Abstract
Significant variability exists in the trajectories of late-life cognitive decline; however, their associated lifestyle factors remain less studied. We examined these trajectories among elderly participants from the recent five waves (at three-year intervals) of the Chinese Longitudinal Healthy Longevity Study (CLHLS) from 2002 to 2014. Participants from this cohort were included if they completed at least four waves of measurements. Mini–Mental State Examination (MMSE) scores, demographics, medical diagnoses (e.g., hypertension, diabetes and heart disease) and lifestyle-related information (e.g., smoking, drinking alcohol and exercise) were collected from participants (N= 2,584; mean age at baseline=73.3) at least four times across 12 years. MMSE scores were entered into a latent class mixed model analysis. Subsequently, demographic, medical and lifestyle predictors were entered into multinomial logistic regression models to predict the trajectories. One of the four emerged classes (no decline) was characterized by an absence of cognitive decline; the other three exhibited various degrees of cognitive decline. The inclusion of lifestyle factors significantly improved the prediction of the different trajectories, above and beyond demographics and medical variables; the ‘no decline’ class was significantly more likely to report exercising regularly. Changes in cognitive functioning across the late-life period are characterized by multiple trajectories. Cognitive decline is not inevitable across the late-life period; the absence of such cognitive decline is partly explained certain lifestyle factors.
Keywords: late-life, cognitive function, lifestyle, latent class mixed model, exercise, cognitive aging
Introduction
There is considerable heterogeneity in the trajectories of cognitive decline during the late-life period. At one extreme, some individuals diagnosed with dementia experience a rapid decline in cognitive functioning [1]. Yet on the other extreme, some individuals exhibited superior cognitive abilities during their late-life period which are comparable or even superior to much younger healthy controls [2], alluding to an absence of age-related cognitive decline among these individuals. Such differences suggest that late-life cognitive trajectories may differ categorically. In relation to this, several growth mixture modeling studies have examined the trajectories of cognitive decline across the late-life period in the general population[3–8], these different trajectories ranged from subgroups of participants who did not evidence a decline in cognitive functioning to those who experienced a sharp and rapid decline, across the late-life period. Some of these studies have also identified biological factors associated with declining trajectories such as APOE ε4 and amyloid-beta positivity status [4] and neuronal density in the locus ceruleus [7].
While these findings were certainly impressive, these trajectories might have been influenced by a significant minority of participants who were experiencing a terminal decline in cognition that is associated with impending death, regardless of its cause [9]. Hence, this terminal decline, arguably, should be considered separately when examining the spectrum of age-related cognitive decline. The inclusion of participants who experienced such terminal decline will skew the trajectories, resulting in a steeper slope of cognitive decline than what would be observed in the average, high life-expectancy individual — typical among populations in the developed countries.
On top of studying these trajectories, it would be helpful from a clinical/public education perspective to look at sociodemographic, health and lifestyle factors that may predispose one to follow a certain trajectory of cognitive decline. In this regard, apart from Yu et al. [7], the lifestyle factors associated with these trajectories were not examined in previous growth mixture modeling studies. In Yu et al.[7], significant differences in cognitive, physical and social activity levels between the different trajectories were observed; the most cognitively resilient group (i.e., absence of decline across time) reported higher levels of cognitive, physical and social activity relative to the other groups. The authors explained that such activity levels are indicators of cognitive reserve— which would be instrumental for the brain to maintain normal levels of functioning despite the cumulative neuropathological burden from various age-related conditions.
The current study aims to build on previous research in two ways. First, we identified the cognitive trajectories, while attempting to minimize the influence of terminal decline on these trajectories. Second, we examined various health-related predictors at baseline, such as pre-existing medical conditions and lifestyle-related variables, that would predict which cognitive trajectory an individual will be on eventually. In this regard, we chose to focus the study of these variables among participants belonging to the most resilient cognitive trajectory. This is motivated by the fact that these factors were relatively less studied on this end of the cognitive functioning spectrum. For the first aim, we hypothesized that changes in cognitive functioning across the late-life period could be characterized by more than one trajectory. As for the latter aim, we hypothesized that lower medical burden (as operationalized by the absence of hypertension, diabetes and heart diseases) and healthier lifestyles (e.g., do not smoke or drink, and exercise regularly) at baseline are associated with the most resilient cognitive trajectory in the late-life period.
Methods
Participants
We used data from the recent five waves of the Chinese Longitudinal Healthy Longevity Study (CLHLS) [10] for the current research. The data was collected from elderly Chinese participants, aged 65 years and above, longitudinally across the five recent waves, each approximately three years apart from the next, from 2002 to 2014. Information regarding participant recruitment and data quality have been described in detail elsewhere [11,12]. From the original dataset of 33,157 participants, we included 2,574 participants who a) had at least four time points of valid data, b) had MMSE scores above normative cut-offs for dementia in the Chinese population [13] and c) did not report a history of stroke. As shown in Table 1, the included and excluded participants differed significantly in all studied variables. Nevertheless, given the large sample sizes, it would be expected that even tiny differences will become statistically significant. Hence it may be more useful to look at the effect sizes. Apart from the relatively large effect sizes from expected age and MMSE scores differences given our inclusion criteria, differences in the other participants’ characteristics were characterized by small effect sizes. Not surprisingly, the included participants were younger, more educated and healthier compared to the excluded ones, given the need to fulfill at least four waves of data collection spanning across nine years or more.
Table 1.
Characteristics of included and excluded participants
| Participants’ Characteristics at baseline | Included (N= 2,584) | Excluded (N=30,573) | Between-group difference statistics |
|---|---|---|---|
| Mean Age at baseline (SD) | 73.3 (7.3) | 88.5 (11.4) | t= 67.9; p< .001; d=.1.30 |
| Sex | |||
| Male (%) | 1,274 (49.3) | 12,622 (41.3) | χ2 =63.2; p<.001; φ = .04 |
| Female (%) | 1,310 (50.7) | 17,951 (58.3) | |
| Mean years of education (SD) | 2.7 (3.7) | 1.9 (3.4) | t= 12.7; p< .001; d=.23 |
| Mean MMSE score (SD) | 27.2 (2.9) | 19.7 (9.6) | t=.39.9; p< .001; d=.80 |
| Urban residence (%) | 995 (38.5) | 13,099 (57.2) | χ2 =18.4; p<.001; φ = .02 |
| Lifestyle | |||
| Smoke (%) | 696 (26.9) | 5,336 (17.5) | χ2 =144.2; p<.001; φ =.07 |
| Drink (%) | 665 (25.7) | 5,638 (18.4) | χ2 =96.0; p<.001; φ = .05 |
| Exercise (%) | 949 (36.7) | 8,385 (27.4) | χ2 =102.2; p<.001; φ = .06 |
| Medical Diagnoses | |||
| Hypertension (%) | 328 (12.7) | 4,401 (14.4) | χ2 =5.6; p=.018; φ = .01 |
| Diabetes (%) | 41 (1.6) | 872 (2.9) | χ2 =14.2; p<.001; φ = .02 |
| Heart Disease (%) | 150 (5.8) | 2,491 (8.1) | χ2 =17.3; p<.001; φ =.02 |
Each of the included participants was assessed an average of 4.35 times. In order to minimize the influence of terminal cognitive decline on the trajectories, for each participant, we removed the most recent time point. This would mean that participants remained alive for at least three years approximately after the final included time point.
Ethical approval for the Chinese Longitudinal Healthy Longevity Survey study was granted by Duke University Health System’s Institutional Review Board, the National Bureau of Statistics of China, and the Ethical Committee of the Social Science Division of Peking University. Written consent was obtained from all participants. The procedures involving experiments on human subjects are done in accord with the ethical standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accord with the Helsinki Declaration of 1975.
Measures
Cognitive functioning was assessed via the MMSE [14]. The MMSE has been used in previous growth mixture modeling studies on age-related cognitive decline [5,6,8]. The MMSE was scored on a 30-point scale, and higher scores corresponded to better cognitive status. It includes 24 items assessing orientation, attention, calculation, recall, and language.
Participants were also administered a questionnaire, in which we extracted sociodemographic information such as age, sex, education(years) and urban residence (city vs. rural), self-reported medical history— specifically relating to previous hypertension, diabetes, and heart disease diagnoses, as well as yes/no responses from three questions: 1) Did you smoke in the past (smoke), 2) Do you drink alcohol at the present time (drink) and 3) Do you do exercises regularly at present (exercise).
Statistical Analyses
Participants’ cognitive functioning trajectories were modeled using latent class mixed models (LCMM). LCMM is an extension of linear mixed models. This linear mixed modeling approach makes it possible to model data from participants with different baseline age and number of time points[15]. In our LCMM, we included age as both as a fixed and random effect, without any baseline covariates. Random intercepts and slopes for age were assumed in the model to allow variability in the baseline MMSE scores and the rate of change. The quadratic splines link function was used to capture the distribution and account for possible ceiling effects[16].
The LCMM analyses were carried out using the R package lcmm to fit one to six latent-class solutions to the data. The best solution was selected after examining fit indices such as the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and considering the solution’s interpretability and parsimony. Lower criterion values indicate better model fit. The adjusted Lo-Mendell-Rubin Likelihood Ratio Test (LMR LRT) was used to assess if adding a class to the model significantly improved the model fit [17]. Next, using the R package nnet, we carried out multinomial logistic regressions on four models in a hierarchical manner, to examine the various predictors of the trajectories. The first model included only the intercept. In the second model, sociodemographic variables such as MMSE scores and participants’ age at baseline, sex, education levels, and urban residence were entered. In the third model, medical diagnoses such as hypertension, diabetes, and heart disease were entered. In the final model, lifestyle variables such as drinking, smoking, and exercise were entered. The likelihood ratio test (LRT) was used compared between the different regression models. All analyses were carried out in R 3.4.0. Statistical significance was set at p<.05.
Results
Late-life cognitive trajectories
Overall, the average rate of decline in MMSE scores across all participants was .39 points/year (SD= .84). The fit statistics of the LCMM solutions are presented in Table 2. The LMR LRT statistic was not significant beyond the four-class model. Hence a six-class model was not tested. The four-class model was selected due to the fact that it had low information criterion values in general, relative to other models As shown in Figure 1, the first class (going from top to bottom) did not evidence a decline in MMSE scores across time; MMSE scores remained relatively high throughout the studied period. On average, the MMSE scores for participants in this class increased at the rate of .06 points/year (SD= .29). To facilitate subsequent reference to this class, this class is henceforth labeled as ‘no decline.’ The second and largest class was characterized by relatively high scores after which the scores appeared to dip slightly. MMSE scores for these participants declined at an average rate of .20 points/year (SD= .60). This class is labeled as ‘minimal decline.’ The third class or ‘moderate decline’ is characterized by a moderate drop in MMSE scores, with the majority of the participants’ scores going below 20 subsequently. The average rate of decline in MMSE scores for this class was .65 points/year (SD=1.00). The final and smallest class is characterized by a rapid drop in MMSE scores. These participants’ MMSE scores declined at an average rate of 1.22 points/year (SD= 1.22). This class was thus labeled as ‘rapid decline.’
Table 2.
Model fit of LCMM solutions
| No. of classes | AIC | BIC | PLMR LRT | Class Proportions (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 41444 | 41502 | - | 100 | ||||
| 2 | 41369 | 41445 | <.001 | 73.8 | 26.2 | |||
| 3 | 41329 | 41422 | <.001 | 54.7 | 38.1 | 7.2 | ||
| 4 | 41313 | 41425 | <.001 | 51.0 | 36.3 | 8.3 | 4.5 | |
| 5 | 41319 | 41448 | ~1 | 45.1 | 25.5 | 24.7 | 4.5 | .1 |
Note. AIC=Akaike Information Criterion; BIC= Bayesian Information Criterion; LMR-LRT = Lo-Mendell-Rubin Likelihood Ratio Test.
Figure 1.
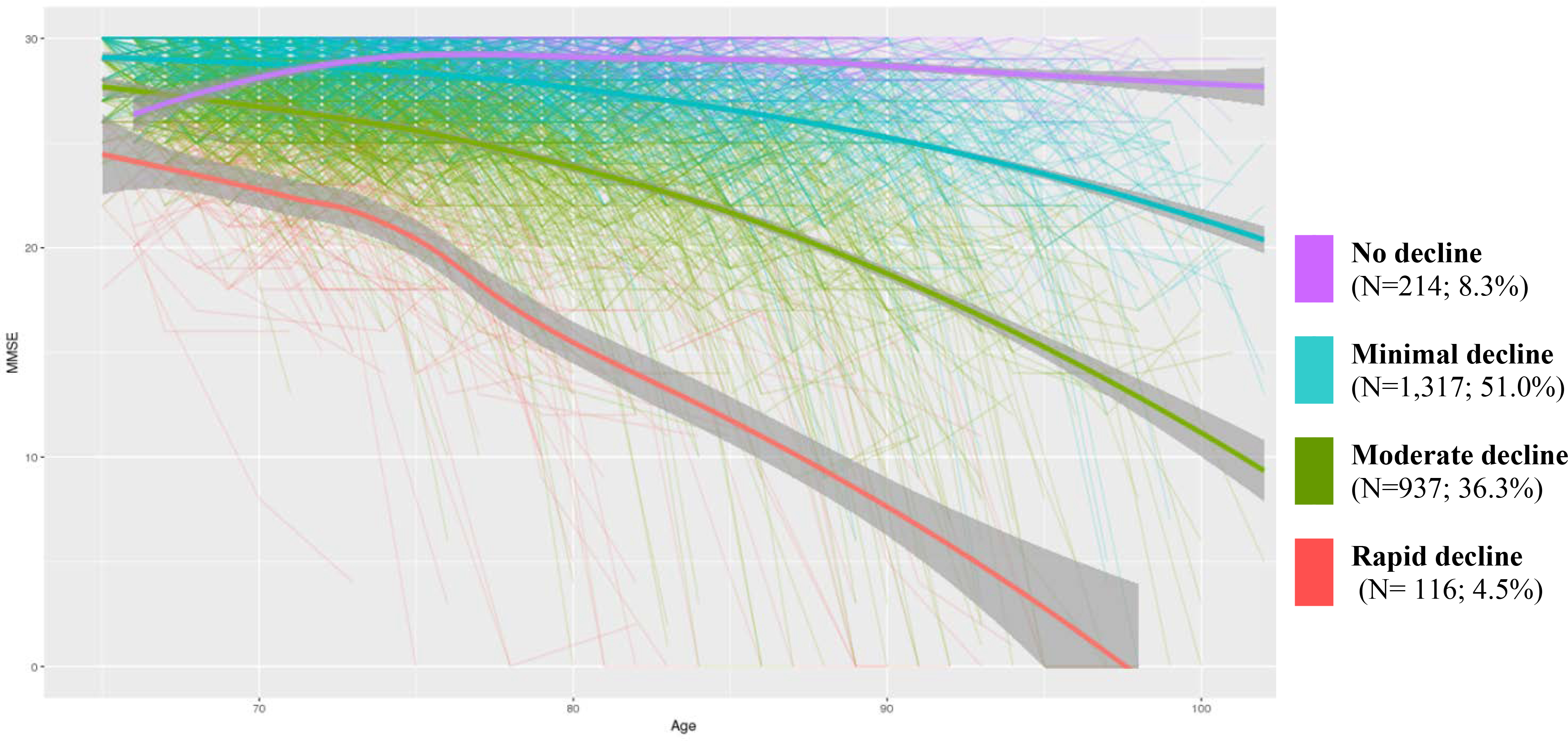
Trajectories of cognitive functioning as assessed on the MMSE. The thin lines are the trajectories of each participant. The thick lines are loess-smoothed, calculated growth trajectories. The gray area enclosing these thick lines represent the standard error.
The estimates of the modeled fixed effects are shown in table 3. At the class-membership level, using the ‘no decline’ class as a baseline, the intercepts of the rapid and minimal decline groups were significant (p <.05), while that of the moderate decline group was marginally significant (p = .066). At the longitudinal model level, the intercepts of all classes except ‘no decline’ were not statistically significant (p>.05). Furthermore, age had significantly predicted MMSE scores for all classes (p<.01) except ‘no decline.
Table 3.
Fixed effects estimates.
| Level | Class | Parameter | Estimates | SE |
|---|---|---|---|---|
| Class-membershipa | ||||
| Rapid decline | Intercept | −1.20** | .37 | |
| Moderate decline | Intercept | .54 | .29 | |
| Minimal decline | Intercept | .71* | .33 | |
| Longitudinal model | ||||
| Rapid decline | Intercept | Not estimated | ||
| Moderate decline | Intercept | .12 | .72 | |
| Minimal decline | Intercept | −1.03 | .76 | |
| No decline | Intercept | −4.76*** | 1.01 | |
| Rapid decline | Age | −.11*** | .01 | |
| Moderate decline | Age | −.09*** | .003 | |
| Minimal decline | Age | −.07** | .005 | |
| No decline | Age | −.01 | .01 |
Note. SE= Standard error.
No decline was used as class of reference.
p <.05.
p <.01.
p <.001.
For the purpose of sensitivity analyses, another set of LCMM analyses were carried out on the same group of participants, without excluding data from their last time point. The four-class solution had similarly emerged as the optimal model. This model had produced similar trajectories and fixed-effects estimates (see figure S1 and table S1 in the supplementary materials).
Predictors of cognitive trajectories
We entered various predictors into multinomial logistic regression models to predict the different LCMM classes. The results of these analyses are presented in table 4. The inclusion of MMSE scores, age, sex, education and urban residence in model 2 resulted in a significant LRT statistic (p<.001). Using the no decline class as a reference, all included predictors significantly predicted the other three classes (p<.001). Next, the inclusion of medical diagnoses such as hypertension, diabetes and heart disease in model 3, did not result in a significant LRT statistic (p = .482). In this model, none of these newly added predictors significantly predicted the other three classes. Finally, the inclusion of lifestyle factors such as smoke, drink, and exercise resulted in a significant LRT statistic (p<.030). In this model, ‘exercise’ significantly predicted the moderate and minimal decline classes (p<.05). Additionally, ‘smoke’ significantly predicted the ‘rapid decline’ class (p<.05). The descriptive and between-group statistics of all studied variables are presented in table S2 in the supplementary materials.
Table 4.
Multinomial logistic regressions of baseline variables
| Predictors | Unstandardized regression coefficients (SE) | LRTModel K vs. Model K-1 | |||||
|---|---|---|---|---|---|---|---|
| Rapid decline | Moderate decline | Minimal decline | |||||
| Model 1 | - | ||||||
| Intercept | −0.61*** | (0.12) | 1.48*** | (0.08) | 1.82*** | (0.07) | |
| Model 2 | 1188.23*** | ||||||
| Intercept | 51.43*** | (1.09) | 30.7*** | (1.10) | 15.77*** | (1.16) | |
| MMSE | −1.09*** | (0.04) | −0.62*** | (0.03) | −0.21*** | (0.04) | |
| Age | −0.35*** | (0.02) | −0.19*** | (0.01) | −0.12*** | (0.01) | |
| Sex | 1.21*** | (0.32) | 1.04*** | (0.20) | 0.70*** | (0.19) | |
| Education | −0.31*** | (0.07) | −0.20*** | (0.03) | −0.07*** | (0.02) | |
| Urban residence | 0.89** | (0.32) | 0.61*** | (0.18) | 0.21 | (0.16) | |
| Model 3 | 8.52 | ||||||
| Intercept | 51.69*** | (1.09) | 30.88*** | (1.10) | 15.87*** | (1.16) | |
| MMSE | −1.09*** | (0.04) | −0.62*** | (0.03) | −0.21*** | (0.04) | |
| Age | −0.35*** | (0.02) | −0.19*** | (0.01) | −0.12*** | (0.01) | |
| Sex | 1.23*** | (0.33) | 1.07*** | (0.20) | 0.72*** | (0.19) | |
| Education | −0.31*** | (0.07) | −0.19*** | (0.03) | −0.07*** | (0.02) | |
| Urban residence | 0.87** | (0.32) | 0.58** | (0.18) | 0.19 | (0.17) | |
| Hypertension | 0.36 | (0.42) | 0.18 | (0.28) | 0.25 | (0.25) | |
| Diabetes | −0.43 | (1.25) | −0.95 | (0.62) | −0.90 | (0.51) | |
| Heart disease | −1.31 | (0.86) | −0.33 | (0.38) | −0.02 | (0.33) | |
| Model 4 | 18.45* | ||||||
| Intercept | 51.09*** | (1.11) | 30.08*** | (1.13) | 15.27*** | (1.19) | |
| MMSE | −1.09*** | (0.04) | −0.62*** | (0.04) | −0.21*** | (0.04) | |
| Age | −0.35*** | (0.02) | −0.19*** | (0.01) | −0.12*** | (0.01) | |
| Sex | 1.15** | (0.37) | 1.05*** | (0.22) | 0.70*** | (0.20) | |
| Education | −0.30*** | (0.07) | −0.18*** | (0.03) | −0.06** | (0.02) | |
| Urban residence | 0.66* | (0.33) | 0.43* | (0.19) | 0.10 | (0.17) | |
| Hypertension | 0.39 | (0.43) | 0.24 | (0.28) | 0.29 | (0.25) | |
| Diabetes | −0.38 | (1.26) | −0.86 | (0.62) | −0.84 | (0.51) | |
| Heart disease | −1.40 | (0.86) | −0.35 | (0.38) | −0.03 | (0.33) | |
| Smoke | 0.77* | (0.39) | 0.07 | (0.21) | −0.02 | (0.19) | |
| Drink | −0.63 | (0.34) | −0.07 | (0.20) | 0.03 | (0.18) | |
| Exercise | 0.51 | (0.32) | 0.59** | (0.19) | 0.35* | (0.17) | |
Note. no decline was used as class of reference. SE = Standard Error; LRT = Likelihood Ratio Test. MMSE = Mini–Mental State Examination.
p <.05.
p <.01.
p <.001.
Discussion
The current report examined the trajectories of cognitive decline across the late-life period in a large cohort of elderly Chinese participants while minimizing the effects of terminal decline by excluding data from the final included time point in each participant. We found four distinct cognitive trajectories ranging from participants who did not evidence a decline, to those who exhibited various degrees of decline. The profiles of these trajectories are largely similar to those documented in previous growth mixture modeling studies [3–8]. Perhaps a more interesting finding may relate to the discovery of a group of participants, who despite their relatively higher baseline age, not only did not exhibit a decline in cognitive functioning but had instead experienced a slight increase in cognitive functioning across time. In relation to this, one other growth mixture modeling studies had observed a similar cognitive trajectory [4]. This group of participants would very much exemplify the idea of successful cognitive aging [18].
In our examination of factors related to such resilient cognitive aging, we note a few interesting findings. First, we found that the inclusion of chronic medical conditions, such as hypertension, diabetes and heart disease, did not significantly improve the prediction of the different cognitive trajectories; This suggests that these chronic conditions have a non-significant influence on the late-life cognitive trajectories. Such findings are inconsistent with previous longitudinal research [19–21] which reported that these illnesses would increase the risk of future cognitive impairment. Though, it should be noted that these findings were typically associated with small effect sizes. It is possible that the MMSE, a screening measure for global cognition, may not be sensitive enough to pick up the small effects associated with these illnesses. Furthermore, given that these medical conditions were assessed via subjective self-reports, they may not accurately reflect the participant’s actual medical comorbidities.
Next, the inclusion of lifestyle factors significantly improved the prediction of the different cognitive trajectories. Specifically, participants within the ‘no decline’ class were significantly more likely, relative to the other three groups, to report exercising regularly, even after controlling for other lifestyle, medical and demographic variables. This is not surprising, given that previous meta-analyses of exercise-based interventions had revealed significant exercise-associated improvements on cognitive functions [22]. Relatedly, long term physical exercise is known to increase the circulating levels of brain-derived neurotrophic factor, insulin-like growth factor 1, and vascular endothelial growth factor. These factors promote gliogenesis, neurogenesis, synaptogenesis, and angiogenesis, which induces structural and functional changes in the brain that benefit cognitive health [24].
Finally, we observed that a larger proportion of participants in the ‘no decline’ group had indicated having smoked in the past, relative to those of the ‘rapid decline’ group. This appears to be inconsistent with previous meta-analytic findings [25]. Nevertheless, it should be noted the pooled estimates from these meta-analyses, though significant, were minute in magnitude. Previous research has shown that smoking tobacco has significant socio-cultural implications in China. Smoking may be seen as a sign of social status among the mainland Chinese; they are also often being offered as gifts or being used to facilitate social interactions [26]. We speculate that in the current study, the larger proportion of participants among the ‘no decline’ class who had smoked before may allude to them coming from the higher socio-economic strata or having more frequent social interactions; both of which have been documented to be associated with better cognitive outcomes [27,28]. It should also be noted that, in examining the effects of smoking on cognitive decline, it may be more important to look at the frequency of smoking instead of simply whether one had smoked in the past. Thus, having more people in the ‘no decline’ class who have had a smoking experience, does not necessarily mean that they smoked more frequently than the smokers in the ‘rapid decline’ class.
Interestingly, across the four classes, we observed that the proportion of male participants increased as the severity of decline decreased, with males forming a large majority in the no decline class and a small minority in the rapid decline class. It would appear that the male gender confers certain advantages in the maintenance of late-life cognitive functions. There might be a few explanations for such an observation. First, although both genders are of approximately equal risk of developing Alzheimer’s disease[29], females are more vulnerable to the effects of the disease and would experience greater cognitive decline [30]. Second, given that the average lifespan of men is lower than females in general, it is likely that men who survive into their late-life period are more physically and cognitively healthy than their female counterparts [31]. Finally, gender is likely to be confounded with education levels in the current context. Due to cultural factors in the past, the study’s cohort of females was deprived of educational opportunities, especially those residing in rural areas[32]. Consequently, females had much lower education levels which meant that they had much less cognitive reserve [33] to buffer them from age-related neurodegenerative conditions.
The findings of the current study are subjected to some major limitations. First, the use of MMSE to characterize cognitive functioning may be associated with ceiling effects and inadequate sensitivity in detecting subtle cognitive impairment. Second, the lifestyle factors were assessed purely via subjective self-reports, which may bring about concerns relating to the accuracy of participants’ responses; they may be influenced by social desirability biases. These self-reported responses were also made in a binary force choice manner. As such, they may not adequately characterize the variability in these lifestyle factors across the sample. Third, we excluded many participants who were, in general, older and less healthy—in terms of the presence of medical comorbidities, compared to the included participants. It is plausible that if these excluded participants had persisted through at least four waves of data collection in the CLHLS, they would most probably be assigned to the ‘moderate decline’ or ‘rapid decline’ classes. Consequently, it is likely that the current study had underestimated the proportion of participants in these two classes. Furthermore, differences in age, sex, urban residence, lifestyle factors and medical comorbidities between the included and excluded sample, despite many of them being small, may point to limitations in generalizing the study’s findings to the general population. Finally, in our regression models, we did not include any sleep variables which could be an important predictor that differentiates between the different cognitive trajectories. Poor sleep has been linked to decreased tau phosphorylation, increased amyloid deposition, and impaired memory consolidation, which collectively contributes to cognitive decline [34].
Supplementary Material
Acknowledgments
CLHLS is funded by National Natural Science Foundation of China (71110107025, 71233001, 71490732), National Institute of Aging/National Institutes of Health (2P01AG031719) and United Nations Funds for Population Activities. Lei Feng is supported by the National Medical Research Council of Singapore (grant no. NMRC/TA/0053/2016), and the National Innovation Challenge on Active and Confident Ageing Programme, Ministry of Health of Singapore (award no.: MOH/NIC/COG06/2017).
Footnotes
Conflict of Interest
The authors have no conflict of interest to report
References
- [1].Breitve MH, Chwiszczuk LJ, Hynninen MJ, Rongve A, Brønnick K, Janvin C, Aarsland D (2014) A systematic review of cognitive decline in dementia with Lewy bodies versus Alzheimer’s disease. Alzheimers. Res. Ther. 6, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, Bigio EH, Weintraub S, Rogalski E, Mesulam M-M, Geula C (2015) Morphometric and Histologic Substrates of Cingulate Integrity in Elders with Exceptional Memory Capacity. J. Neurosci. 35, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hayden KM, Reed BR, Manly JJ, Tommet D, Pietrzak RH, Chelune GJ, Yang FM, Revell AJ, Bennett DA, Jones RN (2011) Cognitive decline in the elderly: An analysis of population heterogeneity. Age Ageing 40, 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pietrzak RH, Lim YY, Ames D, Harrington K, Restrepo C, Martins RN, Rembach A, Laws SM, Masters CL, Villemagne VL, Rowe CC, Maruff P (2015) Trajectories of memory decline in preclinical Alzheimer’s disease: Results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing. Neurobiol. Aging 36, 1231–1238. [DOI] [PubMed] [Google Scholar]
- [5].Royall DR, Palmer RF, Chiodo LK, Polk MJ (2014) Towards an aging-specific cognitive phenotype: the freedom house study. Exp. Aging Res. 40, 245–65. [DOI] [PubMed] [Google Scholar]
- [6].Terrera GM, Brayne C, Matthews F (2010) One size fits all? Why we need more sophisticated analytical methods in the explanation of trajectories of cognition in older age and their potential risk factors. Int. Psychogeriatr. 22, 291–9. [DOI] [PubMed] [Google Scholar]
- [7].Yu L, Boyle P a, Segawa E, Leurgans S, Schneider J a, Wilson RS, Bennett D a (2015) Residual decline in cognition after adjustment for common neuropathologic conditions. Neuropsychology 29, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Small BJ, Bäckman L (2007) Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: A growth mixture modeling analysis. Cortex 43, 826–834. [DOI] [PubMed] [Google Scholar]
- [9].Small BJ, Fratiglioni L, von Strauss E, Bäckman L (2003) Terminal decline and cognitive performance in very old age: does cause of death matter? Psychol. Aging 18, 193. [DOI] [PubMed] [Google Scholar]
- [10].Zeng Y (2004) Chinese Longitudinal Healthy Longevity Survey and some research findings. Geriatr. Gerontol. Int. 4, S49–S52. [Google Scholar]
- [11].Gu D (2008) General Data Quality Assessment of the CLHLS BT - Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions. In, Yi Z, Poston DL, Vlosky DA, Gu D, eds. Springer; Netherlands, Dordrecht, pp. 39–60. [Google Scholar]
- [12].Zeng Y (2004) Chinese Longitudinal Healthy Longevity Survey and some research findings. Geriatr. Gerontol. Int. 4, S49–S52. [Google Scholar]
- [13].Li H, Jia J, Yang Z (2016) Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J. Alzheimer’s Dis. 53, 487–96. [DOI] [PubMed] [Google Scholar]
- [14].Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [15].Proust-Lima C, Philipps V, Diakite A, Liquet B (2017) lcmm: Extended Mixed Models Using Latent Classes and Latent Processes. [Google Scholar]
- [16].Verlinden VJA, Van Der Geest JN, De Bruijn RFAG, Hofman A, Koudstaal PJ, Ikram MA (2016) Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimer’s Dement. 12, 144–153. [DOI] [PubMed] [Google Scholar]
- [17].Lo Y, Mendell NR, Rubin DB (2001) Testing the Number of Components in a Normal Mixture. Biometrika 88, 767–778. [Google Scholar]
- [18].Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, Launer LJ, Yaffe K, Cuthbert BN, Edwards E, Wagster MV (2006) The NIH Cognitive and Emotional Health Project. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2, 12–32. [DOI] [PubMed] [Google Scholar]
- [19].Cheng G, Huang C, Deng H, Wang H (2012) Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern. Med. J. 42, 484–491. [DOI] [PubMed] [Google Scholar]
- [20].Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, DeKosky ST, Kuller LH (2005) Dementia and Alzheimer’s Disease Incidence in Relationship to Cardiovascular Disease in the Cardiovascular Health Study Cohort. J. Am. Geriatr. Soc. 53, 1101–1107. [DOI] [PubMed] [Google Scholar]
- [21].Tzourio C, Dufouil C, Ducimetière P, Alpérovitch A, Group EVAS (1999) Cognitive decline in individuals with high blood pressure A longitudinal study in the elderly. Neurology 53, 1948. [DOI] [PubMed] [Google Scholar]
- [22].Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S (2014) The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res. Rev. 16, 12–31. [DOI] [PubMed] [Google Scholar]
- [23].Zheng G, Xia R, Zhou W, Tao J, Chen L (2016) Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 50, 1443 LP–1450. [DOI] [PubMed] [Google Scholar]
- [24].El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ (2018) Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neurosci. 25, 65–85. [DOI] [PubMed] [Google Scholar]
- [25].Anstey KJ, von Sanden C, Salim A, O’Kearney R (2007) Smoking as a Risk Factor for Dementia and Cognitive Decline: A Meta-Analysis of Prospective Studies. Am. J. Epidemiol. 166, 367–378. [DOI] [PubMed] [Google Scholar]
- [26].Rich ZC, Xiao S (2012) Tobacco as a Social Currency: Cigarette Gifting and Sharing in China. Nicotine Tob. Res. 14, 258–263. [DOI] [PubMed] [Google Scholar]
- [27].Xu S, Xie B, Song M, Yu L, Wang L, An C, Zhu Q, Han K, Zhao X, Zhang R, Dong L, Chai N, Gao Y, Zhang Q, Wang X (2014) High Prevalence of Mild Cognitive Impairment in the Elderly: A Community-Based Study in Four Cities of the Hebei Province, China. Neuroepidemiology 42, 123–130. [DOI] [PubMed] [Google Scholar]
- [28].Haslam C, Cruwys T, Haslam SA (2014) “The we’s have it”: Evidence for the distinctive benefits of group engagement in enhancing cognitive health in aging. Soc. Sci. Med. 120, 57–66. [DOI] [PubMed] [Google Scholar]
- [29].Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang L-S, Romero K, Arneric SP, Redolfi A, Orlandi D, Frisoni GB, Au R, Devine S, Auerbach S, Espinosa A, Boada M, Ruiz A, Johnson SC, Koscik R, Wang J-J, Hsu W-C, Chen Y-L, Toga AW (2017) Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 74, 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, Papp KV, Jacobs HIL, Burnham S, Hanseeuw BJ, Doré V, Dobson A, Masters CL, Waller M, Rowe CC, Maruff P, Donohue MC, Rentz DM, Kirn D, Hedden T, Chhatwal J, Schultz AP, Johnson KA, Villemagne VL, Sperling RA (2018) Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimer’s Dement. 14, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mielke MM, Vemuri P, Rocca WA (2014) Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol. 6, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou DF, Wu CS, Qi H, Fan JH, Sun XD, Como P, Qiao YL, Zhang L, Kieburtz K (2006) Prevalence of dementia in rural China: impact of age, gender and education. Acta Neurol. Scand. 114, 273–280. [DOI] [PubMed] [Google Scholar]
- [33].Roe CM, Xiong C, Miller JP, Morris JC (2007) Education and Alzheimer disease without dementia. Neurology 68, 223 LP–228. [DOI] [PubMed] [Google Scholar]
- [34].Scullin MK, Bliwise DL (2015) Sleep, Cognition, and Normal Aging: Integrating a Half Century of Multidisciplinary Research. Perspect. Psychol. Sci. 10, 97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen J-C, Espeland MA, Brunner RL, Lovato LC, Wallace RB, Leng X, Phillips LS, Robinson JG, Kotchen JM, Johnson KC, Manson JE, Stefanick ML, Sarto GE, Mysiw WJ (2016) Sleep duration, cognitive decline, and dementia risk in older women. Alzheimer’s Dement. 12, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


