Abstract
Background
Atopic dermatitis (AD) has been clarified that imbalance of bacterial and fungal communities in the skin and gut play key roles in immunologic dysfunction. Atopic keratoconjunctivitis (AKC), one of severe ophthalmic manifestation of AD, could be related with dysbiosis as same as AD.
Objective
In this case-control study, the roles of conjunctival microbial communities in AKC were evaluated by a comparative analysis with healthy controls (HCs).
Methods
16S rRNA sequencing was used to construct libraries of compositional information for a total of 30 volunteers including 20 patients with AKC and 10 HCs.
Results
In the results, variation in the conjunctival taxonomic composition was higher in patients with AKC than in the HC group. In an analysis of relative abundance at the genus level, some taxa significantly differed between groups, including Ralstonia, Staphylococcus, Pseudomonas, Proteus, Haemophilus, and Bifidobacterium (p<0.05). Beta diversity was significantly higher in patients with AKC than in HCs (PERMANOVA, p=0.004).
Conclusion
The results indicated that the diversity and composition of the microbiome differs between patients with AKC and HCs.
Keywords: Atopic keratoconjunctivitis, Atopy, Dysbiosis, Microbiota
INTRODUCTION
Atopic keratoconjunctivitis (AKC) is a chronic allergic disease involving the ocular surface in patients with a history of atopic dermatitis (AD). AKC typically begins in the late adolescence and early adulthood and the peak incidence period of AKC is between 30 and 50 years old. Patients usually complain of symptoms such as pain, redness of conjunctiva, and blurred vision, and have chronic symptoms rather than seasonal symptoms1. As a subtype of allergic conjunctivitis, proper treatment of AKC is important to prevent severe damage to the ocular area. AKC shares some immunological properties with AD, including type IV hypersensitivity reactions and the involvement of type 1 hypersensitivity. Additionally, patients with AKC exhibit dysfunctional cell-mediated immunity and sensitivity against specific allergens2,3. Alterations in conjunctival epithelial layer thickness and numbers of mast cells, eosinophils, and other T cells suggest that AKC is related to mucosal barrier dysfunction and local immune processes4.
Previous studies of the bacterial microbiome have suggested that a floral imbalance or alterations in composition may lead to some diseases. In addition to genetic factors, the dysbiosis of the skin and intestinal mucosa plays a pivotal role in AD5,6,7,8. Our previous studies have also proved that the composition of the fungal community, including the abundance of Malassezia, is altered in the skin of patients with AD9.
The impact of the microbiome on ocular disorders has been established in some studies. For example, conjunctival microbial changes induced by lens use leads to conjunctivitis10. Infectious diseases, such as trachomatous disease and pseudomonas keratitis, are related to alterations in the conjunctival microbiome11,12. Additionally, immunological effects of dysbiosis in allergic eye diseases have been established13,14. Based on results of relation between microbiome and certain diseases, we performed a comparative analysis of taxonomic compositions using 16S rRNA gene sequences to clarify the status of the mucosal flora and confirm that alterations in environmental conditions induced by dysbiosis are related to AKC.
MATERIALS AND METHODS
Subjects and sample collection
A total of 64 volunteers were recruited at Konkuk University Medical Center between March and December 2018, including 27 patients with AKC and 37 healthy controls (HCs). The inclusion criteria were as follows: (1) patients were diagnosed with AKC by an ophthalmologist at Konkuk University Medical Center; (2) no history of concomitant systemic (within 4 weeks of enrollment) or topical (within 2 weeks of enrollment) treatments that could affect the microbiome results, particularly antimicrobial and antifungal agents, anti-inflammatory drugs, and immunomodulators, including steroids; (3) no systemic immune-associated diseases. Institutional review board (IRB) approval was obtained from the Konkuk University Medical Center, Seoul (KUH1120101). The study was performed in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from each individual.
Conjunctival sample collection
Skin swab samples were obtained from all individuals according to a protocol approved by the IRB. After anesthesia with a proparacaine hydrochloride ophthalmic solution, two sterilized cotton swabs, one to left and the other to right, were used to wipe gently more ten times at the lower bulbar conjunctiva and fornices. The swab heads were then separated and stored in 500 µl of sterile swab solution (100 mM Tris-HCl [pH 8.0], 30 mM EDTA [pH 8.0], and 0.5% sodium dodecyl sulfate) in conical tubes at -70℃ until DNA extraction. DNAs were isolated and processed according to previously described protocols15,16.
Polymerase chain reaction amplification and sequencing
Universal polymerase chain reaction (PCR) primers targeting the V3 to V4 regions of the 16S rRNA gene were used. Secondary amplification was performed to attach the Illumina NexTera barcode to the primary PCR products using i5 and i7 primers under identical primary PCR conditions, but only 8 amplification cycle sets. The products were purified using a QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) after confirmation by 2% agarose gel electrophoresis and visualized under a Gel Doc system (BioRad, Hercules, CA, USA). Short fragments (<200 bp, non-target products, which can influence sequencing results) were removed using an AMPure Bead Kit (Agencourt Bioscience, Beverly, MA, USA). Product size and quality were checked using a Bioanalyzer 2100 (Agilent, Palo Alto, CA, USA) and a DNA 7500 chip. Finally, an amplicon library was prepared and sequenced at ChunLab (Seoul, Korea). The Illumina MiSeq Sequencing System (Illumina, San Diego, CA, USA) was used according to the manufacturer's instructions.
Raw reads were processed for quality control (QC) and low-quality reads (mean quality value, Q<25) were removed using Trimmomatic 0.321. After passing QC, PandaSeq217 was used to merge paired-end sequences. Primers for PCR that were not "sequenced" were trimmed using a ChunLab in-house program, applying a similarity cut-off of 0.8. Then, de-noising was performed using DUDE-Seq18 and identical sequences were de-replicated.
PCR protocols and the primers for the secondary amplification of fungi were the same as those used for bacteria, except for the primers targeting the internal transcribed spacer 2 (ITS2) regions: UTS3-Mi and ITS4-Mi.
Sequence data processing and analysis
Taxonomic assignments were obtained using USEARCH, which calculates sequence similarity against reads in the EzBioCloud database (https://www.ezbiocloud.net)19. A cut-off value of 97% was used for species-level profiling and other previously established cut-offs were applied for higher taxonomic rankings20. Using the UCLUST8 tool with a sequence matching the EzBioCloud database and a 97% cutoff, the remaining clustered sequences were aggregated into the final set of operational taxonomic units (OTUs).
Secondary analyses were performed to estimate taxonomic compositions, alpha- and beta-diversity, and relative abundance using the web-based analysis program BIOiPLUG provided by ChunLab.
RESULTS
After QC for the microbiome taxonomic profiling analysis, sequence data for 50 samples were included in subsequent analyses. Finally, 30 samples (20 for the AKC group and 10 for the HC group) were used for bacterial library construction. Taxonomic profiling of the fungal community was not performed because the fungal library for AKC samples was not successfully constructed (Supplementary Fig. 1).
Average taxonomic compositions
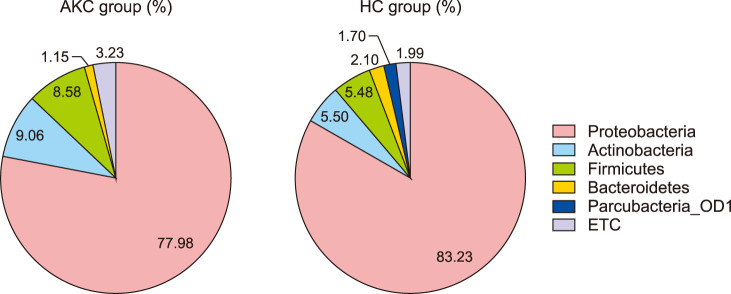
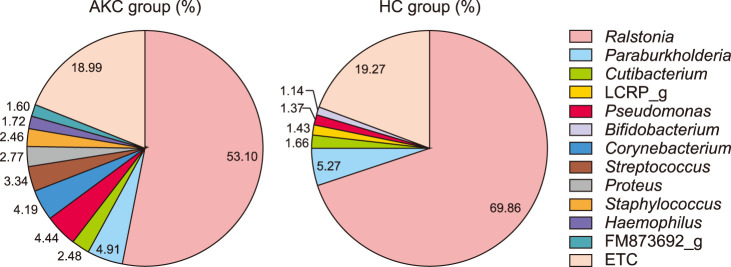
We compared the compositions of the ocular surface microbiota between the two groups. Three bacterial phyla, Proteobacteria, Actinobacteria, and Firmicutes, accounted for more than 95% of the AKC set and 94% of the HC set. Other phyla, including Bacteroidetes and Parcubacteria_OD1, were detected. Despite a similar composition with respect to rank order at the phyla level, Parcubacteria_OD1 was detected only in the HC group (Fig. 1). Ralstonia accounted for the largest portion (53.10% vs. 68.86%), followed by Paraburkholderia (4.91% vs. 5.27%), Pseudomonas (4.44% vs. 1.37%), and Cutibacterium (2.48% vs. 1.66%) in compositional rank of genus. At the genus level, there were relatively large differences between the two groups. Corynebacterium (4.19%), Streptococcus (3.34%), Proteus (2.77%), Staphylococcus (2.46%), and Haemophilus (1.72%) were only detected in the AKC group, while LCRP_g (1.43%), and Bifidobacterium (1.14%) were found in the HC group (Fig. 2).
Fig. 1. Proposition of average composition in phylum presents major component of microbial community in both groups. They showed almost same propositional rank suggesting that Proteobacteria, Actinobacteria, and Firmicutes were accounted over 95% of composition. AKC: atopic keratoconjunctivitis, HC: healthy control.
Fig. 2. Proposition of average composition in genus showed certain difference between two groups. Atopic AKC group were accounted more various than HC group and significant difference in proposition. AKC: atopic keratoconjunctivitis, HC: healthy control.
Comparative diversity analysis
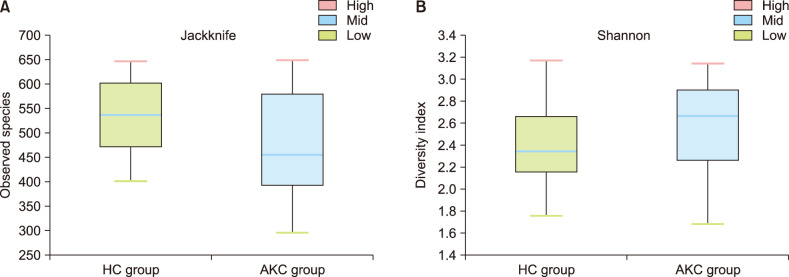
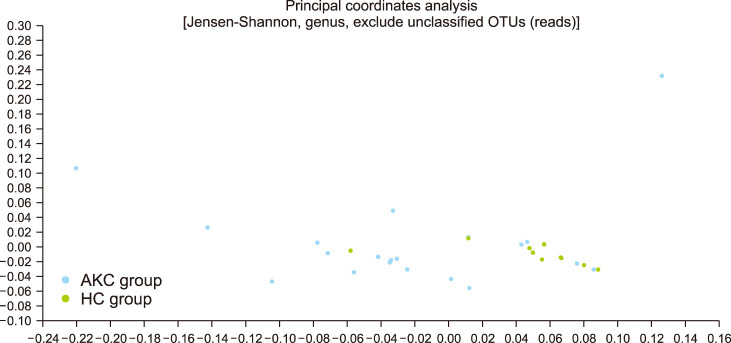
Alpha-diversity was evaluated by the Wilcoxon rank-sum test. Jackknife analyses was performed to evaluate species richness (Fig. 3A) and Shannon's index was used to evaluate diversity indices (Fig. 3B). There were no significant differences between two groups (Jackknife, p=0.253; Shannon, p=0.173). Beta diversity differed significantly between the two groups. A principal coordinates analysis showed that taxa could generally be classified into two distinct groups (Fig. 4; PERMANOVA, p=0.004).
Fig. 3. Alpha diversity including species richness (A) and diversity indices (B) were performed to analyze within sample diversity. There was no significant difference between two groups calculated by the Wilcoxon rank-sum test. HC: healthy control, AKC: atopic keratoconjunctivitis.
Fig. 4. Principle coordinates analysis for beta diversity presenting significant difference. It suggested that the distribution and abundances of two groups were different. OUT: operational taxonomic units, AKC: atopic keratoconjunctivitis, HC: healthy control.
Relative abundance analysis
We analyzed the relative abundance of each taxon. Parcubacteria_OD1 showed a significant difference between groups (p=0.001) but others did not differ between groups (Table 1). In an analysis of relative abundance at the genus level, some taxa differed between groups, including Ralstonia (p=0.002), Staphylococcus (p=0.039), Pseudomonas (p=0.008), Proteus (p=0.041), Haemophilus (p=0.049), LCRP_g (p=0.002), and Bifidobacterium (p=0.014) (Table 2). As shown in a heatmap, the composition of genera showed greater variation in the AKC group than in the HC group (Supplementary Fig. 2).
Table 1. Relative abundance of phylum composition.
| Phylum | Relative abundance, median (Q1, Q3) | p-value | |
|---|---|---|---|
| AKC group (%) | HC group (%) | ||
| Proteobacteria | 81.84 (71.89, 88.75) | 83.9 (79.38, 86.66) | 0.379 |
| Actinobacteria | 6.64 (3.16, 10.23) | 4.18 (2.50, 7.56) | 0.253 |
| Firmicutes | 5.58 (3.85, 9.37) | 5.42 (2.40, 6.55) | 0.481 |
| Bacteroidetes | 1.06 (0.61, 1.62) | 1.61 (0.74, 2.86) | 0.078 |
| Parcubacteria_OD1 | 0.60 (0.35, 1.07) | 1.80 (1.68, 1.98) | 0.001* |
AKC: atopic conjunctivitis, HC: healthy control. *Statistically significance (p<0.05).
Table 2. Relative abundance of genus composition.
| Phylum | Relative abundance, median (Q1, Q3) | p-value | |
|---|---|---|---|
| AKC group (%) | HC group (%) | ||
| Ralstonia | 56.04 (43.34, 61.08) | 70.23 (66.01, 73.9) | 0.002* |
| Paraburkholderia | 4.51 (4.16, 5.80) | 5.11 (4.75, 5.41) | 0.356 |
| Cutibacterium | 2.21 (1.24, 3.32) | 1.26 (0.72, 2.24) | 0.235 |
| LCRP_g | 0.00 (0.00, 0.94) | 1.5 (1.37, 1.76) | 0.002* |
| Pseudomonas | 4.63 (1.33, 6.9) | 1.11 (0.46, 1.66) | 0.008* |
| Bifidobacterium | 0.03 (0.00, 0.14) | 0.19 (0.06, 0.25) | 0.014* |
| Corynebacterium | 0.71 (0.29, 2.49) | 0.40 (0.15, 1.44) | 0.253 |
| Streptococcus | 0.89 (0.24, 2.03) | 0.62 (0.46, 1.39) | 0.725 |
| Proteus | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.09) | 0.041* |
| Staphylococcus | 1.26 (0.39, 2.88) | 0.39 (0.18, 0.66) | 0.039* |
| Haemophilus | 0.35 (0.08, 0.96) | 0.08 (0.00, 0.16) | 0.049* |
| FM873692_g | 0.05 (0.00, 0.33) | 0.03 (0.00, 0.56) | 0.792 |
AKC: atopic conjunctivitis, HC: healthy control. *Statistically significance (p<0.05).
DISCUSSION
Defining the normal microbiome of conjunctival bacterial communities is important for understanding its role in the maintenance of immunologic homeostasis and to identify causative pathogens leading to infectious eye diseases. Furthermore, some diseases of the eye, such as conjunctivitis, are associated with an imbalance of ocular surface homeostasis21.
Our aim was to identify the microbiome and mycobiome in the conjunctival mucosa; however, all AKC samples that passed QC failed to produce a fungal library and therefore the mycobiome could not be characterized. Compared to the skin surface, the detection of fungi on the ocular surface is more difficult by culture-based and PCR-based methods22. We used a PCR-based approach based on previous reports indicating that it is superior to cultivation methods, but we were unable to generate a fungal library for the ocular surface. It is possible that the proparacaine hydrochloride ophthalmic solution, which is a topical anesthetic agent, and other ingredients could explain the lack of results in this study. Furthermore, we used conventional cotton swabs with saline, while a previous study of the fungal community on the human ocular surface22 used a commercially available Isohelix swab for higher yields.
The composition of bacterial phyla and genera on the ocular surface differed between patients with AKC and HCs. At the phylum level, the results of our study were highly similar to those of previous studies. Proteobacteria accounted for the largest portion of sequencing reads, followed by Actinobacteria and Firmicutes; these are considered the three most abundant bacterial taxa on the ocular surface23,24,25. Parcubacteria_OD1 was detected in the HC group but has previously been identified in a broad range of anoxic environments. Although none of these species have been isolated in the laboratory, several genome sequences have been reconstructed from metagenomics data26. Accordingly, the role of the phylum is unclear.
At the genus level, there were various differences between the two groups. We analyzed compositional ranks and relative abundance. The dominant genera were analyzed by proportional rank, which showed high variation in the bacterial composition in patients with AKC. We detected Ralstonia, which belongs to Proteobacteria, previously included in the genus Pseudomonas, consistent with previous studies12,25,27. Corynebacterium (4.19%), Streptococcus (3.34%), Proteus (2.77%), Staphylococcus (2.46%), and Haemophilus (1.72%) accounted for over 1% of the composition but were not detected in the HC group. Bifidobacterium was also observed only in the HC group. We did not consider LCRP_g as a meaningful result for the same reason applied to Parcubacteria_OD1.
We also analyzed relative abundance for compositional genera. Significant difference between two groups including Ralstonia, Pseudomonas, Staphylococcus, Haemophilus, Bifidobacterium, and Proteus were detected. It is unclear yet whether the over-representation of various genera, including Pseudomonas, Staphylococcus, and Haemophilus, and the under-expression of Bifidobacterium are related to AKC. In previous studies, some genera have been reported as causative factors in ocular diseases. For example, increases in the diversity of coagulase-negative Staphylococci species have been observed in non-autoimmune dry eye disease14. Alterations of ocular surface microbial compositions with higher abundances of Pseudomonas have been reported in patients with keratitis28. Other finding was obtained showing that Staphylococcus and Haemophilus are more abundant in contact lens wearers than in controls, which suggested that alterations of the ocular bacterial composition plays a pivotal role in the maintenance of the homeostasis of the ocular environment10,14,29.
Allergic conjunctivitis has an underlying allergic mechanism, and each ocular surface condition, varying in severity, involves different cellular responses. Using models of experimental allergic conjunctivitis, crosstalk between conjunctival epithelial cells related to conjunctival immunity, including mast cells and T cells, seems to be a significant factor. Allergic eye disease leads to changes mucosal immunity at the conjunctival surface. In seasonal allergic conjunctivitis the epithelial layer appears normal, whereas in AKC, the epithelial layer tends to be thicker, reflecting the ongoing inflammatory response13. Based on this theory, dysbiosis leads to an altered host immune response followed by impaired barrier dysfunction associated with changes in microbial diversity. The alteration would result in a vicious cycle causing immunological stimulation.
Owing to the relatively low abundance and diversity of the ocular microbiota, it is tempting to infer that the microbial community has little impact on ocular immunity and health. However, our PCR-based analysis of the microbial community supported the stimulatory role of the bacterial community on local immune responses of the eye. Relationships between the ocular microbiota and immunological environment have been reported.
Our study has some limitations. There is lack of agreement regarding the causative pathogen in allergic conjunctivitis as well as a lack of consensus regarding the typical bacterial community on the ocular surface may leading to misinterpretation. Furthermore, technical errors, such as contamination and low sample quantities, can lead to changes in the composition of the bacterial community. Additionally, we did not obtain results for the fungal community on the ocular surface; our follow-up studies should reconsider the method to evaluate the composition of fungal taxa.
To our knowledge, this is the first study to investigate the conjunctival microbial communities of AKC using PCR-based gene sequencing metagenomics analysis. The alteration of the average composition, relative abundance, and diversity of the conjunctival microbiome in patients with AKC provides new clues to the etiology of AKC.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: The work was supported by Konkuk University Medical Center Research Grant 2018.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-33-163-s001.pdf.
Flow chart of the study process.
Heatmap of composition in genus level supported the result which suggested that composition of atopic keratoconjunctivitis (AKC) patients were more various than healthy control (HC) group.
References
- 1.Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323–336. doi: 10.1016/j.iac.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Bonini S. Atopic keratoconjunctivitis. Allergy. 2004;59 Suppl 78:71–73. doi: 10.1111/j.1398-9995.2004.00570.x. [DOI] [PubMed] [Google Scholar]
- 3.Braude LS, Chandler JW. Atopic corneal disease. Int Ophthalmol Clin. 1984;24:145–156. [PubMed] [Google Scholar]
- 4.Leonardi A, De Dominicis C, Motterle L. Immunopathogenesis of ocular allergy: a schematic approach to different clinical entities. Curr Opin Allergy Clin Immunol. 2007;7:429–435. doi: 10.1097/ACI.0b013e3282ef8674. [DOI] [PubMed] [Google Scholar]
- 5.Isolauri E. Intestinal involvement in atopic disease. J R Soc Med. 1997;90 Suppl 30:15–20. doi: 10.1177/0141076897090030s04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park KD, Pak SC, Park KK. The pathogenetic effect of natural and bacterial toxins on atopic dermatitis. Toxins (Basel) 2016;9:3. doi: 10.3390/toxins9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pike MG, Heddle RJ, Boulton P, Turner MW, Atherton DJ. Increased intestinal permeability in atopic eczema. J Invest Dermatol. 1986;86:101–104. doi: 10.1111/1523-1747.ep12284035. [DOI] [PubMed] [Google Scholar]
- 8.Wollina U. Microbiome in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:51–56. doi: 10.2147/CCID.S130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han SH, Cheon HI, Hur MS, Kim MJ, Jung WH, Lee YW, et al. Analysis of the skin mycobiome in adult patients with atopic dermatitis. Exp Dermatol. 2018;27:366–373. doi: 10.1111/exd.13500. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Zhao F, Hutchinson DS, Sun W, Ajami NJ, Lai S, et al. Conjunctival microbiome changes associated with soft contact lens and orthokeratology lens wearing. Invest Ophthalmol Vis Sci. 2017;58:128–136. doi: 10.1167/iovs.16-20231. [DOI] [PubMed] [Google Scholar]
- 11.Kugadas A, Christiansen SH, Sankaranarayanan S, Surana NK, Gauguet S, Kunz R, et al. Impact of microbiota on resistance to ocular Pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 2016;12:e1005855. doi: 10.1371/journal.ppat.1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Holland MJ, Makalo P, Joof H, Roberts CH, Mabey DC, et al. The conjunctival microbiome in health and trachomatous disease: a case control study. Genome Med. 2014;6:99. doi: 10.1186/s13073-014-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Offiah I, Calder VL. Immune mechanisms in allergic eye diseases: what is new? Curr Opin Allergy Clin Immunol. 2009;9:477–481. doi: 10.1097/ACI.0b013e3283303e2e. [DOI] [PubMed] [Google Scholar]
- 14.Kugadas A, Gadjeva M. Impact of microbiome on ocular health. Ocul Surf. 2016;14:342–349. doi: 10.1016/j.jtos.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2:6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellemain E, Carlsen T, Brochmann C, Coissac E, Taberlet P, Kauserud H. ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol. 2010;10:189. doi: 10.1186/1471-2180-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics. 2012;13:31. doi: 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee B, Moon T, Yoon S, Weissman T. DUDE-Seq: fast, flexible, and robust denoising for targeted amplicon sequencing. PLoS One. 2017;12:e0181463. doi: 10.1371/journal.pone.0181463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarza P, Yilmaz P, Pruesse E, Glöckner FO, Ludwig W, Schleifer KH, et al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol. 2014;12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 21.Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013;117:99–105. doi: 10.1016/j.exer.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Shivaji S, Jayasudha R, Sai Prashanthi G, Kalyana Chakravarthy S, Sharma S. The human ocular surface fungal microbiome. Invest Ophthalmol Vis Sci. 2019;60:451–459. doi: 10.1167/iovs.18-26076. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Yang B, Li W. Defining the normal core microbiome of conjunctival microbial communities. Clin Microbiol Infect. 2016;22:643.e7–643.e12. doi: 10.1016/j.cmi.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Ozkan J, Nielsen S, Diez-Vives C, Coroneo M, Thomas T, Willcox M. Temporal stability and composition of the ocular surface microbiome. Sci Rep. 2017;7:9880. doi: 10.1038/s41598-017-10494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Q, Brulc JM, Iovieno A, Bates B, Garoutte A, Miller D, et al. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci. 2011;52:5408–5413. doi: 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson WC, Stegen JC. The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle. Front Microbiol. 2015;6:713. doi: 10.3389/fmicb.2015.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daroy ML, Lopez JS, Torres BC, Loy MJ, Tuaño PM, Matias RR. Identification of unknown ocular pathogens in clinically suspected eye infections using ribosomal RNA gene sequence analysis. Clin Microbiol Infect. 2011;17:776–779. doi: 10.1111/j.1469-0691.2010.03369.x. [DOI] [PubMed] [Google Scholar]
- 28.Ge C, Wei C, Yang BX, Cheng J, Huang YS. Conjunctival microbiome changes associated with fungal keratitis: metagenomic analysis. Int J Ophthalmol. 2019;12:194–200. doi: 10.18240/ijo.2019.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sankaridurg PR, Sharma S, Willcox M, Naduvilath TJ, Sweeney DF, Holden BA, et al. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J Clin Microbiol. 2000;38:4420–4424. doi: 10.1128/jcm.38.12.4420-4424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of the study process.
Heatmap of composition in genus level supported the result which suggested that composition of atopic keratoconjunctivitis (AKC) patients were more various than healthy control (HC) group.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.