Summary
Blunted reward responsivity is associated with anhedonia in humans and is a core feature of depression. This protocol describes how to train the common marmoset, Callithrix jacchus, on an appetitive Pavlovian conditioning paradigm to measure behavioral and cardiovascular correlates of anticipatory and consummatory phases of reward processing. We describe how to use intracerebral infusions to manipulate brain regions whose activity is relevant to impaired reward processing in depression and how the paradigm can be used to test antidepressant efficacy.
For complete details on the use and execution of this protocol, please refer to Alexander et al. (2019).
Subject areas: Model Organisms, Neuroscience, Behavior
Graphical abstract

Highlights
-
•
Pavlovian conditioning in marmosets to model reward processing deficits in depression
-
•
Combining behavior and cardiac physiology to measure reward-related arousal in monkeys
-
•
Using targeted intracerebral infusions to manipulate non-human primate brain areas
-
•
Describing use of the model to test the efficacy of antidepressants
Blunted reward responsivity is associated with anhedonia in humans and is a core feature of depression. This protocol describes how to train the common marmoset, Callithrix jacchus, on an appetitive Pavlovian conditioning paradigm to measure behavioral and cardiovascular correlates of anticipatory and consummatory phases of reward processing. We describe how to use intracerebral infusions to manipulate brain regions whose activity is relevant to impaired reward processing in depression and how the paradigm can be used to test antidepressant efficacy.
Before you begin
The model organism and its welfare
The model organism we use is the common marmoset, Callithrix jacchus, a type of new-world primate. Our marmosets are bred on-site. Their utility in understanding the neurobiology of psychiatric disorders lies in their prefrontal cortex which has similar cytoarchitectonic subdivisions to the human prefrontal cortex. The marmoset brain is also lissencephalic facilitating easy surgical targeting. Very importantly, their relatively small size makes them ideal for keeping in captivity in a manner that fully supports their biological and social needs. Nevertheless, the step-by-step methods of this protocol are also relevant to other non-human primate species.
CRITICAL: Marmoset studies must be performed with the approval of a research ethics committee.
-
1.Appropriate conditions must be in place to ensure animal welfare, which is of the utmost priority. For marmosets, this includes:
-
a.Paired housing wherever possible. Marmosets are social animals and are at their happiest when they are with a partner. Breeding groups are housed as families. Experimental pairs are housed mixed sex, with males vasectomized.
-
b.A spacious home cage with enrichment objects (Figure 1A).
-
i.Our home cages measure 280 × 120 × 98 cm, with stainless-steel backing and high-pressure laminate side walls.
-
ii.At the top of the cage are clear plastic verandas (Figure 1B).
- iii.
-
iv.Plastic trays form the bottom of the cage and are filled with sawdust (Figure 1E) to promote natural foraging behavior for dropped food and encourages full use of the enclosure.
-
i.
-
c.Appropriate environmental conditions.
-
i.We use a 12-h light-dark cycle with dawn and dusk transition periods (lights on at 07:00 and lights off at 19:00) in a controlled environment of 22°C ± 1°C and 50 ± 1% humidity.
-
i.
-
d.A nutritious and varied diet.
-
i.All marmosets have ad libitum access to water.
-
ii.Since the marmosets will be trained on a task involving food reward, we fed them a restricted diet on testing days. Marmosets are tested on five days out of seven per week (typically weekend breaks). In a five-day block, their diet included 20 g of pellets (Special Diet Services, Witham, Essex, UK) with a slice of orange after testing on the first testing day and pellets and carrots after testing on the second to fourth testing days.
-
iii.On the fifth testing day in a five-day block, animals were given rusk (Farley’s Rusk, Heinz Foods Ltd., UK), pear and a sandwich with a filling of Nutrica Complan Original (Complan, Trowbridge, Wiltshire, UK), boiled egg, Mazuri Powder (Special Diet Services), multivitamin drops (Abidec; Omega Pharmaceuticals Ltd., London, UK) and vitamin D3 drops (Special Diet Services) after testing.
-
iv.On the first rest day, animals were given pieces of banana and a sandwich. On the second rest day (immediately before the start of the next block), animals were fed with pellets and carrots.
-
i.
-
e.Access to veterinary surgeon and animal care & welfare team.
-
i.Our animal care & welfare team ensure that the marmosets are fed and watered; that the cage atmosphere is harmonious; that the cages are regularly washed; that marmosets’ enrichment is changed; and that no marmosets have any signs of illness. They regularly assist with pre-operative preparation and post-operative monitoring.
-
ii.We also have access to an on-call veterinary surgeon who is available if there are any medical or surgical concerns with the marmosets. The veterinary surgeon visits the colony most days to review both experimental and breeding groups.
-
i.
-
a.
Figure 1.
The home cage
The home cage housed the marmosets in pairs
(A) Entire home cage, dimensions: 280 × 120 × 98 cm. Enrichment items were placed throughout the cage including ropes, logs, ladders, and toys.
(B) Clear plastic verandas forming the roof of the home cage.
(C) Nest box. The entrance to the nest box included a flap to prevent the cannula implants catching on the doorway.
(D) Food tray, where marmosets were fed each day.
(E) Plastic tray forming the floor of the home cage.
Selecting an appropriate cohort size
The study is carried out using a within-subjects repeated measures design and an appropriately sized cohort must be selected.
-
2.
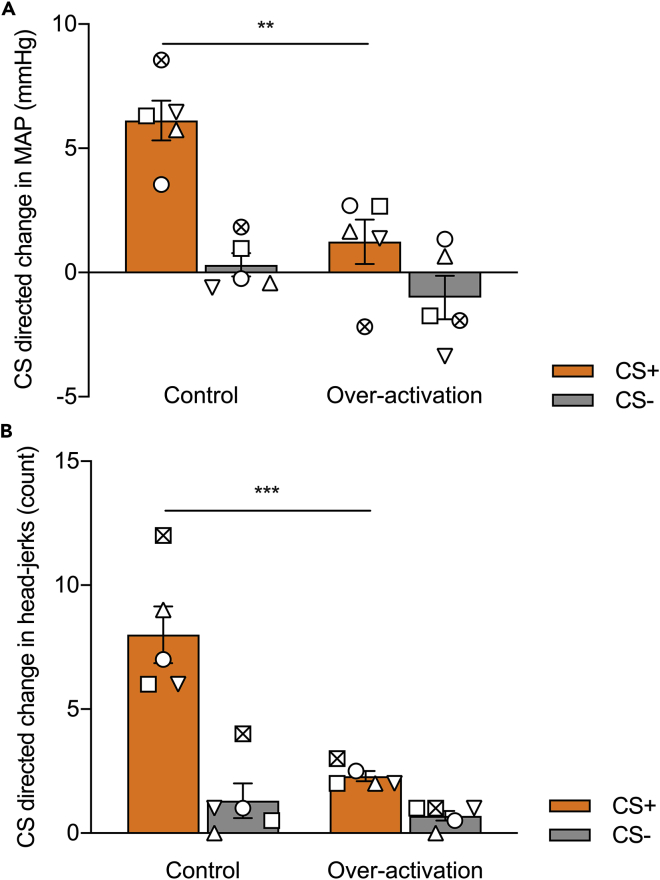
The anticipated effect size we used was based on results published by Braesicke et al. (2005) examining the effects of amygdala lesions on anticipatory (CS directed) arousal using a very similar paradigm (Braesicke et al., 2005). In Braesicke et al., a between-subjects design was used. The control (sham lesion) group showed an approximate 6 mmHg anticipatory increase in blood pressure with a standard deviation of approximately 2 mmHg, whereas the amygdala lesion group showed only a 2 mmHg increase in blood pressure with a standard deviation of approximately 2 mmHg.
-
3.
The α value is set at 0.05. Using a statistical table, the Z1-α/2 value is therefore 1.96.
-
4.
The β value is set at 0.2 (therefore power, 1-β, is 0.8). Using a statistical table, the Z1-β value is therefore 0.84.
-
5.
The formula used for calculating sample size, n, for a between-subjects design is:
where σ refers to the standard deviation and M refers to the sample mean. The n per arm in a between-subjects design would be:
Given this protocol uses a within-subjects design rather than a between-subjects design (and therefore there is a reduced inter-subject contribution to the standard deviation), we used this figure as a guide for the minimum number of subjects needed.
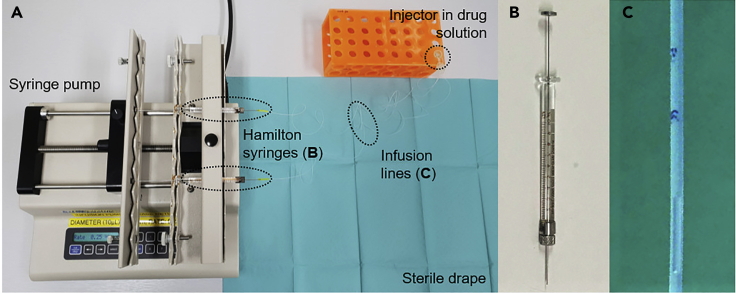
The behavioral testing apparatus and telemetry system
We use a specially designed sound-attenuated testing chamber for the marmoset. The chambers have a modular design with a removable floor. This means the same chamber can be used for multiple different paradigms requiring different sets of equipment. We call the module used for the appetitive Pavlovian conditioning task the ‘food box’ apparatus. For the full specifications of the testing chamber and food box apparatus, please contact Cambridge University Biotronix Workshop at biotronix@psychol.cam.ac.uk. We describe the key components here.
-
6.The appartus (Figure 2) consists of the following:
-
a.A sound attenuating box containing the testing chamber (Figure 2A).
-
b.LED strips (RS Components, Corby, UK) on the ceiling consisting of individually controllable red, blue, and green lights which can be turned on together to create a white ‘houselight’ (Figure 2B-h).
-
c.A computer-controlled speaker system to play auditory cues (Figure 2B-s). Speakers are located at one end of the chamber behind a plastic wall to obscure them from view. Their loudness is the same at each food box.
-
d.Three video cameras (CPC Farnell, Preston, UK) to continuously monitor the animal during testing (Figure 2B-c). These are connected to the acquisition computer using CyberLink PowerDirector software (CyberLink Corp., Vaals, ND) although other recording software would work too.
-
i.It is important to have more than one view of the animal: we use a top-down view (especially good for counting head-jerks, a conditioned orienting response directed at auditory conditioned stimuli, as described in Reekie et al., 2008) and two oblique views facing either food box to identify when animals retrieve the food reward (in order to measure latency).
-
i.
-
e.The telemetry system receiver, which is concealed beneath the white floor panel (Figure 2B-t).
-
a.
-
7.The food box module (Biotronix, Cambridge, UK; Figure 2B-fb and Figure 2C) includes the following:
-
a.Two food boxes.
-
i.The food boxes (Figure 2C-fb) are electrically controlled, attached to the left and right walls of the internal frame of the apparatus. Each food box is cylindrical measuring 52 mm in diameter and 51 mm in length.
-
ii.When the carry box (used to transport the marmoset) is fitted to the internal frame of the apparatus, the positions of the windows are aligned with the food boxes (Figure 2C-d).
-
iii.A small extension to the food box cylinder contains a 28V, 0.04W encased light bulb above the chamber.
-
iv.A latch to secure the carry box is located next to the left food box (Figure 2C-l).
-
i.
-
b.Two doors in front of each food box – one opaque and one transparent.
-
i.The doors can be opened remotely to grant access.
-
ii.When only the opaque door is opened, the transparent door remains in place to allow ‘sight’ of an empty food box without access (Figure 2D).
-
iii.When both the opaque and transparent doors are opened the animal has access to the food box containing reward.
-
i.
-
c.A central clearing for the carry box to be inserted.
-
a.
-
8.The testing chamber and module is controlled by the Whisker control system (Cardinal and Aitken, 2010) and in-house software (our code is available on request). The apparatus’ function is best understood by correlating its operation with the phases of the appetitive Pavlovian conditioning task, namely (a) an inter-trial interval (ITI); (b) a CS period (either an auditory CS+ predicting food reward or an auditory CS- predicting no reward); (c) a US period (either a US+ consisting of marshmallow [Mini Mallows, Frisia, Spain] as a food reward or a US- consisting of no reward); and (d) an end.
-
a.During the ITI, the LED houselight is on, the food box lights are off, and the doors are closed concealing the contents of the food boxes.
-
b.During the CS period, the food box apparatus remains unchanged compared to the ITI period, but an auditory cue plays from the computer-controlled speakers.
-
c.During the US-, the food box light turns on and the opaque door opens to reveal an empty food box (as in Figure 2D). The transparent door remains shut to prevent the animal reaching in and trapping its hand. During the US+, the food box light turns on and both the opaque and transparent door open to reveal marshmallow. Since this is always the final trial, the doors do not close at the US+ offset.
-
d.At the end of the session, the food box light turns off.
-
a.
Figure 2.
The Pavlovian discrimination testing chamber and food box apparatus
(A) The testing chamber, with a sound attenuating external casing.
(B) The doors of the testing chamber opened to visualize the food box apparatus. The houselight (h) consists of two strips of LED lights either side of the main chamber. The cameras (c) record r using three views: top-down, left food box and right food box. The food box (fb) module has a left and right food box and a central clearing for the carry box and marmoset. The telemetry receiver (t) is concealed beneath the floor of the apparatus. A speaker system (s) is used to play auditory cues (CS- and CS+).
(C) A close-up view of the internal frame of the food box module. The left food box (fb) is filled with marshmallow, whereas the right food box (fb) is empty. Doors (d) obstruct access to both food boxes – the opaque black door is visible, behind which is a transparent door. The latch (l) is used to attach the carry box into the internal frame of the apparatus.
(D) The right opaque door has opened to reveal an empty food box. The food box light is also on. The transparent door still obstructs access to the empty food box chamber. This is equivalent to the US- period.
A screenshot of the software we use to program the task is shown in Figure 3.
-
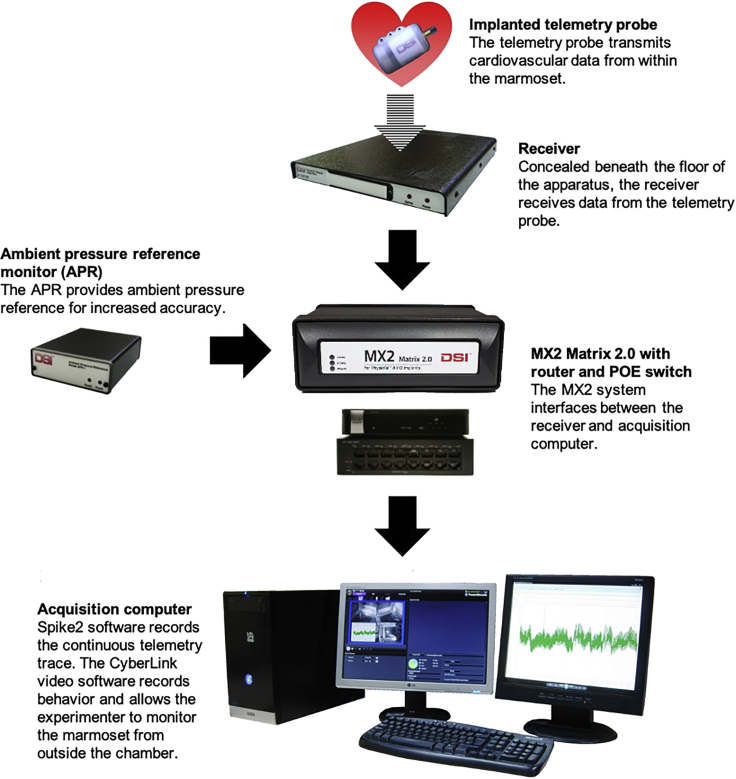
9.Beneath the removeable floor of our testing chambers, a telemetry receiver receives continuous transmission of heart rate and blood pressure information from telemetry probes implanted into animals (see telemetry surgery below). This is part of the PhysioTel Telemetry System (Data Sciences International, St. Paul, MN, USA) which uses short range telemetry to record data from the telemetry probes of untethered animals. A schematic of the PhysioTel telemetry system is shown in Figure 4.
-
a.An ambient pressure reference monitor (APR) continuously records ambient air pressure.
-
b.An MX2 Matrix 2.0 with router and power over ethernet switch gathers pressure information from the animal via a telemetry receiver and from the APR and interfaces with the acquisition computer.
-
c.The acquisition computer transfers the recorded data to a Spike2 datafile using the Spike2 software (version 8.12; Cambridge Electronic Design [CED], Cambridge, UK).
-
d.The computer generates a raw blood pressure trace by comparing the absolute pressure measured by the probe and the ambient pressure from the APR. This datafile can then be used for offline analysis.
-
a.
-
10.
Each animal’s telemetry probe has individual pressure settings, which are registered and stored using PhysioTel software.
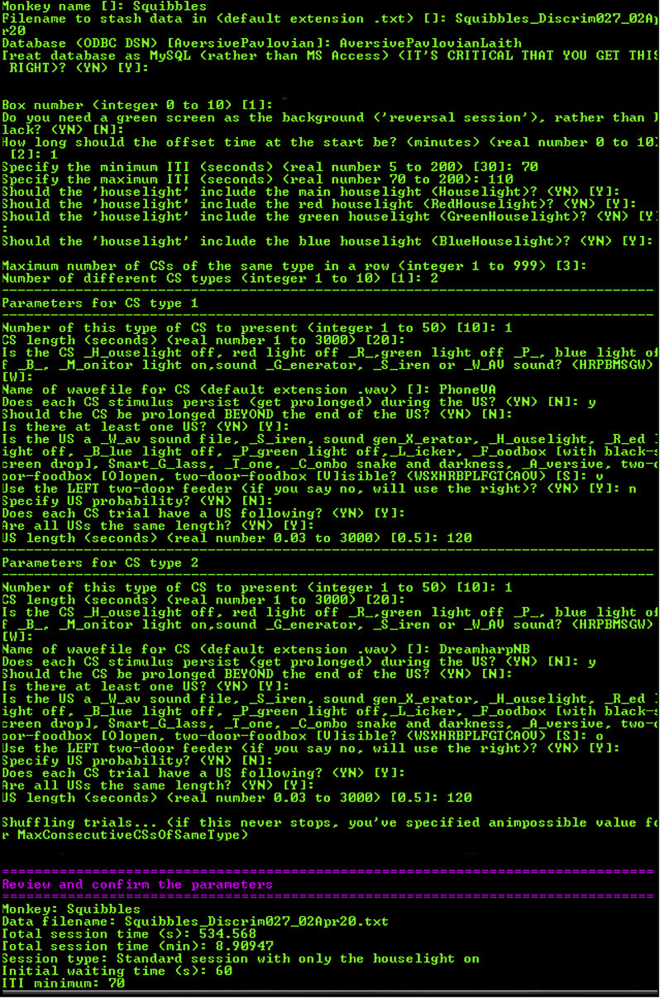
Figure 3.
Screenshot of the bespoke software interface used to control the testing chamber
Software is available upon request from the lead author. The options for each prompt are shown in brackets ( ) and the default entry for each prompt is shown in square brackets [ ]. This screenshot is an example of a session containing a single CS-/US- trial followed by a single CS+/US+ trial. The first set of prompts concern global task parameters: the monkey name and file name; the name of an SQL database which stores the task parameters (in this case called AversivePavlovianLaith, storing data for both appetitive and aversive Pavlovian tasks we run in the apparatus); the initial offset time at the start of the session (one minute); the inter-trial interval (ITI, set to vary between 70 and 110 s); the houselight (set to comprise of the red, blue, and green LED lights to create a white light); and the number of CS types (two: CS- and CS+). The first CS type describes the CS-/US- trial. The CS duration is 20 s and is set as a ‘wav’ file with the name ‘PhoneVA’ (the sound of a phone ringing). The CS is specified to continue playing during the US, but not to carry on beyond the end of the US. The US is set to ‘food box visible’ (US-) and to last 120 s. The second CS type describes the CS+/US+ trial. The CS parameters are identical except the ‘wav’ file is now ‘DreamharpNB’ (the sound of a harp playing). The US is set to ‘food box open’ (US+) and to last 120 s. Finally, the user is prompted to review and confirm the parameters before beginning the session (not fully shown).
Figure 4.
The PhysioTel Telemetry System and Spike2/video recording software
The MX2 Matrix system integrates pressure information from the receiver and the ambient pressure reference monitor, and both the absolute and ambient pressure readings are transferred to the acquisition computer which generates a continuous telemetry readout.
Establishing the testing schedule
-
11.We used the following rules when designing the schedule:
-
a.No more than one session per day.
-
b.No more than one CS+/US+ trial per session.
-
c.No more than two trials of any type per session.
-
d.No more than five CS+/US+ trials over a two-week period (i.e. 10 days of testing).
-
e.Infusions only happen on days with a CS-/US- trial first and CS+/US+ trial second.
-
f.We set the ITIs to pseudo-randomly vary between 70 and 110 s.
-
a.
Example schedules are shown in Table 1. Each trial lasts approximately 210–250 s (depending on the ITI). Sessions with a single trial therefore last 210–250 s (after which the marmoset is removed from the apparatus); sessions with two trials last 420–500 s.
CRITICAL: Remember to have some sessions with just one CS+/US+ trial and other sessions with two CS-/US- trials, to avoid marmosets associating a longer time in the apparatus with reward. It is also important not to ‘overload’ the marmosets with reward; the US+ needs to be an infrequent event to maximize the cardiovascular and behavioral arousal that is generated.
Note: It is worth having two parallel testing schedules established in advance – for example, we had one schedule with reward sessions on Tuesdays and Thursdays, and another with reward on Wednesdays and Fridays (as shown in Table 1). This is useful if your cohort is relatively large and avoids having too many infusions on any one day.
-
12.
Within your cohort, pseudo-randomly allocate a rewarded side to each marmoset (right food box or left food box).
Note: We found marmosets conditioned more rapidly and reliably when we used a single rewarded side compared to varying from one side to the other. However, the conditioned response directed to the CS (orienting head-jerks) is often greater if marmosets can’t predict which food box the marshmallow will be found in – if a single rewarded side is used, conditioned responses directed towards the US (for example, rearing towards the food box) can compete with those directed towards the CS. In some cases, CS directed conditioned responses are seen early on during the CS, progressing to US directed conditioned responses later on in the CS as food anticipation becomes greater (Holland, 1977).
-
13.
Check you have the telemetry probes registered on the acquisition computer’s PhysioTel software for each animal.
Table 1.
Example testing schedules
| Day | Schedule 1 | Schedule 2 |
|---|---|---|
| Monday | nn | nn |
| Tuesday | np | n |
| Wednesday | n | np |
| Thursday | np | n |
| Friday | p | np |
| Saturday | Rest | Rest |
| Sunday | Rest | Rest |
| Monday | n | p |
| Tuesday | np | nn |
| Wednesday | nn | np |
| Thursday | np | nn |
| Friday | n | np |
| Saturday | Rest | Rest |
| Sunday | Rest | Rest |
‘n’ = CS-/US-, ‘p’ = CS+/US+. Indicated in bold are the days where infusions would typically take place. Having two parallel testing schedules is useful to avoid having too many infusions on any one particular day of the week. There were no more than five sessions containing ‘p’ in two weeks. ‘nn’ trials were included to diminish the association between time spent in the apparatus and likelihood of obtaining reward.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Citalopram HBr | Sigma-Aldrich | Cat #C7861, CAS 59729-32-7 |
| Dexamethasone | Hameln Pharma GmbH | Cat #DEXA3.3; CAS 50-02-2 |
| Dihydrokainic acid | Tocris | Cat #0111, CAS 52497-36-6 |
| Enrofloxacin (Baytril) | Elanco | CAS 93106-60-6 |
| Isoflurane (AErrane) | Baxter International | Cat #FDG9623, CAS 26675-46-7 |
| Ketamine HCl (Ketavet) | Tocris | Cat #PFKET03, CAS 1867-66-9 |
| Lidocaine (Intubeaze) | Dechra | CAS 73-78-9 |
| Meloxicam (Metacam) | Boehringer Ingelheim | Cat #86998653, CAS 71125-38-7 |
| Oxygen cylinders | N/A | CAS 7782-44-7 |
| Experimental models: Organisms/strains | ||
| Common marmosets (Callithrix jacchus) aged between 18 months and 2 years at the start of training; eight females and three males in original cohort | University of Cambridge Marmoset Breeding Colony | N/A |
| Software and algorithms | ||
| PhysioTel software | Data Sciences International | N/A |
| PhysioTel-Spike2 interface software | Cambridge Electronic Design and Data Sciences International | N/A |
| PowerDirector | CyberLink Corporation | Version 11.0.0.2027 |
| Prism | GraphPad | Version 8.00.178, RRID: SCR_002798 |
| Spike2 | Cambridge Electronic Design | Version 8.11a, RRID: SCR_000903 |
| Whisker | Cambridge University Technical Services Ltd. | Version 4.6 |
| Other | ||
| −20°C freezer | N/A | N/A |
| 1 mL, 5 mL, and 10 mL slip-tip disposable syringes | Becton Dickinson | N/A |
| 70% Isopropyl cleaning alcohol for injections (Alcotip) | 365 Healthcare | Cat #UNG602 |
| 8 × 480 mm 12V 200 mA LED light bars | RS Components | Cat #ZRS-8480-CW |
| Acrodisc syringe filters | Sigma-Aldrich | Cat #Z259942 |
| Anesthetic machine | Vet-Tech Solutions Ltd. | Cat #AN001 |
| Anistel unfragrenced cleaning spray | Tristel Solutions Ltd. | Cat #TR105 |
| Biogel surgical sterile gloves | Mölnlycke Health Care | Cat #96170 |
| Blackcurrant squash | Tesco | N/A |
| Cannula blockers | Plastics One | Cat #C235DCS (specify pedestal height and center-center distance) |
| Cannula caps | Plastics One | Cat #303DC/1A |
| Carry box | Cambridge University Biotronix Workshop | N/A |
| Chlorhexidine (ChloraPrep SEPP) applicators | Becton Dickinson | Cat #MRB302 |
| Decibel meter | CADRIM | Cat #NT612021 |
| Dental broach (without handle) | Micro-Mega | N/A |
| Dental drill | Eickemeyer | Cat #174028 (drill), #174026 (drill head) |
| Dental drill bits | Henry Schein | Cat #9794056 |
| Desktop computer | N/A | N/A |
| Digital multi-syringe infusion and withdrawal pump | KD Scientific | Cat #780230V |
| Endotracheal (ET) tube | Vygon | Cat #0310.08 |
| Eppendorf microcentrifuge tubes | Thermo Fischer Scientific | Cat #10451043 |
| Ethilon non-absorbable suture | Ethicon Inc., Johnson & Johnson | Cat #W1621T |
| Fine-tip permanent marker pen | N/A | N/A |
| Fischerbrand ZX3 Vortex Mixer | Thermo Fischer Scientific | Cat #13214789 |
| Food box module | Biotronix, Cambridge | N/A |
| Gas-tight 10 μL Model 701RN Hamilton syringe | Hamilton | N/A |
| Heat mat | N/A | N/A |
| Home cage | N/A | N/A |
| ICFP26 Portable Radio | Sony | N/A |
| Incubator | N/A | N/A |
| Intracerebral cannulae (supplier will require specification of pedestal height and center-center distance) | Plastics One | Cat #C235GS |
| Ioban-2 antimicrobial incision drape | 3M | Cat #6635 |
| LICG24SM security cameras | CPC Farnell UK | Cat #SR08748 |
| Magnet | N/A | N/A |
| MicroFine Sterile Needles (23-gauge, 25-gauge) | Becton Dickinson | Cat #PMC0736 |
| MicroFine+ Insulin Syringe | Becton Dickinson | Cat #230-45094 |
| Mini mallows | Frisia | N/A |
| Paladur dental cement | Kulzer GmbH/ AgnThos | Cat #203097 |
| PhysioTel cellulose patch (cut to size) | Data Sciences International/ADInstruments | Cat #DSI00652 |
| PhysioTel HD-S10 telemetric implant | Data Sciences International | N/A |
| PhysioTel Small Animal Telemetry system | Data Sciences International | N/A |
| Plastic dividers | N/A | N/A |
| Plastic tubing (0.3 mm diameter) | Harvard Apparatus | Cat #72-1042 |
| Pressure Implant Re-gel Syringe | Data Sciences International/ADInstruments | Cat #DSI00611 |
| Pulse oximeter/capnograph (MicroCap Handheld Capnograph) | Oridion Capnography Inc. | N/A |
| Q-tips | Johnson & Johnson | N/A |
| Sharps bin | N/A | N/A |
| Shaver | N/A | N/A |
| Spatula | N/A | N/A |
| Speaker system (Z120 USB-powered speakers) | Logitech | N/A |
| Steel screws | Plastics One | Cat #0-80 × 1/16 |
| Stereotaxic frame | David Kopf Instruments | N/A |
| Sterile drapes | N/A | N/A |
| Sterile gauze | 365 Healthcare | Cat #7170 |
| Sterile saline | Baxter International | Cat #FKE1324, CAS 7647-14-5 |
| Stopwatch | N/A | N/A |
| Super-Bond C+B dental adhesive | Sun Medical | N/A |
| Surgical instruments including scalpel, scissors, retractors, hemostat, and forceps | N/A | N/A |
| TES-1319 K-type digital rectal thermometer | TES Electrical Electronic Corp. | N/A |
| Testing chamber | Cambridge University Biotronix Workshop | N/A |
| Thread | N/A | N/A |
| Vetbond tissue adhesive | 3M | Cat #1469sb |
| Vicryl absorbable suture | Ethicon Inc., Johnson & Johnson | Cat #W9444 |
| Weighing scales | N/A | N/A |
| Yellow bung tubing | Harvard Apparatus | Cat #73-1855 |
Materials and equipment
Below are directions for preparing the drugs we use for intracerebral infusions.
Preparing dihydrokainic acid (DHK) for intracerebral infusion
The concentration of DHK we use for infusions is 6.25 nmol/μL diluted in sterile 0.9% saline. We receive a 1 mg sterile vial of DHK from Tocris, stored at 23°C–24°C.
-
•
The molecular weight of DHK is 215.25 g/mol so 1 mg contains 4646 nmol.
-
•
To create a 6.25 nmol/μL solution, you therefore must add 4646 ÷ 6.25 = 743 μL of sterile saline.
-
•
Add 743 μL of sterile saline to the DHK vial.
-
•
Vortex the vial.
-
•
Syringe-filter the contents of the vial (using Acrodisc 0.2 μL syringe filters, Sigma Aldrich, MO, USA).
-
•
Using a pipette with sterile tips, aliquot the solution into sterile Eppendorf tubes.
-
•
Store in a −20°C (or cooler) freezer. We store the solution for up to one month.
Preparing ketamine for intramuscular injection
The concentration of ketamine we use for intramuscular injection is 0.5 mg/kg. The stock solution has a concentration of 100 mg/mL and is stored at 24°C and typically has a shelf life of 3 years. Once opened, the vial and/or any diluted stock solution is disposed of within 28 days.
-
•
Measure the animal’s mass in kilograms.
-
•
Calculate the mass of ketamine needed using mg ketamine = 0.5 × mass of animal in kg.
-
•
We inject no more than 0.1 mL intramuscularly to avoid muscle damage, therefore the concentration of ketamine solution needed is Cinjection = mg ketamine ÷ 0.1 where Cinjection refers to the concentration of the ketamine solution for injection.
-
•
Dilute 0.1 mL of the 100 mg/mL stock solution in 1.9 mL of sterile saline to create 2 mL of 5 mg/mL stock solution, which is easier to dilute to the final concentration.
-
•
We prepare 0.2 mL of the solution for intramuscular injection, even though only 0.1 mL will be injected, so it is easier to draw up. Calculate the final dilution using the formula CstockVstock = CinjectionVinjection where C and V refer to concentration and volume, respectively, and ‘stock’ and ‘injection’ refer to the 5 mg/mL stock solution and the injectable solution, respectively. This formula can be rearranged to work out the volume of stock solution needed: Vstock = (CinjectionVinjection) ÷ (Cstock)
-
•
Using a pipette, draw up the required volume of the 5 mg/mL stock solution and aliquot it into a sterile Eppendorf. Make up to 0.2 mL with sterile saline.
-
•
Using an insulin syringe, draw up 0.1 mL of this injectable solution.
Worked example:
-
•
A marmoset’s mass is 0.456 kg.
-
•
For a 0.5 mg/kg ketamine dose, the mass of ketamine needed is 0.5 × 0.456 = 0.228 mg.
-
•
The Cinjection needed to deliver this in 0.1 mL = 0.228 ÷ 0.1 = 2.28 mg/mL.
-
•
Dilute 0.1 mL of the 100 mg/mL stock solution in 1.9 mL sterile saline to create 2 mL of 5 mg/mL stock solution.
-
•
Prepare 0.2 mL of the 2.28 mg/mL injectable solution using the 5 mg/mL stock solution where Vstock = (CinjectionVinjection) ÷ (Cstock) = (2.28 × 0.2) ÷ (5) = 0.0912 mL = 91.2 μL. Therefore 91.2 μL of 5 mg/mL stock solution provides the same amount of ketamine as 0.2 mL (200 μL) of a 2.28 mg/mL solution.
-
•
Draw up 91.2 μL of 5 mg/mL and aliquot it into a sterile Eppendorf. Make up to 200 μL with 200−91.2 = 108.8 μL of sterile saline.
-
•
Draw up 0.1 mL (100 μL) of this solution for injection.
Preparing citalopram for intramuscular injection
The concentration of citalopram we use for intramuscular injection is 10 mg/kg; again, a volume of 0.1 mL is injected intramuscularly. Citalopram hydrobromide comes as a powder from Sigma-Aldrich and is stored at 23°C–24°C. We keep the powder for no longer than 2 months once opened.
-
•
Measure the animal’s mass in kilograms.
-
•
Calculate the mass of citalopram needed using mg citalopram = 10 × mass of animal in kg.
-
•
Weigh out citalopram using scales and a sterile Eppendorf – usually approximately 6.5–7.0 mg is a reasonable mass, which results in just over 0.1 mL solution (0.1 mL to be injected, with some spare).
-
•
To make the required concentration of 10 mg/kg in 0.1 mL, dissolve the citalopram powder in the Eppendorf in sterile saline using the formula mg citalopram in Eppendorf ÷ saline volume = mg citalopram to be injected ÷ 0.1, rearranged to saline volume = (mg citalopram in Eppendorf × 0.1) ÷ mg citalopram to be injected. This solution will contain the desired concentration of citalopram.
-
•
Using an insulin syringe, draw up 0.1 mL (100 μL) of this injectable solution. Discard any remaining solution after injection.
Worked example:
-
•
A marmoset’s mass is 0.456 kg.
-
•
For a 10 mg/kg citalopram dose, the mass of citalopram needed is 10 x 0.456 = 4.56 mg.
-
•
Weigh out citalopram – in this case, suppose 6.55 mg is weighed out into a sterile Eppendorf.
-
•
To make 4.56 mg/0.1 mL solution, dilute 6.55 mg in (6.55 × 0.1) ÷ 4.56 = 0.144 mL = 144 μL of sterile saline.
-
•
Draw up 0.1 mL (100 μL) of this solution for injection.
Step-by-step method details
Carry box training
Timing: 2 weeks
Marmosets must be ‘box trained’ to enter a carry box in which they can be transported to and from the behavioral testing apparatus. We use a Perspex carry box (dimensions: 24 × 23 × 20 cm). One face of the carry box can be removed and acts as a door, which can be secured with a latch. The latch can also be used to secure the box in the testing chamber. The door and opposite surface have a circular window (diameter 3.5 cm) for food box access together with eight smaller air holes (diameter 1.5 cm).
-
1.
Wipe down the carry box with a scentless cleaning spray and a clean cloth. Allow time to dry.
-
2.
Bring the carry box to the cage, checking against the relevant identification to ensure you are training the correct marmoset.
CRITICAL: always confirm the identity of the animal you are training/collecting against the identification cards affixed to the cage.
-
3.
Open the cage hatch and place the carry box at the opening. Open the box door and attracting the marmoset into the carry box by using small pieces of marshmallow through one of the carry box’s circular windows.
Note: Some marmosets are distracted by the transparent floor of the Perspex carry box – in this case, you can rest the carry box on an opaque surface on your knee.
Note: Now is also a good time to familiarize the marmosets with the sound of your voice.
-
4.
Once marmosets enter the carry box, feed them small pieces of marshmallow to familiarize them with the carry box. Spend the first few days simply familiarizing marmosets with the box.
-
5.
When marmosets readily enter the carry box, transport them to the testing room. You should initially familiarize them with the room itself (by resting the box on a surface in the room) and then only after this with the testing apparatus specifically.
Familiarization with the testing apparatus and “door training”
Timing: 2 weeks
Ultimately, marmosets need to be familiar with being in the testing apparatus for at least 15 min.
-
6.
Ensure the testing chamber has been wiped down with cleaning spray. Allow time to dry.
-
7.Collect the marmoset in the carry box as described above and place the carry box into the testing chamber (secured using a latch; Figure 2C-l).
-
a.For the first two to three sessions, leave the main testing chamber door open so the marmoset can see you.
 CRITICAL: Place marshmallow in the carry box at the same time to make the marmosets comfortable in the testing apparatus.
CRITICAL: Place marshmallow in the carry box at the same time to make the marmosets comfortable in the testing apparatus. -
b.Marmosets should be relatively comfortable after 2–3 sessions, indicated by them sitting still, eating marshmallow, and looking around the chamber.
-
a.
-
8.For the next few sessions, close the testing chamber door and leave the houselight on. We would typically start by doing this for 5 min, gradually extending the duration to ∼20 min.
-
a.For these sessions, start the CyberLink video recording software. Indicate the name, date, and trial type ('habituation') in the file name.
-
b.Carefully monitor the marmoset for any signs of agitation or distress (including stereotyped behaviors such as excessive turning, gnawing and grooming).
-
c.Once the time has elapsed, open the testing chamber door and return the marmoset to its home cage.
-
d.Watch the video and for 20 s at the onset of every minute measure:
-
i.Locomotion duration (seconds).
-
ii.Scanning behaviors (either a count of ‘bouts’ or duration in seconds).
-
iii.A count of stereotyped behaviors including turning, gnawing, and grooming.
-
i.
-
e.Keep a running log of the day-by-day extent of these behaviors and determine if the marmoset is habituating to the test apparatus. Locomotion levels/scanning levels should decrease, and stereotyped behaviors should be minimal.
-
a.
-
9.Next, familiarize marmosets with the operation of the testing apparatus (this is called ‘door training’). The rationale for this step is to familiarize marmosets with the noises made by the opening of the food box doors during the US period.
-
a.Program the behavioral task to run with an initial wait period of 120 s, after which the door will open (‘US’) with no preceding CS, to reveal marshmallow for 120 s.
-
b.Weigh approximately 6 g of marshmallow (US+) and place it in one of the food boxes. You should also count the number of marshmallow pieces this weight is equivalent to. During door training, alternate between filling the left and right food box pseudo-randomly to ensure marshmallow is present for 2–3 days on the left and 2–3 days on the right per week, so marmosets are familiar with the operation of both food boxes.
 CRITICAL: Always use fresh marshmallow – old marshmallow becomes dehydrated meaning the weight will change and the reward will be less appetizing for the marmosets.
CRITICAL: Always use fresh marshmallow – old marshmallow becomes dehydrated meaning the weight will change and the reward will be less appetizing for the marmosets. -
c.Collect the marmoset as before and secure the carry box in the testing chamber. Once the chamber door is closed start the video recording software and the behavioral task.
-
d.Monitor the marmoset carefully. They should look very calm and relatively still during the first 120 s (as this period is similar to previous habituation sessions). They may initially be hesitant to reach for the marshmallow.
-
e.When animals start consuming marshmallow within 40 s of the door opening over two consecutive sessions, the marmosets are ‘door trained.’ This took our marmosets on average 10 sessions but varied between 4 and 16 sessions depending on how ‘timid’ the marmoset was.
-
a.
-
10.
Once marmosets are door trained, they are ready to undergo surgical procedures. Note that on testing sessions after door training, the marshmallow reward will be present on one side (varied across animals).
Surgical procedures
Timing: 4 weeks to complete all surgical procedures
Animals will undergo at least two aseptic surgical procedures: one to implant a telemetric blood pressure monitor (HD-S10 Implant, Data Sciences International [DSI], St. Paul, MN, USA) into the descending aorta, and one to implant intracerebral cannulae targeting the brain region of interest. Telemetric blood pressure monitors are always implanted first, followed by intracerebral cannulae. Both can be implanted sequentially provided there is adequate recovery time between operations of at least ten days. Alternatively, intracerebral cannulae can be implanted further on in the protocol when marmosets have learnt the appetitive Pavlovian conditioning task.
-
11.In advance of the day of surgery:
-
a.Weigh marmosets the day before surgery. Surgery should not take place if there is a 10% or greater reduction in weight compared to the previous week.
-
i.Additionally, 24 h before telemetry surgery, give marmosets prophylactic antibiotic treatment with oral enrofloxacin (Baytril; 0.2 mL of a 25 mg/mL oral solution; Elanco, IN, USA) mixed with 0.4 mL diluted blackcurrant squash (Tesco, UK).
-
ii.It is also important to check the to-be-implanted telemetry probe is functioning properly and document the relevant settings on the DSI software as per the manufacturer’s guidance.
-
i.
-
b.Ensure marmosets are nil-by-mouth at least 12 h prior to surgery to minimize the risk of aspiration during general anesthetic.
-
c.Ensure you have everything you need – in particular, double check that you have enough isoflurane and oxygen cylinders.
-
a.
-
12.On the day of surgery:
-
a.Pre-medicate the marmoset with i.m. ketamine hydrochloride (Ketavet; 0.1 mL of a 100 mg/mL solution; Henry Schein, Melville, NY, USA) by cleaning an area of the lateral thigh with alcohol and injecting at a 90° angle. Place the marmoset in an incubator (warmed to 34°C) until fully sedated.
-
b.Once sedated, we administer meloxicam s.c. (Metacam; 0.075 mL of a 2 mg/mL solution; Boehringer Ingelheim) for all surgeries. For telemetry surgery, we additionally administer enrofloxacin s.c. (Baytril; 0.2 mL of a 25 mg/mL injectable solution). Administer s.c. medications by cleaning the skin, lifting a small area of skin and injecting at a shallow angle.
-
c.Shave the relevant incision sites.
-
i.For telemetry surgery, shave the abdomen from the inguinal ligament to the xiphoid process of the sternum.
-
ii.For intracerebral cannulation surgery, shave the scalp taking care to avoid the ear tufts as these act as important social signals.
-
i.
-
d.Weigh and record the marmoset’s weight.
-
a.
-
13.Once sedated, shaved, and weighed, transfer the animal to the surgical suite. Our surgical suite consists of an operating table with a heat mat, an anesthetic machine (Compact Anesthesia Systems; VetTech Solutions Ltd., Cheshire, UK) and a scavenger system.
-
a.Induce anesthesia using a facemask covering the mouth and nose which delivers the inhalational anesthetic isoflurane (4% in 0.6–0.8 L/min O2; Baxter International, IL, USA).
-
i.Assess the depth of anesthesia using deep-tendon reflexes: when there is no reflex in the ankle extensors, the animal is sufficiently anesthetized.
-
i.
-
b.Intubate the marmoset. This requires at least two people. A third person should ideally be available to prepare the anesthetic circuit after intubation. A good description of small non-human primate intubation is provided in the reference Morris et al. (1997).
-
i.One person uses one hand to hold the marmoset at the angle of the jaw. The index finger of the other hand is hooked onto the marmoset’s canine, opening the marmoset’s mouth.Note: ensure that you have good lighting shining into the marmoset’s pharynx – you should be able to see the epiglottis beyond the root of the tongue and the opening into the larynx.
-
ii.A second person prepares the endotracheal (ET) tube and a Q-tip sprayed with the anesthetic lidocaine (IntuBeaze 20 mg/mL oromucosal spray; Dechra, Shrewsbury, UK).
-
iii.Apply the Q-tip with lidocaine onto the epiglottis to relax it for at least 15 s.
-
iv.Insert the ET tube into the trachea. The ET tube is in the right position if the marmoset has an effective cough upon insertion.
-
v.The gas supply is then switched from the facemask to the ET tube. The anesthetic flow is modified for the new circuit (2.25%–2.75% isoflurane in 0.3–0.4 L/min O2). The marmoset is laid down on a heat mat.
-
i.
-
c.Attach the monitoring equipment (Figure 5).
-
i.Attach a pulse-oximeter (Figure 5A) to the marmoset’s paw (Figure 5B) to monitor O2 saturations (>95%) and pulse rate (200–250 beats per minute) and a capnograph to monitor end-tidal CO2 (35–55 mmHg) and respiratory rate (20–45 breaths per minute; Figure 5C). We use a combined pulse-oximeter and capnograph (MicroCap Handheld Capnograph; Oridion Capnography Inc., MA, USA).
-
ii.Monitor core body temperature (36°C–38°C) using a lubricated rectal thermometer (TES-1319 K-type digital thermometer; TES Electrical Electronic Corp., Taiwan; Figure 5D).
-
i.
-
a.
-
14.In telemetry surgery (Figure 6), marmosets undergo a laparotomy to implant an HD-S10 transmitter probe which continuously detects and transmits an arterial blood pressure trace via radio-frequency signals. This telemetry implant is suitable for small animals including marmosets, rats, ferrets, guinea pigs and others. This procedure needs three people: a surgeon, an anesthetist, and an assistant. The anesthetist should record observations every 5 min from the onset of anesthesia.
-
a.Once the animal is intubated and under stable anesthesia with monitoring in place, place a sterile drape on the heat mat (lifting the marmoset up) and rest the marmoset back down in a supine position.
-
b.Secure the upper and lower limbs using small pieces of measuring tape.
 CRITICAL: the surgery should be carried out under aseptic conditions. The surgeon and their assistant must now wash their hands using iodine or chlorhexidine and must don sterile gloves, a sterile gown and a surgical mask.
CRITICAL: the surgery should be carried out under aseptic conditions. The surgeon and their assistant must now wash their hands using iodine or chlorhexidine and must don sterile gloves, a sterile gown and a surgical mask. -
c.Clean the abdomen using chlorhexidine (Chloraprep SEPP applicators; BD, Berkshire, UK) and cover the abdomen with an Ioban-2 antimicrobial incision drape (3M, St. Paul, MN).
-
d.The initial stage of the laparotomy to isolate the abdominal aorta is performed as follows:
-
i.Cut the skin and rectus sheath using a scalpel, with a single vertical incision extending from the xiphoid process to an imaginary horizontal line at the level of the anterior superior iliac spines.
-
ii.Cut through the linea alba using sterile scissors and make an incision into the peritoneal cavity.
-
iii.The intestines are exposed using blunt dissection and positioned to the sides of the peritoneal cavity, held in place using metal retractors. The abdominal aorta should be visible as a large vessel in the retroperitoneum. Ensure it is exposed from the level of the coeliac trunk to its bifurcation in the common iliac arteries.Note: in some cases, the intestines are particularly voluminous and will need to be exteriorized to visualize the abdominal aorta – if this is necessary, keep them moist by placing the bowel loops in between swabs soaked in sterile saline.
-
iv.Separate this portion of the aorta from the surrounding viscera, fat, and connective tissue using blunt dissection with soft pressure swabs.
-
v.Once separated, pass an 8 cm piece of thread under the aorta approximately 1 cm superior to its bifurcation. Use a hemostat to clamp the thread and apply gentle tension to the aorta in preparation for implantation.
-
i.
-
e.In parallel an assistant should be preparing the insertion needle used to insert the telemetry probe catheter into the aorta, and the telemetry probe.
-
i.The needle used to insert the telemetry probe catheter is a 23-gauge needle bent at 60° and needs to be sealed with 10 μL of Vetbond tissue adhesive (3M Animal Care Products, St. Paul, MN, USA) pipetted into the needle chamber (to prevent blood leakage once the aorta is punctured).
-
ii.Before insertion, the telemetry probe catheter is examined for any bubbles using a surgical microscope. If there are bubbles, these can be removed by flushing the catheter probe with pre-supplied catheter gel (pressure implant pre-gel syringe, DSI, St. Paul, MN, USA). The telemetry probe signal should be rechecked by switching the probe on using a magnet and using a radio to test the signal. The probe can remain on for the remainder of the surgery.
-
i.
-
f.To insert the telemetry probe:
-
i.The assistant should use their index finger to apply pressure to the aorta immediately superior to the coeliac trunk.
-
ii.The anesthetist should begin a timer to ensure pressure is maintained for no more than three minutes (with a two-minute warning) to minimize the risk of lower limb ischemia.
-
iii.Puncture the aorta approximately 1 cm above the bifurcation using the 23-gauge bent insertion needle.
-
iv.Insert the catheter of the telemetry probe into the vessel using a grooved introducer (Figure 6B) until a small length of tubing (approximately 1.25 cm) is inserted into the vessel.
-
v.Once inserted, the assistant’s index finger is gradually released over ten seconds.
-
vi.Apply 10 μL of Vetbond adhesive to the puncture site. Leave the glue to dry for 30 s and check for any bleeding around the insertion site.
-
vii.Use the radio to check for correct placement of the catheter.
 CRITICAL: A clear fluctuating tone (corresponding to the cardiac cycle) indicates correct placement. See the troubleshooting section (problem 2) if the signal is weak or absent.
CRITICAL: A clear fluctuating tone (corresponding to the cardiac cycle) indicates correct placement. See the troubleshooting section (problem 2) if the signal is weak or absent. -
viii.Apply a small 0.25 cm2 cellulose patch onto the puncture site together with a further 10 μL of Vetbond adhesive to ensure integrity of the seal.
-
i.
-
g.Following insertion, remove the thread and retractors, gently move the intestines back into position and gently rest the probe on top with the catheter directed as rostrally as possible to minimize any kinks.
-
i.Suture the tabs on the probe body into the abdominal wall using non-absorbable sutures (Ethilon 3-0W; Ethicon Inc., Puerto-Rico, USA).
-
ii.Close the muscular abdominal wall using non-absorbable sutures.
-
iii.Check the signal of the probe again using the radio; if the signal remains strong and fluctuant, the probe is working. It can be switched off using the magnet and it is not turned on again until behavioral testing after recovery.
-
iv.Close the skin using an absorbable suture (Vicryl 3-0 W9444; Ethicon Inc., Puerto-Rico, USA) and glue the skin by opposing the wound edges either side of the suture using Vetbond.
-
v.Stop the anesthetic by turning off the isoflurane (but leave the oxygen on).
-
vi.Inject 1.0–2.5 mL of sterile saline subcutaneously to replace fluid loss.
-
vii.Once the animal starts to rouse with a cough, remove the ET tube and supply the animal with wafted O2 until it can maintain saturations of 95%–100% independently (usually after 3–5 min).
-
viii.Place the animal in the incubator and monitor regularly during recovery.
-
i.
-
h.Once the animal has recovered, return them to their home cage with food and water.
-
i.Administer 0.1 mL meloxicam (Metacam; 1.5 mg/mL solution; Boehringer Ingelheim) each morning for three days following surgery.
-
j.Animals will need a minimum of ten days recovery following surgery.
-
a.
-
15.In cannulation surgery, marmosets are implanted using 26-gauge double-pronged guide cannulae (Plastics One; Roanoke, Virginia, USA) targeting bilateral subgenual anterior cingulate cortex (sgACC/Brodmann Area 25) alone (7.0 mm long, 1.0–1.4 mm apart) or both bilateral sgACC/25 and bilateral perigenual anterior cingulate cortex (pgACC/Brodmann Area 32; 2.0–3.5 mm long, 1.0–1.2 mm apart).
-
a.Once the marmoset is under stable anesthesia, it is secured into a stereotaxic frame modified for the marmoset (Figure 7; David Kopf Instruments; Los Angeles, CA, USA).
-
i.Carefully position the ear bars into each ear canal to prevent lateral movements and adjust their position in the lateromedial (LM) direction until the marmoset is centered in the frame.
-
ii.Place the mouth bar on the hard palate of the marmoset.
-
iii.Position the eye bars in the supraorbital notches of the eye sockets.
 CRITICAL: Apply Lacrilube ointment (Allergan, Bucks, UK) around the eyes and eye bars to prevent the eyes from drying out during surgery.
CRITICAL: Apply Lacrilube ointment (Allergan, Bucks, UK) around the eyes and eye bars to prevent the eyes from drying out during surgery. -
iv.Ensure the marmoset’s head is fixed rigidly in place in the stereotaxic frame by applying gentle pressure to the lateral area of the skull and checking for the absence of movement.
-
i.
-
b.Clean the shaved area of the marmoset’s scalp using chlorhexidine as above and place an Ioban-2 antimicrobial drape onto the scalp.
-
c.Using a sterile scalpel blade, create a longitudinal incision on the scalp and use retractors to create a clear field of view of the skull surface.
-
d.The coordinate system (in mm) uses the inter-aural line as the anteroposterior (AP) zero coordinate (positive anteriorly) and the superior sagittal sinus at AP +17.5 as the LM zero coordinate (positive to the animal’s right).
-
e.Positioning of the guide cannulae to target sgACC/25 and/or pgACC/32 needs to be adjusted in-situ according to cortical depth to account for variation in brain size between marmosets.
-
i.We achieve this by measuring a cortical depth at a standard coordinate (AP +17.5 LM −1.5) called a depth-check. The depth-check is done using a very fine, smooth dental broach (Micro-Mega, Besancon, France) lowered vertically through the brain.
-
ii.At this coordinate, create a burr hole in the skull (approximately 4 mm in diameter) using a dental drill (Dental Micro Motor, Eickemeyer, UK) to expose the dura mater adherent to the brain surface.
-
iii.Remove the dura gently using a small needle, bent to a 45° angle, which you can use to hook and remove pieces of dura.
-
iv.Using the broach, take measurements at the cortical surface and at the base of the skull (detected as the broach is lowered and then deflects slightly). The depth is the difference between the surface and the base, and our accepted depth is 5.8–6.8 mm (based on the expected depth from the atlas (Paxinos et al., 2011) and previous depth checks in our colony).
-
v.If the depth at this position falls outside of the range of 5.8–6.8 mm, adjust the AP measurement in steps of 0.5 mm (anteriorly if the depth was >6.8 mm, and posteriorly if the depth was <5.8 mm), and reassess the depth until it falls within the 5.8–6.8 mm range. The burr hole can be extended using the dental drill.
-
vi.Note all AP alterations and adjust subsequent coordinates for guide cannulae insertion accordingly.
-
i.
-
f.To target pgACC/32, we combine any adjustment from the depth-check together with the co-ordinate from the atlas. For sgACC/25, we use an additional depth-check performed at AP +14.0 LM -1.0 (location according to the atlas, ± any correction from the 'general' depth-check), 0.3 mm lateral to where the injection will be made, because of previous variability in cannula positioning in this region. This allows us to more accurately determine the depth around sgACC/25. We consider a depth of 8.9–9.3 mm to be acceptable, and in a similar fashion we adjust the AP measurement in steps of 0.5 mm until the depth is in this range.
-
g.A burr hole is created at the correct target locations, bilaterally. If the target region is close to the midline, as is the case with these prefrontal regions, then a single burr hole is created that spans the midline to accommodate a bilateral cannula.
-
h.Fasten steel screws (0.80 mm, 1/16”; Plastics One) to the skull surrounding the guide cannulae to facilitate adhesion of cement to the skull.
-
i.Paint a layer of Super-Bond dental adhesive (Sun Medical, Shiga, Japan) onto the skull surface, to provide an optimal bonding surface for the dental acrylic mount used to fashion the cannula implant.
-
j.Once the coordinate with the correct depth has been determined, remove the dura mater with a bent 23-gauge needle.
-
k.Insert the double guide cannula.
-
i.Using the guide prongs of the double lumen cannulae, take another cortical surface measurement.
-
ii.For sgACC/25: lower the cannula vertically downwards until the surrounding skull obstructs you from lowering them further. An ‘estimated’ appropriate length of guide cannula is approximately two-thirds of the depth of the brain at the sgACC/25 co-ordinate, minus 1.0 mm (which is the typical length the injector must protrude beyond the end of the guide cannula).
-
iii.For pgACC/32: angle the cannula to 25° with the tips facing posteriorly and lower them in the same way.
 CRITICAL: you must record the depth you reached with the guide cannulae: the projection of the injectors depends on how much deeper they have to go to reach the target area.
CRITICAL: you must record the depth you reached with the guide cannulae: the projection of the injectors depends on how much deeper they have to go to reach the target area. -
iv.Once the guide cannula is in position, apply a small amount of Vetbond adhesive into the hole of the skull containing the cannulae. This is to create a barrier between the brain and the dental acrylic mount.
-
i.
-
l.Insert cannula blockers, used to maintain patency of the guide cannulae (they do not project beyond the end of the guide cannulae).
-
m.Apply dental acrylic (Paladur, Kulzer GmbH, Germany) onto the Super-Bond ensuring to go underneath skull screws and around the guide cannulae.Note: use a small spatula to smoothen the acrylic cement as it dries to avoid any sharp edges.
-
n.Once the cement has cured, suture the loose skin in front and behind the implant using absorbable sutures (Vicryl 3-0 W9444; Ethicon Inc., Puerto Rico, USA).
-
o.At the end of surgery, give marmosets 0.7 mg intramuscular dexamethasone (0.18 mL of a 3.8 mg/mL solution; Hameln pharma GmbH, Hameln, Germany) to reduce any cerebral edema.
-
p.Place stainless steel ‘dummy’ cannulae blockers into the guide cannulae to maintain patency, and screw on brass/aluminum protective caps using the thread on the guide cannulae.
-
q.Once surgery is completed, switch off the anesthetic, remove the ET tube and recover the animal as above.
-
r.Injectors (for subsequent intracerebral infusions) extend beyond the ends of the guide cannulae. Record the injector lengths for the brain regions that were cannulated. We typically aimed for a depth 2/3 down from the cortical surface for sgACC/25 injectors, whereas we used a fixed projection of +1.5 mm beyond the end of the guide cannula for pgACC/32.
-
s.Administer oral meloxicam (Metacam; 0.1 mL of 1.5 mg/mL solution; Boehringer Ingelheim) for three days after surgery.
-
a.
-
16.
Marmosets will need at least ten days of recovery after each surgical procedure.
CRITICAL: caps and cannula blockers should be changed at least once per week, to ensure guide cannula patency. The implant should be cleaned with chlorhexidine and inspected for any signs of infection.
CRITICAL: Following surgery and recovery, you should check that the marmosets are still comfortable with the Pavlovian apparatus by repeating ‘door training’ (see familiarization with the testing apparatus and ‘door training’).
-
17.
Once the marmosets have re-stabilized, the next step is to counterbalance the auditory cues (‘to-be-CS+’ and ‘to-be-CS-,’ such that the cue with the least intrinsic arousal becomes the CS+ and vice versa) and then commence appetitive Pavlovian conditioning. The next sections describe how to set up and carry out both the one-off counterbalancing session and the subsequent Pavlovian conditioning sessions.
Figure 5.
Monitoring equipment for surgical procedures
Adapted from Jackson (2016).
(A) Capnograph/pulse oximeter, showing clockwise from top left: expired CO2 partial pressure (EtCO2), oxygen saturation (SpO2%), pulse rate, breathing profile, and respiratory rate. The tube with orange connector seen on the top left of the photograph samples gases from inside the intubation tube which is not shown here.
(B) Pulse oximetry sensor applied to hand of marmoset during surgery.
(C) Close-up view of a capnograph readout.
(D) Rectal thermometer with lubricated plastic sheath covering the probe.
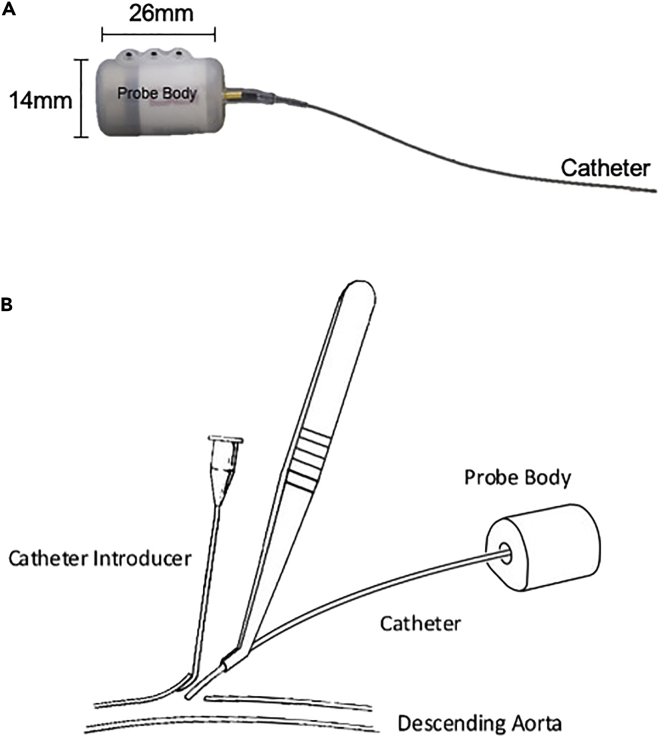
Figure 6.
The telemetry probe and insertion of the probe during telemetry surgery
(A) Telemetry probe. The probe body contains a battery, signal emitter and sensor. The catheter is filled with a non-compressible fluid and bio-compatible gel at the catheter tip. The tip is coated with an anti-thrombogenic film.
(B) Implantation procedure. Blood flow in the descending (abdominal) aorta is briefly occluded and a small incision is made in the arterial wall. A catheter introducer with a grooved tip is used to lift a flap at the incision site. The catheter is introduced using forceps and is guided into the vessel through a groove in the catheter introducer. Once the catheter was in place and an adequate signal is detected, the catheter is secured in place with glue and a cellulose patch.
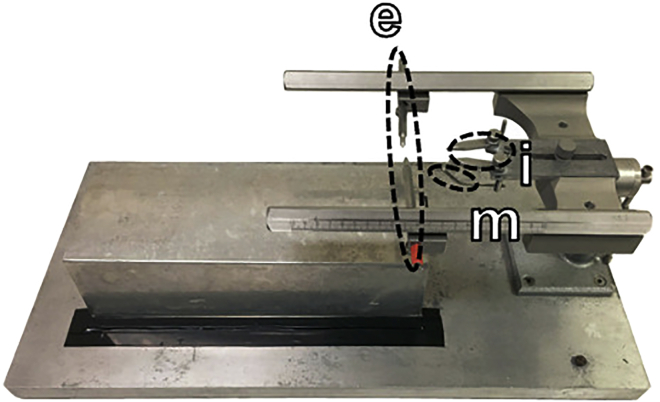
Figure 7.
Stereotaxic frame for cannulation surgery
The stereotaxic frame was specially modified for the marmoset. The marmoset was secured on the frame using ear bars (e), eye bars (i) and a mouth bar (m).
Training on the appetitive Pavlovian discrimination task
Timing: several weeks
Prepare the testing chamber and software for appetitive Pavlovian conditioning sessions
Timing: 30 min per day with 10 min to reset the apparatus in between animals.
-
18.Prepare the testing chamber.
-
a.Wipe down the testing chamber with cleaning spray and clean out the food boxes using a damp cloth, ensuring that you dry them afterward.
-
b.Before testing any marmosets, use the Whisker software (Cardinal and Aitken, 2010) to check that all the components of the testing chamber are working, in particular the house light, food box lights, doors, and sounds.
-
c.Place approximately 6 g of fresh marshmallow in the rewarded food box. You should also count the number of marshmallow pieces you place in the food box. There should be marshmallow in this food box regardless of whether or not there is a rewarding trial scheduled that day. This is to ensure that the marmosets cannot use the smell of the marshmallow to predict whether they will receive marshmallow on that session or not. In so doing, you are ensuring the onset of the CS+ is the only predictor. You can skip this step for the 'counterbalancing auditory cues' session, described below.
-
d.At least twice per week, place a decibel meter in a carry box and play the auditory cues through the speaker system. Ensure that the auditory cues for the CS+ and CS- measure 70 ± 2dB.
-
a.
-
19.Prepare the testing software.
-
a.Using your behavioral task software, prepare the session for the marmoset according to your testing schedule.
-
i.We name the files ‘marmosetname_sessionnumber-sessiontype_date’ where ‘sessiontype’ is either n, nn, p, np (n = CS-/US- and p = CS+/US+).
-
ii.The key variables for Pavlovian conditioning sessions are the number of trials, the types of trial and the ITI, CS and US duration.
-
iii.For a CS-/US- (unrewarded) trial, there should be an ITI of 70–110 s; a 20 s auditory cue (CS-) played through the speaker; a 120 s period of the food box light switching on and the opaque door opening revealing an empty food box (US-); and finally closure of the opaque door at the end of the US-.
-
iv.For a CS+/US+ (rewarded trial), there should be an ITI of 70–110 s; a 20 s auditory cue (CS+, distinct) played through the speaker; a 120 s period of the food box light switching on and both the opaque and transparent door opening revealing a full food box (US+); and then termination of the session (the CS+/US+ trial is always the final trial).
-
v.For the single session to counterbalance auditory cues, name the file ‘marmosetname_counterbalance_date’. Program the task to present each auditory cue three times in a random order, with an ITI of 70–110 s and a duration of 20 s each.
-
i.
-
b.Using the Spike2 software, load the telemetry acquisition software which will record the telemetry trace.Note: include a textbox where you can name the animal, date, and session number so it is visible on the video when you record.
-
c.Load the CyberLink software for video recording. We typically have a single screen split into four: three camera views (left food box, right food box and top-down) and one with a screen view of the Spike2 telemetry trace and textbox (as seen in Methods video S1).Methods video S1. Example of an appetitive Pavlovian discrimination session showing a CS-/US- trial and CS+/US+ trial, Related to steps 32–36The video has been shortened to include only the two trials, without the inter-trial interval (ITI) period. The lower right panel of the video shows the Spike2 telemetry trace and the Spike2 session timer. The CS- trial consists of the baseline period (00:10 to 00:30 on the video; 40 s to 60 s on the Spike2 timer), the CS- cue (00:30 to 00:50 on the video; 60 s to 80 s on the Spike2 timer) and the US- (00:50 to 02:50 on the video; 80 s to 200 s). During the US- period, the non-rewarded food box light is switched on and the opaque door opens to reveal no food reward. The CS+/US+ trial consists of the baseline period (283 s to 303 s), the CS+ period (303 s to 323 s) and the US+ period (323 s to 443 s). During the US+ period, the rewarded food box light is switched on and both the opaque and transparent doors are opened to enable access to marshmallow reward. 443 s marks the end of the session; typically, a two-trial session lasts just over 7 min.Download video file (87.3MB, flv)
-
a.
-
20.Prepare the telemetry system.
-
a.Use the DSI software to select the telemetry probe settings of the animal you are going to test.
-
a.
Gathering the marmoset for testing
Timing: 5–10 min per day
-
21.
Collect the marmoset from the cage, checking the identity card. Limit the amount of marshmallow you use to entice the marmoset into the box – try and use only one marshmallow.
-
22.
Bring the marmoset into the testing room.
-
23.Turn on the telemetry probe by holding the carry box above a radio set to AM 600 Hz frequency.
-
a.Turn on the telemetry probe by placing a magnet through the carry box circular opening. We use a small bar magnet attached to a spatula.
-
b.Listen for the signal of the probe – the telemetry probe is now on – and switch the radio off.
-
a.
-
24.
Enter the testing room and secure the marmoset with the carry box in the testing chamber as described above. Close the testing chamber door.
Counterbalancing auditory cues
Timing: 10 min (once only)
-
25.
Begin the Spike2 software and behavioral program simultaneously. The Spike2 software has a timer built in, which you will be able to see on the screen.
-
26.
Begin the video recording.
-
27.During testing, watch the animal carefully on the monitor and make sure the session is running smoothly.
-
a.The auditory cues should present in a random order, with a duration of 20 s and an ITI of 70–110 s. The doors should not be opening as there is no US.
-
a.
-
28.At the end of the sixth auditory cue presentation, the session has finished.
-
a.Open the testing box door and remove the marmoset from the testing chamber, remembering to switch off the telemetry probe with the magnet and radio.
-
b.Return the marmoset to its home cage.
-
c.Save the telemetry and video data, backing up the files to a readily accessible drive for subsequent analysis.
-
a.
-
29.
Clean the carry box and testing chamber using cleaning spray and a cloth, and prepare the testing chamber, software, and telemetry system for the next animal by repeating the steps in Prepare the testing chamber and software for appetitive Pavlovian conditioning sessions.
-
30.Later in the day, use the onset times of the auditory cues to measure the cardiovascular arousal response to each auditory cue, paying particular attention to the blood pressure (mean arterial pressure, MAP) and heart rate.
-
a.For each auditory cue calculate the average MAP and heart rate during the 20 s CS period and the 20 s preceding ‘baseline’ period.
-
b.Calculate the change for each auditory cue using a (CS – baseline) difference measure – we call this ‘CS directed’ (although, technically speaking, the auditory cues are not CSs yet). This will give you six CS directed MAP/heart rate responses; three for each cue type.
-
c.Use a paired t-test to see if there is a significant difference in the MAP response between the two cues.
-
i.If there is, counterbalance the cues such that the cue provoking the smallest MAP response becomes the CS+ and the cue provoking the largest MAP response becomes the CS-.
-
ii.If there is not, check whether there is a significant difference in the heart rate response and counterbalance the cues in the same way.
-
iii.If there is no difference in MAP or heart rate response, randomly allocate the CS+ and CS- cue taking into account the remainder of your cohort. Alternatively, calculate a mean MAP response for each cue and counterbalance the cues based on the mean MAP.
-
i.
-
a.
Note: We have found over numerous studies and testing of individual marmosets that the conditioned MAP response is far more stable than the conditioned HR response.
-
31.
Record the file name of the auditory cue for each animal’s CS+ and CS-.
Carrying out standard testing sessions
Timing: 10 min per day
Methods video S1 shows a video of a standard testing session.
-
32.
Begin the Spike2 software and behavioral program simultaneously.
-
33.
Begin the video recording.
-
34.
Watch the animal carefully on the monitor and make sure the session is running smoothly. Ensure the CS cues are of the correct duration; the US period is of the correct duration and the doors are operating correctly; and that the houselight is working correctly.
-
35.During the US+, record the latency (in seconds) for the animal to reach and grab the marshmallow from the food box. At the end of the US+, the food box light will turn off to signal termination of the session.
-
a.Open the testing box door and remove the marmoset from the testing chamber, remembering to switch off the telemetry probe.
-
b.Return the marmoset to its home cage.
-
c.Return to the testing room. Weigh the remaining marshmallow and calculate the mass of marshmallow consumed. Record this value.
-
a.
-
36.
Clean the carry box and testing chamber using cleaning spray and a cloth, and prepare the testing chamber, software, and telemetry system for the next animal by repeating the steps in Prepare the testing chamber and software for appetitive Pavlovian conditioning sessions.
Analyzing your cardiovascular data
Timing: 1 h per day
-
37.Pre-processing of the Spike2 trace will produce a tab-separated text file separated into rows of time, systolic blood pressure, diastolic blood pressure and heart rate (Figure 8). The second-by-second mean arterial pressure (MAP) can be calculated using the formula: diastolic blood pressure + ⅓(systolic blood pressure – diastolic blood pressure).
-
a.Outliers (systolic blood pressure values >200 mmHg or <0 mmHg) are removed.
-
b.Recording failures <0.4 s are replaced using cubic spline interpolation. Failures ≥ 0.4 s are treated as missing values, although data collection is highly reliable overall.
-
a.
CRITICAL: Movement of the animal should not cause noise in the telemetry signal, other than in the rare event that the catheter briefly kinks with abdominal flexion. This causes a brief signal drop-out, which is treated as a recording failure (37b).
-
38.The onset times and durations of the events in the task, including CS onset and US onset, should be outputted from the behavioral testing program controlling the apparatus.
-
a.We developed our own simple in-house software to ‘read’ the onset time file and ‘read’ the corresponding Spike2 tab-separated result file of the same name and calculate average absolute systolic blood pressure, diastolic blood pressure, MAP and heart rate for:
-
i.The 20 s baseline period (before CS- or CS+)
-
ii.The 20 s CS period
-
iii.The 120 s US period (additionally separated into 20 s windows)The software also calculated the difference between:
-
iv.CS and baseline (CS directed)
-
v.US and CS (US directed)Additional measures may include:
-
vi.The maximum heart rate and/or blood pressure during the CS-/CS+/US-/US+ periods
-
vii.The heart rate and/or blood pressure values during 10 s windows of the CS and US periods
-
i.
-
a.
-
39.
Create a file with a running summary of each animal’s heart rate and blood pressure data day-by-day. The main data points you will be using are the CS directed and US directed values for MAP and heart rate.
Note: record absolute blood pressure values too, to see if there are any baseline differences in cardiovascular function from day-to-day (or following an infusion).
-
40.To determine whether the marmosets have successfully acquired conditioned cardiovascular discrimination between the CS- and the CS+, perform a paired t test over the previous four consecutive sessions containing both CS-/US- and CS+/US+ (np).
-
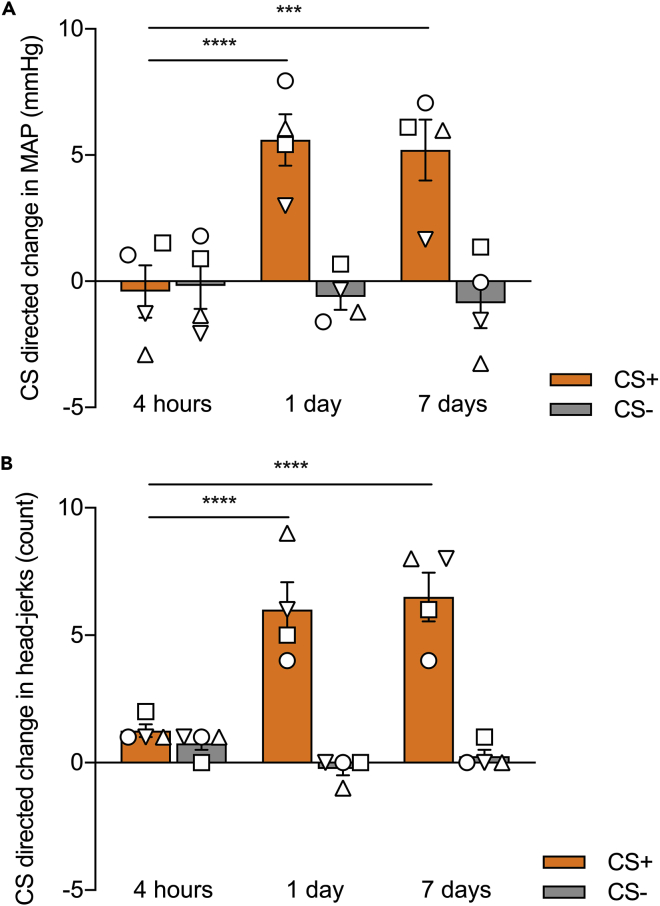
a.If there is a significant difference between the CS directed MAP responses to the CS+ and CS- with increased CS directed responses to the CS+, the marmosets are deemed to have successfully discriminated. In our cohort of marmosets, cardiovascular conditioning was most reliably evidenced on MAP measures, although there was also a significant difference in heart rate responses.
-
a.
-
41.
Continue to analyze data in this way, including on your run sheet a column where you can signify which days marmosets had a mock infusion, control infusion or drug infusion (see mock infusion sessions and intracerebral infusions and testing following infusions).
CRITICAL: It is also important to check that the marmosets are also showing US directed cardiovascular responses. We found marmosets would show increases in MAP over-and-above the CS+ during the US+ period, resulting in a positive US directed response.
Note: Generally speaking, discrimination should be stable once acquired, but make sure you are aware of any ‘blips’ in marmosets arousal response. There’s usually a reason for this, like disruption in the home cage or in the home room. If a marmoset does have an ‘off day,’ we necessitated two more CS+ sessions with stable performance (including the CS+/US+ single trial sessions) before re-commencing infusions.
Figure 8.
Output from pre-processing of the raw telemetry trace
The tab-separated text file contains seven columns: time of the local maximum of the cardiac cycle used for RR interval/heart rate measurement; RR interval as the time between the local maximum and the previous cardiac cycle maximum (s); heart rate (beats per minute); a second time column corresponding to the local maximum of the cardiac cycle used for systolic blood pressure measurement (identical to the first column); systolic blood pressure (mmHg); a third time column corresponding to local minimum of the cardiac cycle used for diastolic blood pressure measurement; and the diastolic blood pressure (mmHg). Note that the first row of data in the output is ignored during further analysis as the RR interval is calculated using t=0 as the reference point for the previous cardiac cycle maximum (because there is no cardiac cycle preceding the first cycle) generating an erroneous result. Mean arterial pressure (MAP) values for each cycle were calculated later in the analysis.
Analyzing your behavioral data
Timing: 1 h per day
-
42.Watch the video recording of the day’s session and score the behaviors. The same time windows are important as mentioned above in Analyzing your cardiovascular data. The behaviors to be scored include:
-
a.Locomotion (duration in seconds) during the baseline and CS periods.
-
b.Head-jerks (rapid side to side flicks of the head, best visualized from the top-down view with blurring of the ear tufts – the head moves very fast indeed) during the baseline and CS periods.Note: Head-jerks vary considerably between animals. Whilst some animals make very fast orienting movements, others show a slower head orienting response to sound cues which nevertheless distinguish the CS+ from the CS-. It is important to carefully compare the CS+ and the CS- during training to determine the behavior the animal makes to the CS+ vs. CS-.
-
c.The latency to grab the food reward during the US+ period if you haven’t already recorded this. Along with the mass of marshmallow consumed.
-
a.
-
43.
Alongside the animal’s running summary of cardiovascular data, create a running log of their behavioral performance too.
-
44.
Determine whether marmosets show significant behavioral discrimination using head-jerk data, in a similar way to determining cardiovascular discrimination described above. Record behaviors as a running summary alongside your cardiovascular data.
Note: Generally, behavioral measures displayed by the marmosets tend to discriminate between the CS+ and CS- before cardiovascular ones, but successful discrimination requires both significant behavioral and cardiovascular discrimination.
Mock infusion sessions
Timing: 30 min per day on 4–5 days over the course of 2 weeks
Once marmosets are discriminating based on behavioral and cardiovascular measures, you can carry out mock infusions before testing sessions. This acclimatizes marmosets to the infusion procedure, ensuring that it does not disrupt their subsequent performance or confound interpretation of intracerebral infusions. Mock infusions should be carried out in a separate minor procedures room, in the same place where you will carry out the intracerebral infusions. You will need two people for this.
-
45.
Using plastic laminate dividers, divide the marmoset into a quadrant of its home cage. Try and remove as much equipment from this quadrant as possible to make it easier for another researcher to catch the marmoset.
-
46.
In the infusion room, all you will need is a drape, an infusion pump, non-sterile gloves and a timer. Place the drape on the table and ensure the infusion pump is on – no Hamilton syringes need to be in situ; the pump is only there to make a noise.
-
47.
Ask your colleague to catch the marmoset and bring it to the infusion room.
Note: It is better if someone other than the experimenter catches the marmoset, so the experimenter maintains as good a relationship with the marmoset as possible.
-
48.
Using non-sterile gloves, handle the marmoset’s caps mimicking their removal. Marmosets will likely already be accustomed to this, because their caps and cannulae blockers will have been changed in a similar way.
-
49.
Press the infusion pump and start a timer for two minutes, the duration over which infusions run. In so doing, you are acclimatizing marmosets to the sounds of an infusion procedure.
-
50.
Once the timer has finished, handle the marmoset’s caps again, mimicking their reinsertion, and ask the researcher to return the marmoset to the home cage, pulling out the dividers so it can move freely around the entire home cage.
-
51.
Leave the marmoset in the home cage for a time period equivalent to the action time of the drug (usually between 10–30 min).
-
52.
Test the marmoset in the usual way and analyze the data from the session to see if the mock infusion has affected their performance in any way. We would wait for 2–3 sessions of stable performance following mock infusions before commencing real infusions.
Intracerebral infusions and testing following infusions
Timing: Intracerebral infusions typically take 20 min to set up and 15 min to perform. Each drug will have its own ‘wait time’ for pharmacological action before you should test the animals. Typically, the procedure will take approximately 45 min.
Once marmosets have maintained stable discrimination following mock infusions, they can undergo intracerebral infusions prior to testing sessions. A single experimenter can set up the infusion equipment, but for actual infusions two experimenters are needed.
-
53.
Check the wait time of your drug. If the wait time is less than 10 min, set up the testing apparatus in advance as described in Prepare the testing chamber and software for Pavlovian conditioning sessions. If the wait time is greater than 10 min, you can set up during the wait time.
-
54.
First, spray down the area in the infusion room with cleaning spray and wipe with a cloth. Spray the infusion pump and an Eppendorf holder and wipe these down, too.
-
55.
Remove an Eppendorf containing the drug solution from the −20°C freezer and place it in the Eppendorf holder, allowing it to defrost and equilibrate to room temperature whilst the rest of the infusion system is being set up.
CRITICAL: leave the Eppendorf closed as the drug solution contained within it is sterile and should be exposed to air for the minimal amount of time possible.
-
56.Lay down a sterile drape, ensuring only to handle the very edges of the drape to maintain the rest of the field as sterile. Make sure there is a sharps bin next to the drape to dispose of sharps. Empty the following onto the sterile drape:
-
a.Two sterile Hamilton syringes (gas-tight 10 μL Model 701RN syringes; Hamilton, Reno, NV, USA).
-
b.The relevant injector (these will be different lengths depending on the surgical coordinates of the animal the brain region being infused). The injectors for sgACC/25 and pgACC/32 are double lumen injectors to infuse into the left and right brain region simultaneously. Each lumen is attached to a separate Hamilton syringe via a separate infusion line.
-
c.Two loops of approximately 70 cm long sterile 0.3 mm plastic tubing (VWR International Ltd., UK).
-
d.Two pairs of sterile 1 cm long yellow bung tubing to attach the plastic tubing to the Hamilton syringes at one end and the injector at the other end.
-
e.A sterile 1 mL syringe and a small 23-gauge needle, for filling the Hamilton syringes and plastic tubing.
-
f.Sterile gauze.
-
g.Sterile gloves.
-
h.Sterile caps and cannulae blockers to replace the caps and blockers that will be removed during the infusion. Have a tube filled with detergent nearby, into which the old caps and cannulae blockers can be placed during the infusion for subsequent sterilization.
-
i.Have a marker pen that has been wiped down to the side of the sterile drape. This will be used to mark air bubbles at the end of the set-up.
-
j.You will also need a vial of sterile saline to fill the 1 mL syringe used to fill the Hamilton syringes and infusion lines. The vial itself is not sterile so do not place it on the drape; place it to the side within easy reach. It will need to be handled with sterile gauze.
-
a.
-
57.
Fill the 1 mL syringe with sterile saline, using gauze to open and handle the vial.
-
58.Set up the infusion pump, syringes, and tubing on top of the drape. See Figure 9 for the complete set up.
 CRITICAL: Infusions are aseptic procedures. Experimenters must wash their hands and don sterile gloves.
CRITICAL: Infusions are aseptic procedures. Experimenters must wash their hands and don sterile gloves.-
a.We use an infusion pump (Kd Scientific, Holliston, MA, USA) set to a diameter for the 10 μL Hamilton syringe (0.485 mm) and a rate between 0.25 μL/min and 0.5 μL/min depending on the drug infused. The pump is positioned to the left-hand side of the sterile drape.
-
b.Set up the Hamilton syringes.
-
i.Remove the plunger of the Hamilton syringe and insert the saline filled syringe.
-
ii.Inject saline into the Hamilton syringe, withdrawing the needle tip out of the syringe at the same time to fill the Hamilton with saline without any bubbles.
-
iii.Place the plunger into the Hamilton syringe. Gently push the plunger in, tapping with your thumb. You should feel a gentle impulse with resistance and a droplet of saline will appear from the end of the Hamilton syringe.Note: If there is no resistance and the plunger moves too easily, try refilling the Hamilton syringe with saline as there is most likely an air bubble.
-
iv.Use a piece of sterile gauze to handle the infusion pump and place the syringe in the infusion pump. We find that two syringes placed symmetrically apart from the center works best.Note: try to leave a droplet of saline at the end of each Hamilton syringe (by pushing on the plungers slightly when the syringes are mounted in the pump) as this helps establish an airtight seal when the infusion lines are attached.
-
i.
-
c.Set up the infusion lines.
-
i.Attach the yellow bung tubing to either end of the plastic tubing.
-
ii.Attach the relevant injector to one end of the infusion tubing using the yellow bung tubing.
-
iii.Using the sterile saline-filled syringe, inject saline into the two free ends of the infusion tubing and withdraw as you do so, creating an airtight saline-filled line in the same way the Hamilton syringes were prepared. A jet of saline should be visualized from the two prongs (left and right) of the injector.Note: if there is resistance as you fill the lines with saline, or no jet emerges, the injector could be blocked. Try a new sterile injector.
-
iv.Attach the free end of the tubing onto the Hamilton syringes, and lie the injector onto the sterile drape.
-
v.Withdraw both Hamilton syringes very slightly, one at a time, to create an approximately 0.25 μL air bubble visualized in the tubing near the injector.
-
vi.Now, place the free end of the injector into the defrosted drug solution (or defrosted 0.9% saline solution in the case of a control session). Take care not to touch the injector tips to anything other than the sterile drape or drug solution.
-
vii.Withdraw the Hamilton syringe plungers to draw up drug solution into the line. From Hamilton syringe to injector, the lines now contain saline → air bubble → drug (or saline) solution.
-
viii.Mark the air bubble in each infusion line with a permanent marker. This will allow you to verify that the infusion set up is working during the infusion by seeing the bubble move.
-
i.
-
a.
CRITICAL: you should test the system is working by pressing RUN on the infusion pump for a few seconds and see that (1) the bubble in each infusion line moves and (2) fluid comes out from the tips of the injector. Remark the bubble after this and wipe away excess solution with sterile gauze, ready for the infusion.
-
59.Once you are satisfied the infusion system is working, ask another person (to whom the marmoset is accustomed to being held by) to catch the marmoset from the cage and bring the marmoset into the infusion room.
-
a.Unscrew the marmoset’s cap and remove the cannulae blockers, placing them into a detergent solution.
-
b.Use an alcohol or chlorhexidine wipe to wipe the top of the exposed guide cannulae.
-
c.Check that the infusion pump settings are correct for the drug you are infusing.
-
d.Change into sterile gloves and hold the injector ensuring that the prongs do not touch anything. Ask the holder to gently secure the marmoset’s head.Note: a bib can be worn by the holder which the marmoset can chew as a distraction during the infusion.
-
e.Insert the injector tip into the lumen of the guide cannulae.
-
f.Press RUN on the infusion pump, and simultaneously start the timer for 2 min.
-
g.Check that the bubble is moving against the mark. If you have drawn up a bubble of approximately 0.25 μL, it should move two lengths per minute if the infusion rate is 0.5 μL/min.
 CRITICAL: if the bubbles do not move (or move less than expected), you may have a partial or complete blockage in the line. See the troubleshooting section (problem 7) for advice.
CRITICAL: if the bubbles do not move (or move less than expected), you may have a partial or complete blockage in the line. See the troubleshooting section (problem 7) for advice. -
h.Once the 2 min timer has finished, stop the pump and time for an additional 1 min to allow the drug to diffuse.
-
i.Once the 1 min timer has finished, remove the injector from the guide cannulae lumen, detach it from the tubing and place it into a tube of detergent solution for subsequent sterilization.
 CRITICAL: now start a timer for the ‘wait time’ of your drug.
CRITICAL: now start a timer for the ‘wait time’ of your drug. -
j.Replace the guide cannulae and caps and ask the holder to return the animal to its home cage, withdrawing the plastic dividers so the animal can roam freely.
-
a.
Pause point: (10–15 min) In the wait time, set up the testing apparatus if needed and check that the apparatus is working. If you are infusing a new drug or infusing a drug for the first time, check in on the marmoset during the wait time to ensure there are no obvious side-effects.
-
60.
Approximately two minutes before the wait time is over, gather the marmoset from its home cage as described in Gathering the marmoset for testing.
-
61.
Carry out the testing session as described in Carrying out standard testing sessions. Infusion sessions will always be carried out on a session with both a CS-/US- and CS+/US+ trial.
-
62.
After the infusion and testing session is complete, ensure you document the date of the infusion and the drug that was infused.
-
63.
Analyze the cardiovascular and behavioral data as described in Analyzing your cardiovascular data and Analyzing your behavioral data. Remember that all behavioral data must be scored by a blinded observer.
-
64.
We would recommend stable performance on at least one session with a CS-/US- and CS+/US+ trial before doing another drug infusion.
Figure 9.
Intracerebral infusion set-up
(A) Infusion set-up. Hamilton syringes are connected to an injector with PTFE infusion lines. The Hamilton syringes are mounted onto a syringe pump with an adjustable rate.
(B) Hamilton syringe.
(C) Infusion line after an infusion. Prior to an infusion, the syringes and lines are filled with 0.9% sterile saline. A bubble is manually drawn up, and then drug (or saline control) is drawn up so that the fluid after the bubble is the substance to be infused. The bubble is marked with permanent marker. This is used to assess fluid movement – as can be seen, the bubble has moved beyond the initial markings.
Measuring the efficacy of antidepressants
Timing: variable.
In Alexander et al., 2019, we use this paradigm coupled with intracerebral infusions of the drug dihydrokainic acid (DHK, which reduces glutamate reuptake in astrocytes) into sgACC/25 to show that over-activity in this area is sufficient to blunt the anticipatory (CS directed) arousal to reward. This provided causal evidence for the correlative findings in humans that over-activity in this region (identified using functional neuroimaging) was associated with blunted reward responsivity seen in anhedonia and depression. This blunting of anticipatory appetitive responses allowed us to test the ability of different antidepressants to reverse this effect. We describe this below.
-
65.
Select the antidepressant you wish to test. We tested both racemic ketamine, an NMDA receptor antagonist, and citalopram, an SSRI.
-
66.
Determine the dose of drug to use. For ketamine, we used an i.m. dose of 0.5 mg/kg akin to that used in i.v. humans (Berman et al., 2000). For citalopram, we were guided by a previous study in our laboratory showing an acute dose of 10 mg/kg i.m. citalopram can reduce anxiety-like behavior displayed by marmosets to a human intruder (Santangelo et al., 2016).
-
67.Determine the timepoint to test therapeutic efficacy. For ketamine, we chose 4 h, 1 day, 7 days and 21 days (Figure 10). These were chosen to coincide with work in humans showing a rapid (4 h) and sustained (1 day and 7 day) effect of ketamine to improve depression scores (Abdallah et al., 2015; Berman et al., 2000). For citalopram we chose 30 min to match with the previous study in our laboratory mentioned above. In humans, citalopram is known to have rapid effects on brain reactivity to fearful faces (Murphy et al., 2009).
-
a.Ideally, we would have liked to administer citalopram chronically to achieve greater face validity with its use as a first-line antidepressant in humans, although this is logistically challenging. Options would include using an osmotic minipump system (more invasive but more precise dosing) or dissolving citalopram in drinking water (less invasive but imprecise dosing).
-
a.
-
68.Administer the antidepressant. In the case of i.m. administration, two people are needed – one holder and one injector. We recommend carrying out i.m. injections in a separate room to intracerebral infusions, and acclimatizing marmosets to this procedure with mock injections prior to injections of saline control/antidepressant.
-
a.Divide the marmoset off in the same way described in point 37 of mock infusion sessions.
-
b.Prepare your equipment: a 0.5 mL insulin syringe; the antidepressant to be injected; a sharps bin and alcohol spray.
-
c.Draw up the required volume in the insulin syringe, ensuring there are no bubbles.
 CRITICAL: Ketamine is a controlled substance and may require special documentation prior to its administration.
CRITICAL: Ketamine is a controlled substance and may require special documentation prior to its administration. -
d.Ask the holder to collect the marmoset from the home cage, holding the leg to be injected between the fourth and fifth finger to expose the lateral aspect of the quadriceps muscle.
-
e.Spray a small amount of alcohol onto the lateral quadriceps where you will inject.
-
f.Use the insulin syringe to inject at a 45° angle to the surface of the skin, into the body of the quadriceps muscle.
 CRITICAL: do not inject large volumes of fluid via the i.m. route; we used a maximum volume of 0.1 mL. If multiple i.m. injections are required, use alternate legs.
CRITICAL: do not inject large volumes of fluid via the i.m. route; we used a maximum volume of 0.1 mL. If multiple i.m. injections are required, use alternate legs. -
g.Return the marmoset to the home cage. If this is the first time injecting a drug, monitor carefully for side effects.
-
a.
-
69.
In the wait time between administration and the onset of therapeutic efficacy, prepare the testing chamber and software as described in Prepare the testing chamber and software for appetitive Pavlovian conditioning sessions, and prepare the infusion system as described in intracerebral infusions and testing following infusions.
-
70.
Approximately 10 min before the timepoint to test therapeutic efficacy, infuse either saline or DHK into sgACC/25 as described in intracerebral infusions and testing following infusions. For example, after the one-off i.m. ketamine injection, we infused DHK 10 min before the 4 h, 1-day, 7-day and 28-day timepoints post-injection as described above. Ketamine successfully reversed the anticipatory blunting at the 1-day and 7-day timepoints, but not at 4 h (no rapid effect of ketamine) or 21 days (chosen to establish that ketamine’s effect had worn off).
-
71.
After 10 min – the wait time for DHK – test the marmoset on the Pavlovian conditioning test. Analyze the cardiovascular and behavioral data and determine whether the antidepressant successfully reverses the blunted CS+ induced arousal expected from sgACC/25 over-activation.
CRITICAL: before further testing, it is critical to establish that the antidepressant’s effect has worn off by re-testing the effect of DHK – particularly relevant to ketamine, which is known to have sustained efficacy.
Figure 10.
Testing the efficacy of ketamine to ameliorate blunted reward processing
Adapted from (Alexander et al., 2019). Marmosets receive a single i.m. injection of 0.5 mg/kg racemic ketamine at t = 0, followed by over-activation of sgACC/25 using DHK 4 h, 1 day and 7 days later. Marmosets also received an infusion of DHK 21 days later to determine if the effect of ketamine had abrogated (not shown). 10 min before each timepoint, DHK was infused into sgACC/25 and marmosets were tested on the appetitive Pavlovian discrimination paradigm (CS-/US-, CS+/US+ session which lasts approximately 7 min).
Expected outcomes
Outcome one: successful discrimination performance as evidenced by a rise in blood pressure during the CS+/US+, but not during the CS-/US-. This is shown in Figure 11.
Figure 11.
Anticipated outcome of the protocol: successful discrimination performance as evidenced by a rise in blood pressure during the CS+/US+, but not during the CS-/US-
(A) Typical performance on the Pavlovian appetitive task, shown for one animal over 30 sessions. The x axis represents the session number, and the y axis represents the CS directed change in mean arterial pressure (MAP). The control (saline) infusion session is indicated by the black arrow.
(B) Within-session performance following the saline infusion. The CS+/US+ trial (orange) is plotted on the same axis as the CS-/US- trial (gray) with baseline (BL), CS and US phases shaded. The x axis represents time within the trial (with 0 s being CS onset) and the y axis represents change in MAP at any one point compared to the average mean arterial pressure during the baseline period. The MAP rises during the CS+ period and then rises further during the US+ period.
Outcome two: the within-subject repeated measures design facilitates the comparison of performance during control infusions versus drug infusions. Our data comparing infusions of saline control vs. infusions of dihydrokainic acid (DHK, over-activation) into sgACC/25 on CS directed anticipatory arousal are shown in Figure 12 as an example. Over-activation of sgACC/25 blunted CS directed cardiovascular and behavioral anticipatory arousal. These graphs are adapted from Alexander et al., 2019.
CRITICAL: Some brain manipulations may uncouple cardiovascular and behavioral responses on this paradigm. For example, our laboratory has shown that orbitofrontal cortex lesions can uncouple cardiovascular and behavioral performance during the reversal of Pavlovian reward contingencies (Reekie et al., 2008).
Figure 12.
Anticipated outcome of the protocol: the effect of intracerebral infusions on anticipatory (CS directed) arousal, in this case infusing dihydrokainic acid (DHK) into sgACC/25
Adapted from Alexander et al., 2019. n=5. Symbols represent individual subjects.
(A) CS directed change in mean arterial pressure (MAP) values during the CS+ (orange) and CS- (gray) periods comparing control (saline) and over-activation (DHK) infusions into sgACC/25. Over-activation blunted anticipatory blood pressure arousal in a CS-dependent manner (manipulation × CS, F1,4 = 10.63, p = 0.031), decreasing responding to the CS+, but not the CS− (effect of manipulation: CS+, p = 0.006; CS−, p = 0.301).
(B) CS directed change in head-jerk values during the CS+ (orange) and CS- (gray) periods comparing control (saline) and over-activation (DHK) infusions into sgACC/25. Over-activation blunted anticipatory behavioral arousal in a CS-dependent manner (manipulation × CS, F1,4 = 72.25, p = 0.001), decreasing responding to the CS+, but not the CS− (effect of manipulation: CS+, p < 0.001; CS−, p = 0.407).
Outcome three: measuring the efficacy of antidepressants in alleviating blunted anticipatory arousal. The effects of ketamine to ameliorate DHK-induced blunting of CS directed anticipatory arousal are shown in Figure 13 as an example. These graphs are adapted from Alexander et al., 2019.
Figure 13.
Anticipated outcome of the protocol: measuring the efficacy of the antidepressant ketamine
Adapted from Alexander et al., 2019. n=4. Symbols represent individual subjects.
(A) Ketamine had a time-dependent effect to reverse the blunted blood pressure response induced by sgACC/25 over-activation using DHK (time point × CS, F2,12 = 14.71, p < 0.001). Compared to control infusions of saline (not shown), sgACC/25 over-activation 4 h after ketamine administration still resulted in significant blunting of blood pressure responses (manipulation × CS, F1,3 = 60.46, p = 0.004; effect of manipulation on CS+, p = 0.003). Over-activation 1 day following ketamine administration evidenced amelioration of the blood pressure impairment (4 h versus 1 day: CS+, p < 0.0001; CS−, p = 0.863) as did over-activation 7 days following ketamine administration (4 h versus 7 days: CS+, p < 0.001; CS−, p = 0.704).
(B) Ketamine had a time-dependent effect to reverse the blunted head-jerk response induced by sgACC/25 over-activation using DHK (time point × CS, F2,12 = 19.59, p < 0.001). Compared to control infusions of saline (not shown), sgACC/25 over-activation 4 h after ketamine administration still resulted in significant blunting of behavioral arousal (manipulation × CS, F1,3 = 25.59, p = 0.015; effect of manipulation on CS+, p = 0.012). Over-activation 1 day following ketamine administration evidenced amelioration of the behavioral impairment (4 h versus 1 day: CS+, p < 0.0001; CS−, p = 0.371) as did over-activation 7 days following ketamine administration (4 h versus 7 days: CS+, p < 0.0001; CS−, p = 0.767).
Quantification and statistical analysis
The infusion experiments of this protocol rely upon a within-subjects repeated-measures design, comparing ‘control’ infusions of saline vehicle to drug infusions of, for example, DHK into specific brain regions. Table 2 is an example of a dataset published in Alexander et al., 2019 comparing the effect of infusions of saline to infusions of DHK into sgACC/25 on the CS directed blood pressure response to the CS+ and CS-.
Table 2.
Comparing the effects of infusions of saline control vs. DHK into sgACC/25 on CS directed blood pressure responses to the CS+ and CS-
| Blood pressure (MAP) responses | CS+ |
CS- |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S1 | S2 | S3 | S4 | S5 | |
| Saline control | 3.54 | 6.31 | 5.74 | 6.45 | 8.55 | −0.24 | 0.97 | −0.41 | −0.60 | 1.84 |
| DHK over-activation | 2.69 | 2.67 | 1.65 | 1.37 | −2.18 | 1.35 | −1.74 | 0.66 | −3.37 | −1.93 |
Sx refers to an individual subject: each subject had two measurements of the CS+ response (saline control/DHK over-activation) and two measurements of the CS- response (saline control/DHK over-activation). This design is therefore a within-subject repeated-measures design.
Given that there are two factors in this analysis ([drug type] and [CS type]) and the measures are repeated in the same subject, a two-way repeated-measures ANOVA is the appropriate statistical test. Running this is very straightforward using GraphPad Prism:
-
1.
Input your data into a GraphPad Prism sheet as in Table 2.
-
2.
Click Analyze and under Grouped analyses select Two-way ANOVA. Analyze both CS+ and CS- datasets.
-
3.
Under RM Design, match by both column (CS type) and row (drug type), as matched values are stacked against both rows and columns.
-
4.
Under RM Analysis, select Repeated measures ANOVA (based on GLM) as the data should not have any missing values. If there are missing values, a mixed-effects model is needed.
-
5.
Under Factor Names, the Column Factor is CS type, and the Row Factor is Drug type.
-
6.
Under Multiple Comparisons, select Compare each cell mean with the other cell mean in that column: this will compare CS+ arousal under drug vs. saline, and CS- arousal under drug vs. saline.
-
7.
Under Options, you should correct for multiple comparisons using Sidak’s correction.
-
8.These are all the relevant parameters that need to be specified, once you have done this click OK and the ANOVA will run.
-
a.The first tab will report the result of the main two-way repeated-measures ANOVA (Figure 14A). Under Source of Variation, the two main effects will be reported (Drug type and CS type) as well as the Drug type × CS type interaction. A significant interaction necessitates checking the multiple comparisons results to see which differences are significant.
-
b.The second tab reports the results of the multiple comparisons tests (Figure 14B). This will show you which specific comparisons are significant – in this case, over-activation (using DHK) causes a significant reduction in CS+ responses with no significant effect on CS- responses.
-
a.
Note: The same statistical framework applies for measuring the effect of antidepressants. For example, in the case of the ketamine experiment, the ANOVA has two factors: CS type and time window. The time window has three levels: 4 h, 1 day and 7 days.
Figure 14.
Two-way repeated-measures ANOVA readout from GraphPad Prism using infusion data from the protocol
The raw data are shown in Table 1.
(A) The results of the two-way ANOVA. There is a significant Drug type × CS type interaction (p=0.0311) indicating a multiple comparisons test should be performed to understand which specific comparisons are significant.
(B) The results of Sidak’s multiple comparisons test. The comparison of interest was between saline and drug for both CS- and CS+ arousal responses. There is a significant difference in the response to the CS+ (p=0.0064) but not the CS- (p=0.2995).
Limitations
First, by virtue of the small number of animals, studies in primates are always vulnerable to type II errors through being underpowered. The within-subject design goes some way to mitigating this as each animal acts as its own control. Second, marmosets are sensitive and temperamental animals whose behavior is sensitive to slight changes in their social and physical environment. It is absolutely critical to maintain conditions in their home cage (and home room) as constant as possible, and when this is not possible, to suspend experimental manipulations until conditions return to normal. Third, the appetitive Pavlovian discrimination paradigm does not measure every aspect of anhedonia-like symptoms: whilst the CS+ period is analogous to anticipation and the US+ period is analogous to consumption, there is no measure of motivation. A separate paradigm, such as the progressive ratio paradigm described in this protocol’s source paper (Alexander et al., 2019) is needed to explore this construct. Finally, the manipulations described here are all acute intracerebral pharmacological infusions which do not fully mimic the chronic depressed state. Technologies such as Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) may yet prove a tractable means to chronically over-activate sgACC/25 to more closely reflect the brain state in people with depression.
Troubleshooting
Problem 1
There is variation in the weight of each marshmallow. (steps 9b and 18c)
Potential solution
Marshmallow can vary in weight due to manufacturing differences but also as they dry out with age. The latter contributes to substantially more weight variation, so always use fresh marshmallow. Drier marshmallow is also harder to chew, increasing the time for consumption and reducing overall consumption.
Problem 2
After inserting the telemetry probe into the aorta, the signal is weak or absent. (step 14f (vii))
Potential solution
In the rare event that the telemetry probe signal is weak or absent, it may suggest the catheter lumen is occluded (by a bubble or clot); that the lumen is opposed to the vessel wall; or that the telemetry probe has low battery. Remove the catheter by gently removing the layer of Vetbond, re-seal the puncture site with Vetbond and check the catheter lumen. If the lumen is obstructed, flush the catheter with gel and reattempt insertion. If the battery is low, switch to a new telemetry probe, ensuring to record the details of the probe for subsequent registration with the PhysioTel software.
Problem 3
The marmoset is not acquiring the behavioral or cardiovascular discrimination between the CS+ and CS-. (step 40)
Potential solution
Firstly, check that the marmosets are on the correct dietary regimen and are not receiving too many treats. In the early stages of trialing the task, we realized that the reward really has to be something special for marmosets to learn the response: marshmallow effectively has to only be accessible after the CS+ plays. Marmosets receiving marshmallow ‘collaterally’ because their cage mate was being treated was a common issue in the early stages.
Next, check that the marmoset isn’t being startled by the US period. Most commonly this was because in the initial iterations of the task the houselight was turning off during the US period. Our initial rationale for the houselight turning off was to make the food box light more noticeable, but for some marmosets (particularly high-anxious ones) it hampered their conditioning because the darkness was off-putting. We therefore adapted the paradigm to leave the houselight on during the US period.
Problem 4
The marmoset’s blood pressure is increasing before the onset of the CS+, leading to artificially high baseline blood pressures and artificially low CS directed values. (step 40)
Potential solution
In this case, the marmoset is (incorrectly) learning that the silence following the US- will lead to reward (predicting a session structure of a CS-/US- trial followed by CS+/US+ trial). The first step in tackling this problem is identifying that it is taking place: it highlights the importance of monitoring the blood pressure in each phase of the task on your run sheets. Including day-by-day absolute blood pressure values for the baseline period (20 s pre-CS) may highlight this trend to you. Carefully designing your testing schedule can prevent this. Ensure that there are regular sessions with two CS-/US- trials, together with sessions that contain only one CS+/US+ trial. An additional preventative measure is to leave marmosets in the apparatus for approximately 60–70 s after the end of a session with a single CS-/US- trial.
Problem 5
The marmoset seems stressed by the auditory cues selected as the CS+ and CS-. (step 42)
Potential solution
Spend enough time prior to your experiments to find out auditory cues which marmosets are comfortable with. If a marmoset is persistently bothered by an auditory cue, you might have to switch out the cues for something else – and it is better to do this sooner rather than later. We have a library of auditory cue .wav files which most marmosets find ‘neutral’ which we are happy to share on request. We have found that marmosets typically find white noise aversive.
Problem 6
The animal’s conditioned responses begin to decay across testing sessions. (steps 40 and 44)
Potential solution
Check to see whether the US+ response is also declining. If so, change the reward from marshmallow to malt-loaf or another highly preferred food. Alternatively, introduce variety in the content of the food box across sessions. With the latter strategy, it is preferable to keep the same reward type for infusion sessions for comparison purposes in case some foods are more preferred than others.
Problem 7
During the infusion, the air bubble in the line is moving less than expected. (step 59g)
Potential solution
There is either a partial or total blockage in the infusion line. Pause the infusion and allow 1 min for any solution that may have entered the brain to diffuse. Withdraw the injector and check if any solution emerges from the injector tips by pressing RUN. If no solution is coming through one or both of the injector tips, then remove the blocked injector and insert another sterile injector onto the ends of the lines. Press RUN and check if the bubble is moving and fluid is emerging from the injector tips; if it is, reinsert the injector and restart the infusion. If there is still an apparent blockage, then there is likely a problem further up the infusion line. In this case, return the marmoset to the home cage set up the infusion system again. Allow the marmoset sufficient rest time of 1–2 h before reattempting the infusion. Make a note of any blockage in a logbook.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the technical contact, Laith Alexander (laith.alexander@nhs.net), or the lead contact, Angela C Roberts (acr4@cam.ac.uk).
Materials availability
Most of the materials used in this protocol are available commercially. The marmosets were bred on site, and we are happy to provide husbandry advice upon request. The testing chambers are bespoke, and the plans for the chamber are available upon request.
Data and code availability
Code is available upon request from the technical contact, Laith Alexander (laith.alexander@nhs.net), or the lead contact, Angela C Roberts (acr4@cam.ac.uk).
Acknowledgments
The development of this protocol and the experiments reported were supported by long-term funding from the MRC (A.C.R.) and the Wellcome Trust (A.C.R.), and the protocol was performed within the Behavioral and Clinical Neuroscience Institute, University of Cambridge, funded jointly by the MRC and the Wellcome Trust. We particularly thank Dr Katrin Braesicke, Dr John Parkinson, and Yvonna Reekie for helping to develop this paradigm in its early iterations.
Author contributions
Conceptualization, L.A. and A.C.R.; methodology design, L.A., C.M.W., and A.C.R.; methodology implementation, L.A., R.B.-T., and C.M.W.; data analysis and statistical design, L.A., R.B.-T., and C.M.W.; writing—original draft, L.A.; writing—review and editing, L.A., R.B.-T., C.M.W., and A.C.R.; visualization, L.A.; funding acquisition, A.C.R.; resources, A.C.R.; supervision, A.C.R.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100454.
Contributor Information
Laith Alexander, Email: laith.alexander@nhs.net.
Angela C. Roberts, Email: acr4@cam.ac.uk.
References
- Abdallah C.G., Sanacora G., Duman R.S., Krystal J.H. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu. Rev. Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L., Gaskin P.L.R., Sawiak S.J., Fryer T.D., Hong Y.T., Cockcroft G.J., Clarke H.F., Roberts A.C. Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron. 2019;101:307–320.e6. doi: 10.1016/j.neuron.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Braesicke K., Parkinson J.A., Reekie Y., Man M.-S., Hopewell L., Pears A., Crofts H., Schnell C.R., Roberts A.C. Autonomic arousal in an appetitive context in primates: a behavioural and neural analysis. Eur. J. Neurosci. 2005;21:1733–1740. doi: 10.1111/j.1460-9568.2005.03987.x. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Aitken M.R.F. Whisker: a client-server high-performance multimedia research control system. Behav. Res. Methods. 2010;42:1059–1071. doi: 10.3758/BRM.42.4.1059. [DOI] [PubMed] [Google Scholar]
- Holland P.C. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J. Exp. Psychol. Anim. Behav. Process. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Jackson S. Modelling the Neuropsychopharmacology of Obsessive-Compulsive Disorder in the Common Marmoset (Callithrix jacchus) Unpublished PhD thesis. 2016 [Google Scholar]
- Morris T.H., Jackson R.K., Acker W.R., Spencer C.K., Drag M.D. An illustrated guide to endotracheal intubation in small non-human primates. Lab Anim. 1997;31(2):157–162. doi: 10.1258/002367797780600143. [DOI] [PubMed] [Google Scholar]
- Murphy S.E., Norbury R., O’Sullivan U., Cowen P.J., Harmer C.J. Effect of a single dose of citalopram on amygdala response to emotional faces. Br. J. Psychiatry. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C., Petrides M., Rosa M., Tokuno H. Academic Press; 2011. The Marmoset Brain in Stereotaxic Coordinates. [Google Scholar]
- Reekie Y.L., Braesicke K., Man M.S., Roberts A.C. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc. Natl. Acad. Sci. U S A. 2008;105:9787–9792. doi: 10.1073/pnas.0800417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo A.M., Ito M., Shiba Y., Clarke H.F., Schut E.H., Cockcroft G., Ferguson-Smith A.C., Roberts A.C. Novel primate model of serotonin transporter genetic polymorphisms associated with gene expression, anxiety and sensitivity to antidepressants. Neuropsychopharmacology. 2016;41:2366–2376. doi: 10.1038/npp.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video has been shortened to include only the two trials, without the inter-trial interval (ITI) period. The lower right panel of the video shows the Spike2 telemetry trace and the Spike2 session timer. The CS- trial consists of the baseline period (00:10 to 00:30 on the video; 40 s to 60 s on the Spike2 timer), the CS- cue (00:30 to 00:50 on the video; 60 s to 80 s on the Spike2 timer) and the US- (00:50 to 02:50 on the video; 80 s to 200 s). During the US- period, the non-rewarded food box light is switched on and the opaque door opens to reveal no food reward. The CS+/US+ trial consists of the baseline period (283 s to 303 s), the CS+ period (303 s to 323 s) and the US+ period (323 s to 443 s). During the US+ period, the rewarded food box light is switched on and both the opaque and transparent doors are opened to enable access to marshmallow reward. 443 s marks the end of the session; typically, a two-trial session lasts just over 7 min.
Data Availability Statement
Code is available upon request from the technical contact, Laith Alexander (laith.alexander@nhs.net), or the lead contact, Angela C Roberts (acr4@cam.ac.uk).