Summary
Circulating tumor cells (CTCs) are precursors of metastasis in various cancer types. Many aspects regarding CTC biology remain poorly understood. Here, we describe mass spectrometric analysis of CTCs from a breast cancer xenograft mouse model, including procedures comprising CTC enrichment, separation of different CTC subpopulations, and their quantitative proteomic assessment. This protocol aims to facilitate the identification of protein content dynamics in human CTCs that are physiologically shed from tumor-bearing xenografts, providing a framework for investigating metastasis biology.
For complete details on the use and execution of this protocol, please refer to Donato et al. (2020).
Subject areas: Cell Biology, Cell isolation, Flow Cytometry/Mass Cytometry, Cancer, Model Organisms, Proteomics, Mass Spectrometry
Graphical abstract

Highlights
-
•
Method for mass spectrometry analysis of circulating tumor cells from xenografts
-
•
Procedure to stain and separate subpopulations of circulating tumor cells
-
•
Methods to perform data analysis from mass spectrometry of circulating tumor cells
Circulating tumor cells (CTCs) are precursors of metastasis in various cancer types. Many aspects regarding CTC biology remain poorly understood. Here, we describe mass spectrometric analysis of CTCs from a breast cancer xenograft mouse model, including procedures comprising CTC enrichment, separation of different CTC subpopulations, and their quantitative proteomic assessment. This protocol aims to facilitate the identification of protein content dynamics in human CTCs that are physiologically shed from tumor-bearing xenografts, providing a framework for investigating metastasis biology.
Before you begin
This protocol aims at performing a quantitative proteomic analysis of CTCs enriched from xenograft models of breast cancer. The selection of a suitable cell line and the strain of the murine host represent critical choices for the success of this experiment as abundant quantities of spontaneously generated CTCs are necessary for mass spectrometric analysis. For instance, cancer cells transplanted in the context of a specific murine host should be evaluated not only for their ability to engraft and form a growing primary tumor, but more specifically for their ability to spontaneously metastasize to distant sites. For this protocol, MDA-MB-231 LM2 breast cancer cell line (LM2) and the immunocompromised Nod-SCID-gamma (NSG) strain of mice were used. All the procedures concerning animals were performed in compliance with institutional and cantonal guidelines (approved mouse protocols #2781 and #3053, Cantonal Veterinary Office of Basel-City).
It is crucial to know ahead what the expected yield of CTCs from each injected mouse will be before starting with this protocol. Further, it is important to carefully evaluate the chosen CTC enrichment technology to avoid loss of important CTC subpopulations. Lastly, stringent gating while FACS sorting and high-purity standards for separation of singled versus clustered CTCs may result in relatively high abort rates, which may influence the final number of successfully sorted cells. As a minimum of 10’000 cells per sample are necessary to perform mass spectrometry analysis, a pilot experiment is strongly recommended to calculate the final yield of CTCs per mouse.
Pooling of CTCs enriched from multiple biological replicates (e.g., CTCs enriched from two to three mice are pooled together as one sample) could compensate for a low CTC isolation yield.
Cell line preparation and injection
Timing: 2–3 weeks
-
1.Infection of LM2 cells with a fluorescent probe.
-
a.Using Lipofectamine, stably transduce LM2 cancer cells, previously expanded in DMEM F-12 with 10% FBS until reaching 80% confluence, with lentiviral vectors expressing a fluorophore of choice (e.g., mCherry-T2A-Luc) or a fluorescent Tag for a specific target (e.g., HIF1α activity reporter) (Donato et al., 2020).
-
b.Following BSL-2 practices and approved guidelines, expand the infected cells for 2-3 passages to wash out the virus and increase the number of cells.
-
c.Positively select successfully transduced cells with antibiotic treatment (when the lentiviral vector contains an antibiotic resistance gene, e.g., Puromycin resistance gene) or by sorting with flow cytometry.
-
a.
CRITICAL: Make sure to start the selection, either via antibiotic treatment or sorting, with a large number of cells (e.g., 1-2 million cells) to avoid clonal enrichment.
Note: Prior to antibiotic selection, make sure to perform a killing curve on the cell line in use to establish the best concentration of antibiotics, starting number of cells and duration of the treatment.
-
2.LM2 cells expansion for injection.
-
a.Expand LM2-fluorophore positive (+) cells.
-
b.Use multiple flasks in order to reach 1.5–2 times the number of cells needed for the day of injection (i.e., injection of 1 million cells/mouse. For 5 mice, seed cells in order to obtain 7.5–10 million cells on the day of injection).
-
a.
CRITICAL: It is necessary to know ahead the expected yield of CTCs from each injected mouse before expanding the cells for injection. A pilot experiment with three to five mice allows to calculate the possible yield per mouse and to plan accordingly.
-
3.
Inject 1 million LM2 cells in a solution 1:1 of PBS in BME orthotopically into the mammary fat pad of NSG female mice in the age range of 8–12 weeks (Donato et al., 2020).
Sample preparation
Timing: 4.5–5 weeks and 2-–3 h per cohort
-
4.Blood withdrawal of LM2-(+) tumor-bearing mice (Figure 1).
-
a.4.5–5 weeks following the orthotopic injection of 1 million LM2-(+), and upon the first signs of mouse distress, perform a cardiac puncture to withdraw up to 1.5 mL of central line blood containing CTCs (Donato et al., 2019).
-
a.
CRITICAL: Incubate the blood-containing tubes only for a short time period (up to 1h) at 20°C–25°C (room temperature; RT) on a rocking shaker at 40 osc/min to minimize cell death.
-
5.Enrichment of CTCs (Figure 1).
-
a.Using the isolation method of choice, preferably in an antigen-agnostic fashion (e.g., exploiting physical features of CTCs), enrich for single CTCs and CTC clusters from fresh whole blood.Optional: If conceivable with the CTC isolation method of choice, built-in staining for white blood cells (e.g., CD45) or CTCs populations (e.g., EpCAM, EGFR, HER2) can be performed here (Donato et al., 2019).Note: Consider that different CTC isolation technologies may be more suitable for the isolation of single CTCs or CTC clusters, depending on their driving principles.
-
b.Release CTCs in a cold 1× DPBS solution.Note: Check the cassette for residual CTCs. Release multiple times to ensure maximum retrieval of CTCs from the enrichment technology.
-
c.Proceed immediately to the next step.
-
a.
CRITICAL: The CTC-containing solution must stay on ice during manipulation.
Figure 1.
Workflow of circulating tumor cells collection (CTCs)
From left to right, NSG female mice bearing LM2 mCherry/Luc tumors and expressing the HIF1α activity reporter (eYFP(+)); yellow cells) are used. Upon blood sampling, CTCs are enriched from whole blood with microfluidic devices, and subsequently stained in cassette (if applicable) or directly released in solution.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD45-AF647 (anti mouse) | BioLegend | Cat# 103124, RRID:AB_493533 |
| DAPI | Sigma-Aldrich | Cat# D9542-1MG |
| CD298-PE | BioLegend | Cat# 341704 |
| Chemicals, peptides, and recombinant proteins | ||
| Lipofectamine | Invitrogen | Cat# 11668-019 |
| EF1a_Firefly Luciferace-T2A-mCherry (mCherry/Luc) | Biosettia | GlowCell 15-10 |
| 1× DPBS | Gibco | Cat# 14190-094 |
| Sodium deoxycholate (SDC) | Thermo Scientific | Cat# 89904 |
| Trizma® base (TRIS) | Sigma | Cat# T6791 |
| Tris(2-carboxyethyl)phosphine hydrochlorid (TCEP) | Sigma | Cat# 646547 |
| Ammonium bicarbonate | Sigma | Cat# 09830 |
| Chloroacetamide | Sigma | Cat# C0267 |
| Trifluoroacetic acid (TFA) | Thermo Scientific | Cat# 28904 |
| Porcine trypsin | Promega | Cat# V5113 |
| 2-Propanol | Merck | Cat# 109634 |
| Ammonium hydroxide solution | Sigma | Cat# 17093 |
| HPLC water | Fisher Scientific | Cat# W6-212 |
| Acetonitrile | Thermo Scientific | Cat# A955-212 |
| Formic acid | Sigma | Cat# 94318 |
| BME | R&D Biosystems | Cat# 3533-010-02 |
| Critical commercial assays | ||
| GOLD BSA removal kit | Abcam | Cat# ab204912 |
| Reducing agent compatible BCA assay | Thermo Fisher | Cat# 23250 |
| SDB-RPS cartridges | PreOmics | Cat# P.O.00013 |
| Software and algorithms | ||
| Progenesis QI | Waters | n/a |
| Mascot | Matrix Science | n/a |
| SafeQuant R package | GitHub | n/a |
| Experimental models: cell lines | ||
| MDA-MB-231 LM2 | Massague Lab, MSKCC | n/a |
| Experimental models: organisms/strains | ||
| Mouse: NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ | The Jackson Laboratory | Cat# 5557 |
| Other | ||
| Benchtop centrifuge | n/a | n/a |
| Thermomixer | Eppendorf | Cat #EP5382000023 |
| Biruptor Pico | Diagenode | Cat# B01060010 |
| VialTweeter sonicator | Hielscher | model UIS250v |
| Vacuum concentrator | Labconco | Cat# 7810016 |
| Nozzle Tip, 200 Micron | BD Biosciences | Cat# 645895 |
| Plate reader/ nanodrop | BMG Labtech | SpectroStar nano |
| EASY-nLC 1200 system | Thermo Scientific | Cat# LC140 |
| Orbitrap Fusion Lumos Tribrid Mass Spectrometer | Thermo Scientific | n/a |
Materials and equipment
Buffers and solution that need to be prepared in advance
-
•
50 μL of 0.75 M Chloroacetamide, store at −20°C for up to 1 year
-
•
5 mL of 100 mM Ammonium bicarbonate, store at −20°C for up to 1 year
-
•
50 mL 5% TFA, store in glass container at 4°C for up to 1 year
-
•
Lysis buffer (pH = 8.5)
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium deoxycholate (SDC) | 1% | 0.5 g |
| TCEP | 10 mM | 1 mL of 0.5 M solution (entire vial) |
| Tris | 100 mM | 5 mL of 1 M stock solution |
| ddH2O | 44 mL |
CRITICAL: Adjust pH with NaOH/HCl, do not use KOH for pH adjustments.
-
•
50 mL Peptide desalting wash 1 buffer: 1% TFA in 2-propanol, store in glass container at 4°C, for up to 1 year
-
•
50 mL Peptide desalting wash 2 buffer: 0.2% TFA in water, store in glass container at 4°C, for up to 1 year
-
•
50 mL of Elution buffer: 1% ammonium hydroxide, 80% acetonitrile, 19% water, store in glass container at 4°C, for up to 1 year
| Reagent | Final concentration | Amount |
|---|---|---|
| Ammonium hydroxide | 1% | 1.6 mL |
| Acetonitrile | 80% | 40 mL |
| miliQ water | 8.4 mL |
-
•
100 mL of LC-MS sample buffer: 0.1% formic acid in water, store in glass container at 4°C, for up to 1 year
-
•
100 mL of nanoLC mobile phase A: 0.1% formic acid in water, store in glass container at 4°C, for up to 1 year
-
•
100 mL of nanoLC mobile phase B: 0.1% formic acid, 19.9% water, 80% acetonitrile, store in glass container at 4°C, for up to 1 year
| Reagent | Final concentration | Amount |
|---|---|---|
| Acetonitrile | 80% | 80 mL |
| Formic acid | 0.1% | 0.1 mL |
| miliQ water | 19.9 mL |
CRITICAL: De-gas buffers for nanoLC using ultrasonication prior use.
LC gradient for MS
| Time (min) | Duration (min) | Flow (nL/min) | % nanoLC mobile phase B |
|---|---|---|---|
| 0 | 0 | 200 | 5 |
| 5 | 5 | 200 | 12 |
| 70 | 65 | 200 | 35 |
| 90 | 20 | 200 | 50 |
| 92 | 2 | 200 | 95 |
| 110 | 18 | 200 | 95 |
MS data acquisition parameters
| Method Node | Parameter | Settings |
|---|---|---|
| MS Scan Properties | Detector Type | Orbitrap |
| Orbitrap Resolution | 120,000 | |
| Mass Range | Normal | |
| Use Quadrupole Isolation | True | |
| Scan Range (m/z) | 375-1500 | |
| RF Lens (%) | 30 | |
| AGC Target | custom | |
| Normalized AGC Target | 250% | |
| Maximum Injection Time Mode | custom | |
| Maximum Injection Time (ms) | 50 | |
| Microscans | 1 | |
| Data Type | Profile | |
| Polarity | Positive | |
| Source Fragmentation | False | |
| MIPS Properties | Monoisotopic Peak Determination | Peptide |
| Intensity Properties | Filter Type | Intensity Threshold |
| Intensity Threshold | 5.0e3 | |
| Charge State Properties | Include charge state(s) | 2–5 |
| Include undetermined charge states | False | |
| Dynamic Exclusion Properties | Exclude after n times | 1 |
| Exclusion duration (s) | 60 | |
| Mass tolerance | ppm | |
| Low | 10 | |
| High | 10 | |
| Exclude isotopes | True | |
| Performed dependent scan on single charge state per precursor only | False | |
| Data-Dependent MS2 Scan Properties | Isolation mode | Quadrupole |
| Isolation Window (m/z) | 1.4 | |
| Isolation Offset | Off | |
| Activation Type | HCD | |
| Collision Energy Mode | Fixed | |
| CID Collision Energy (%) | 35 | |
| Detector Type | Ion Trap | |
| Ion Trap Scan rate | Rapid | |
| Mass Range | Normal | |
| Scan Range Mode | Auto | |
| AGC Target | Standard | |
| Maximum Injection Time Mode (ms) | 35 | |
| Data Type | Centroid |
Step-by-step method details
CTC live immunostaining
Timing: 45 min
This staining procedure discriminates between CTCs (e.g., EpCAM-positive), mouse blood cells (e.g., CD45-positive) and dead cells (DAPI-positive). Further staining for Tags or taggable markers (e.g., EGFR, HER2) can be added to refine the cell populations. For instance, some cancer cells express low levels of epithelial surface markers, such as EpCAM. In this case, when these are not stably transduced with a tagging fluorophore, CD298 (antibody recognizing β3 Na+/K+ ATPase protein and abundantly present on the membrane of several cancer models, such as LM2 cells) could be used to discriminate between cancer cells and contaminant cells (Lawson et al., 2015).
In this protocol, we added CD298-PE staining of LM2 mCherry/Luc expressing HIF1α activity reporter (eYFP(+)).
-
1.BSA removal from antibody solutions (work at RT).
-
a.Mix the chosen antibody cocktail: for 100 μL staining solution, add 1:15 dilution CD298-PE, 1:10 mouse CD45-AF647 and 1× DPBS up to 100 μL.
-
b.Apply the GOLD BSA removal kit according to the manufacturer’s protocol.
-
a.
-
2.CTCs live immunostaining (work on ice).
-
a.Gently centrifuge the CTC suspension at 72 × g for 4 min in a pre-cooled centrifuge to reduce the volume.
-
b.Gently resuspend the pellet in cold 1× DPBS and add the BSA-free antibody mix in a total volume of 500 μL.
-
c.Incubate for 30 min at RT and protect from light.
-
d.Wash the CTCs suspension using 1 mL of cold 1× DPBS and centrifuge at 72 × g for 4 min in a pre-cooled centrifuge. Repeat this wash twice to ensure total elimination of excess antibodies.
-
e.Resuspend stained cells in 1–2 mL of cold 1× DPBS solution and transfer on ice.
-
a.
Note: When working with single CTCs and CTC clusters, it is important to reduce the steps of centrifugation to the minimum. Therefore, the use of fluorescent labeling of cancer cells and in-cassette staining procedures are strongly advised.
Fluorescent-activated cell sort
Timing: 2–3 h
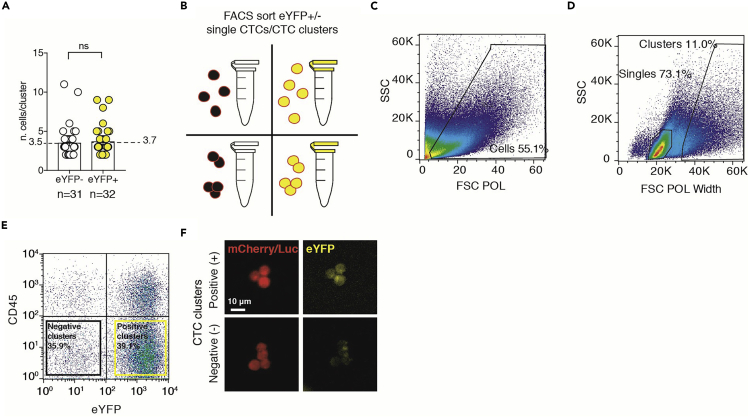
FACS sorting of CTCs separates different populations of interest. Single CTCs and CTC clusters are separated by physical properties via Side SCatter (SSC) and Forward SCatter (FSC). The use of specific FACS sorting technology that allow to mount 200 μm nozzles (e.g., Influx, BD) and to set the minimum fluidic pressure to five pounds per square inch (psi), permits the separation of CTC clusters from single CTCs without damaging CTC clusters (Figure 2A).
Figure 2.
FACS strategy to separate eYFP(+) CTCs from eYFP(-) CTCs, and single CTCs from CTC clusters
(A) The plot shows the average cluster size of eYFP(+) and eYFP(-) CTC clusters after FACS sort. The number of independent biological replicates (n) is shown and the Error bars represent SEM.
(B) Schematics of experimental design.
(C) The plot shows the separation of cells from debris based on Forward SCatter polarized (FSC POL, X Axis) and Side SCatter (SSC, Y Axis).
(D) The plot shows the separation and gating strategy of single cells from clustered cells based on FSC POL Width (X Axis) and SSC (Y Axis).
(E) The plot shows the gating strategy of cells based on the expression of eYFP (X Axis) and CD45 (white blood cells marker, Y Axis).
(F) Representative pictures of sorted CTC clusters positive (top) or negative (bottom) for the expression of eYFP.
A higher level of separation is achieved when fluorescent staining of cell markers is combined to the stable labeling of the cells of interest. Single CTCs or CTC clusters can be separated for further characteristics such as their hypoxic status (i.e., HIF1α activity reporter, eYFP (+)) based on the combination of fluorescent signals (Figure 2B).
Lastly, while for single-cell analysis a micromanipulation of single CTCs and CTC clusters is significantly superior to FACS due to high levels of purity and membrane integrity of micromanipulated CTC clusters, for bulk analysis such as quantitative protein assessment a single-cell micromanipulation would be time consuming. Therefore, FACS represents the best compromise between CTC cluster integrity, sorted CTC yield and time commitment.
-
3.FACS gating strategy.
-
a.Define the gating strategy in order to separate cells from debris and red blood cells (RBCs) based on their physical properties (Figure 2C).
-
b.Use both SSC and FCS Width (W) and Length (L) to separate single cells (left) from clustered cells (right) (Figure 2D).
-
c.Negatively gate single cells for CD45 cells while positively gate the same population for the fluorophore of choice (e.g., eYFP +/-) (Figure 2E). Repeat the same action for the other cell compartment (clustered cells).
-
d.Sort a small quantity of cells in a plate or 1.5 mL tube to assess the purity of the sorted samples under a fluorescent microscope (Figure 2F).
-
a.
CRITICAL: When sorting for 4 populations simultaneously (Single CTCs eYFP (+), Single CTCs eYFP (-), Clusters eYFP (+), Clusters eYFP (-)), make sure to check the purity of the sample before starting each sort, as changes in the charge of the deflection plates could significantly affect the purity of sorted cells.
-
4.CTC sort of single CTCs or CTC clusters, positive or negative for eYFP fluorophore tag.
-
a.Place 1.5 mL tubes in the collection casket with 0.5 mL of cold 1× DPBS.
-
b.Add DAPI 1:1000 to the sample to discriminate between alive and dead cells.Note: Add DAPI right before starting the sort as prolonged incubation might cause leakage of DAPI in live cells.
-
c.Start and monitor the sort.Note: Clogs due to big clusters or clamps can contaminate, and in extreme cases, abort the sort. It is advised to not leave the machine unsupervised.
-
d.For single CTC samples, collect a minimum of 10’000 events. For CTC clusters, collect based on the average cluster size calculated during a trial sort. For example, with 3.5 cells per cluster ratio, collect a minimum of 2’860 events.
-
e.Collect sorted samples and incubate on ice. Proceed immediately to the next step.
 Pause point: Storage of sorted CTCs
Pause point: Storage of sorted CTCs -
f.Gently centrifuge the CTC suspension at 72 × g for 4 min in a pre-cooled centrifuge to pellet the sorted CTCs.
-
g.Remove as much supernatant as possible and snap freeze the tubes containing the sorted samples in liquid nitrogen
-
h.Store samples at −80° C for up to 1 month. For longer storage (up to 3 months), store samples in liquid nitrogen.
-
a.
Sample preparation for mass spectrometry (MS) analysis
For a successful proteomic experiment, efficient cell lysis, digestion, and peptide purification are required. In the presented protocol, the protein extraction is performed in the presence of sodium deoxycholate (SDC) that facilitates the extraction of membrane proteins and does not inhibit the enzymatic activity of trypsin. In addition, as a detergent precipitating at pH below 4, it can be easily removed from the sample by acidification. Finally, tryptic peptides are purified using the SDB-RPS spin columns (PreOmics, #P.O.00013), which allow for stringent washes with organic solvents. Importantly, for this protocol, at least 1 μg of starting material is required. In case such yields cannot be achieved, we recommend to use protocols that are optimized for sub-microgram amounts of starting material, for example Single-Pot Solid-Phase-enhanced sample preparation procedure known as SP3 (Hughes et al., 2019).
Protein extraction from whole cells and protein alkylation
Timing: 1–2 h
-
6.
Thaw sample tubes at RT and shortly spin to re-pellet.
-
7.
Add 50 μL of lysis buffer to cell pellets, ideally corresponding to 1–100 μg.
-
8.
Sonicate samples using Bioruptor (Diagenode, 30 s on, 30 s off, 10 cycles at 4° C).
Note: After sonication, samples should look completely translucent. If not, repeat the sonication step.
-
9.
Shortly spin samples for 10 s at 2000 × g.
-
10.
Heat samples at 300 rpm in a Thermomixer for 10 min at 95°C.
CRITICAL: Pressure can develop during the heating phase, which can cause the opening of the lid and consequent sample loss. To avoid this, insert the samples in the heating block with the lid opened and close after one minute.
-
11.
Cool down samples at 25°C and subsequently spin down at 2000 × g for 10 s.
Optional: If the protein content is not known, measure protein concentration using Reducing Agent compatible BCA assay, according to the manufacturer’s protocol.
-
12.
Add 1 μL of 0.75 M Chloroacetamide solution and incubate at 500 rpm in a Thermomixer for 30 min at 37° C.
-
13.
Short spin samples at 2000 × g for 10 s.
-
14.
Immediately continue with the next step.
Protein digestion
Timing: 12 h
-
16.
Measure pH of the samples. pH should be kept between 8 and 9. If adjustments are required, use 1M Ammonium bicarbonate.
-
17.
Add trypsin to a final enzyme/protein ratio of 1:50 and digest for 12 h at 37°C.
Note: Up to 100 μg of protein can be used for the digestion (which is the maximum capacity of SDB-RPS cartridges). The remaining sample can be stored at −80°C.
Solid-phase extraction (SDB-RPS purification)
Timing: 2–3 h
-
18.
The next day, spin samples at 2000 × g for 10 s.
-
19.
Add 50 μL of 5% TFA solution.
Note: After addition of the TFA, a white precipitate should be visible. If not, check the pH to be < 2. If not, add additional 10 μL of 5% TFA.
-
20.
Add 100 μL of Wash 1 buffer.
-
21.
Vortex for 10 s.
Note: The precipitate should now be dissolved.
-
22.
Shortly spin samples at 2000 × g for 10 s
-
23.
Place SDB-RPS cartridges (PreOmics, #P.O. 00013) in 2 mL flow through collection tubes.
-
24.
Add peptide samples to the cartridges and spin at 2’000 × g for 4 min at 25°C .
-
25.
Discard the flow-through.
Note: Increase the speed by 200 × g and spin again if the liquid does not go through completely
-
26.
Add 200 μL of Wash 1 buffer and spin down at 2’000 × g for 4 min at 25°C.
-
27.
Repeat step 26.
-
28.
Discard the flow-through.
-
29.
Add 200 μL of Wash 2 buffer and spin down at 2’000 × g for 4 min at 25°C.
-
30.
Repeat step 29.
-
31.
Discard the flow-through.
-
32.
Place cartridges in clean and labeled 2 mL tubes.
-
33.
To elute the peptides, add 100 μL of the Elution buffer and spin down at 2’000 × g for 2 min at 25°C.
-
34.
Repeat step 33.
-
35.
Dry eluted peptides in a vacuum concentrator at 50°C.
Note: The temperature of the vacuum is not crucial and it can be run at different temperatures depending on the type of speedvac.
Pause point: Dried peptides can be stored in −20°C, up to 1 year.
Mass spectrometry analysis
Timing: 2 h per sample
Different quantitative approaches can be applied to study proteomes of CTCs. The appropriate method should be selected based on the total amount of extracted proteins and number of samples to be compared. In this protocol, we focus on a label-free quantification approach that can be used when the yield of extracted proteins is limited (less than 5 μg). If larger quantities of starting material are available, the quantification accuracy and the proteome coverage can be further increased by employing the tandem mass tagging (TMT) approach (Thompson et al., 2003) followed by peptide fractionation (Wang et al., 2011).
-
36.Reconstitute peptides in 20 μL of LC-MS buffer:
-
a.Add 20 μL of buffer to each sample.
-
b.Sonicate in Vial Tweeter sonicator (5 pulses, Amplitude 100%, cycle 0.5).
-
c.Shake at 1400 rpm in a Thermomixer for 5 min at 25°C.
-
a.
-
37.
Determine peptide concentration using a NanoDrop (280 - 340 nm wavelengths). 30%–40% of initial protein amount is expected to be purified as peptides.
-
38.
Dilute samples with LC-MS buffer to the same concentration, ideally 0.25 ug/ul.
-
39.
Transfer samples to the LC-MS vials.
-
40.
Analyze by LC-MS using the gradient and instrument settings indicated in the materials and equipment section. Inject up to 0.5 μg of peptide per run.
Note: The rest of the peptide can be stored at −20°C in case the analysis needs to be repeated.
Expected outcomes
CTCs are key players in the metastatic process, and a better understanding of CTC biology is likely to foster new treatment opportunities for metastatic cancers (Aceto et al., 2014; Gkountela et al., 2019; Pantel and Alix-Panabieres, 2019; Szczerba et al., 2019). In this protocol, we describe how to perform quantitative proteomic analysis of CTCs, previa separation of single CTCs and CTC clusters and according to a fluorescent reporter using FACS sorting and mass spectrometry. The output list of upregulated and downregulated molecular functions represents the biological processes that define the compared samples (e.g., CTCs eYFP (+) and CTCs eYFP (-)). A view of the top molecular functions is usually sufficient to highlight the main processes that are ongoing at a given point in time. However, it is important to consider that each function could identify a high number of proteins involved in the process, often overlapping with other functions. For this reason, generally, results validation is crucial to test the reliability of the findings, for instance adopting in independent settings. As previously described, for example, direct immunofluorescence staining can inform on candidate protein expression and localization (e.g., overlap of the signal between the protein of interest and eYFP fluorophore) and a knockdown of the candidate protein of interest can validate its functional role (Donato et al., 2020).
Quantification and statistical analysis
The quantified CTC protein content holds several information that require deep analysis of the results for their interpretation. The performance of quality checks (reproducibility of LC separation, mass accuracy) and the setting of exclusion criteria for technical outliers (rejection/reanalysis of MS runs with significantly lower intensity and identification rates) are crucial to obtain reliable results. Further analysis, based on relative protein abundances of the samples (e.g., singles CTCs versus CTC clusters) provides the identity of the most abundant proteins, allowing to infer the activity of biological pathways and various molecular functions. Data interpretation is dependent on the comparisons of the datasets and the control used. For instance, in this experiment, Single CTCs eYFP (+), Single CTCs eYFP (-), Clusters eYFP (+), Clusters eYFP (-) could be compared, resulting in the identification of upregulated or downregulated protein signatures.
Mass spectrometry data analysis
The acquired data can be analyzed with any method of choice suitable for the label-free quantification. For this protocol, it is recommended to use the following approach:
-
1.
Use the Progenesis QI software (v2.0) to align MS1 signals from different raw files (using an “automatic processing” function) and export the “.mgf” files for peptide identification.
-
2.Search generated “.mgf” files using MASCOT against a selected database (containing forward and reverse sequences and lists of common Mass Spectrometry (MS) contaminants) using the following search criteria:
-
a.Full tryptic specificity required.
-
b.Up to 3 missed cleavages allowed.
-
c.Set Carbamidomethylation (C) as fixed modification; set Oxidation (M) and Acetyl (protein N-term) as variable modification.
-
d.Set mass tolerance to 10 ppm for precursor and 0.6 Da for fragment ions.
-
a.
-
3.
Import search results (xml-format) into Progenesis QI software (v2.0) and export the .csv file containing precursor intensities for identified peptides.
-
4.Perform the quantification using the SafeQuant R package (v2.3.2) (Ahrne et al., 2016) to obtain the relative abundances. A standard label-free quantification obtained with the suggested tool includes:
-
a.Data normalization.
-
b.Missing data imputation using the knn algorithm (Arian et al., 2020).
-
c.Calculation of protein intensities as a sum of the peak areas of peptides specific to a given protein.
-
d.Statistical testing between conditions using e-Bayes moderated t-Test implemented in a limma package (Ritchie et al., 2015).
-
e.Multiple testing corrections using the Benjamini-Hochberg approach.
-
a.
Further MS data analysis for the comparison of CTCs eYFP (+) versus CTCs eYFP (-)
The final lists show all the proteins detected by the MS ranked by q and p value obtained by comparing the protein abundances among samples (relative abundances). Relative abundances could be analyzed with several methods. In his protocol, we used Microsoft Excel.
-
5.
Accurately screen for mislabeling of the protein names due to the import into Microsoft Excel (i.e., numbers and specific gene names could be automatically renamed in months of the year or dates).
-
6.
Set a significance q value cutoff at < 0.1.
-
7.
Re-filter all the significant proteins for a unique entry name.
-
8.
Repeat steps 6 and 7 for each list of proteins obtained from the MS analysis. Each list represents the upregulated proteins in a given sample (e.g., CTCs eYFP (+) or CTCs eYFP (-)).
-
9.Run gene ontology (GO) analysis on gProfiler web server.
-
a.Set “organism” to “Homo Sapiens”.
-
b.Within the advanced options, set “only annotated genes” in the “statistical domain scope”.
-
c.Set “g:SCS threshold” in the “significance threshold”
-
d.Set “0.05” in the “user threshold”Note:P value of 0.05 is widely accepted for significance threshold. However, a more stringent threshold could be used if required.
-
e.Set “ENTREZGENE_ACC” in the “numeric IDs treated as”.
-
a.
Note: For R users, use gProfileR package v 0.6.7.
-
10.
The results could be plotted by adjusted p value (e.g., Bonferroni correction) to rank the significance of the biological functions, and by assigning an intersection score to show the protein abundance per biological pathway (Donato et al., 2020).
Limitations
Together with the availability of a mass spectrometer, the main limitation of this protocol remains the number of CTCs collected from xenograft models of breast cancer and successfully processed through FACS. Low number of cells submitted for MS analysis may result in the identification of only the most abundant proteins expressed in the cells at a given point in time (e.g., ribosomal proteins or fluorophore tags) and miss a more global overview on the proteome.
Troubleshooting
Problem 1
4-way sort contaminates one or more samples (steps 3 and 4 in “step-by-step method details”)
Potential solution: Sequential sorting
Perform a 2×2-way sort until the desired number of cells per sample is reached. Then, switch to another 2×2-way sort for the second condition. For instance, start with sorting single CTCs eYFP (+) versus single CTCs eYFP (-) until 10’000 cells are sorted. Subsequently, switch to sort CTC clusters eYFP (+) versus CTC clusters eYFP (-). As CTC clusters are generally less abundant than single CTCs, sorting first single CTCs would allow to quickly retrieve living cells that could be already stored for the following steps.
Problem 2
4-way sort significantly slows down the sort (steps 3 and 4 in “step-by-step method details”)
Potential solution: Sequential sorting
The abort rate of the sorter should be kept under control: if it increases, changes in the sorting strategy should be applied. As described above, perform a 2×2-way sort until the desired number of cells per sample is reached. Then, switch to another 2×2-way sort for the second condition. Alternatively, stop the sort and re-mix the sample to avoid artificial cell aggregation that could slow down the machine’s flow.
Problem 3
Contamination of mouse cells in the MS analysis (step 1 in “quantification and statistical analysis”)
Potential solution: Negative selection of mouse cells during FACS sorting
Human CTCs are isolated from the blood of xenograft models, i.e., murine blood. For this reason, contamination with mouse cells can occur. The proteomes of the two organisms are highly similar and a large fraction of peptide sequences is shared, therefore a mixture cells from human and mouse should be avoided. Thus, it is critical to ensure that murine cells are sorted out during the FACS sorting step, for instance by using mouse-specific antibodies against common cell-surface antigens (e.g., CD45).
Problem 4
Contamination of mouse cells in the FACS sort (step 3 and 4 in “step-by-step method details”)
Potential solution: Negative selection of mouse cells during FACS sorting
Human CTCs are isolated from the blood of xenograft models, i.e., murine blood. For this reason, contamination with mouse cells can occur and the FACS sort must be planned to negatively or positively sort out murine blood contaminant cells. Mouse contaminant blood cells can be negatively sorted out by using multiple markers for human CTCs such as EpCAM. However, when epithelial surface markers are not present (e.g., LM2 cancer cells express low EpCAM levels), other markers such as CD298 could be added (Lawson et al., 2015). In addition, mouse contaminant blood cells can be positively sorted out by using mouse CD45 labeling.
Problem 5
Insufficient yield of CTCs at week 5 (step 4 and 5 in “before you begin”)
Potential solution: Pilot study and delayed harvest of CTCs
When LM2 are used in NSG, CTCs should appear in the blood circulation from week 3, with high peaks from week 4 (refer to Donato et al., 2020 for further details). If during the pilot this trend is not observed, consider to perform a delayed harvest proportional to the tumor growth curve.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nicola Aceto (Nicola.Aceto@biol.ethz.ch).
Materials availability
The protocol does not describe newly generated materials.
Data and code availability
The protocol does not include datasets generated or analyzed during this study.
Acknowledgments
We thank Dr. Joan Massagué (MSKCC, NY, USA) for donating cell lines. We thank all members of the Aceto Lab for feedback and discussions. We thank the members of the flow cytometer core (DBM of University of Basel) for performing flow cytometer analysis. Calculations were performed at the sciCORE (https://scicore.unibas.ch/) scientific computing center at the University of Basel. Research in the Aceto Lab is supported by the European Research Council (678834 and 840636), the European Union (801159-B2B), the Swiss National Science Foundation (PP0P3_163938, PP00P3_190077, IZLIZ3_182962), the Swiss Cancer League (KFS-3811-02-2016, KLS-4222-08-2017, KLS-4834-08-2019), the Basel Cancer League (KLbB-4173-03-2017, KLbB-4763-02-2019), the two Cantons of Basel through the ETH Zürich (PMB-01-16), and the University of Basel, Switzerland.
Author contributions
C.D. and N.A. designed the study, performed the experiments, analyzed the data, and drafted the manuscript. K.B. and A.S. provided the protocols, performed the mass spectrometry analysis, performed preliminary data analysis, and drafted the manuscript. All authors have read, commented, and approved the manuscript in its final form.
Declaration of interests
N.A. and C.D. are listed as inventors in patent application EP 19188215.8, ‘‘Angiogenesis promoting agents for prevention of metastatic cancer.’’ N.A. is a paid consultant for companies with an interest in liquid biopsy.
References
- Aceto N., Bardia A., Miyamoto D.T., Donaldson M.C., Wittner B.S., Spencer J.A., Yu M., Pely A., Engstrom A., Zhu H. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrne E., Glatter T., Vigano C., Schubert C., Nigg E.A., Schmidt A. Evaluation and improvement of quantification accuracy in isobaric mass tag-based protein quantification experiments. J. Proteome Res. 2016;15:2537–2547. doi: 10.1021/acs.jproteome.6b00066. [DOI] [PubMed] [Google Scholar]
- Arian R., Hariri A., Mehridehnavi A., Fassihi A., Ghasemi F. Protein kinase inhibitors' classification using K-Nearest neighbor algorithm. Comput. Biol. Chem. 2020;86:107269. doi: 10.1016/j.compbiolchem.2020.107269. [DOI] [PubMed] [Google Scholar]
- Donato C., Kunz L., Castro-Giner F., Paasinen-Sohns A., Strittmatter K., Szczerba B.M., Scherrer R., Di Maggio N., Heusermann W., Biehlmaier O. Hypoxia triggers the intravasation of clustered circulating tumor cells. Cell Rep. 2020;32:108105. doi: 10.1016/j.celrep.2020.108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato C., Szczerba B.M., Scheidmann M.C., Castro-Giner F., Aceto N. Micromanipulation of circulating tumor cells for downstream molecular analysis and metastatic potential assessment. J. Vis. Exp. 2019 doi: 10.3791/59677. [DOI] [PubMed] [Google Scholar]
- Gkountela S., Castro-Giner F., Szczerba B.M., Vetter M., Landin J., Scherrer R., Krol I., Scheidmann M.C., Beisel C., Stirnimann C.U. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176:98–112.e14. doi: 10.1016/j.cell.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.S., Moggridge S., Muller T., Sorensen P.H., Morin G.B., Krijgsveld J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- Lawson D.A., Bhakta N.R., Kessenbrock K., Prummel K.D., Yu Y., Takai K., Zhou A., Eyob H., Balakrishnan S., Wang C.Y. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel K., Alix-Panabieres C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019;16:409–424. doi: 10.1038/s41571-019-0187-3. [DOI] [PubMed] [Google Scholar]
- Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba B.M., Castro-Giner F., Vetter M., Krol I., Gkountela S., Landin J., Scheidmann M.C., Donato C., Scherrer R., Singer J. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- Thompson A., Schafer J., Kuhn K., Kienle S., Schwarz J., Schmidt G., Neumann T., Johnstone R., Mohammed A.K., Hamon C. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 2003;75:1895–1904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang F., Gritsenko M.A., Wang Y., Clauss T., Liu T., Shen Y., Monroe M.E., Lopez-Ferrer D., Reno T. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics. 2011;11:2019–2026. doi: 10.1002/pmic.201000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The protocol does not include datasets generated or analyzed during this study.