Abstract
Introduction: In 2019, an alarming number of cases coined as e-cigarette, or vaping, product use-associated lung injury (EVALI) were described in adolescents ranging from mild respiratory distress to fulminant respiratory failure. Limited data have been published on outcomes at short-term follow-up. We aimed to describe pulmonary manifestations, function, and radiologic findings after corticosteroid therapy in a cohort of adolescent patients.
Methods: A retrospective chart analysis of all patients presenting to our institution between July 2019 and December 2019 with EVALI was conducted. Patients who had pulmonary follow-up were included. Spirometry was performed before discharge from the hospital and during outpatient follow-up. A paired t-test was used to compare serial spirometry data between visits.
Results: Eight patients (6 males) were included. Two patients required intubation with mechanical ventilation, 2 required bilevel positive airway pressure, and 3 required oxygen supplementation. All patients received glucocorticoids (3 receiving pulse dosing). Initial spirometry revealed decreased forced expiratory volume in one second and forced vital capacity with clinically and statistically significant improvement at follow-up (mean follow-up was 46.5 days). Radiographic manifestations also improved after vaping was discontinued.
Conclusion: In our cohort of patients with EVALI, at short-term follow-up, all normalized their spirometry parameters and showed clinical resolution of symptoms.
Keywords: EVALI, vaping, e-cigarettes, spirometry
Introduction
An epidemic of e-cigarette, or vaping, product use-associated lung injury (EVALI) has affected thousands of people in the United States.1,2 Vaping involves the inhalation of concentrated, noxious chemicals mixed with attractive flavorings. It has been increasingly popular with adolescents and young adults as an alternative method of consuming nicotine and non-nicotine products such as tetrahydrocannabinol (THC). The process in which e-cigarette liquid is aerosolized involves heating the liquid using an atomizer made of metal. Another common practice of aerosolization is dabbing, which involves placing concentrated marijuana as wax or hashish onto an extremely hot metallic surface and inhaling the vapor produced. This aerosolization releases toxic chemicals and metallic nanoparticles that deposit into the user's lungs following inhalation and cause lung injury.3
This disease was initially described in August 2019 with a preliminary report outlining a cluster of patients presenting to hospitals with a recent history of vaping and subsequent pulmonary and constitutional symptoms.1 As of February 18, 2020, the Centers for Disease Control and Prevention (CDC) reported 2,807 hospitalized cases in the United States with 68 deaths.2 The initial presenting symptoms of EVALI have now been well characterized by a series of publications describing patients presenting with fever, respiratory distress (cough, dyspnea, tachypnea), nausea, vomiting, malaise, and weight loss.1,2,4–6 Characteristic radiographic findings on computerized tomography (CT) scan include bilateral consolidation, centrilobular distribution, ground-glass opacities, septal thickening, crazy-paving, and lymphadenopathy.7,8 Patients present with varying degrees of severity ranging from mild symptoms not requiring respiratory support to acute respiratory failure requiring increased respiratory support, mechanical ventilation, and in rare cases, leading to death.6 Histopathological studies of acute lung injury as a result of EVALI include organizing pneumonia, diffuse alveolar damage, and acute fibrinous pneumonitis.9
As the number of new cases has declined since August 2019,2,6,10 there have been limited reports describing outcomes and follow-up. There have been subjective data on clinical resolution of symptoms following discontinuation of vaping and e-cigarette use and treatment with corticosteroids. However, data on pulmonary function testing on patients diagnosed with EVALI have been limited to outpatient follow-up with qualitative reports on spirometry.4,5,11 Kalininskiy et al. reported resolution in spirometry and radiographic findings in their cohort of patients during follow-up,4 and Blagev et al. reported mild abnormalities during follow-up spirometry in 6/9 patients.5 Recently, a report by Carroll et al. described residual abnormalities in pulmonary function testing in 7/11 patients during short-term follow-up,11 and a report by Wang et al. from our institution described complete or near-complete resolution of radiographic abnormalities in nearly all cases with a median follow-up period of 114 days.8 In this study, we aim to describe changes over time in quantifiable spirometry values, clinical findings, and radiographic findings on follow-up in pediatric patients who were hospitalized for EVALI at our medical center.
Materials and Methods
Study design
We performed a retrospective medical records review of patients diagnosed with EVALI admitted to our pediatric tertiary care hospital. Approval for the study was obtained from the Baylor College of Medicine Institutional Review Board with a waiver of informed consent.
Study subjects and selection criteria
A retrospective chart review of the electronic medical record in a single pediatric tertiary care hospital was performed for patients who were diagnosed with a confirmed or probable EVALI1 between August 2019 to February 2020. A confirmed case is described as (1) use of e-cigarettes (vaping) or dabbing within 90 days of symptom onset; (2) pulmonary infiltrate on chest X-ray (CXR) or CT scan; (3) absence of pulmonary infection on initial workup; and (4) no evidence in the medical record of alternative plausible diagnoses. A probable case would be defined as a patient who fulfills the aforementioned criteria but instead has an identified respiratory infection by polymerase chain reaction or culture and the clinical team caring for the patient does not believe that infection was the only etiology for the onset of symptoms.1 Inclusion criteria for our study included the following: (1) age 18 years or younger, who were seen at our medical center with a confirmed or probable case of EVALI; and (2) had at least 2 spirometry tests performed separated by an interval period of at least 21 days (mean: 46.5 days), with the first test occurring after diagnosis of EVALI. Details abstracted from the electronic medical record included patient demographic information, e-cigarette and other illicit drug use, duration and frequency of use, clinical symptoms upon presentation, treatment course, clinical progression, spirometry, imaging studies, and bronchoalveolar lavage results.
Spirometry
Pulmonary function testing was performed on our patients admitted to the hospital before discharge upon clinical stabilization (extubated, off supplementary oxygen, and hemodynamically and clinically stable to perform appropriate pulmonary function maneuvers). Follow-up pulmonary function testing was obtained during outpatient visits at the pediatric pulmonary clinic separated by an interval period of time following discharge from the hospital. Pulmonary function tests were performed by respiratory therapists using a spirometer adherent to the ATS/ERS (American Thoracic Society/European Respiratory Society) standard guidelines.12,13 Predicted values were based off the ERS Global Lung Function Initiative standardization of spirometry based on average multiracial variability in height, age, and gender.14 Diffusion capacity of the lung for carbon monoxide results was corrected for alveolar volume and hemoglobin concentration in the blood (mL/mmHg/min).12
Bronchodilator response was determined by administering 4 puffs of albuterol (90 mcg/puff) via metered dose inhaler with a spacer. Spirometry repeated 15 min after bronchodilator administration. Primary outcomes measured in the study were the changes in forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and forced expiratory flow at 25–75% between spirometry measurements. Differences in pulmonary function test values comparing baseline in hospital with outpatient follow-up were determined using a paired t-test. Statistical significance was accepted as a 2-tailed P value <0.05.
Imaging analysis
CXR and CT scans were obtained during the patient's hospital course and outpatient follow-up. Available imaging was evaluated independently by 2 board-certified pediatric radiologists who were blinded to demographic information and clinical presentation.8 Interobserver agreement was analyzed using the chance-correlated index Kleiss weighted κ (kappa) statistic based on the Landis and Koch benchmarks.15,16
Results
Clinical presentation
We identified 11 patients with a diagnosis of EVALI who were admitted to our hospital, 3 did not follow up outpatient and were excluded from the study. All 8 patients (6 male, 2 female; mean age 16.25 years old) reported vaping THC containing products, with 5 of the 8 also vaping nicotine containing products. Seven patients also reported smoking marijuana, 1 patient reported use of combustible tobacco, and 1 patient reported other substance abuse. Drug screens were positive for THC on 7 patients, and positive for methamphetamine for 2 patients.
Our patients presented with symptoms of fever (8/8), cough (8/8), nausea and vomiting (7/8), weight loss (4/8), and respiratory distress (4/8) (Table 1). Seven patients were hospitalized and placed on respiratory support; 1 patient was seen in the emergency department without necessitating respiratory support. Two patients were intubated and required ventilatory support, 2 patients needed bilevel positive airway pressure, and 3 patients required supplemental oxygen (Table 1). All patients received systemic corticosteroids during their acute illness, with 5 patients receiving methylprednisolone intravenously and the remaining 3 receiving oral prednisone. Two patients received pulse corticosteroid dosing (1 g daily for 3 days) with the rest of the patients receiving regular dosing (maximum of 2 mg/kg daily). One patient's course was complicated by hypotensive shock requiring vasopressor support. That patient received a longer course of intravenous steroids (500 mg for 8 days) with a prolonged taper of oral steroids for 3 more weeks. Upon clinical stabilization and completion of steroid courses, all patients performed pulmonary function testing before discharge from the hospital. All patients who were admitted were subsequently discharged with no reported short-term adverse side effects to steroid treatment. During outpatient follow-up, 7 patients reported to have discontinued vaping, while 1 patient reported stopping vaping THC but continued vaping nicotine. One patient reported exercise-induced dyspnea ∼1 month after discontinuing vaping and the rest of the patients reported complete resolution of initial clinical symptoms such as fever, dyspnea, nausea, vomiting, weight loss, and respiratory distress during outpatient follow-up. Patients who endorsed weight loss reported regaining the weight back after discontinuing vaping.
Table 1.
Demographics, Medical and Vaping History, and Clinical Course of the Adolescents Affected with E-Cigarette, or Vaping, Product Use-Associated Lung Injury
| Case | Age | M/F | Vaping substance | Length | Other drugs | Presenting symptoms | Comorbidities | Highest respiratory support | Steroids | Pertinent laboratories | Clinical outcome on follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | F | THC nicotine | 2 years | Marijuana | Fever, nausea, vomiting, cough, weight loss, malaise | Anxiety, ADHD | Intubated | Methylprednisolone IV 1 g × 3 days | THC+; BAL− | Symptoms resolved; stopped vaping |
| 2 | 17 | M | THC nicotine | 1 week | Marijuana cigarettes | Fever, nausea, vomiting, cough, weight loss, malaise, dyspnea | ADHD | BPAP | Methylprednisolone IV 1 g × 3 days | THC+, methamphetamine+ | Remained vaping nicotine, symptoms resolved |
| 3 | 16 | M | THC | 3 years | Marijuana | Fever, nausea, vomiting, cough, dyspnea | None | BPAP | Methylprednisolone IV 80 mg then prednisone PO taper; total 24 days | THC+; Clostridium difficile+ | Symptoms resolved; stopped vaping |
| 4 | 18 | M | THC nicotine | 2 years | Marijuana | Fever, nausea, vomiting, cough, weight loss, malaise, back pain, abdominal pain | PMH of neurocardiogenic syncope | HFNC | Prednisone PO 60 mg × 5 days | THC+, methamphetamine+ | Symptoms resolved; stopped vaping |
| 5 | 15 | F | THC | 3 weeks | None | Fever, nausea, vomiting, cough, dyspnea, malaise | Obesity, ADHD | HFNC | Methylprednisolone IV 120 mg × 3 days | THC+, methamphetamine+, elevated liver enzymes | Stopped vaping; mild exercise-induced dyspnea after 1 month |
| 6 | 16 | M | THC nicotine | 3–4 months | Marijuana | Fever, nausea, vomiting, tachypnea | Mild OSA | HFNC | Prednisone PO 60 mg × 3 days | THC+, elevated D-dimer, CRP, ESR, liver enzymes | Symptoms resolved; stopped vaping |
| 7 | 15 | M | THC | >1 year | Marijuana cocaine benzodiazepine promethazine | Fever, diarrhea, abdominal pain, myalgia, cough, weight loss | Behavioral issues, anxiety, RIJ vein thrombus on enoxaparin | Intubated, hypotensive shock on norepinephrine | Methylprednisolone IV 500 mg × 8 days then prednisone PO taper × 3 weeks; total 29 days | THC+, stool adenovirus+, elevated ESR, CRP, Mycoplasma IgM+ IgG+ | Stopped vaping, continued drugs and cigarettes |
| 8 | 17 | F | Nicotine | 1.5 years | Marijuana | Fever, nausea, vomiting, cough, myalgia, headache | ADHD, allergic rhinitis on immunotherapy | Room air | Prednisone PO 80 mg × 5 days | Elevated D-dimer | Symptoms resolved; stopped vaping |
ADHD, attention deficit hyperactivity disorde; BAL, bronchoalveolar lavage; BPAP, bilevel positive airway pressure; CRP, C reactive protein; HFNC, high flow nasal cannula; IV, intravenous; OSA, obstructive sleep apnea; PMH, past medical history; PO, per os; RIJ, right internal jugular; THC, tetrahydrocannabinol.
Spirometry
Serial spirometry for each patient was performed and measured between hospital discharge and outpatient follow-up (mean interval 46.5 days). Initial spirometry measurements showed moderately to severely reduced FVC (mean volume 3.46 L, 80.2% predicted) and FEV1 (mean volume 2.89 L, 76.39% predicted), with all improving to normal range on follow-up with FVC (mean volume 4.70 L, 108.26% predicted; P < 0.01) and FEV1 (mean volume 3.82, 105.7% predicted; P < 0.01) (Table 2). Corrected diffusing capacity of lung for carbon monoxide (DLCO) was measured initially for 6 patients (4/6 normal, 1/6 mildly decreased, and 1/6 moderately decreased) and on follow-up, 5 patients underwent DLCO testing (1/5 normal and 4/5 mildly decreased).
Table 2.
Spirometry Measurements Including Mean Forced Expiratory Volume in One Second, Forced Vital Capacity, and Forced Expiratory Flow at 25–75% Values, Percent Predicted, and z-Scores During Hospitalization (Initial) and During Outpatient Follow-Up
| Case | Days to FU | FVC (L) | FVC (%) | FVC (z) | FEV1 (L) | FEV1 (%) | FEV1 (z) | FEV1/FVC (%) | FEF 25–75 (L) | FEF 25–75 (%) | FEF 25–75 (z) | DLCO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||||||
| Initial | 31 | 2.01 | 53.7 | −3.98 | 1.76 | 53.0 | −3.87 | 87 | 2.03 | 53.5 | −2.49 | 75.2 |
| FU | 3.19 | 85.1 | −1.23 | 2.82 | 84.8 | −1.29 | 88 | 3.22 | 84.8 | −0.90 | 69.1 | |
| 2 | ||||||||||||
| Initial | 29 | 4.45 | 84.6 | −1.34 | 3.71 | 82.5 | −1.48 | 83 | 3.73 | 75.8 | −1.13 | 76.9 |
| FU | 5.24 | 99.4 | −0.05 | 4.03 | 89.4 | −0.90 | 77 | 3.36 | 68.1 | −1.53 | N/A | |
| 3 | ||||||||||||
| Initial | 27 | 3.37 | 60.5 | −3.47 | 3.04 | 64.2 | −2.94 | 90 | 4.58 | 90.2 | −0.44 | 77.4 |
| FU | 5.59 | 99.8 | 0.70 | 5.03 | 105.5 | 0.47 | 90 | 6.72 | 131.4 | 1.29 | N/A | |
| 4 | ||||||||||||
| Initial | 43 | 4.03 | 77.5 | −1.97 | 3.35 | 75.4 | −2.11 | 83 | 3.91 | 79.8 | −0.92 | N/A |
| FU | 5.81 | 111.5 | 0.99 | 4.50 | 113.1 | 1.15 | 77 | 3.88 | 79.1 | −0.96 | 71.4 | |
| 5 | ||||||||||||
| Initial | 26 | 2.30 | 74.4 | −2.27 | 1.82 | 64.9 | −2.89 | 79 | 1.70 | 48.6 | −2.62 | N/A |
| FU | 3.26 | 105.1 | 0.44 | 3.03 | 107.7 | 0.66 | 93 | 4.24 | 120.9 | 0.95 | 61.9 | |
| 6 | ||||||||||||
| Initial | 92 | 3.90 | 94.8 | −0.48 | 3.22 | 89.3 | −0.89 | 83 | 3.80 | 91.8 | −0.37 | 100.7 |
| FU | 5.01 | 121.0 | 1.90 | 4.01 | 110.5 | 0.89 | 80 | 3.92 | 91.0 | −0.26 | N/A | |
| 7 | ||||||||||||
| Initial | 78 | 4.32 | 114.8 | 1.34 | 3.25 | 98.6 | −0.12 | 75 | 2.59 | 67.9 | −1.55 | 64.2 |
| FU | 5.71 | 150.5 | 4.56 | 3.97 | 119.3 | 1.61 | 70 | 2.72 | 70.7 | −1.40 | 87.3 | |
| 8 | ||||||||||||
| Initial | 48 | 3.29 | 81.3 | −1.55 | 2.97 | 83.2 | −1.43 | 90 | 3.43 | 82.7 | −0.83 | 54.1 |
| FU | 3.80 | 93.7 | −0.51 | 3.20 | 89.5 | −0.89 | 84 | 3.17 | 76.4 | −1.15 | 64.8 | |
| FVC |
FEV1 |
Mean FEV1/FVC (%) | FEF 25–75 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (L) | Mean (%) | Mean (z) | Mean (L) | Mean (%) | Mean (z) | Mean (L) | Mean (%) | Mean (z) | ||||
| Initial |
3.46 |
80.20 |
−1.72 |
2.89 |
76.39 |
−1.97 |
83.75 |
3.22 |
73.79 |
−1.29 |
||
| FU |
4.70 |
108.26 |
0.85 |
3.82 |
102.48 |
0.21 |
82.38 |
3.90 |
90.30 |
−0.50 |
||
| P | 0.0004 | 0.0001 | 0.0002 | 0.0022 | 0.0015 | 0.0014 | 0.6836 | 0.1317 | 0.1224 | 0.1473 | ||
Each individual flow-volume loop can be found in the Supplementary Appendix Figure S1.
DLCO, diffusing capacity of lung for carbon monoxide; FEF, forced expiratory flow; FEV1, forced expiratory volume in one second; FU, follow-up; FVC, forced vital capacity.
Imaging
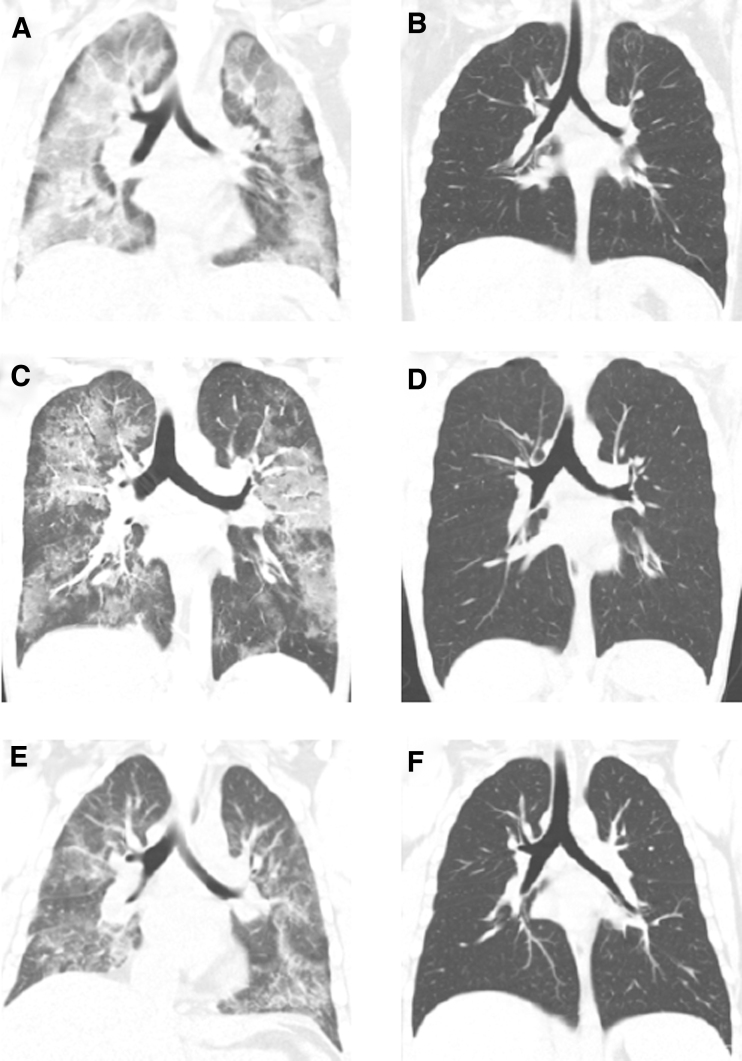
Chest imaging was performed on each patient during hospitalization and at outpatient follow-up to aid in the assessment of severity and progression of the disease. All patients underwent a CXR and 7/8 patients had a chest CT scan at presentation. Five out of 8 patients had a repeat CXR at follow-up, and 4 out of 7 patients had a repeat chest CT scan. All chest CT scans obtained at presentation were abnormal and 7/8 CXRs at presentation were abnormal. Repeat CXR findings from all 5 patients showed significant improvement or resolution of the initial findings. Chest CT scans revealed bilateral ground-glass opacities, consolidation, and mediastinal lymphadenopathy in most patients at presentation. Repeat chest CT findings revealed complete or near-complete resolution of the initial chest CT findings (Fig. 1) with the exception of 1 patient having widespread CT abnormalities (Fig. 1D) attributed to continued vaping of nicotine products on outpatient follow-up. A more detailed description of the imaging findings for 7 of these patients was previously reported in the radiology literature by Wang et al.8
FIG. 1.
Representative chest CT scans of 3 patients affected by EVALI during hospitalization and outpatient follow-up. (A) Initial chest CT showed extensive consolidation and ground-glass opacities. (B) Follow-up chest CT of the same patient showed near-complete resolution of the prior opacities with residual apical ground-glass opacities. (C) Initial chest CT showed sharply demarcated ground-glass opacities with areas of subpleural sparing. (D) Follow-up chest CT showed widespread patchy ground-glass opacities. (E) Initial chest CT showed bilateral patchy ground-glass opacities. (F) Follow-up chest CT of the same patient showed complete resolution of the previous opacities. CT, computerized tomography; EVALI, e-cigarette, or vaping, product use-associated lung injury.
Discussion
We present a cohort of adolescents diagnosed with EVALI and followed-up in our hospital after clinical resolution of symptoms. All of the adolescents in our cohort describe vaping THC containing products and reported discontinuation of THC containing products on follow-up.
We found that spirometry parameters were all moderately to severely abnormal at baseline, and improved to normal range on follow-up. Our data demonstrate clinically important and statistically significant improvement in spirometry values with respect to FEV1 and FVC. In comparing with previous reports characterizing the symptoms and clinical course of patients affected by EVALI, our patients experienced similar clinical resolution.4,5,11,17 A majority of patients affected by EVALI show significant improvement by clinical,4,5,17 radiologic,8 and spirometric assessment.18
The radiographic findings in our patients closely follow the improvement in the patient's pulmonary function and clinical status following discontinuation of the offending agent, expanding upon the earlier observation reported by Wang et al.8 The 1 patient with significant abnormalities on follow-up CT scan had continued vaping nicotine (not THC products), suggesting that cessation of vaping product use may contribute to optimal resolution of EVALI disease. Further larger-scale studies are needed to determine whether the cessation of vaping is directly linked to reversal or resolution of EVALI symptoms. Several of the adolescents in our case series had evidence of other drug abuse, suggesting that assessment for other substance abuse is also an important part of the evaluation of EVALI patients.
In vaping products, THC or nicotine is mixed with flavoring substances and oils such as diacetyl, propylene glycol, glycerin, benzaldehyde, vitamin E, and other unknown substances.3,19–21 These flavored substances are heated up releasing toxic chemicals and potential carcinogens into the alveoli causing lung injury. Previous reports have demonstrated acute and subacute inhalation injury with compounds such as diacetyl causing bronchiolitis obliterans, colloquially known as “popcorn lung.”20 Recently, vitamin E acetate was found in the bronchoalveolar lavage samples of EVALI patients. This was linked as one of the potential toxic agents that led to acute lung injury in this recent epidemic.19
With our available data, we find that patients affected with EVALI have recovered clinically, radiographically, and functionally on spirometry with little or no residual disease. Further follow-up will provide additional understanding of the residual effects of acute injury caused by these inhaled toxic vapors.
Limitations
This study involves a small series of patients who had follow-up pulmonary function testing performed at our center as part of their routine clinical care. Due to the limited sample size, it is difficult to extrapolate generalizable conclusions with short-term spirometric changes. We described a cohort of patients who continue to add and reinforce what we currently know about the acute, reversible lung findings in EVALI. Furthermore, the inclusion criteria were subject to selection bias, as this was a nonrandom sample and was retrospectively obtained from chart review. Patients who were hospitalized and subsequently attended their follow-up appointment were more likely to have severe symptoms, have persistence of disease, or have better access to social support and care. Furthermore, patients with EVALI are mainly adolescents and young adults, and given that the offending agent was THC and/or nicotine, there is an aspect of patient dishonesty in completely disclosing their true vaping habits. Furthermore, the chemical(s) used in the vaping products that caused these patients' severe lung disease is not known.
The incidence of EVALI has significantly decreased since February 2020 according to the CDC. Further research is needed to describe long-term outcomes of EVALI patients and to identify substances that contribute to the development of EVALI. Although there is good evidence suggesting a role of vitamin E acetate, other inhaled toxins may also contribute to the development of the severe lung disease observed.
Conclusion
Follow-up of patients presenting with severe respiratory disease from EVALI reveals normalization of spirometry parameters in all patients. This study further lends support to previous reports suggesting that EVALI is reversible from a pulmonary and radiographic perspective upon discontinuation of vaping.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin—final report. N Engl J Med 2020; 382:903–916 [DOI] [PubMed] [Google Scholar]
- 2. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed September1, 2020)
- 3. Farber HJ, Conrado Pacheco GM, Galiatsasos P, et al. Harms of E-cigarettes: what the healthcare provider needs to know. Ann Am Thorac Soc 2020. [Epub ahead of print]; DOI: 10.1513/AnnalsATS.202009-1113CME [DOI] [PubMed] [Google Scholar]
- 4. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med 2019; 7:1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blagev DP, Harris D, Dunn AC, et al. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet 2019; 394:2073–2083 [DOI] [PubMed] [Google Scholar]
- 6. Chatham-Stephens K, Roguski K, Jang Y, et al. Characteristics of hospitalized and nonhospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep 2019; 68:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med 2019; 381:1486–1487 [DOI] [PubMed] [Google Scholar]
- 8. Wang KY, Jadhav SP, Yenduri NJS, et al. E-cigarette or vaping product use-associated lung injury in the pediatric population: imaging features at presentation and short-term follow-up. Pediatr Radiol 2020; 50:1231–1239 [DOI] [PubMed] [Google Scholar]
- 9. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med 2019; 381:1780–1781 [DOI] [PubMed] [Google Scholar]
- 10. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep 2020; 69:90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel DA, Jatlaoui TC, Koumans EH, et al. Update: interim guidance for health care providers evaluating and caring for patients with suspected E-cigarette, or vaping, product use associated lung injury—United States, October 2019. MMWR Morb Mortal Wkly Rep 2019; 68:919–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968 [DOI] [PubMed] [Google Scholar]
- 13. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200:e70–e88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kundel HL, Polansky M. Measurement of observer agreement. Radiology 2003; 228:303–308 [DOI] [PubMed] [Google Scholar]
- 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159. [PubMed] [Google Scholar]
- 17. Kass AP, Overbeek DL, Chiel LE, et al. Case series: adolescent victims of the vaping public health crisis with pulmonary complications. Pediatr Pulmonol 2020; 55:1224–1236 [DOI] [PubMed] [Google Scholar]
- 18. Carroll BJ, Kim M, Hemyari A, et al. Impaired lung function following e-cigarette or vaping product use associated lung injury in the first cohort of hospitalized adolescents. Pediatr Pulmonol 2020; 55:1712–1718 [DOI] [PubMed] [Google Scholar]
- 19. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med 2020; 382:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes. JAMA 2014; 312:2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kosmider L, Sobczak A, Prokopowicz A, et al. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 2016; 71:376–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.