toxicity studies because pabinafusp alfa recognizes cynomolgus monkey TfR with a similar affinity range as that with hTfR [10].
2.6.1. Four-week repeat dose toxicity studies using sexually mature monkeys
Sexually mature 4- to 8-year-old male and female cynomolgus monkeys were used. Pabinafusp alfa was administered intravenously once a week for 4 weeks (five doses in total on days 0, 7, 14, 21, and 28) to the monkeys, specifically three males and three females from each of the 3 and 10 mg/kg groups and five males and five females from each of the 0 (vehicle control) and 30 mg/kg groups. To determine the reversibility of toxicity, two males and two females from each of the 0 and 30 mg/kg groups were allowed a 4-week recovery period following the last dose. Pabinafusp alfa was administered through the cephalic vein at a rate of 10 mL/kg over 1 h. In the control group, the vehicle (saline) was administered in the same manner. Necropsy was performed after euthanization by exsanguination under anesthesia with intravenous sodium pentobarbital, 2 days after the last dose or after the 4-week recovery period.
To assess general toxicity, clinical signs, body weight, organ weight, food consumption, ophthalmic parameters, and rectal temperature were measured. Further, urinalysis, hematology (blood samples collected during the acclimatization period, on days 22 and 27, and at the end of the recovery period), blood chemistry (plasma samples collected during the acclimatization period, on day 27, and at the end of the recovery period), necropsy, and histopathological analyses were performed. Anti-pabinafusp alfa antibody testing was also performed using plasma samples collected during the acclimatization period, at day 28 pre-dose, and after the 4-week recovery period.
For safety pharmacology, electrocardiography (telemetry), blood pressure measurement, respiratory rate measurement, and arterial blood gas analysis (PaO2, PaCO2, pH, and hemoglobin oxygen saturation) were performed during the acclimatization period, at the second or third dose, and during the recovery period. Effects on the CNS were evaluated using the functional observational battery (FOB) during the acclimatization period, at the fourth dose, and during the recovery period. The FOB included body position, consciousness, behavior, gait, motor power/movement of the hand, motor power/movement of the leg, palpebral closure, CNS excitation, vomiting, lacrimation, salivation, visual response, sound response, muscle tone, touch response, eyelid reflex, pinna reflex, pupillary reflex, and pain response.
2.6.2. Four-week repeat dose toxicity studies using juvenile monkeys
Three- to 5-month-old male cynomolgus monkeys were used. Pabinafusp alfa was administered intravenously once a week for 4 weeks (five doses in total, on days 0, 7, 14, 21, and 28) to the juvenile monkeys, specifically three males from each of the 3 and 10 mg/kg groups and five males from each of the 0 (vehicle control) and 30 mg/kg groups. To determine the reversibility of toxicity, two males from each of the 0 and 30 mg/kg groups were allowed a 4-week recovery period following the last dose. Pabinafusp alfa was administered through the cephalic vein at 10 mL/kg over approximately 10 min. In the control group, the vehicle (saline) was administered in the same manner. Necropsy was performed after euthanization by exsanguination under anesthesia with intravenous sodium pentobarbital, 2 days after the last dose or after the 4-week recovery period.
To assess general toxicity, clinical signs, body weight, organ weight, and ophthalmic parameters were measured. Further, electrocardiography, hematology (blood samples collected during the acclimatization period, on day 27, and at the end of the recovery period), blood chemistry (plasma samples collected during the acclimatization period, on day 27, and at the end of the recovery period), necropsy, and histopathological analyses were performed. Anti-pabinafusp alfa antibody testing was also performed using plasma samples collected on day 0 and 28 pre-dose and after the 4-week recovery period.
2.6.3. Twenty-six-week repeat dose toxicity study
Two- to 4-year-old male and female cynomolgus monkeys were used. Pabinafusp alfa was administered intravenously once a week for 26 weeks (27 doses in total, on days 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, 84, 91, 98, 105, 112, 119, 126, 133, 140, 147, 154, 161, 168, 175, and 182) to the monkeys, specifically four males and four females from each of the 3 and 10 mg/kg groups and six males and six females from each of the 0 and 30 mg/kg groups. To determine the reversibility of toxicity, two males and two females from each of the 0 and 30 mg/kg groups were allowed an 8-week recovery period following the last dose. Pabinafusp alfa was administered through the cephalic vein at a rate of 10 mL/kg over 1 h. In the control group, the vehicle was administered in the same manner. Necropsy was performed after euthanization by exsanguination under anesthesia with intravenous sodium pentobarbital, 2 days after the last dose or after the 8-week recovery period.
To assess general toxicity, clinical signs, body weight, organ weight, food consumption, and ophthalmic parameters were evaluated. Further, electrocardiography, urinalysis, hematology (blood samples collected during the acclimatization period, on days 90 and day 181 and at the end of the recovery period), blood chemistry (plasma samples collected during the acclimatization period, on days 90 and 181 and at the end of the recovery period), necropsy, and histopathological analyses were performed. Anti-pabinafusp alfa antibody testing was performed using the plasma samples collected at pre-dose of the first, 13th, and last dose and at the end of the recovery period. In addition, drug concentrations were measured in the CSF and central nervous tissues (cerebral cortex, cerebellum, hippocampus, medulla oblongata, and spinal cord) collected at necropsy.
To assess safety pharmacology parameters, effects on the CNS were evaluated by the FOB during the acclimatization period, at the 25th dose, and during the recovery period.
2.6.4. Toxicokinetics (TK)
For the above-mentioned 4-week toxicity studies, plasma samples were collected at the indicated time points following the first and last doses. For the study with adult monkeys, three males and three females each from the 3 and 10 mg/kg groups and five males and five females each from the 0 and 30 mg/kg groups were used. For the study with juvenile monkeys, three males each from the 3 and 10 mg/kg groups and five males each from the 0 and 30 mg/kg groups were used. For the 26-week toxicity study, plasma samples were also collected following the first and last doses and week 13 (four males and four females each from the 3 and 10 mg/g groups and six males and six females each from the 0 and 30 mg/kg groups). The plasma concentration of pabinafusp alfa was determined by a sandwich electrochemiluminescence assay with anti-hIDS antibody and anti-hIgG antibody as described previously [10].
2.6.5. Anti-pabinafusp alfa antibody analysis
Anti-pabinafusp alfa antibody was analyzed using an electrochemiluminescent assay. Serially diluted plasma samples were mixed with biotinylated- and ruthenylated-pabinafusp alfa at room temperature, and the mixtures were added to the appropriate wells of a casein-blocked streptavidin-coated plate (Meso Scale Diagnostics, Rockville, MD). After washing with a wash buffer containing 0.05% Tween 20, 2× Read Buffer T (Meso Scale Diagnostics) was added to each well, and the response was measured by SECTOR Imager (Meso Scale Diagnostics). To determine the neutralizing antibody against TfR or mannose-6-phasphate receptor (M6PR), serially diluted plasma samples were mixed with biotinylated pabinafusp alfa and ruthenylated TfR or M6PR at room temperature, and the mixtures were added to the wells of the casein-blocked streptavidin-coated plate. After washing with the wash buffer, 2× Read Buffer T was added to each well, and the response was measured by SECTOR Imager. The data were analyzed using SoftMax Pro (Molecular Devices).
2.7. Biodistribution
The biodistribution of pabinafusp alfa was investigated at the Pharmacokinetics and Bioanalysis Center, Shin Nippon Biomedical Laboratories, Ltd. (Approval No.: 16036). Pabinafusp alfa was labeled with 125I by the iodogen method. Cynomolgus monkeys aged 4 to 6 years (10 animals in total: one male and one female per group for each time of necropsy) were administered a single intravenous dose of 125I-labeled pabinafusp alfa (1 mg/2.5 MBq/kg). Plasma samples were obtained at pre-dose and at 20 min and 1, 3, 8, 24, and 72 h after the initiation of administration, and radioactivity was measured to calculate the pharmacokinetic parameters. For quantitative whole-body autoradioluminography (QWBA), the animals were euthanized with sodium pentobarbital at 2, 8, 24, 48, and 72 h after the initiation of administration for necropsy. Whole-body sections (40 μm thick) at the midline, middle of the kidney, and middle of the eyeball were prepared using a cryomicrotome (Leica CM3600, Leica, Wetzlar, Germany). The sections were freeze-dried at −20 °C. The dried sections were covered with Mylar sheets, kept in close contact with the imaging plate, and exposed for 24 h. The imaging plates were analyzed using a bioimaging analyzer (BAS-2500, GE Healthcare).
2.8. Placental transportability
On day 139 of gestation, pabinafusp alfa at a dose of 10 mg/kg was administered via continuous infusion to 5- to 6-year-old cynomolgus monkeys (3 and 2 animals undergoing cesarean sections at 8 and 24 h, respectively, after the initiation of administration). Plasma concentrations of pabinafusp alfa in the dams and fetuses (umbilical vein and artery) were determined as described for TK measurement. Time points for blood collection from dams were pre-dose and at 20 min and 1, 3, 8, and 24 h after the initiation of administration. Time points for blood collection in the fetuses were 8 and 24 h after the initiation of administration (at the time of cesarean section).
2.9. Statistical analyses
Data on body weight, food consumption, electrocardiography, blood pressure, respiratory rate, body temperature, blood gas analysis, urinalysis, hematology, blood chemistry, and organ weights were analyzed for homogeneity of variance using Bartlett's test. When the variance was homogeneous, Dunnett's test was performed for multiple comparisons between the control group and each test group. When the variance was heterogeneous by Bartlett's test, Miller's test was performed for multiple comparisons between the control group and each test group. For urinalysis, gradable data were analyzed by Wilcoxon's rank sum test, and urine color was analyzed by Fisher's exact test between the control group and each test group. The MiTOX System (Mitsui E&S Systems Research, Chiba, Japan) was used for statistical analyses at a significance level of 5% for Bartlett's test or at a two-sided significance level of 5% for other tests.
3. Results
3.1. Effector function of pabinafusp alfa
Antibody-based therapeutics may activate immunologic effector functions mediated by the fragment crystalizable (Fc) of antibodies. As pabinafusp alfa has an IgG1 moiety, its effector functions were examined by assessing CDC and ADCC activities. For the CDC assay, TfR-expressing hematopoietic cells (CCRF-CEM human acute lymphocytic leukemia cell line) were used as target cells. The humanized anti-hTfR mAb, which was used to produce pabinafusp alfa, induced cytotoxicity in the target cells in a concentration-dependent manner (Fig. 1A). In contrast, pabinafusp alfa exhibited almost no cytotoxicity at any of the concentrations tested (0.0244–100 μg/mL) (Fig. 1A), indicating that CDC activity of the antibody was lost by the fusion of the enzyme to the antibody. The ADCC activity of pabinafusp alfa was examined using TfR-expressing hematopoietic cells (CCRF-CEM, K562 human myelogenous leukemia cell line and HEL92.1.7 human erythroleukemia cell line) as target cells and engineered Jurkat cells stably expressing the FcγRIIIa receptor V158 (high affinity) variant and an NFAT response element driving the expression of firefly luciferase as effector cells. Neither the humanized anti-hTfR antibody nor pabinafusp alfa induced luminescent signals in the target cell systems (Fig. 1B), indicating that no ADCC activity occurred under these conditions. Rituximab, used as a positive control, showed ADCC in this reporter system (Fig. S1), thus validating the assay system. These results suggest that pabinafusp alfa possesses no significant Fc-mediated effector functions.
Fig. 1.
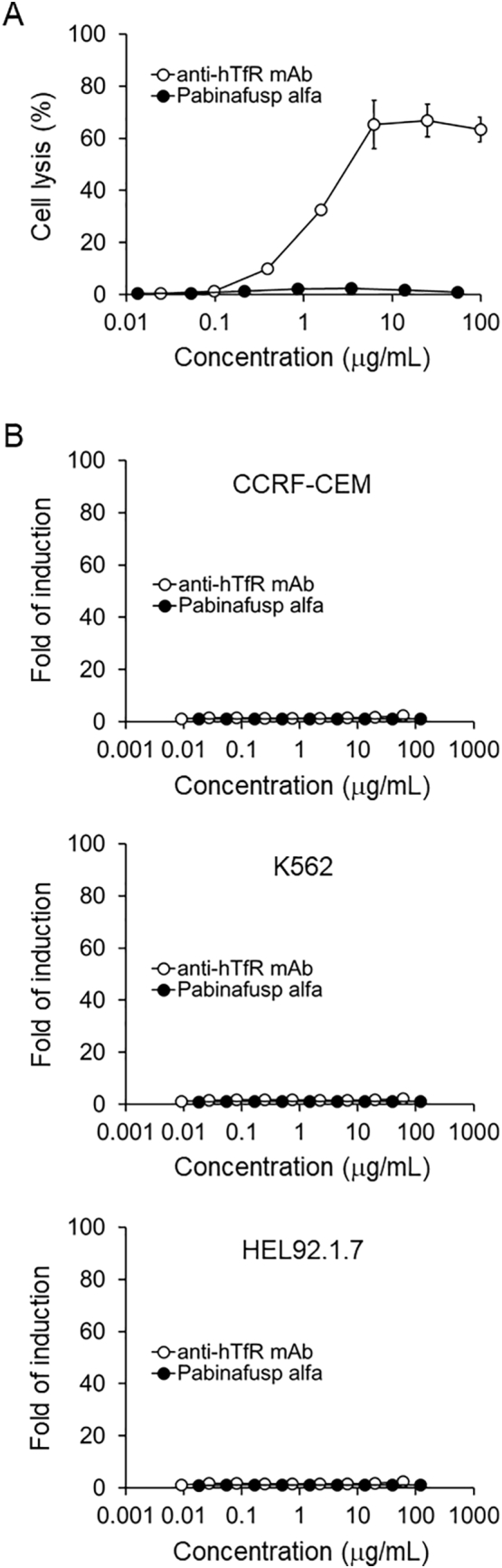
Evaluation of the effector functions of pabinafusp alfa. (A) Complement-dependent cytotoxicity (CDC). The TfR expressing CCRF-CEM human T lymphoblast cell line was used to examine CDC activity. Anti-hTfR mAb is the antibody used to produce pabinafusp alfa. (B) Antibody-dependent cellular cytotoxicity (ADCC). TfR-expressing hematopoietic cells (CCRF-CEM, K562 human myelogenous leukemia cell line and HEL92.1.7 human erythroleukemia cell line) were used as the target cells, and engineered Jurkat cells stably expressing the V158 FcγRIIIa receptor variant were used as the effector cells. Data are means ± S.D. bars (n = 3).
3.2. Effect of pabinafusp alfa on Tf-TfR interaction
TfR is involved in cellular iron transport through its interaction with Tf. We therefore examined whether pabinafusp alfa interferes with Tf-TfR binding. The Tf-TfR interaction was determined in the presence of pabinafusp alfa, anti-hTfR mAb (the parent antibody of the humanized anti-hTfR mAb used in pabinafusp alfa), or anti-hTfR pAb (as a positive control), by surface plasmon resonance. The relative Tf-TfR binding responses measured for the negative control (human IgG1) were not decreased by the addition of pabinafusp alfa or anti-hTfR mAb, whereas anti-hTfR pAb reduced the signal by approximately 60% (Table 1). These results indicate that pabinafusp alfa does not interfere with the Tf-TfR interaction, and thus, it is unlikely that pabinafusp alfa affects cellular iron transport and systemic iron metabolism.
Table 1.
Tf-TfR binding in the presence or absence of test samples.
| Test sample | Resonance units (RU) | % of negative control |
|---|---|---|
| hIgG1 (negative control) | 339.8 ± 1.0 | 100% |
| Pabinafusp alfa | 348.1 ± 4.7 | 102% |
| Anti-hTfR mAb | 362.8 ± 1.5 | 107% |
| Anti-hTfR pAb (positive control) | 133.9 ± 0.5 | 39.4% |
Data are means ± S.D. (n = 3).
3.3. TK and biodistribution of pabinafusp alfa in cynomolgus monkeys
3.3.1. TK
Prior to conducting detailed safety assessments, TK and biodistribution of pabinafusp alfa were evaluated in cynomolgus monkeys. Following administration of the first and last doses during the 4-week repeated intravenous dose toxicity study in sexually mature animals, plasma samples were collected, and drug concentrations were measured. The plasma concentration of pabinafusp alfa over time was slightly lower after the last dose than after the first dose (Fig. S2). The maximum plasma concentration (Cmax) and area under the plasma concentration-time curve from zero to the last measurable point (AUC0-t) also tended to be lower after the last dose (Table 2). The decline in these parameters may be accounted for by the production of anti-drug antibodies (ADA) (Table S1). Pabinafusp alfa was barely detectable just before the last dose in all groups. No marked sex-based differences were noted in the TK properties (Fig. S2 and Table 2). Similar results were obtained from TK measurements in the 4-week repeated intravenous dose toxicity study using juvenile male (3 to 5 months of age) cynomolgus monkeys (Fig. S2, Table 3, and Table S2).
Table 2.
Toxicokinetic parameters in cynomolgus monkeys given repeated intravenous doses of pabinafusp alfa for 4 weeks.
| Parameter | Dose (mg/kg) | Male |
Female |
||
|---|---|---|---|---|---|
| First does | Last dose | First dose | Last dose | ||
| Cmax (μg/mL) | 3 | 48.4 ± 12.9 | 35.0 ± 14.8 | 45.1 ± 10.4 | 33.2 ± 6.75 |
| 10 | 252 ± 66.0 | 161 ± 6.43 | 205 ± 20.6 | 176 ± 34.0 | |
| 30 | 784 ± 119 | 662 ± 104 | 699 ± 61.3 | 555 ± 97.7 | |
| AUC0-t (μg·h/mL) | 3 | 418 ± 137 | 180 ± 108 | 317 ± 97.1 | 125 ± 53.5 |
| 10 | 2320 ± 307 | 994 ± 307 | 1980 ± 332 | 1110 ± 568 | |
| 30 | 9100 ± 2130 | 5860 ± 1980 | 6800 ± 933 | 5160 ± 1440 | |
Data are means ± S.D. (n = 3 for 3 and 10 mg/kg groups, n = 5 for 30 mg/kg groups).
Table 3.
Toxicokinetic parameters in juvenile cynomolgus monkeys given repeated intravenous doses of pabinafusp alfa for 4 weeks.
| Parameter | Dose (mg/kg) | First does | Last dose |
|---|---|---|---|
| Cmax (μg/mL) | 3 | 51.9 ± 1.44 | 34.9 ± 4.11 |
| 10 | 166 ± 35.7 | 158 ± 22.5 | |
| 30 | 518 ± 32.0 | 538 ± 31.1 | |
| AUC0-t (μg·h/mL) | 3 | 212 ± 8.19 | 91.2 ± 24.1 |
| 10 | 851 ± 117 | 728 ± 244 | |
| 30 | 3550 ± 469 | 3280 ± 546 |
Data are means ± S.D. (n = 3 for 3 and 10 mg/kg groups, n = 5 for 30 mg/kg groups).
TK measurements were also conducted in the 26-week repeated intravenous dose toxicity study. Plasma concentrations of pabinafusp alfa were measured following the first, 13th, and last doses. The results showed that plasma concentrations of pabinafusp alfa over time were slightly lower after the 13th and last doses than after the first dose (Fig. S2). The Cmax and AUC0-t tended to decrease after repeated dosing (Table 4). The decline in these parameters may be accounted for by the production of ADA (Table S3). Pabinafusp alfa was barely detectable just before the last dose in all groups.
Table 4.
Toxicokinetic parameters in cynomolgus monkeys given repeated intravenous doses of pabinafusp alfa for 26 weeks.
| Parameter | Dose (mg/kg) | Male |
Female |
||||
|---|---|---|---|---|---|---|---|
| First dose | 13th dose | Last dose | First dose | 13th dose | Last dose | ||
| Cmax (μg/mL) | 3 | 44.5 ± 4.92 | 27.6 ± 13.8 | 20.7 ± 14.0 | 42.2 ± 5.40 | 24.6 ± 9.04 | 9.68 ± 3.78 |
| 10 | 189 ± 24.5 | 104 ± 47.8 | 81.9 ± 47.4 | 169 ± 16.7 | 130 ± 63.8 | 97.1 ± 58.3 | |
| 30 | 540 ± 69.1 | 546 ± 77.7 | 432 ± 44.9 | 574 ± 79.3 | 442 ± 97.2 | 358 ± 71.7 | |
| AUC0-t (μg·h/mL) | 3 | 316 ± 56.3 | 115 ± 89.4 | 113 ± 133 | 256 ± 25.6 | 108 ± 100 | 23.5 ± 13.0 |
| 10 | 1640 ± 350 | 526 ± 420 | 411 ± 454 | 1210 ± 194 | 712 ± 500 | 599 ± 592 | |
| 30 | 5900 ± 824 | 3710 ± 1130 | 3260 ± 985 | 5380 ± 990 | 2620 ± 1130 | 1870 ± 732 | |
Data are means ± S.D. (n = 4 for 3 and 10 mg/kg groups, n = 6 for 30 mg/kg groups).
From these results, we conclude that intravenous pabinafusp alfa showed dose-dependent drug exposure and no accumulation after repeated dosing up to 30 mg/kg/week for up to 26 weeks. Moreover, when the AUC values of the individual animals after the first dose in the toxicity studies were plotted against dose, the drug exposure increased proportionally with dose (Fig. 2), indicating a linear dose-exposure relationship.
Fig. 2.
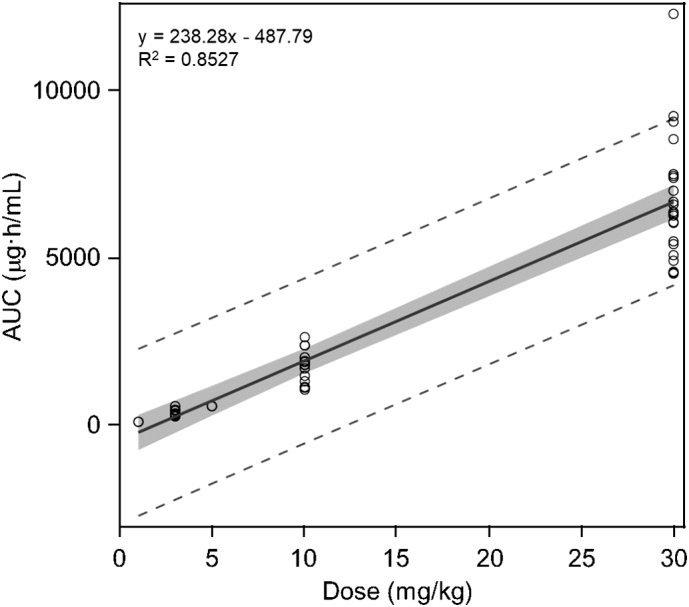
Relationship between dose and drug exposure in cynomolgus monkeys administered pabinafusp alfa intravenously. The AUC values obtained from individual animals after the first dose of the 4- and 26-week studies are plotted against dose. Results from a single-dose study (1 and 5 mg/kg) are also included. The gray area indicates 95% confidence interval (y-intercept: −1036.21 to 60.64). The dashed lines indicate the range of 95% prediction interval. Statistical analyses, including linear regression analysis, were performed using SAS version 9.4 statistical software package (SAS Institute, Cary, NC).
3.3.2. Biodistribution
CNS delivery of pabinafusp alfa across the BBB has been confirmed in cynomolgus monkeys as reported previously [10]. To determine whole-body tissue distribution, 125I-labeled pabinafusp alfa was administered intravenously to cynomolgus monkeys at a single dose (1 mg/kg) by continuous infusion, and radioactivity was measured using QWBA. The plasma radioactivity reached Cmax at the end of the infusion and gradually decreased over time (Fig. S3). The QWBA results revealed radioactivity (pabinafusp alfa) in all the tissues examined (Table S4). There were no apparent sex-based differences in the tissue distribution of radioactivity except in the reproductive organs.
3.4. Placental transportability
As maternal IgG antibodies are known to be transferred across the placenta [18], placental transportability of pabinafusp alfa was evaluated using pregnant cynomolgus monkeys. Following pabinafusp alfa administration at 10 mg/kg via continuous intravenous infusion over 1 h to pregnant monkeys on day 139 of gestation, no changes in the clinical signs and symptoms were observed in the dams. Pabinafusp alfa was not detected in the umbilical cord venous or arterial plasma at 8 or 24 h after the initiation of infusion, indicating little, if any, placental penetration of pabinafusp alfa.
3.5. Safety assessments of pabinafusp alfa in cynomolgus monkeys
3.5.1. Four-week repeat-dose toxicity studies in sexually mature animals
A 4-week repeat-dose toxicity study including safety pharmacology core battery studies was conducted using sexually mature male and female cynomolgus monkeys. Pabinafusp alfa was administered intravenously to the monkeys at 3, 10, or 30 mg/kg once a week. No animals died in any group, and no changes attributable to pabinafusp alfa were observed in terms of clinical signs, body weight, food consumption, ophthalmic parameters, urinalysis parameters, blood pressure, electrocardiographic measurements, respiratory rate, blood gas, rectal temperature, FOB results, necropsy findings, or organ weights. No histopathological changes attributable to pabinafusp alfa were observed in either the peripheral or CNS tissues. Hematological analysis showed a lower red blood cell (RBC) count on day 22 in male animals in the 3 mg/kg treated group, and a lower hematocrit (Ht) on day 27 in male and female animals in the 30 mg/kg treated group than in the control group (Table S5). However, these changes were considered unrelated to the administration of pabinafusp alfa for the following reasons: the changes were mild (within the normal range of background data), unrelated to the dose, and not accompanied by any apparent changes in other erythrocytic parameters (Table S5). No substantial changes in blood chemistry attributable to pabinafusp alfa were observed (Table S6). There were no histopathological changes in the male and female reproductive organs, and the drug did not affect the weight of these organs, suggesting that pabinafusp alfa does not adversely affect male and female fertility. Although most of the animals produced ADA, most of which had neutralizing activity against TfR binding or M6PR binding (Table S1), no pabinafusp alfa-related changes were observed irrespective of ADA production. The no-observed-adverse-effect level (NOAEL) for pabinafusp alfa in this study was estimated to be 30 mg/kg.
3.5.2. Four-week repeat-dose toxicity studies in juvenile animals
A 4-week repeat-dose toxicity study was conducted on juvenile (unweaned male) cynomolgus monkeys. Pabinafusp alfa was administered intravenously to the monkeys at a dosage of 3, 10, or 30 mg/kg once a week. No animals died in any group, and no changes attributable to pabinafusp alfa were observed in terms of clinical signs, body weight, ophthalmic parameters, electrocardiographic measurements, necropsy findings, or organ weights. No histopathological changes attributable to pabinafusp alfa were observed in either the peripheral or CNS tissues. Hematological analysis revealed lower hemoglobin (Hb) levels and mean corpuscular hemoglobin concentrations (MCHC) and higher reticulocyte (Ret) rates in the 30 mg/kg treated group than in the control group (Table S7). These changes were not considered toxicologically significant because the changes were mild and were not accompanied by any apparent changes in other erythrocytic parameters (Table S7). No substantial changes in blood chemistry attributable to pabinafusp alfa were observed (Table S8). Although most of the animals produced ADA, most of which had neutralizing activity against TfR binding or M6PR binding (Table S2), no pabinafusp alfa-related changes were observed irrespective of ADA production. The NOAEL of pabinafusp alfa in juvenile cynomolgus monkeys was estimated to be 30 mg/kg.
3.5.3. Twenty-six-week repeat-dose toxicity studies
A 26-week repeat-dose toxicity study including safety pharmacology core battery studies was conducted using male and female cynomolgus monkeys. Pabinafusp alfa was administered intravenously to the monkeys at a dose of 3, 10, or 30 mg/kg once a week. No animals died in any group, and no changes attributable to pabinafusp alfa were observed in terms of clinical signs, body weight, food consumption, ophthalmic parameters, urinalysis parameters, blood pressure, electrocardiographic measurements, respiratory rate, blood gas, FOB results, necropsy findings, or organ weights. No histopathological changes attributable to pabinafusp alfa were observed in either the peripheral or CNS tissues. In the 3 mg/kg treated group, higher RBC, Hb, and Ht levels were observed in males at week 26 compared with those in the control group. In the 10 mg/kg treated group, higher RBC and Ht were observed in males at week 13 than in the control group. In the 30 mg/kg treated group, higher RBC in males at week 26, lower Hb and Ht in females at week 13, and lower mean corpuscular hemoglobin (MCH) and MCHC in males at weeks 13 and 26 were observed (Table S9). However, the changes were mild, with the direction of change (increase or decrease) being inconsistent across the parameters (Table S9), indicating no relationship with pabinafusp alfa dose. No substantial changes in blood chemistry attributable to pabinafusp alfa were observed (Table S10). Although the effects of pabinafusp alfa on the male reproductive organs were not determined because the male animals were still immature at the end of the study, pabinafusp alfa did not affect the reproductive tract of sexually mature females. Although most of the animals produced ADA, most of which had neutralizing activity against TfR binding or M6PR binding (Table S3), no pabinafusp alfa-related changes were observed irrespective of ADA production. The NOAEL of pabinafusp alfa in this study was estimated to be 30 mg/kg. In addition, pabinafusp alfa did not affect CNS function after long-term use up to 30 mg/kg, as assessed by the FOB in the safety pharmacology study.
4. Discussion
Based on the results of a comprehensive nonclinical safety assessment, pabinafusp alfa, a BBB-penetrating fusion protein for intravenous ERT of neuronopathic MPS II, was found to have low toxicity and few adverse effects. In particular, Fc-mediated effector function assessed by CDC and ADCC was almost eliminated, and consistent with this finding, anemia with decreased blood reticulocyte number, which can be caused by suppression of erythroid precursor cells (rich in TfR expression), was not observed in cynomolgus monkeys after chronic treatment with pabinafusp alfa.
CDC, an effector function of IgG (and IgM) antibodies, is a cytolytic cascade triggered by binding of the complement C1q to the constant region of cell-bound antibodies followed by activation of a series of complement proteins [19,20]. Our results showed that, while the anti-hTfR mAb used in pabinafusp alfa elicited CDC, the fusion protein did not. Thus, the fusion of the enzyme molecule to the mAb possibly interferes with the access of complement proteins to the antibody moiety by steric hindrance, resulting in the loss of CDC activity of pabinafusp alfa.
ADCC, another effector function of IgG antibodies, is the process of killing opsonized cells (cells coated with antibodies) by effector cells of the immune system, such as natural killer cells [19,20]. Thus, ADCC requires an interaction between the antibody CDR domains and the antigens on target cells as well as binding of the antibody Fc domains to Fcγ receptors on effector cells. Our results showed that neither the humanized anti-hTfR mAb nor pabinafusp alfa exerted ADCC under the conditions used in this study. Because the carbohydrate structure of the Fc region is known to be critical for the binding of antibodies to the Fcγ receptors [19,20] and the structure of the anti-TfR mAb moiety in pabinafusp alfa is similar to that of natural IgG1, the binding ability to Fcγ receptors may be retained at least to some degree. In this case, it is possible that the unique binding angle of the mAb to TfR makes it difficult for Fcγ receptors on the effector cells to access the Fc domain of the antibody bound to the target cells, which is an essential step for bridge formation in these cells to initiate ADCC.
The TfR-binding property of pabinafusp alfa raises concerns about its influence on the Tf-TfR interaction, which may lead to abnormal iron metabolism. In this regard, our studies show that pabinafusp alfa does not interfere with the binding of Tf to TfR, probably because the TfR epitope recognized by the anti-TfR antibody used in pabinafusp alfa is distinct from the Tf binding site. More importantly, in vivo toxicity studies in cynomolgus monkeys showed no abnormalities in iron-related parameters such as serum iron, unsaturated iron binding capacity (UIBC), ferritin, haptoglobin, and total iron binding capacity (TIBC) (Tables S5-S7). Therefore, pabinafusp alfa has minimal potential to produce toxicity related to perturbation of iron metabolism.
A recent study reported that chronic treatment of rhesus monkeys with an mAb against TfR (up to 30 mg/kg, for 4 weeks, twice weekly) caused anemia associated with lower blood reticulocyte number and glial activation in the brain [21], which were not observed in our study. The reason for this discrepancy is considered to be based on the different nature of the mAbs, including the binding property for TfR and the target epitope. In fact, a variety of mAbs against TfR with different properties have been reported so far; some mAbs block the growth of cells expressing high levels of TfR, and others do not [22]. Their effect on Tf-TfR binding varies among different mAbs [22,23]. Therefore, the toxicity of anti-TfR mAbs cannot be generalized and should be determined individually for each mAb.
Pabinafusp alfa has been shown to be effective in a mouse model of MPS II at a dose of 2 mg/kg/week in terms of substrate reduction and neurobehavioral improvement [11]. This regimen has also been shown to be effective in humans in clinical studies [13,14]. Considering that the NOAEL in our toxicity studies was 30 mg/kg, the safety margin seems to be sufficient for the treatment of MPS II. Although these preclinical results suggest that pabinafusp alfa is unlikely to cause serious adverse effects in patients with MPS II, the drug safety profile in humans can only be established via clinical trials. To this end, a 52-week phase 2/3 clinical study in Japanese patients was conducted and showed that drug-related adverse events were mild or moderate in severity, transient, and manageable, and most of them were infusion-associated reactions commonly observed in ERT [13,14], indicating that pabinafusp alfa is suitable for the long-term treatment of individuals with MPS II. Nevertheless, as patients with MPS II depend on life-long ERT, the safety profile of pabinafusp alfa should be investigated cautiously in clinical practice.
Funding
This study was sponsored by JCR Pharmaceuticals.
Declaration of Competing Interest
R.Y., E.Y., N.T., M.K., A.I., T.H., and K.M. received compensation as employees of JCR Pharmaceuticals.
Acknowledgements
We thank Mitsuaki Masuyama, Hiroyuki Yamashita, Hitomi Goto, and Taishi Tateishi of Shin Nippon Biomedical Laboratories (Kagoshima, Japan) for the monkey studies, and Hideaki Hirai of JCR Pharmaceuticals for the statistical analysis with SAS software. Special thanks to Mathis Schmidt of JCR Pharmaceuticals for his immense editorial help. We also would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2021.100758.
Contributor Information
Tohru Hirato, Email: hirato-t@jcrpharm.co.jp.
Kohtaro Minami, Email: minami-k@jcrpharm.co.jp.
Appendix A. Supplementary data
Supplementary material
References
- 1.Wilson P.J., Morrism C.P., Anson D.S., Occhiodoro T., Bielicki J., Clements P.R., Hopwood J.J. Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc. Natl. Acad. Sci. U. S. A. 1990;87(21):8531–8535. doi: 10.1073/pnas.87.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tylki-Szymańska A. Mucopolysaccharidosis type II, Hunter’s syndrome. Pediatr. Endocrinol. Rev. 2014;12(Suppl. 1):107–113. [PubMed] [Google Scholar]
- 3.Neufeld E.F., Muenzer J. The Mucopolysaccharidoses. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic & Molecular Bases of Inherited Disease. McGraw Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 4.Al Sawaf S., Mayatepek E., Hoffmann B. Neurological findings in Hunter disease: pathology and possible therapeutic effects reviewed. J. Inherit. Metab. Dis. 2008;31(4):473–480. doi: 10.1007/s10545-008-0878-x|. [DOI] [PubMed] [Google Scholar]
- 5.Muenzer J., Beck M., Giugliani R., Suzuki Y., Tylki-Szymanska A., Valayannopoulos V., Vellodi A., Wraith J.E. Idursulfase treatment of Hunter syndrome in children younger than 6 years: results from the Hunter Outcome Survey. Genet. Med. 2011;13(2):102–109. doi: 10.1097/GIM.0b013e318206786f. [DOI] [PubMed] [Google Scholar]
- 6.Muenzer J., Beck M., Eng C.M., Giugliani R., Harmatz P., Martin R., Ramaswami U., Vellodi A., Wraith J.E., Cleary M., Gucsavas-Calikoglu M., Puga A.C., Shinawi M., Ulbrich B., Vijayaraghavan S., Wendt S., Conway A.M., Rossi A., Whiteman D.A., Kimura A. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet. Med. 2011;13(2):95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 7.Okuyama T., Tanaka A., Suzuki Y., Ida H., Tanaka T., Cox G.F., Eto Y., Orii T. Japan Elaprase Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II) Mol. Genet. Metab. 2010;99(1):18–25. doi: 10.1016/j.ymgme.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Giugliani R., Hwu W.L., Tylki-Szymanska L., Whiteman D.A., Pano A. A multicenter, open-label study evaluating safety and clinical outcomes in children (1.4–7.5 years) with Hunter syndrome receiving idursulfase enzyme replacement therapy. Genet. Med. 2014;16(6):435–441. doi: 10.1038/gim.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn Y.B., Cho S.Y., Park S.W., Kim S.J., Ko A.R., Kwon E.K., Han S.J., Jin D.K. Phase I/II clinical trial of enzyme replacement therapy with idursulfase beta in patients with mucopolysaccharidosis II (Hunter syndrome) Orphanet J. Rare Dis. 2013;8:42. doi: 10.1186/1750-1172-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonoda H., Morimoto H., Yoden E., Koshimura Y., Kinoshita M., Golovina G., Takagi H., Yamamoto R., Minami K., Mizoguchi A., Tachibana K., Hirato T., Takahashi K. A blood-brain-barrier-penetrating anti-human transferrin receptor antibody fusion protein for neuronopathic Mucopolysaccharidosis II. Mol. Ther. 2018;26(5):1366–1374. doi: 10.1016/j.ymthe.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morimoto H., Kida S., Yoden E., Kinoshita M., Tanaka N., Yamamoto R., Koshimura Y., Takagi H., Takahashi K., Hirato T., Minami K., Sonoda H. Clearance of heparan sulfate in the brain prevents neurodegeneration and neurocognitive impairment in MPS II mice. Mol. Ther. 2021 doi: 10.1016/j.ymthe.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuyama T., Eto Y., Sakai N., Minami K., Yamamoto T., Sonoda H., Yamaoka M., Tachibana K., Hirato T., Sato Y. Iduronate-2-sulfatase with anti-human transferrin receptor antibody for neuropathic Mucopolysaccharidosis II: a phase 1/2 trial. Mol. Ther. 2019;27(2):456–464. doi: 10.1016/j.ymthe.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuyama T., Eto Y., Sakai N., Nakamura K., Yamamoto T., Yamaoka M., Ikeda T., So S., Tanizawa K., Sonoda H., Sato Y. A phase 2/3 trial of pabinafusp alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol. Ther. 2021;29(2):671–679. doi: 10.1016/j.ymthe.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giugliani R., Martins A.M., So S., Yamamoto T., Yamaoka M., Ikeda T., Tanizawa K., Sonoda H., Schmidt M., Sato Y. Iduronate-2-sulfatase fused with anti-human transferrin receptor antibody, pabinafusp alfa, for treatment of neuronopathic and non-neuronopathic mucopolysaccharidosis II: report of a phase 2 trial in Brazil. Mol. Ther. 2021 doi: 10.1016/j.ymthe.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesik-Brodacka M. Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 2018;65(3):306–322. doi: 10.1002/bab.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luria-Pérez R., Helguera G., Rodríguez J.A. Antibody-mediated targeting of the transferrin receptor in cancer cells. Bol. Med. Hosp. Infant. Mex. 2016;73(6):372–379. doi: 10.1016/j.bmhimx.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Leoh L.S., Daniels-Wells T.R., Martínez-Maza O., Penichet M.L. Insights into the effector functions of human IgG3 in the context of an antibody targeting transferrin receptor 1. Mol. Immunol. 2015;67(2 Pt B):407–415. doi: 10.1016/j.molimm.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeSesso J.M., Williams A.L., Ahuja A., Bowman C.J., Hurtt M.E. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit. Rev. Toxicol. 2012;42(3):185–210. doi: 10.3109/10408444.2011.653487. [DOI] [PubMed] [Google Scholar]
- 19.Liu R., Oldham R.J., Teal E., Beers S.A., Cragg M.S. Fc-engineering for modulated effector functions-improving antibodies for cancer treatment. Antibodies (Basel) 2020;9(4):64. doi: 10.3390/antib9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Horst H.J., Nijhof I.S., Mutis T., Chamuleau M.E.D. Fc-engineered antibodies with enhanced Fc-effector function for the treatment of B-cell malignancies. Cancers (Basel) 2020;12(10):3041. doi: 10.3390/cancers12103041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardridge W.M., Boado R.J., Patrick D.J., Ka-Wai Hui E., Lu J.Z. Blood-brain barrier transport, plasma pharmacokinetics, and neuropathology following chronic treatment of the rhesus monkey with a brain penetrating humanized monoclonal antibody against the human transferrin receptor. Mol. Pharm. 2018;15(11):5207–5216. doi: 10.1021/acs.molpharmaceut.8b00730. [DOI] [PubMed] [Google Scholar]
- 22.White S., Taetle R., Seligman P.A., Rutherford M., Trowbridge I.S. Combinations of anti-transferrin receptor monoclonal antibodies inhibit human tumor cell growth in vitro and in vivo: evidence for synergistic antiproliferative effects. Cancer Res. 1990;50(19):6295–6301. [PubMed] [Google Scholar]
- 23.Trowbridge I.S. Transferrin receptor as a potential therapeutic target. Prog. Allergy. 1988;45:121–146. doi: 10.1159/000416377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


