Abstract
Spinal metastases are most commonly osseous and may extend to the epidural space. Less commonly, spinal metastases can be subdural, leptomeningeal, or intramedullary. Among these, subdural metastases are the most rare, with few reported cases. While these lesions are now almost exclusively detected on MRI, they can rarely be apparent on other modalities. It is important to recognize subdural metastases on any modality, because they have a significant impact on patient prognosis and treatment. We report a case of renal cell carcinoma in a 68-year-old male initially presenting with subdural metastases detected on CT myelography, with subsequent confirmation by MRI. The case illustrates, to our knowledge, the first example of subdural metastatic disease seen on CT myelography.
Keywords: Spinal subdural metastases, CT myelography, Intradural metastases, Extramedullary metastases
Introduction
The spine is a relatively common location for metastatic disease. The vast majority of spinal metastases are osseous. However, intramedullary and extramedullary intradural metastases can occur more rarely. This includes subdural metastases, which we define as lesions that are intradural, extramedullary, and associated specifically with the dura rather than intrathecal structures such as nerve roots or leptomeninges. Subdural spinal metastases are exceptionally rare and the least common of spinal metastases. In one study of patients with a total of 220 spinal metastases, fewer than 3% were both intradural and extramedullary, and it is uncertain if any of these were specifically subdural [1]. Very few individual cases of subdural spinal metastatic disease have been previously reported, and prior cases have mainly been demonstrated on MRI [2]. One remote report described subdural spinal metastases on conventional myelography [3]. Another showed a confluent intrathecal tumor on CT myelography; however, this was felt to be related to tumor seeding from prior epidural metastasis resection rather than de novo metastatic disease [4]. To our knowledge, discrete subdural metastases have not previously been reported on CT myelography during the initial presentation of metastatic disease, likely because most patients undergo MRI as the first test of choice. We report the first patient with subdural metastases initially detected on CT myelography.
Case report
A 68-year-old male with two months of shoulder and groin pain and no other significant past medical history presented with acute lower extremity weakness, urinary retention, and severe back pain. Initially, there was clinical concern for cauda equina syndrome. Non contrast CT of the thoracolumbar spine was obtained to exclude any acute abnormality. This showed numerous osseous vertebral lesions suspicious for metastases without any definite spinal canal narrowing (Fig. 1). Subsequent MRI of the thoracolumbar spine was requested, but the patient was unable to tolerate the exam due to pain. Therefore, an emergent CT myelogram was performed. This showed diffuse spinal metastatic disease, including widespread subdural implants in the thoracic spinal canal (Fig. 2). While there was no substantial spinal canal narrowing, corticosteroids were given due to continued clinical concern for cauda equina syndrome. MRI was subsequently performed as the patient improved clinically and was able to tolerate the exam. This showed numerous enhancing subdural spinal lesions, confirming the myelographic findings (Fig. 3). Multiple vertebral body metastases were also present (Fig. 4). CT of the chest, abdomen, and pelvis demonstrated a large solidly enhancing right renal mass as well as diffuse pulmonary metastases (Fig. 5). The patient underwent transbronchial biopsy of an enlarged mediastinal lymph node, which was metastatic renal cell carcinoma. He was referred to medical oncology for further management, having opted not to receive radiation therapy or undergo surgery. He was started on cabozantinib immunotherapy with initial improvement in his back pain but was subsequently lost to follow-up.
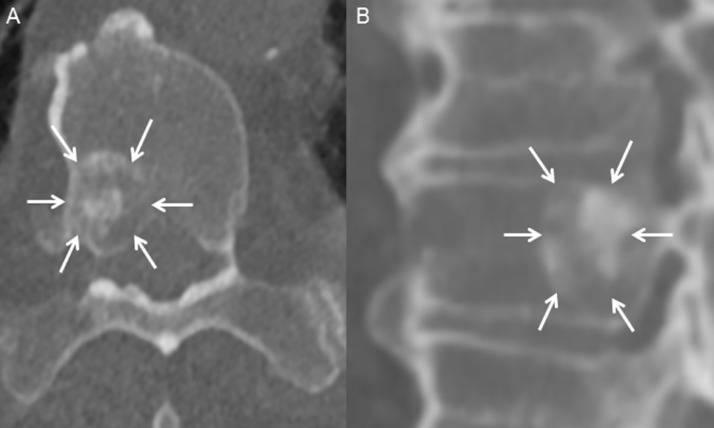
Fig. 1.
Axial (A) and sagittal (B) noncontrast computed tomography images through T11 show a sclerotic vertebral body lesion (A and B, arrows), likely representing a metastasis in this patient with renal cell carcinoma. Multiple similar lesions were present throughout the spine.
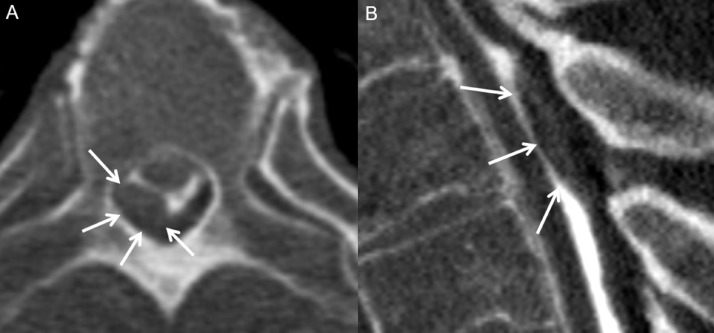
Fig. 2.
Axial (A) and sagittal (B) images through the mid thoracic spine from the patient's CT myelogram show a representative subdural nodular filling defect (A and B, arrows), consistent with a metastasis. Multiple similar lesions were present at other levels.
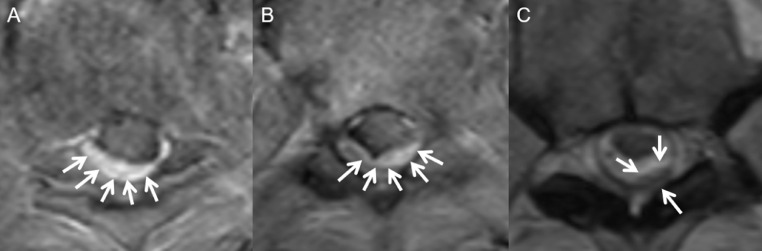
Fig. 3.
Axial T1W postcontrast MR image at two levels in the mid thoracic spine (A and B) show discrete nodular enhancing subdural lesions (A and B, arrows), consistent with metastases. Axial T2W image through the mid thoracic spine at a different level (C) shows a separate T2 hypointense subdural metastasis.
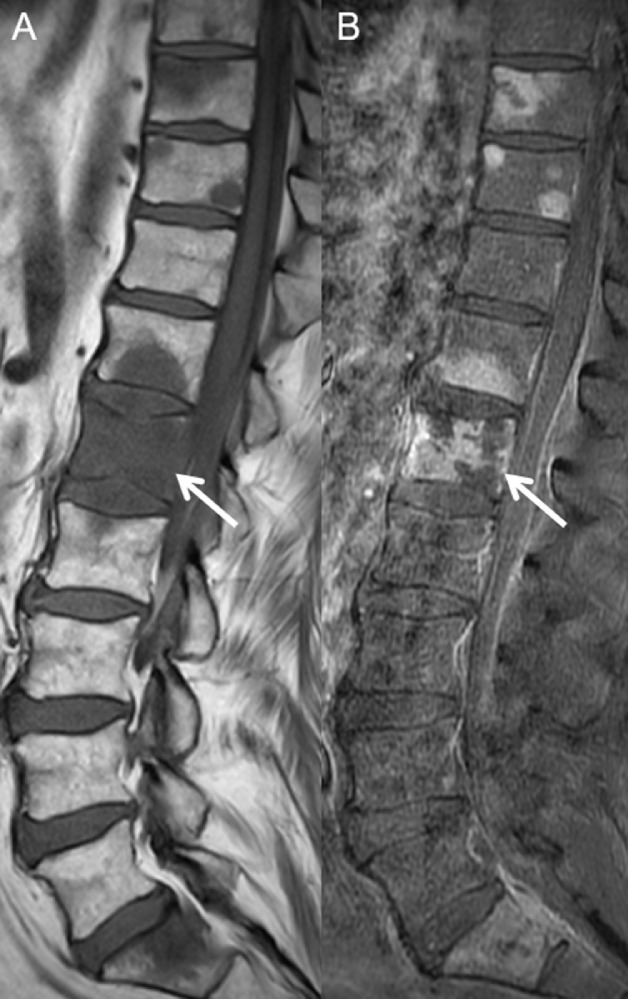
Fig. 4.
Sagittal T1W precontrast (A) and postcontrast (B) MR images of the lumbar spine show T1 hypointense marrow-replacing vertebral body lesions with enhancement, most prominently in L1 (A and B, arrows), consistent with metastases.
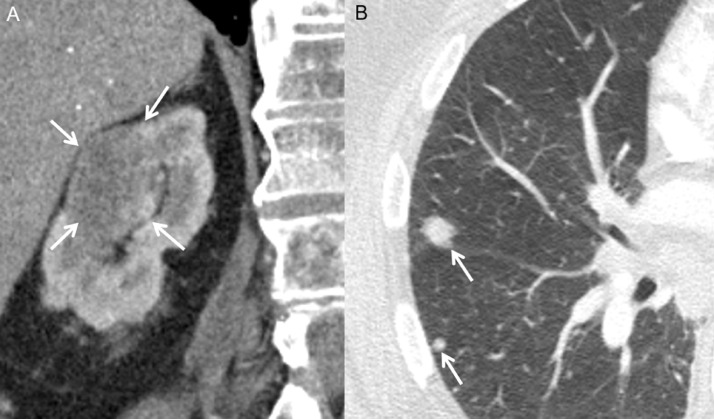
Fig. 5.
Coronal contrast enhanced CT image through the abdomen (A) and axial image through the chest (B) show a large right renal mass (A, arrows) and pulmonary nodules (B, arrows).
Discussion
Subdural metastases are exceptionally rare and not well studied in the literature. They can be caused by a variety of primary tumors, though there is a paucity of data in this regard. For intradural metastases in general, CNS tumors are a common source in young patients [5]. In adults, the most common sources are lung, breast, and renal cell carcinoma. Subdural spinal metastases can have variable clinical presentations. Subdural metastases are most often discovered incidentally, because they usually occur only in patients with advanced metastatic disease. However, mass effect from the lesions can cause myelopathic symptoms, including pain, bilateral weakness, and sensory deficits [6]. Bladder dysfunction has also been noted in several cases of intradural metastases, although several of these were intramedullary [7]. These symptoms may occur gradually or have an acute onset, with the latter being seen in our patient's case. Notably, our patient did not have significant spinal stenosis from his metastases, highlighting that imaging findings do not necessarily correlate with symptoms.
On CT myelography, subdural metastases appear as intradural, extramedullary nodular filling defects separate from adjacent nerve roots. There should not be any displacement of the dura towards the spinal cord or cauda equina, as this finding would be more suggestive of an extradural or trans-spatial lesion. With large lesions, filling defects may be appreciated on conventional myelographic images [3]. On spine MRI, similar intradural, extramedullary lesions are expected. Signal characteristics are variable depending on the primary tumor of origin and the presence and age of intralesional blood products. Gadolinium enhancement is helpful in corroborating the diagnosis when present but can be absent depending on the tumor type [2]. As demonstrated by our patient's case, CT myelography can accurately identify these lesions in patients with a contraindication to or inability to undergo MRI. While there are no data regarding the sensitivity of MRI vs CT myelography for detecting subdural metastases specifically, there is evidence that some intradural lesions are better characterized by CT myelography due to its superior spatial resolution [8].
Multiple subdural spinal lesions encountered on imaging have a limited differential. These include su bdural metastases, syndromic meningiomas, lymphoma, tuberculosis, and sarcoidosis (Table) [9], [10], [11]. Peripheral nerve sheath tumors are not included in this differential as they are not strictly subdural.
Table.
Selected differential diagnoses for multiple spinal subdural lesions.
| Differential Diagnosis | Precontrast MRI (Spine) | Postcontrast MRI (Spine) | Noncontrast CT (Body and Spine) | CT myelography (Spine) |
|---|---|---|---|---|
| Subdural metastases | - Multiple nodular subdural lesions. - Variable T1 and T2 signal depending on primary tumor and presence/age of hemorrhage. |
- Enhancement usually seen, but absence of enhancement does not exclude metastases. | - Osseous lytic or sclerotic metastases often seen in patients with subdural metastases. - Dural-based lesions of variable attenuation may be seen. |
- Nodular subdural filling defects +/- displacement of the spinal cord. |
| Multiple meningiomas | - Broad dural base. - Often T2 hypointense. - Variable T1 signal. |
- Usually homogenously enhance. - Enhancing dural tail. |
- Often hyperattenuating. - Intralesional calcification and adjacent hyperostosis are uncommon in the spine. |
- Filling defects with a broad dural base. |
| Sarcoidosis | - Dural, leptomeningeal, or intramedullary lesions. - Slightly T2 hypointense with variable T1 signal. |
- Frequently enhance in a nodular pattern. | - Hilar or mediastinal adenopathy and perilymphatic nodularity in the chest. - Low attenuation hepatic and splenic lesions. - Spine imaging usually unremarkable, but can see lytic osseous lesions. |
- Dural-based. - Nodular thickening of the cauda equina. |
| Lymphoma | - Dural, leptomeningeal, or intramedullary lesions. - Usually T2 hypointense; can be T2 hyperintense less commonly. - Cervical > thoracic > lumbar spine involvement. |
- Homogenous contrast enhancement. | - Adenopathy variably involving the neck, chest, and abdomen/pelvis. - Often hyperattenuating. - Spine imaging usually unremarkable, but can see lytic osseous lesions. |
- Dural-based. - Nodular thickening of the cauda equina. |
| Tuberculosis | - Thoracolumbar junction most common. - Vertebral and paravertebral involvement are common with characteristic sparing of the intervertebral disc. - Intradural disease, though less common, can occur. - Marrow-replacing vertebral body signal abnormality. |
- Variable enhancement of inflammatory lesions. - Peripheral enhancement of associated abscesses. |
- Low-attenuation lymphadenopathy throughout the body. - Cavitary lesions, consolidation, tree-in-bud micronodularity, or miliary nodularity in the chest. - “Fragmented” vertebral body destruction, often at the highly vascular anterior endplate corners. |
- Nodular dural filling defects and/or cauda equina thickening may be seen in rare cases of intradural involvement. |
Abbreviations: MRI, magnetic resonance imaging; CT, computed tomography
Treatment of subdural metastases depends on the presence and severity of neurologic symptoms and the extent of metastatic disease. For acute presentations with evidence of cauda equina syndrome or cord compression, surgical treatment may be necessary [12,13]. Prior to surgical intervention, corticosteroids are commonly administered as a temporizing measure. In the setting of malignant cord compression, adjunct radiation is also often used [13], [14], [15]. If no acute neurologic deficit is present or if subdural metastases are discovered incidentally, no immediate treatment is necessary [16]. These patients typically undergo multidisciplinary evaluation to determine the best available treatment options, which can include chemotherapy, radiation, or surgery.
The prognosis of subdural metastatic disease is not well studied but generally considered relatively poor, mainly because subdural metastases are indicative of widespread metastatic disease [17]. No large studies of patients with subdural metastases have been performed, but one study of 22 patients with intramedullary spinal cord metastases, which are more common than subdural disease, found a median survival time of 11.6 months [18]. Overall, given the prognostic implications and treatment impact of subdural spinal metastases, it is important to detect them when possible.
Patient Consent: Verbal consent was obtained prior to inclusion of any identifying information or images and informed consent was obtained from the patient to use unidentifiable medical information for research purposes.
Footnotes
Paper previously presented at the Annual Meeting of the American Society of Spine Radiology, Feb 12-16, 2020; Dana Point, CA.
Acknowledgments: No funding was received for this study.
Competing Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Schick U, Marquardt G, Lorenz R. Intradural and extradural spinal metastases. Neurosurg Rev. 2001;24:1–5. doi: 10.1007/pl00011959. discussion 6-7. 2001/05/08DOI: [DOI] [PubMed] [Google Scholar]
- 2.Rumboldt Z, Lambert L, Talan-Hranilovic J. Nonenhancing spinal subdural metastatic tumor. AJNR Am J Neuroradiol. 2005;26:2406–2409. 2005/10/13. [PMC free article] [PubMed] [Google Scholar]
- 3.Dietemann J.L., Babin E., Maitrot D., Medjek L., Edel L., Wackenheim A. Spinal subdural metastasis. A case report. Neuroradiology. 1982;24:115–116. doi: 10.1007/BF00339202. [DOI] [PubMed] [Google Scholar]
- 4.Mak KH, Kwok JC. Intradural spinal metastasis from renal cell carcinoma: a case report. J Orthop Surg (Hong Kong) 2001;9:57–61. doi: 10.1177/230949900100900212. 2002/07/16DOI: [DOI] [PubMed] [Google Scholar]
- 5.Mirimanoff RO, Choi NC. Intradural spinal metastases in patients with posterior fossa brain metastases from various primary cancers. Oncology. 1987;44:232–236. doi: 10.1159/000226484. 1987/01/01DOI: [DOI] [PubMed] [Google Scholar]
- 6.Ralapanawa DM, Jayawickreme KP, Ekanayake EM. Spinal intradural metastasis from scapular Ewing sarcoma. BMC Res Notes. 2015;8:298. doi: 10.1186/s13104-015-1263-0. 2015/07/15DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jost G, Zimmerer S, Frank S. Intradural spinal metastasis of renal cell cancer. Report of a case and review of 26 published cases. Acta Neurochir (Wien) 2009;151:815–821. doi: 10.1007/s00701-009-0358-6. discussion 821. 2009/05/06DOI: [DOI] [PubMed] [Google Scholar]
- 8.Karnaze MG, Gado MH, Sartor KJ. Comparison of MR and CT myelography in imaging the cervical and thoracic spine. AJR Am J Roentgenol. 1988;150:397–403. doi: 10.2214/ajr.150.2.397. 1988/02/01DOI: [DOI] [PubMed] [Google Scholar]
- 9.Smith JK, Matheus MG, Castillo M. Imaging manifestations of neurosarcoidosis. AJR Am J Roentgenol. 2004;182:289–295. doi: 10.2214/ajr.182.2.1820289. 2004/01/23DOI: [DOI] [PubMed] [Google Scholar]
- 10.Moorthy S, Prabhu NK. Spectrum of MR imaging findings in spinal tuberculosis. AJR Am J Roentgenol. 2002;179:979–983. doi: 10.2214/ajr.179.4.1790979. 2002/09/20DOI: [DOI] [PubMed] [Google Scholar]
- 11.Rajgopalan M, Srivastava A, Dhammi IK. Tuberculosis - 'The great masquerader' presenting as a dumb-bell-shaped intradural extramedullary tumor in a 20-year-old female. J Clin Orthop Trauma. 2017;8:168–170. doi: 10.1016/j.jcot.2016.06.011. 2017/07/20DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach F., Larsen B.H., Rohde K., Borgesen S.E., Gjerris F., Boge-Rasmussen T., Agerlin N., Rasmusson B., Stjernholm P., Sorensen P.S. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien) 1990;107:37–43. doi: 10.1007/BF01402610. [DOI] [PubMed] [Google Scholar]
- 13.Al-Qurainy R, Collis E. Metastatic spinal cord compression: diagnosis and management. BMJ. 2016;353:i2539. doi: 10.1136/bmj.i2539. 2016/05/21DOI: [DOI] [PubMed] [Google Scholar]
- 14.Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 2003;15:211–217. doi: 10.1016/s0936-6555(02)00400-4. 2003/07/09DOI: [DOI] [PubMed] [Google Scholar]
- 15.Klimo P, Jr., Kestle JR, Schmidt MH. Treatment of metastatic spinal epidural disease: a review of the literature. Neurosurg Focus. 2003;15:E1. doi: 10.3171/foc.2003.15.5.1. 2004/08/25DOI: [DOI] [PubMed] [Google Scholar]
- 16.Rider IS, Marra EM. StatPearls; Treasure IslandFL: 2019. Cauda Equina And Conus Medullaris Syndromes. [PubMed] [Google Scholar]
- 17.Land C.F., Bowden B.D., Morpeth B.G., DeVine J.G. Intradural extramedullary metastasis: a review of literature and case report. Spinal Cord Ser Cases. 2019;5:41. doi: 10.1038/s41394-019-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payer S., Mende K.C., Westphal M., Eicker S.O. Intramedullary spinal cord metastases: an increasingly common diagnosis. Neurosurg Focus. 2015;39:E15. doi: 10.3171/2015.5.FOCUS15149. [DOI] [PubMed] [Google Scholar]