Abstract
Background: Interstitial lung disease (ILD) has been recently reported in a few patients with pathogenic variants in the Filamin A (FLNA) gene with variable presentation and prognosis. This study evaluated the respiratory manifestations and clinical features in children with FLNA disease.
Methods: We conducted a retrospective review of pediatric patients with variants in FLNA in a tertiary children's hospital. The clinical features, genotype, management, and outcomes were analyzed.
Results: We identified 9 patients with variants in FLNA aged 15 months to 24 years, 4 females and 5 males. Six patients had abnormal chest imaging ranging from mild interstitial prominence to atelectasis, interstitial densities, and hyperinflation. Three patients with ILD presented during the neonatal period or early infancy with respiratory distress or respiratory failure requiring supplemental oxygen or assisted ventilation via tracheostomy. We report male twins with the same FLNA variant and lung disease, but different ages and clinical features at presentation eventually culminating in respiratory failure requiring assisted ventilation. All patients had FLNA variants identified by FLNA sequencing, had abnormal echocardiograms, and none of the patients underwent lung biopsy or lung transplantation. The outcomes were variable and could be as severe as chronic respiratory failure.
Conclusion: The wide spectrum of respiratory manifestations and abnormal chest imaging in our study highlights the importance of evaluation for lung disease in patients with variants in FLNA. FLNA sequencing in suspected cases with ILD may obviate the need for a lung biopsy, prompt surveillance for progressive lung disease, and evaluation for associated clinical features.
Keywords: Filamin A, FLNA, interstitial lung disease, ILD, childhood ILD
Introduction
Pathogenic variants in Filamin A (FLNA) gene are inherited in an X-linked dominant pattern and result in alterations of cytoskeletal structure and cell signaling.1 FLNA protein is essential for the migration of several cell types, and protein dysfunction results in a range of abnormalities that can include periventricular nodular heterotopia (PVNH), cardiovascular disease, pulmonary arterial hypertension (PH), interstitial lung disease (ILD), skeletal dysplasia, hematologic abnormalities, and intestinal pseudo-obstruction.1–10 Some of these phenotypes are the result of losses or reductions in function of the encoded protein, others with a gain-of-function.11 Due to the location of the gene on the X chromosome, gender differences in the expression of pathogenic variants exist. For example, heterozygous loss-of-function FLNA variants in females can result in PVNH, whereas in males, variants of this type are associated with high mortality with only a few reported cases in the literature.4,10,12 FLNA-related lung disease (FLNA-RLD) is a rare and recently reported consequence of pathogenic variation in FLNA that results in ILD with additional syndromic features. To date, only a few cases have been reported.1–3
Childhood ILD is a rare and heterogeneous group of disorders characterized by persistent respiratory symptoms and signs along with hypoxemia and diffuse pulmonary abnormalities on chest imaging.13 Although the specific role of FLNA in the pathogenesis of lung disease is unclear, the FLNA protein is believed to influence alveolar maturation, lung growth, and development.14,15 Most reported patients with FLNA-RLD are females and have variable presentation, management, and outcome. The phenotypes of FLNA-RLD are heterogenous ranging from clinical recovery to progressive respiratory failure and death.1–3,5,6,8,16 It is currently unclear what dictates the presence or severity of lung disease in those with pathogenic variants in FLNA. The incidence and prevalence of FLNA-RLD are unknown, and currently, there are no recommendations on screening FLNA patients for pulmonary disease.14 The aim of our study was to describe the pulmonary manifestations and associated clinical features in patients with variation in FLNA.
Methods
The study is a retrospective review of pediatric patients with pathogenic variants or variants of uncertain significance (VUS) in FLNA treated at Children's Healthcare of Atlanta between 1995 and 2019. Patients were identified by reviewing the medical records of all patients in the cardiology and pulmonary hypertension clinics. De-identified demographic and clinical information were collected for all eligible patients during the study period. The recorded data included the variant of FLNA, family history, clinical features, chest, cardiac, and brain imaging studies, management, and outcome. Genetic tests were performed during routine clinical management by the treating physicians when patients presented with clinical features of FLNA-related cardiovascular or lung disease, and when siblings of patients with previously identified FLNA variants were identified. All FLNA genetic studies were performed through commercial laboratories. Although this included multiple different laboratories, as part of our study, the variants were also classified independently by the same author (K.B.G.) according to current American College of Medical Genetics variant interpretation guidelines.17 The study was approved by the Institutional Review Board at Children's Healthcare of Atlanta.
Results
We identified 9 patients with FLNA pathogenic variants or VUS between 15 months and 24 years of age. This included 2 sets of siblings, of which 2 were male twins. The clinical characteristics of our patients are summarized in Tables 1 and 2 and presented more thoroughly in the Supplementary Data. Abnormalities in chest imaging were identified in 6 (67%) patients (out of 8 total who had chest imaging) and the remainder of this article describes the characteristics of these patients. The clinical features at presentation ranged from recurrent respiratory infections to hypoxemia and respiratory distress with an age at presentation between 1 day and 1 year of age (Table 1). Four patients (1, 3, 7, and 8) presented during the neonatal period or early infancy with respiratory distress or respiratory failure. All 4 of these patients also had abnormal echocardiograms with either patent ductus arteriosus (PDA), PH, valvular, or aortic abnormalities. A fifth case (patient 9) developed respiratory failure during infancy; he failed several attempts of extubation following abdominal trauma at 12 months of age. Of these cases, 3 (7, 8, and 9) developed progressive lung disease and chronic respiratory failure requiring supplemental oxygen or positive pressure ventilation (PPV) via tracheostomy. Six patients were diagnosed with PH that eventually resolved on echocardiography in 3 of them.
Table 1.
Summary of Patient Characteristics and Pulmonary Manifestations
| Patient 1a | Patient 2 | Patient 3a | Patient 4a | Patient 5 | Patient 6 | Patient 7a | Patient 8a | Patient 9a | |
|---|---|---|---|---|---|---|---|---|---|
| Current age (years), gender | 7, F | 24, M | 6, F | 6, F | 12, M | 3, M | 2, F | 1, M | 1, M |
| Race | Caucasian | AA | AA | AA | AA | AA | Caucasian | AA | AA |
| Gestational age (weeks) | 40 | 40 | 38 | 38 | Unknown | 40 | 37 | 38 | 38 |
| FLNA variant (NM_001456.3) | c.1734_1735delGGinsA (p.V579YfsX97) | c.7647C>T (p.A2549A) | c.134A>G (p.Q45R) | c.2190_2193delTTAC: (p.Y731AfsX10) | c.3806-1 G > T (IVS22-1 G>T) | c.3806-1 G > T (IVS22-1 G>T) | c.6303delA (p.E2102SfsX23) | c.278A>G (p.H93R) | c.278A>G (p.H93R) |
| Variant classification | Pathogenic | VUS | VUS | Pathogenic | Likely pathogenic | Likely pathogenic | Pathogenic | VUS | VUS |
| Age at presentation, clinical features | 6 weeks, intermittent tachypnea from large PDA treated with diuretic | 17 years, DE and cardiac murmur | 1 day, PPHN, AI on echo, FH of PVNH, PDA | 1 year, recurrent respiratory infections, poor weight gain | 10 years, chest pain and FH of polyvalvar dysplasia | 2 years, asymptomatic and sibling with FLNA variant | 1 month, cyanosis, apnea, and hypoxemia | 1 day, respiratory distress and hypoxemia | 12 months, abdominal blunt trauma and failure to extubate |
| Respiratory manifestations | Intermittent tachypnea in infancy attributed to CHF, mild OSA in infancy requiring oxygen (resolved); currently asymptomatic | DE attributed to severe valvular insufficiency (resolved); currently asymptomatic | Neonatal respiratory distress requiring oxygen, wheezing with illness; currently asymptomatic | Recurrent respiratory infections, mild OSA s/p AT, wheezing with illness, DE | None | None | Hypoxemia, apnea; CRF since 9-months of age | Hypoxemia, apnea; CRF since 2-months of age, wheezing with illness | CRF since 13-months of age |
| Medications | Tadalafil | Lisinopril | Losartan | Tadalafil, ambrisentan | None | None | Albuterol, azithromycin | Furosemide, chlorothiazide, spironolactone albuterol, fluticasone | Albuterol |
| Respiratory support | None | None | None | None | None | None | Nasal cannula oxygen at 1.5 LPM | PPV via tracheostomy at 8 months of age | PPV via tracheostomy at 14 months age |
| Chest imaging findings and age at imaging | CXR: At 1 year: Mild pulmonary interstitial prominence, cardiomegaly Chest CT: None |
CXR: At 17 years: Cardiomegaly, clear lungs Chest CT: None |
CXR: At 3 months: RUL, LLL atelectasis Chest CT: At 6 months: Scattered multilobar atelectasis of RUL, RML, LLL |
CXR: At 3 years: Right lung hyperinflation with mediastinal shift to left. At 4 and 5 years: Stable right lung hyperinflation with mediastinal shift to left. Chest CT: None |
CXR: At 11 years: Clear lungs Chest CT: None |
CXR: None Chest CT: None |
CXR: At 1 month: normal. At 1 year: CBID, patchy airspace opacities. At 2 years: CBID, RUL atelectasis, hyperinflation of RML. Chest CT: At 2 months: Diffuse GGO, mild septal thickening. At 1 year: RML and LLL hyperinflation, RUL atelectasis, coarse septal thickening |
CXR: At 2 weeks: Increased pulmonary IM, cardiomegaly. At 6 months: Mild increased pulmonary interstitial prominence. At 1 year: LLL hyperinflation, patchy GGO, subsegmental atelectasis. Chest CT: At 5 months: Multifocal atelectasis, septal thickening, subpleural cystic change, heterogenous mixed aeration |
CXR: At 12 months: Mild pulmonary haziness and increased IM. At 15 months: LLL atelectasis, coarse IM. Chest CT: At 15 months: Atelectasis in dependent portions of RUL and LLL |
Patients with abnormal chest imaging.
AA, African American; AI, aortic insufficiency; AT, adenotonsillectomy; CBID, chronic bilateral interstitial densities; CHF, congestive heart failure; CRF, chronic hypoxemic and hypercapnic respiratory failure; CXR, chest radiograph; CT, computed tomography; DE, dyspnea on exertion; F, female; FH, family history; FLNA, Filamin A; GGO, ground glass opacity; IM, interstitial markings; LLL, left lower lobe; LPM, liter per minute; M, male; OSA, obstructive sleep apnea; PDA, patent ductus arteriosus; PPHN, persistent pulmonary hypertension of the newborn; PPV, positive pressure ventilation; PVNH, periventricular nodular heterotopia; RML, right middle lobe; RUL, right upper lobe; s/p, status post; VUS, variants of uncertain significance.
Table 2.
Summary of Nonpulmonary Manifestations
| Patient 1a | Patient 2 | Patient 3a | Patient 4a | Patient 5 | Patient 6 | Patient 7a | Patient 8a | Patient 9a | |
|---|---|---|---|---|---|---|---|---|---|
| Brain MRI | None | None | Thinning of corpus callosum, PVNH | None | None | None | Thinning of corpus callosum, PVNH | PVNH | PVNH, hypoplastic corpus callosum |
| Cardiac manifestations and echocardiogram | PDA s/p repair, mild AI, severely dilated ascending aorta, moderate dilatation of MPA | Severe AI and moderate MR s/p aortic and mitral valve repair, mild TR | PDA s/p repair, bicuspid pulmonary valve, moderately dilated MPA and ascending aorta, trace AI, mild MR | PDA s/p repair, thickened and prolapsed aortic valve with moderate AI, mild aortic dilation | Dysplastic mitral valve with mild MR, DTV with mild TR, dysplastic aortic valve with prolapse and mild AI, dysplastic pulmonic valve with mild PR | Dysplastic thickened mitral and tricuspid valves with mild tricuspid valve prolapse, trace AI | Bicuspid aortic valve, trace AI, dysplastic pulmonary valve, moderate ascending aorta dilation | Small PDA, dysplastic mitral and tricuspid valves with severe mitral stenosis, moderate MR, moderate to severe TR, mild AI | Fenestrated ASD, thickened and DTV with moderate TR |
| Other clinical features | PH since 1-year of age | None | Resolved PH on echocardiogram, resolved dysphagia | PH since 2-years of age, thrombocytopenia, poor growth, pectus excavatum | None | GERD | Resolved PH on echocardiogram, dysphagia requiring gastrostomy feeds, strabismus, joint hypermobility, speech delay | Severe PH, global developmental delay, natal teeth, dysphagia requiring gastrostomy feeds, GERD requiring fundoplication | Resolved PH on echocardiogram, poor weight gain before trauma leading to gastric perforation and ischemic colon s/p left colectomy |
Patients with abnormal chest imaging.
ASD, atrial septal defect; DTV, dysplastic tricuspid valve; GERD, gastroesophageal reflux disease; MPA, main pulmonary artery; MR, mitral regurgitation; MRI, magnetic resonance imaging; PH, pulmonary arterial hypertension; PR, pulmonary regurgitation; TR, tricuspid regurgitation.
The radiographic abnormalities identified among 6 patients ranged from mild interstitial prominence to chronic interstitial densities, atelectasis, and hyperinflation suggestive of diffuse lung disease (Supplementary Fig. S1). Chest computed tomography (CT) was performed in 4 patients (3, 7, 8, and 9) that demonstrated various abnormalities, including diffuse ground glass opacities, septal thickening, lobar hyperinflation, atelectasis, and subpleural cysts. None of the patients underwent pulmonary function tests, lung biopsy, or a referral for lung transplantation. Bronchoscopy was only performed in patient 9 and showed no airway malacia. There were no deaths during the study period.
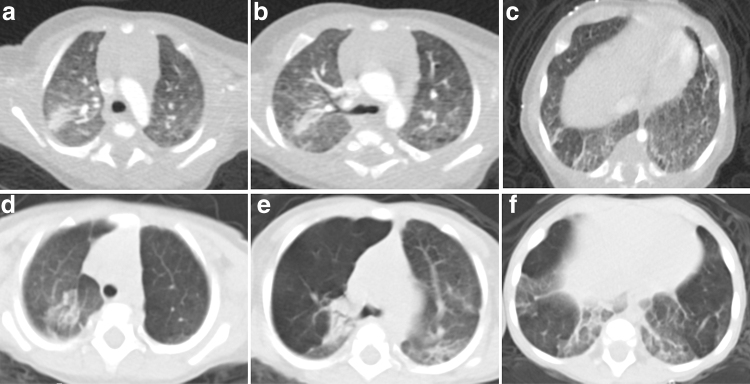
A brief description of the variable respiratory presentations is discussed here with detailed description in the Supplementary Data. Patient 7 presented during early infancy with hypoxemia, normal chest radiograph, and capillary blood gas (Table 1). However, chest CT showed abnormalities suggestive of diffuse lung disease. At 4 months, typical CT findings of lobar hyperinflation and atelectasis were identified (Fig. 1). She developed hypercapnia and progressive increase in oxygen requirement during infancy. At 15 months, she developed mild PH, required 1.5 liter per minute (LPM) oxygen, and thrice-weekly azithromycin was initiated. At 2 years, PH had resolved on echocardiography, however, stable hypercapnia persisted without increasing oxygen requirement.
FIG. 1.
Patient 7: Axial chest CT images at 4 months of age in the upper (a), middle (b), and lower (c) lung zones demonstrate right upper lobe atelectasis, mild diffuse ground glass opacities, and basilar predominant septal thickening. Axial chest CT images at 9 months of age in the upper (d), middle (e), and lower (f) lung zones demonstrate progressive hyperinflation of right upper, left upper, and left lower lobes with persistent atelectasis and basilar septal thickening. CT, computed tomography.
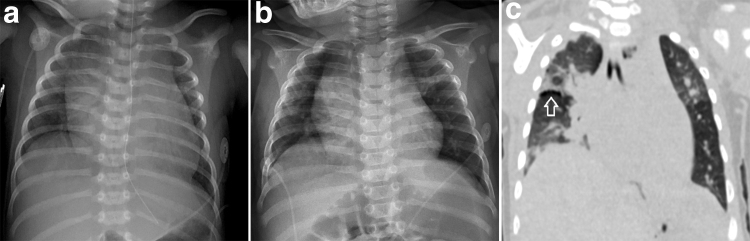
In contrast to patient 7, patient 8 developed PH and respiratory failure in the neonatal period requiring mechanical ventilation. Although he initially tolerated extubation, he developed recurrent episodes of acute respiratory failure requiring mechanical ventilation. Chest CT demonstrated multifocal atelectasis, septal thickening, and subpleural cysts (Fig. 2). Tracheostomy was performed at 8 months for inability to wean off ventilatory support. At 1-year, severe PH persisted, and he required continuous PPV via tracheostomy and diuretics. Patient 9, the twin sibling of patient 8, only presented with a cardiac murmur at birth and was diagnosed with a PDA and dysplastic tricuspid valve. At 12 months of age, he sustained a blunt abdominal trauma requiring abdominal surgery. Following the surgery, he was unable to wean off assisted ventilation and developed mild PH. Hence, tracheostomy was performed at 14 months. At 15 months, PH had resolved on echocardiography; however, he required continuous PPV via tracheostomy.
FIG. 2.
Patient 8: Initial chest radiograph at age 2 weeks (a) demonstrates diffuse pulmonary interstitial prominence and cardiomegaly. Chest radiograph at age 4 months (b) demonstrates persistent interstitial prominence and slight hyperinflation of the upper lobes. Chest CT at 5 months (c) demonstrates mixed aeration throughout the lungs with regions of atelectasis interposed with regions of hyperinflation, thickened interlobular septa, and subpleural cysts along the right minor fissure (→).
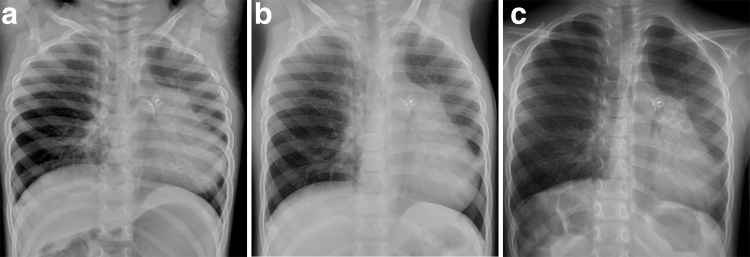
Patient 4 had an uncomplicated infancy and developed recurrent respiratory infections at 1 year of age requiring bronchodilators and antibiotics. At 2 years, a PDA was detected requiring closure and she was also diagnosed with PH. At 3 years, chest radiograph showed right lung hyperinflation. Chest radiograph showed stable right lung hyperinflation at 5 years (Fig. 3). At 6 years, PH persisted requiring medications without hypoxemia.
FIG. 3.
Patient 4: Upright posteroanterior chest radiograph at 3 years (a), 4 years (b), and 5 years (c) of age demonstrate persistent right lung hyperinflation.
In addition to the pulmonary issues described above, all patients also had nonpulmonary manifestations and abnormal echocardiograms (Table 2 and Supplementary Fig. S2). All patients (n = 4) who underwent brain magnetic resonance imaging (MRI) had PVNH (Supplementary Fig. S3). Two patients with PVNH had neurodevelopmental delay and none had seizures.
All patients in our study had a previously undescribed FLNA variant identified on genetic studies. Among these were 3 frameshift variants (patients 1, 4, and 7) that were quite clearly loss-of-function variation and were classified as pathogenic (Table 1). All patients with one of these variants were females and had lung disease. Only one of these individuals (patient 7) had a brain MRI, and it showed PVNH. Two of the other variants were missense VUS (NM_001456.3:c.134A>G (p.Q45R) and NM_001456.3:c.278A>G (p.H93R) in patients 3, 8, and 9). Neither one was found in the Genome Aggregation Database (gnomAD), nor had they been previously reported in individuals with FLNA-related phenotypes.18 Both substitutions were predicted to be deleterious with Combined Annotation-Dependent Depletion scores of 27 and 23.2, respectively.19 The c.278A>G (p.H93R) and c.134A>G (p.Q45R) variants were found in individuals with phenotypes highly suspicious for FLNA-related disease. The c.278A>G (p.H93R) variant was found in a pair of male twin siblings, both with PVNH and early lung disease, whereas c.134A>G (p.Q45R) was found in a female with neonatal lung disease, PVNH, and a PDA. However, due to the lack of further information on either variant, both were classified as VUS.
The NM_001456.3:c.7647C>T (p.A2549A) variant was found in a 17-year old male with dyspnea and severe valvular insufficiency (patient 2). Although this variant was not found in gnomAD, it is predicted to be silent, bringing it into question as a pathogenic variant. Further, the presentation in this patient is less classic for FLNA-related disease and may be due to other causes. The final variant occurs at the canonical splice acceptor site for intron 22 (NM_001456.3:c.3806-1G>T) and was found in 2 male siblings (patients 5 and 6). Although variants of this type alter splicing and often lead to loss of function, the phenotypes of the 2 brothers do not fit with the severe outcome expected for complete loss of FLNA.11 Indeed, looking further at the sequence, skipping exon 23 would be predicted to yield an in-frame product that may have some function. Although we do not have experimental evidence to support this theory, it would help to explain the milder than expected phenotype in these brothers.
Family members were found to have FLNA-related phenotypes in 3 families. This included the 2 sets of male siblings among our subjects (patients 5, 6 and 8, 9), of which only 1 pair (patients 8, 9) had lung disease. Maternal PVNH or cardiac polyvalvar dysplasia (PVD) was identified in 2 families (patients 3 and 5, 6). The mother of patient 3 had mild intellectual disability, PVNH, PDA requiring ligation, aortic root dilatation, and PH. She underwent FLNA sequencing that identified the same VUS that was in her child. Parental genetic studies were either declined, not performed, or not available for review in the remaining patients.
Discussion
We performed a retrospective study of patients with FLNA variation and identified 9 patients with novel FLNA variants, including 2 families of male siblings. There were 6 patients with abnormal findings on chest imaging, indicating a high prevalence of lung disease in the sample. In our sample, the lung disease was variable ranging from asymptomatic to chronic respiratory failure requiring assisted ventilation. Our study adds to the limited literature describing the heterogeneous pulmonary phenotypes in patients with FLNA variants. Indeed, we report on male twin siblings who share an FLNA variant but had different ages and clinical features at presentation, although both ultimately developed respiratory failure requiring PPV via tracheostomy.
Most patients in our study with lung disease presented in the neonatal period or infancy with respiratory manifestations being the presenting feature of this disorder. Our findings are consistent with prior reports where hypoxemia, respiratory distress, and recurrent respiratory infections were the presenting features of FLNA-related disease.1–4 Similar to previous cases, our patients demonstrated typical chest imaging findings of FLNA-RLD, such as lobar hyperinflation, atelectasis, and septal thickening.2,3,5,7,16 Since chest imaging demonstrated pulmonary abnormalities in many patients with FLNA disease, our study highlights the importance of screening patients with suspected FLNA disease for pulmonary involvement. In studies where bronchoscopy was performed in the evaluation of FLNA-RLD, airway malacia has been reported.2–4,7,9 However, only 1 patient in our study underwent bronchoscopy, and airway malacia was not present. In this study, FLNA-RLD was suspected based on typical cardiovascular manifestations, chest, or brain imaging findings, and subsequently confirmed by FLNA genetic studies. In prior studies where lung biopsy/lobectomy was performed for suspected FLNA-related ILD, features of alveolar growth abnormalities such as alveolar simplification, alveolar enlargement, hypertensive pulmonary arteriopathy, and abnormalities of airway cartilage have been reported.2,4,7,16 Our case study demonstrates the utility of FLNA genetic tests in suspected cases that obviated the need for lung biopsy.
The current knowledge of FLNA-RLD is limited to case studies and there is no established genotype and phenotype correlation.7 Consistent with previous studies, our patients had heterogeneous pulmonary phenotypes with variable ages at presentation, severity of lung disease, respiratory support requirements, and outcome.1,2,4,5 Although mortality from progressive lung disease has been reported, there were no deaths in our study.16,20 There are no specific therapies for FLNA-RLD and patients have required supplemental oxygen, assisted ventilation, lobectomy, or lung transplantation.2–4,14 Demirel et al. reported the therapeutic response to systemic steroid in an infant with lung disease due to a pathogenic variant in FLNA and pulmonary interstitial glycogenosis suggesting the utility of steroids in some cases.7 Sasaki et al. reported a 4-week-old female with FLNA-related ILD who was treated with azithromycin and remained clinically stable on supplemental oxygen at 11 months of age.1 In our study, patient 7 developed progressive lung disease during infancy initially requiring only 0.125 LPM oxygen at 7 weeks that later increased to 1.5 LPM at 15 months of age. Following initiation of azithromycin, her oxygen requirement and hypercapnia stabilized. Although the duration of azithromycin therapy was limited to 9 months until the end of the study, our results suggest a beneficial effect of azithromycin in some patients with FLNA-RLD that requires further investigation.
There are only a few reports of males with FLNA-related ILD.1,4,12 A high perinatal mortality has been reported in hemizygous males with FLNA variants and severe clinical features in surviving males.10,12 Gerard-Blanluet et al. reported preterm twin boys with FLNA variant and lung disease.12 Both twins had PVNH and severe bronchopulmonary dysplasia that resulted in the death of 1 sibling at 8 months of age due to respiratory complications. We identified term male twin siblings with FLNA-RLD who had variable phenotypes and ages at presentation eventually requiring PPV via tracheostomy. Although both siblings had the same FLNA variant, the first infant presented in the neonatal period with respiratory failure, whereas the second infant presented at 1 year of age after failure to extubate following blunt abdominal trauma. This suggests that other environmental or genetic factors may influence variable expressivity. The zygosity of the twins was unable to be ascertained. To our knowledge, there are no prior reports of term male twin siblings with FLNA-RLD and variable clinical manifestations. In contrast to the twin males, patients 5 and 6 were male siblings with hemizygous FLNA likely pathogenic variant and relatively less severe phenotypes that could possibly be attributed to residual protein function.1
All patients had cardiovascular manifestations, including PDA, PVD, and/or aortic dilatation. Cardiovascular associations in patients with FLNA variants include PDA, PVD, PH, sinus of Valsalva aneurysm, aortic dilatation, and death due to aortic rupture.1,2,21 While there are no specific guidelines for the management of those with aortic dilatation due to FLNA variants, given the aggressive nature of aortic disease described in this setting, we concur with prior authors that surgical management should mirror guidelines for Loeys-Dietz syndrome.21,22 More specifically, if a patient were to exhibit a rapid rate of aortic growth (>5 mm/year) or an aortic size >4.5cm then elective surgical intervention may be warranted. PH is likely multifactorial and caused by hypoxemia, lung growth abnormality, progressive lung disease, and left sided valvular heart disease. Given the relatively rare nature of PVD, the combination of lung disease and PVD with or without aortic dilatation should trigger an evaluation for FLNA-related disease. Since progressive dilation of the ascending aorta can occur, even in the absence of significant aortic valve involvement, patients should be closely monitored with serial echocardiograms by a pediatric cardiologist.3
PVNH, a common manifestation of FLNA pathogenic variants, was seen in all 4 patients who underwent brain MRI. In a study of 47 patients with FLNA-associated PVNH, Lange et al. identified several subgroups with seizure onset ranging from childhood to adulthood and a subgroup of 10 patients without any seizures.10 Since 5 of our patients lacked neurological symptoms, brain MRI was not performed, and the parents were counseled on seizures and neurologic symptoms requiring further evaluation. The joint hypermobility, natal teeth, and thrombocytopenia seen in our patients are previously reported associations with FLNA disease.4,9,16,20,23 Genetic studies may help identify family members with FLNA-related disease as seen in patients 3, 5, and 6. Since some females with FLNA pathogenic variants only have mild clinical features, genetic tests, and counseling can aid with family planning and assess recurrence risk in future pregnancies.10 For patients with FLNA-related PVNH, screening recommendations include evaluations by a) a neurologist, b) an epileptologist if seizures occur, c) a clinical geneticist, d) a cardiologist along with echocardiogram to assess for congenital cardiac anomalies, aortic, and valvular disease, and e) a pulmonologist to provide general assessment and consideration of imaging to demonstrate the presence of intrinsic lung disease. Based on our review of the literature, during infancy and early childhood, individuals with FLNA variants should be monitored for seizures, abnormal cardiovascular findings, signs of pulmonary disease, and dyslexia.14,24
Our study is limited by the single institution retrospective design. Since patients in our study were identified by reviewing medical records in the cardiology and PH clinics, we acknowledge the possibility of other patients with FLNA-related disease at our institution who were not identified. Although uncommon, clinicians should maintain a high index of suspicion for FLNA-related ILD in infants with progressive lung disease, lobar hyperinflation, and PVD and perform FLNA genetic studies. The early diagnosis of FLNA disease can aid in close clinical monitoring for progressive lung disease, prompt evaluations for associated manifestations, and genetic counseling. Currently, there are no specific recommendations on screening patients with FLNA pathogenic variants for pulmonary disease.14 Based on the wide spectrum of respiratory manifestations and variable prognosis seen in our study and the existing literature reporting potentially fatal outcomes, evaluation for lung disease should be considered in these patients. Long-term pulmonary outcomes for patients with FLNA disease are unknown. Future multicenter prospective cohort studies are required to identify a genotype-phenotype correlation and risk factors for developing ILD to assess therapies and long-term outcomes.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. Sasaki E, Byrne AT, Phelan E, et al. A review of filamin A mutations and associated interstitial lung disease. Eur J Pediatr 2019; 178:121–129 [DOI] [PubMed] [Google Scholar]
- 2. Shelmerdine SC, Semple T, Wallis C, et al. Filamin A (FLNA) mutation-A newcomer to the childhood interstitial lung disease (ChILD) classification. Pediatr Pulmonol 2017; 52:1306–1315 [DOI] [PubMed] [Google Scholar]
- 3. Burrage LC, Guillerman RP, Das S, et al. Lung transplantation for FLNA-associated progressive lung disease. J Pediatr 2017; 186:118–123.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masurel-Paulet A, Haan E, Thompson EM, et al. Lung disease associated with periventricular nodular heterotopia and an FLNA mutation. Eur J Med Genet 2011; 54:25–28 [DOI] [PubMed] [Google Scholar]
- 5. de Wit MCY, Tiddens HAWM, de Coo IFM, et al. Lung disease in FLNA mutation: confirmatory report. Eur J Med Genet 2011; 54:299–300 [DOI] [PubMed] [Google Scholar]
- 6. Lord A, Shapiro AJ, Saint-Martin C, et al. Filamin A mutation may be associated with diffuse lung disease mimicking bronchopulmonary dysplasia in premature newborns. Respir Care 2014; 59:e171–e177 [DOI] [PubMed] [Google Scholar]
- 7. Demirel N, Ochoa R, Dishop MK, et al. Respiratory distress in a 2-month-old infant: is the primary cause cardiac, pulmonary or both? Respir Med Case Rep 2018; 25:61–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pelizzo G, Collura M, Puglisi A, et al. Congenital emphysematous lung disease associated with a novel Filamin A mutation. Case report and literature review. BMC Pediatr 2019; 19:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshii K, Matsumoto H, Hirasawa K, et al. Microdeletion in Xq28 with a polymorphic inversion in a patient with FLNA-associated progressive lung disease. Respir Investig 2019; 57:395–398 [DOI] [PubMed] [Google Scholar]
- 10. Lange M, Kasper B, Bohring A, et al. 47 patients with FLNA associated periventricular nodular heterotopia. Orphanet J Rare Dis 2015; 10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wade EM, Halliday BJ, Jenkins ZA, et al. The X-linked filaminopathies: synergistic insights from clinical and molecular analysis. Hum Mutat 2020; 41:865–883 [DOI] [PubMed] [Google Scholar]
- 12. Gérard-Blanluet M, Sheen V, Machinis K, et al. Bilateral periventricular heterotopias in an X-linked dominant transmission in a family with two affected males. Am J Med Genet A 2006; 140:1041–1046 [DOI] [PubMed] [Google Scholar]
- 13. Kurland G, Deterding RR, Hagood JS, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med 2013; 188:376–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kremer TM, Lindsay ME, Kinane TB, et al. Case 28-2019: a 22-year-old woman with dyspnea and chest pain. N Engl J Med 2019; 381:1059–1067 [DOI] [PubMed] [Google Scholar]
- 15. Calcaterra V, Avanzini MA, Mantelli M, et al. A case report on filamin A gene mutation and progressive pulmonary disease in an infant: a lung tissued derived mesenchymal stem cell study. Medicine (Baltimore) 2018; 97:e13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eltahir S, Ahmad KS, Al-Balawi MM, et al. Lung disease associated with filamin A gene mutation: a case report. J Med Case Rep 2016; 10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17:405–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020; 581:434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ren tzsch P, Witten D, Cooper GM, et al. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res 2019; 47(D1):D886–D894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinane TB, Lin AE, Lahoud-Rahme M, et al. Case 4-2017. A 2-month-old girl with growth retardation and respiratory failure. N Engl J Med 2017; 376:562–574 [DOI] [PubMed] [Google Scholar]
- 21. Chen MH, Choudhury S, Hirata M, et al. Thoracic aortic aneurysm in patients with loss of function Filamin A mutations: clinical characterization, genetics, and recommendations. Am J Med Genet A 2018; 176:337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med 2014; 16:576–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nurden P, Debili N, Coupry I, et al. Thrombocytopenia resulting from mutations in filamin A can be expressed as an isolated syndrome. Blood 2011; 118:5928–5937 [DOI] [PubMed] [Google Scholar]
- 24. Chen MH, Walsh CA. FLNA-related periventricular nodular heterotopia. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews. Seattle: University of Washington, 2015. Available at https://www.ncbi.nlm.nih.gov/books/NBK1213/ (accessed December7, 2020)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.