Abstract
Background: To determine the lung function of children admitted to the intensive care unit (ICU) for a severe asthma exacerbation in the medium- to long-term following hospital discharge.
Methods: We performed a retrospective chart review of children ≥6 years of age admitted to the ICU for a severe asthma exacerbation at a tertiary care center from January 1, 2000, to December 31, 2013. Lung function was ascertained during outpatient follow-up visits at 3–12 months and 12–24 months postdischarge. A total of 72 subjects met the inclusion criteria.
Results: Subjects were predominantly boys (56.9%) and had a mean (standard deviation [SD]) age at admission of 10.3 years (3.4 years). The median (interquartile range) length of stay in the ICU was 1 day (1–3 days). Thirty-eight and 28 subjects performed pulmonary function tests with acceptable technique at the 3–12 months and 12–24 months postdischarge visits, respectively. At 3–12 months, the mean (SD) predicted forced expiratory volume in 1 s (FEV1) and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75) percent were 95.9 (16.7) and 76.7 (25.8), respectively, and 97.4 (17.6) and 70.5 (24.9), respectively, at 12–24 months. FEV1/forced vital capacity (FEV1/FVC) was 81.7 (8.3) at 3–12 months and 79.3 (7.7) at 12–24 months. A paired t-test on 20 subjects who performed acceptable spirometry at both visits showed a significant intraindividual decrease in FEV1 (P = 0.008), FEF25–75 (P = 0.02), and FEV1/FVC (P = 0.01) between the 2 time points.
Conclusion: Although prospective studies are required to confirm our findings, our study suggests that children admitted to the ICU for severe asthma exacerbations may be at risk for declining pulmonary function in the medium- to long-term postdischarge.
Keywords: pulmonary function testing, severe asthma, critical care, respiratory care
Introduction
Asthma is the most common noncommunicable childhood disease,1 affecting 7.5% of children in the United States2 and 9.4% of Canadian children aged 12–17 years in 2018.3 With more than 6,000 hospitalizations for asthma among Canadians younger than 20 years in 2015–2016 and 75 hospitalizations per 100,000 population in 2018, asthma remains a leading cause of hospital admissions, despite a decrease over the past decade.4 Of all children hospitalized for asthma, 6%–34% are admitted to the intensive care unit (ICU) for severe respiratory distress.5–7 The Global Initiative for Asthma (GINA) specifically identifies ICU admission as a risk factor for certain adverse asthma outcomes.8 Specifically, children admitted to the ICU for asthma are at higher risk for subsequent readmissions for asthma7,9 and asthma-related death10 and have a shorter time to readmission.11
Multiple studies documented genetic associations with severe asthma exacerbations,12,13 suggesting that children with severe exacerbations may represent a distinct clinical phenotype. It is well known that severe asthma exacerbations can lead to complications such as respiratory failure, pneumothorax, circulatory compromise, or death.14 Furthermore, recent studies suggest that the occurrence of severe asthma exacerbations is associated with an accelerated loss of lung function over time.15,16 However, little is known about the direct long-term effect of a single severe asthma exacerbations requiring ICU admission on subsequent pulmonary function tests. Indeed, despite the known high morbidity and mortality of children with a previous asthma-related ICU admission, few studies have examined the long-term functional outcomes of these children, particularly with regard to lung function. Given that disturbed lung function noted within the first 6 years of life tends to persist into adulthood17 and that repeated exacerbations may further decrease lung function, it is important to ascertain the trajectory of the pulmonary function of these children. Timely multifaceted interventions may be implemented to avoid further decline.
In this study, we documented the lung function of children following a severe asthma exacerbation requiring ICU admission at 3–12 months and 12–24 months after discharge. We also examined the changes in pulmonary function between the 2 time points.
Methods
Patients and study design
We retrospectively reviewed the medical records of all children ≥6 years of age admitted to the ICU of a tertiary care university hospital (CHU Sainte-Justine, Montreal, Canada) for a severe asthma exacerbation from January 1, 2000, to December 31, 2013 (defined as the index hospitalization). Our institution follows the Canadian Paediatric Society guidelines regarding criteria for admission to the ICU for asthma exacerbations.18 These patients were identified by their primary hospital discharge diagnosis of asthma (International Classification of Diseases-10 code J45 or J46). The minimum age criterion was chosen to restrict the study population to children who were most likely able to cooperate for spirometry testing.8 Children with complex medical conditions19 were excluded. This study was approved by the local institution's Research Ethics Board.
Ascertainment of lung function
The primary outcome was lung function as measured by forced expiratory volume in 1 s (FEV1) at 3–12 months and 12–24 months following the index ICU admission. Secondary outcomes include other spirometric measures, including FEV1/forced vital capacity (FEV1/FVC) and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75), at the same time points. These pragmatic time points were chosen as most patients had an outpatient clinic visit at 3–12 months and 12–24 months postdischarge. Spirometry was routinely performed during outpatient clinic visits by trained respiratory therapists according to the American Thoracic Society (ATS) guidelines.20 Reference values from the study of Stanojevic et al. were used.21
Statistical analysis
A descriptive analysis of the demographic and clinical characteristics at ICU admission of the study cohort and the pulmonary function (FEV1, FEV1/FVC, and FEF25–75) at 3–12 months and 12–24 months postdischarge was performed. We performed a Student's t-test to compare pulmonary function measures between the 2 follow-up visits. Furthermore, a paired t-test was performed to evaluate intraindividual changes among those who performed spirometry at both visits. To assess selection bias, subjects who performed acceptable spirometry at both visits were compared with those who performed acceptable spirometry at 1 visit only. The Student's t-test, Fisher's exact test, or the Mann–Whitney U test was used depending on the nature and normality of the data.
Results
Eighty-five children were admitted to the ICU for a severe asthma exacerbation during the study period. Thirteen patients with complex medical conditions were excluded. Baseline characteristics of the remaining 72 subjects are presented in Table 1. The mean age at admission was 10.3 years with a standard deviation (SD) of 3.4, and with 56.9% of patients being male. The median length of stay in the ICU and the hospital was 1 and 5 days, respectively, with interquartile range (IQR) of 1–3 and 3–7, respectively. The majority of our patients were already known for asthma (91.7%) and had a history of allergies (69.4%). There were few complications with 13.9% of subjects needing intubation with mechanical ventilation and 4.2% having experienced a pneumothorax. There were no deaths in our cohort. Furthermore, every patient left the hospital with a prescription of daily inhaled corticosteroids alone or in conjunction with a long-acting β-agonist (Supplementary Table S1).
Table 1.
Demographic and Clinical Characteristics of Children Admitted to the Intensive Care Unit for Asthma Meeting the Study Criteria
| Total (N = 72) | |
|---|---|
| Demographic and patient characteristics | |
| Male, n (%) | 41 (56.9) |
| Age, years, mean (SD) | 10.3 (3.4) |
| Previous diagnosis of asthma, n (%) | 66 (91.7) |
| Eczema, n (%) | 23 (31.9) |
| Allergies, n (%) | 50 (69.4) |
| Maternal asthma, n (%) | 22 (30.6) |
| Paternal asthma, n (%) | 18 (25.0) |
| Clinical characteristics during hospitalization | |
| Intubation, n (%) | 10 (13.9) |
| Pneumothorax, n (%) | 3 (4.2) |
| Death, n (%) | 0 (0) |
| Daily ICS alone or with LABA at discharge, n (%) | 72 (100) |
| Length of stay, days, median (IQR) | 5 (3–7) |
| Length of stay in the ICU, days, median (IQR) | 1 (1–3) |
ICS, inhaled corticosteroids; IQR, interquartile range; LABA, long-acting β-agonist; SD, standard deviation.
A total of 48 (67%) patients had an outpatient follow-up visit in the respiratory medicine clinic of our institution between 3 and 12 months after their initial ICU admission for severe asthma exacerbation. The median (IQR) follow-up for this visit was 3.7 months (2.0–4.8 months). Of these children, 38 (79%) were able to perform a spirometry meeting the ATS acceptability and repeatability criteria. The median (IQR) follow-up for the second visit at 12–24 months is 15.0 months (13.7–17.6 months). Of the 38 patients who had a 12–24 months visit after the initial hospitalization, 28 (74%) performed spirometry meeting the criteria. A total of 20 subjects had acceptable spirometry at both visits. The ICU admissions and follow-up visits were distributed fairly equally between the seasons (Supplementary Table S2).
FEV1 and other spirometric measures
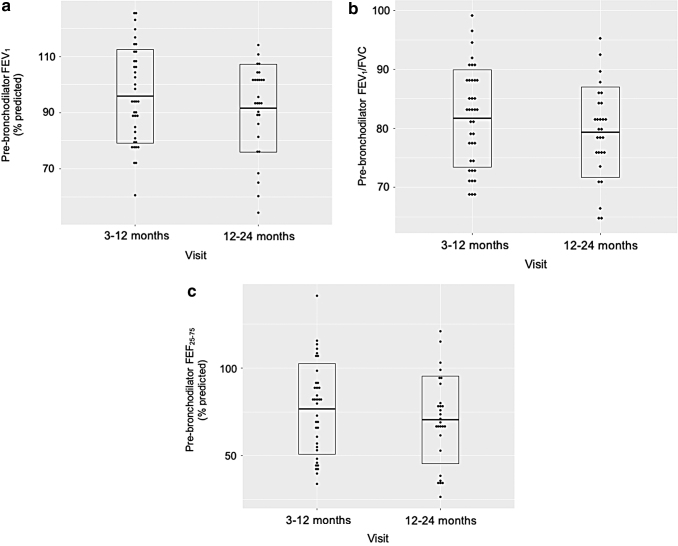
Spirometry results of subjects are shown for both visits in Table 2 (see Supplementary Table S3 for individual values). The mean (SD) FEV1 percent predicted at 3–12 months and 12–24 months were 95.9 (16.7) and 97.4 (17.6), respectively. Eight (21.0%) patients and 6 (21.4%) patients had an FEV1 <80% predicted at the 2 follow-up visits, respectively. While the change in FEV1 between the 2 visits was not significant when subjects were grouped (P = 0.29), there was a significant intraindividual decrease in FEV1 for the 20 subjects who had a measure at both time points (P = 0.008; Fig. 1a).
Table 2.
Spirometry of Patients with Follow-Up Visits at 3–12 Months and 12–24 Months
| All subjects |
Subjects with spirometry at both visits |
|||||
|---|---|---|---|---|---|---|
| 3–12 Months (n = 38) | 12–24 Months (n = 28) | Group P-value (nonpaired) | 3–12 Months (n = 20) | 12–24 Months (n = 20) | P-value (paired) | |
| FEV1 | ||||||
| Percent predicted FEV1, mean (SD) | 95.9 (16.7) | 97.4 (17.6) | 0.29 | 96.7 (18.1) | 89.7 (17.1) | 0.008 |
| FEV1 <80%, n (%) | 8 (21.0) | 6 (21.4) | — | 4 (20.0) | 5 (25.0) | — |
| FEV1/FVC | ||||||
| FEV1/FVC, mean (SD) | 81.7 (8.3) | 79.3 (7.7) | 0.24 | 83.9 (7.0) | 78.3 (7.8) | 0.01 |
| FEV1/FVC <80%, n (%) | 16 (42.1) | 14 (50.0) | — | 5 (25.0) | 10 (50.0) | — |
| FEF25–75 | ||||||
| Percent predicted FEF25–75, mean (SD) | 76.7 (25.8) | 70.5 (24.9) | 0.33 | 80.9 (23.3) | 66.2 (24.2) | 0.02 |
| FEF25–75 <70%, n (%) | 16 (42.1) | 14 (50.0) | — | 6 (30.0) | 11 (55.0) | — |
FEF25–75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expiratory volume in 1 s; FEV1/FVC, FEV1/forced vital capacity; ICU, intensive care unit; IQR, interquartile range.
FIG. 1.
Trajectory and distribution of (a) pre-bronchodilator FEV1; (b) pre-bronchodilator FEV1/FVC; and (c) pre-bronchodilator FEF25–75 in patients with acceptable spirometry at both follow-up visits at 3–12 months and 12–24 months (n = 20). FEF25–75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expiratory volume in 1 s; FEV1/FVC, FEV1/forced vital capacity.
The mean (SD) FEV1/FVC was 81.7 (8.3) at 3–12 months and 79.3 (7.7) at 12–24 months, with 16 (42.1%) and 14 (50%) subjects having an FEV1/FVC <80% at the 2 visits, respectively. The mean (SD) FEF25–75 percent predicted was 76.7 (25.8) at 3–12 months and 70.5 (24.9) at 12–24 months, with 16 (42.1%) and 14 (50.0%) subjects having an FEF25–75 <70% at the 2 visits, respectively. Similar to FEV1, the changes in FEV1/FVC and FEF25–75 between the 2 visits were not significant when subjects were grouped (P = 0.24 for FEV1/FVC and P = 0.33 for FEF25–75). However, significant intraindividual decreases in FEV1/FVC (P = 0.01) and FEF25–75 (P = 0.02) were observed between the 2 follow-up visits (Fig. 1b, c). The baseline characteristics of children who had acceptable spirometry at both visits (n = 20) were comparable to those of children who only had 1 acceptable spirometry and thus not included in the paired analysis (Supplementary Table S4). The baseline characteristics were also comparable between children who had stable or improving vs declining lung function between the two visits (Supplementary Table S5).
Discussion
In this study, we retrospectively examined the pulmonary function of children ≥6 years of age admitted to the ICU for a severe asthma exacerbation at a tertiary care center over a period of 14 years, at 3–12 months and 12–24 months postdischarge. We noted that a large proportion of patients had an obstructive pattern with an FEV1/FVC ratio <80% at both follow-up visits (42.1% and 50% at the respective visits). Furthermore, we documented a significant intraindividual decrease in FEV1, FEV1/FVC, and FEF25–75 between these 2 time points.
As a group, children in our study had a normal mean FEV1 of 95.9% and 97.4% predicted at 3–12 months and 12–24 months, respectively, and ∼80% of children had an FEV1 ≥80% predicted at both time points. However, a large proportion of children had an FEV1/FVC <80% and FEF25–75 <70% at both visits, suggesting that these may be a more sensitive measures of airway obstruction than FEV1 alone.22 While there were no significant changes in the lung function when children were compared as a group across the 2 time points, intraindividual trajectories showed a declining lung function. This observation may reflect the large range of lung function among the subjects within each time point and the fact that the mean lung function measures across the time points may not be sensitive enough to detect intraindividual changes. While children with lower lung function at the 3–12 months visit may be preferentially followed thereafter, baseline characteristics of children who had and did not have a 12–24 months visit are similar. This finding supports the hypothesis of declining lung function in the long-term following a severe asthma exacerbation requiring ICU admission.
Several studies support our results of a decline in lung function following a severe exacerbation. Two studies in adults suggested a strong association between the frequency of severe asthma exacerbations and an excess decline in lung function over time, associated with more severe airway obstruction15,23 and reduced post-bronchodilator response.23 Furthermore, 2 small studies performed serial spirometry measurements in children with asthma following an acute moderate-to-severe exacerbation. An initial improvement in lung function, as measured by FEV1 and FEF25–75 percent predicted24,25 and peak expiratory flow,24 was followed by a plateau within 1–4 weeks after hospital discharge. Having a severe exacerbation was associated with a longer time to recovery of lung function.24 As part of a large randomized controlled trial of budesonide versus placebo in early asthma, a subgroup analysis also documented a statistically significant decline in mean post-bronchodilator FEV1 over a 3-year period in children aged 5–10 years who had at least 1 severe asthma-related event, compared with those who did not.26 It is important to note that these studies defined severe exacerbations as an event requiring hospitalization or emergency treatment due to worsening of asthma. However, our study focused on those admitted to the ICU. Thus, the children in our study may be even more vulnerable to subsequent morbidity as they represent the sickest children with severe exacerbations.
Various factors have been correlated with an excessive decline in lung function among asthmatic patients, including male gender, older age, smoking history, and longer duration of disease.27 However, the excess loss of lung function following severe exacerbation could be explained by a pathological enhancement of airway remodeling caused by the intermittent worsening of airway inflammation occurring during asthma exacerbations.16 However, given that patients admitted to the ICU for severe asthma exacerbation may be more prone to having a more severe chronic underlying inflammation,15 we cannot ascertain that the decline in lung function seen in follow-up visits is solely due to the exacerbation, as the severity of their asthma and other patient factors may have a role to play in this finding. More importantly, independent of whether lung function deteriorated following ICU admission or ICU admission being a marker of severe illness, our data suggest that patients admitted to the ICU for severe asthma exacerbations are at higher risk of lung function decline and need to be identified to optimize their care.
Little is known about the trajectory of other physiological and more objective measures such as pulmonary function in the medium- to long-term after their discharge from the initial admission to the ICU asthma. In addition to being an objective measure, airflow limitation within the first 6 years of life persists into adulthood17 and has been directly linked to increased mortality in adults with asthma.28 Furthermore, repeated exacerbations, for which these children are at risk, significantly predict excess FEV1 decline in adults with asthma15 and lead to impaired maximally attained lung function.29 Patients with an early decline in their lung function are at risk for early development of chronic obstructive pulmonary disease (COPD) or asthma-COPD overlap syndrome.30,31 Thus, the identification of children at risk for lower lung function may provide an opportunity to implement multifaceted and timely interventions targeted at preventing further lung function decline. In addition to lung function, other patient-oriented outcomes such as quality of life may be interesting to explore in children following a severe exacerbation requiring ICU admission.
Our study has strengths and limitations. Despite the limited sample size of our cohort, we were able to document the trajectory of lung function in a subset of children up to a median of 15 months following the initial ICU hospitalization. While other studies are needed to confirm our findings, the decreasing lung function over time in these children is concerning. Notable limitations of this study include a potential selection bias, as only children followed at our hospital were studied. However, it is important to note that our center is the referring institution for a large catchment area, and therefore, children admitted to the ICU for asthma are systematically referred to our respirology team for follow-up. Additionally, due to the retrospective nature of this study, important factors potentially affecting lung function could not be analyzed, including medication prescription and adherence, clinical and socioeconomic characteristics, baseline lung function, and environmental triggers. Furthermore, we did not have data on pre- and post-hospitalization asthma control, patient-oriented outcomes such as quality of life, and post-bronchodilator spirometry results, which could be correlated with pulmonary function tests following ICU admission. Finally, the lack of a control group in our study does not allow us to attribute the lung function decline to the severe asthma exacerbation. However, adult studies have demonstrated an excess in lung function decline following a severe exacerbation, thus corroborating our findings.
Conclusion
Our study suggests that children admitted to the ICU for a severe asthma exacerbation may be at risk for declining lung function several months to years following their initial hospitalization. If our findings are confirmed by larger prospective studies, these children would warrant a long-term follow-up and may benefit from targeted interventions to prevent subsequent complications.
Supplementary Material
Authors' Contributions
S.M. contributed to the data analysis and writing of the article. K.V. contributed to the data collection, data analysis, and writing of the article. S.M.T. contributed to the design, analysis, writing of the article, and supervision of the research. All the authors contributed to the review of the article. The authors alone are responsible for the content and writing of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. World Health Organization. 2020 Asthma. https://www.who.int/news-room/fact-sheets/detail/asthma (accessed September1, 2020)
- 2. Centers for Disease Control and Prevention. 2020 Most Recent National Asthma Data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed September1, 2020)
- 3. Statistics Canada. 2020 Asthma, by age group. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009608 (accessed September1, 2020)
- 4. Asthma Canada. 2018 Asthma hospitalizations drop for children and youth, but inequalities persist. https://asthma.ca/asthma-hospitalizations-drop-for-children-%EF%BB%BFand-youth-but-inequalities-persist (accessed September1, 2020)
- 5. Hasegawa K, Ahn J, Brown MA, et al. Underuse of guideline-recommended long-term asthma management in children hospitalized to the intensive care unit: a multicenter observational study. Ann Allergy Asthma Immunol 2015; 115:10–16 e1. [DOI] [PubMed] [Google Scholar]
- 6. Kenyon CC, Melvin PR, Chiang VW, et al. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr 2014; 164:300–305 [DOI] [PubMed] [Google Scholar]
- 7. Triasih R, Duke T, Robertson CF. Outcomes following admission to intensive care for asthma. Arch Dis Child 2011; 96:729–734 [DOI] [PubMed] [Google Scholar]
- 8. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020. www.ginasthma.org (accessed May9, 2020)
- 9. Visitsunthorn N, Lilitwat W, Jirapongsananuruk O, et al. Factors affecting readmission for acute asthmatic attacks in children. Asian Pac J Allergy Immunol 2013; 31:138–141 [DOI] [PubMed] [Google Scholar]
- 10. To T, Dell S, Dick P, et al. The burden of illness experienced by young children associated with asthma: a population-based cohort study. J Asthma 2008; 45:45–49 [DOI] [PubMed] [Google Scholar]
- 11. Tse SM, Samson C. Time to asthma-related readmission in children admitted to the ICU for asthma. Pediatr Crit Care Med 2017; 18:1099–1105 [DOI] [PubMed] [Google Scholar]
- 12. Bonnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 2014; 46:51–55 [DOI] [PubMed] [Google Scholar]
- 13. Xu M, Tantisira KG, Wu A, et al. Genome Wide Association Study to predict severe asthma exacerbations in children using random forests classifiers. BMC Med Genet 2011; 12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDowell KM, Kercsmar CM, Huang B, et al. Medical and social determinants of health associated with intensive care admission for asthma in children. Ann Am Thorac Soc 2016; 13:1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai TR, Vonk JM, Postma DS, et al. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 2007; 30:452–456 [DOI] [PubMed] [Google Scholar]
- 16. Matsunaga K, Ichikawa T, Oka A, et al. Changes in forced expiratory volume in 1 second over time in patients with controlled asthma at baseline. Respir Med 2014; 108:976–982 [DOI] [PubMed] [Google Scholar]
- 17. Grad R, Morgan WJ. Long-term outcomes of early-onset wheeze and asthma. J Allergy Clin Immunol 2012; 130:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ortiz-Alvarez O, Mikrogianakis A, Canadian Paediatric Society, et al. 2012 Managing the paediatric patient with an acute asthma exacerbation. Paediatr Child Health 2012; 17:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26:319–338 [DOI] [PubMed] [Google Scholar]
- 21. Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med 2008; 177:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riley CM, Wenzel SE, Castro M, et al. Clinical implications of having reduced mid forced expiratory flow rates (FEF25–75), independently of FEV1, in adult patients with asthma. PLoS One 2015; 10:e0145476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsunaga K, Hirano T, Oka A, et al. Progression of irreversible airflow limitation in asthma: correlation with severe exacerbations. J Allergy Clin Immunol 2015; 3:759–764.e1. [DOI] [PubMed] [Google Scholar]
- 24. Yilmaz O, Bakirtas A, Ertoy Karagol HI, et al. Allergic rhinitis may impact the recovery of pulmonary function tests after moderate/severe asthma exacerbation in children. Allergy 2014; 69:652–657 [DOI] [PubMed] [Google Scholar]
- 25. Debley JS, Cochrane ES, Redding GJ, et al. Lung function and biomarkers of airway inflammation during and after hospitalization for acute exacerbations of childhood asthma associated with viral respiratory symptoms. Ann Allergy Asthma Immunol 2012; 109:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Byrne PM, Pedersen S, Lamm CJ, et al. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med 2009; 179:19–24 [DOI] [PubMed] [Google Scholar]
- 27. Lee JH, Haselkorn T, Borish L, et al. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: insights from the TENOR Study. Chest 2007; 132:1882–1889 [DOI] [PubMed] [Google Scholar]
- 28. Huang S, Vasquez MM, Halonen M, et al. Asthma, airflow limitation and mortality risk in the general population. Eur Respir J 2015; 45:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med 2016; 374:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bui DS, Burgess JA, Lowe AJ, et al. Childhood lung function predicts adult chronic obstructive pulmonary disease and asthma-chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med 2017; 196:39–46 [DOI] [PubMed] [Google Scholar]
- 31. Tai A, Tran H, Roberts M, et al. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax 2014; 69:805–810 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.