Abstract
Scaevola spinescens is endemic to Australia and traditionally used as a medicinal plant. While its bioactive compounds have been studied, their concentrations in different parts of the plant have not been reported. This study compared total phenolic content (TPC), flavonoids, saponins and antioxidant properties, as well as major individual phytochemical compounds in the whole root, root bark, root wood, whole stem, stem bark, stem wood, and leaf of S. spinescens. The results showed the leaf had significantly highest concentrations of TPC followed by the root bark and stem bark (47.34, 12.24 and 10.20 mg GAE/g, respectively). Flavonoids concentrations were also significantly higher in the leaf compared to the root bark and stem bark (20.95, 6.22 and 4.19 mg CE/g, respectively). For saponins, the root bark contained significantly highest concentrations (112.58 mg EE/g). Luteolin 7-glucoside was isolated and identified in the leaf of S. spinescens. Eight major compounds were identified with the leaf displaying the highest diversity of major compounds, and in higher concentrations, compared to the other plant constituents. As the leaf and root bark contained the highest concentrations of phytochemicals, these plant parts are recommended as starting material for future studies, to further isolate and identify the major compounds from S. spinescens and investigate their biological properties for use in pharmaceutical and food applications.
Keywords: Scaevola spinescens, Maroon bush, Bioactive compounds, Antioxidants, Phytochemicals, Plant parts, Roots, Stem, Leaf, Bark
Scaevola spinescens, Maroon bush, Bioactive compounds, Antioxidants, Phytochemicals, Plant parts, Roots, Stem, Leaf, Bark.
1. Introduction
Scaevola spinescens R. Br. (also known as maroon bush, murin murin, boogawee, currant bush or prickly fan flower) from the Goodeniaceae family is a rigid shrub that occurs naturally in drier regions of mainland Australia (Ghisalberti, 2004). Indigenous peoples of Australia have traditionally used the roots, stems and leaves of S. spinescens to treat various diseases; a decoction of the roots was used for treatment of urinary disorders and stomach pain, a decoction of the crushed stem was used to treat skin disease such as rashes and boils, and sores were treated by exposure to the steam from the leaves and twigs (Lassak and McCarthy, 2011).
Previous studies have linked the leaves of S. spinescens with various therapeutic benefits. For example, S. spinescens leaf extracts have been shown to exhibit antiviral activity against human cytomegalovirus (HCMV) and MS2 bacteriophage, as well as displaying antibacterial activity (Cock and Kukkonen, 2011; Semple, Reynolds, O'Leary and Flower, 1998). Leaf extracts of S. spinescens also contain relatively high antioxidant properties, and exhibit anti-cancer activity against a panel of cancer lines including cancers of the pancreas, breast, ovary, lung, colon, skin and brain (Vuong, Sadeqzadeh, et al., 2015).
While the beneficial characteristics of the leaves have been studied, the phytochemical characteristics of the various parts of the plant have not been reported. Given that the traditional applications of S. spinescens included not only the leaves, but the roots and stems of the plant, further investigation of the bioactive compounds and antioxidant capacity of the these plant parts and their constituents are warranted. Further, the various plant parts and their constituents, such as the stem bark and stem wood, may have individual, differing characteristics that may also have synergistic effects when combined. Therefore, the aim of this study was to analyse and compare the total phenolic compounds (TPC), flavonoids and saponins, as well as the 2,2-diphenyl-1-picrylhydrazyl (DPPH∗) free radical scavenging capacity, 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS∗+) radical scavenging capacity and ferric reducing antioxidant power (FRAP), from various constituents of S. spinescens including the whole root, root bark, root wood, whole stem, stem bark, stem wood, and leaf of the plant. In addition, this study also aimed to isolate and identify a phytochemical compound in S. spinescens for use as a standard for comparing the major phytochemical compounds among the different plant constituents, using HPLC.
2. Materials and methods
2.1. Materials
All plant materials, including the roots, stems and leaves of S. spinescens were collected approximately 100 km west of Nyngan, NSW, Australia (31°34′49.2″S 145°54′52.3″E) in February 2018. The plant materials were randomly collected from established plants in the region, sealed in plastic bags, stored in large ice boxes and transported directly to the laboratory of the University of Newcastle, Ourimbah, NSW, Australia. A voucher specimen of the plant has been stored in the Don McNair herbarium, University of Newcastle, Callaghan, NSW, Australia, voucher/accession number: 10799. In the laboratory, the roots, stems and leaves were dried in a hot-air oven (LABEC Laboratory Equipment Pty Ltd, Marrickville, NSW, Australia) at 110 °C for 3 h as described by Nguyen et al. (2018). These drying conditions were selected as they have been found to be the optimal method for drying S. spinescens to retain bioactive compound yield and antioxidant properties (Nguyen et al., 2018). The dried material was stored in sealed bags and kept at -18 °C until use. Prior to extraction, the plant materials were ground into small particles using a mortar and pestle.
2.2. Extraction
Ultrasound assisted extraction (UAE) was applied for extraction of 7 different constituents of S. spinescens including: whole root, root bark, root wood, whole stem, stem bark, stem wood and leaves. UAE was conducted using an ultrasonic bath (Soniclean 220 V, 250 W and 50 KHz, Soniclean Pty Ltd., Thebarton, Australia) with fixed conditions: temperature of 40 °C, time of 1 h and power of 150W. The dried plant material (1 g) was added to 100 mL of 1:1 (v/v) ethanol - water solution, placed in the ultrasonic bath and vortexed every five minutes. Following sonication, the mixture was immediately cooled to room temperature and filtered using filter paper (Whatman no. 1) for further examination.
2.3. Analysis of total phenolic content, flavonoids, and saponins
2.3.1. Total phenolic content (TPC)
The TPC was determined as described by Škerget et al. (2005), with the sample extract diluted 1:5 (v/v) with distilled water. Gallic acid standard was used for the calibration curve and the measurements expressed as mg of gallic acid equivalents per gram of dried plant material (mg GAE/g).
2.3.2. Flavonoids
The flavonoids content was determined as described by Zhishen et al. (1999). Catechin standard was used for the calibration curve and the measurements expressed as mg of catechin equivalents per gram of dried plant material (mg CE/g).
2.3.3. Saponins
The saponins content was determined as described by Hiai et al. (1976). Escin standard was used for the calibration curve and the measurements expressed as mg of escin equivalents per gram of dried plant material (mg ESE/g).
2.4. Determination of DPPH∗ radical scavenging, ABTS∗+ radical scavenging and ferric reducing antioxidant power
As each in vitro assay has advantages and limitations, more than one assay was employed to increase rigor and confidence of data. This study applied three assays to determine the 2,2-diphenyl-1-picrylhydrazyl (DPPH∗) free radical scavenging capacity, 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS∗+) radical scavenging capacity and ferric reducing antioxidant power (FRAP) of the S. spinescens constituent extracts. The DPPH∗, ABTS∗+ radical scavenging capacity and FRAP assays were determined as described by Thaipong et al. (2006). Trolox standard was used for each calibration curve and the measurements expressed as mM trolox equivalents per gram of sample (mM TE/g).
2.5. Isolation and identification of luteolin 7-glucoside and analysis of major components using HPLC
2.5.1. Isolation and identification of luteolin 7-glucoside from S. spinescens leaf extract
Dried leaf (10 g) was extracted in 100 mL 1:1 (v/v) acetone – water solution using UAE, set at similar conditions as previously described in the extraction section. The extract was then evaporated to remove acetone under reduced pressure in a rotary evaporator and mixed with n-butanol in a separation funnel to remove the aqueous layer. The saponin enriched butanol fraction was then evaporated under reduced pressure to remove butanol and re-diluted with 20 ml 1:1 (v/v) ethanol-water solution. The extract was then analysed using a Shimadzu HPLC system (LC-20A, Shimadzu Australia, Rydalmere, NSW, Australia) and fractionated using an automatic fraction collector. The HPLC conditions are described in the next sub-section.
Identification of the unknown compound was based on (1) matching retention time with a standard and (2) mass spectrometry. For matching retention time, an unknown compound was found to match with luteolin 7-glucoside. For further confirmation, the unknown compound was subjected to a Shimadzu LC/MS (LCMS-2020, Shimadzu Australia, Rydalmere, NSW, Australia) combined with an electrospray ionization interface (ESI). The mobile phases were similar to the HPLC analysis with a flow rate of 0.1 mL/min. The mass spectrometer was run in negative mode with selected ion monitoring (SIM) and the following parameters: nebulizing gas (nitrogen) flow 1.5 L/min, CDL temperature 250 °C, block heater temperature 200 °C and drying gas pressure 0.15 MPa. The sample injection volume was 10 μL.
2.5.2. High performance liquid chromatography (HPLC)
Using HPLC system coupled with PDA detector, we found that most of the major phytochemical compounds were well absorbed at 320nm. Therefore, this wavelength was chosen for comparison of major phytochemical compounds in different constituents of S. spinescens. As luteolin 7-glucoside was previously identified in S. spinescens, this compound was used as a standard for comparison. The extracts of S. spinescens were then filtered using a 0.45 μm Phenex syringe filter (Phenomenex) and then subjected to the Shimadzu HPLC system coupled with a Luna 5u Phenyl-Hexyl 250 × 3.00 mm 5u micron column (Phenomenex Australia Pty. Ltd., Lane Cove, NSW, Australia), which was maintained at 30 °C. The mobile phases consisted of solvent systems A: deionized water - formic acid, 98:2 (v/v), and solvent B: acetonitrile.
The gradient elution schedule was as follows: 100% A from 0 to 5 min; a linear gradient from 100% A to 75% A from 5 to 15 min; from 75% A to 70% A from 15 to 20 min; from 70% A to 60% A from 20 to 35 min; from 60% A to 20% A from 35 to 40 min and before returning to 100% A at 45 min with a post-run equilibration time of 5 min with 100% A prior to the following injection. The flow rate was 1 mL/min and the extract injection volume was 40 μL. Each peak depicted a single compound and its concentration was calculated based on a luteolin 7-glucoside standard curve and expressed by mg luteolin 7-glucoside equivalents per gram of dried plant material (mg LGE/g).
2.6. Statistical analyses
Differences in the phytochemicals and antioxidant properties among the various plant constituents were examined using one-way analysis of variance (ANOVA) and Tukey post-hoc tests. Differences among mean levels were considered to be significantly different at p < 0.05. The statistical analyses were completed using statistical software IBM SPSS Statistics 24 (version 24.0.0.1). Principal Component Analysis (PCA) was performed to detect sample grouping and explore the sources of variation among the plant constituents. The PCA analysis is presented by a PCA biplot using BioVinci software (BioTuring Inc., San Diego, CA, USA).
3. Results and discussion
3.1. Total phenolic compounds in different constituents of S. spinescens
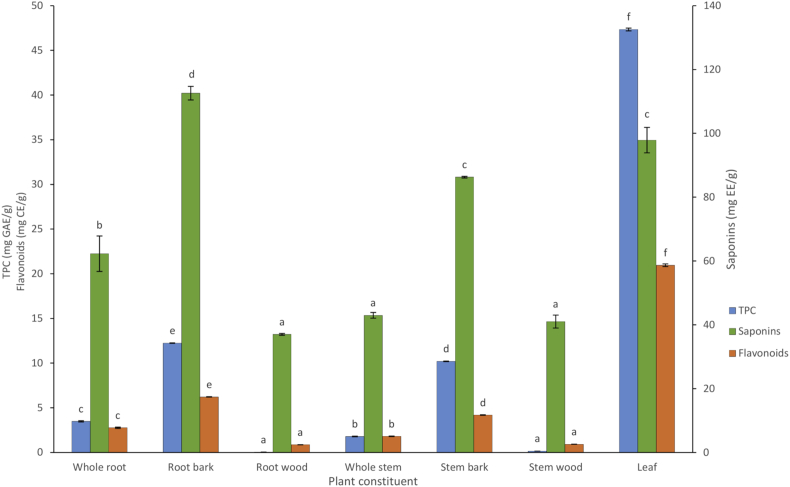
The results showed that the TPC levels varied significantly among the different constituents of S. spinescens (p < 0.05) (Figure 1), with the leaf extracts containing the highest TPC (47.34 mg GAE/g), followed by the root bark and stem bark extracts (12.24 and 10.20 mg GAE/g, respectively). The root wood and stem wood contained the least TPC (0.04 and 0.15 mg GAE/g, respectively). These results show that a drastically higher level of TPC is contained in the leaves of S. spinescens compared to other plant constituents. These findings are supported by previous studies which have found that the TPC can vary among various parts of the plant, and that the leaves can contain the highest concentration. For example, Larbat et al. (2014) found that the leaves of nine tomato cultivars contained a higher diversity and concentration of phenolics compared to the stems and roots of the plants. Similarly, Martin-Puzon and Rivera (2015) also found that Glinus oppositifolius L. leaf contained the highest levels of phenolics, followed by the root and stem.
Figure 1.
Total phenolic content (TPC), flavonoids and saponins in S. spinescens whole root, root bark, root wood, whole stem, stem bark, stem wood, and leaf extracts. Values are means (n = 3) ± SE. Values in the same category (either TPC, flavonoids or saponins) not sharing a letter at each column are significantly different from each other (p < 0.05).
3.2. Flavonoids in different constituents of S. spinescens
The flavonoid content also varied significantly among the different constituents of S. spinescens (p < 0.05) (Figure 1), following a similar pattern to TPC. The leaf extracts had significantly highest concentration of flavonoids (20.95 mg CE/g), followed by the root bark and stem bark extracts (6.22 and 4.19 mg CE/g, respectively). As with TPC, the root wood and stem wood extracts contained the least flavonoid content (0.87 and 0.92 mg CE/g, respectively). These results are supported by previous studies that found the leaves of the Salacia chinensis L. were higher in flavonoids content than the stems (Dharmadasa, 2016). Similarly, the leaves of Glinus oppositifolius L have been found to contain higher concentrations of flavonoids when compared to the roots and stems of the plant (Larbat et al., 2014). Of note is the higher quantitative value of the flavonoids compared to the TPC in the root wood (0.87 mg CE/g and 0.04 mg GAE/g, respectively) and stem wood (0.92 mg (CE/g and 0.15 mg GAE/g, respectively). This higher value of flavonoids than TPC only appears in these two plant constituents. While flavonoids form a large group of naturally occurring phenolic compounds (Bhat et al., 2005), the TPC and flavonoid concentrations in this study were measured using standard curves of different compounds: gallic acid for TPC and catechin for flavonoids. The standard used and unit of measure is different for flavonoids (mg CE/g) and TPC (mg GAE/g), which may explain the higher concentration value of flavonoids compared to TPC in the root wood and stem wood. Similar findings have been reported in previous studies (Vuong et al., 2013; Ngo Van et al., 2017; Vu et al., 2017; Pham et al., 2017; Rajan et al., 2020).
3.3. Saponins in different constituents of S. spinescens
As with TPC and flavonoids, saponins content varied significantly among the different constituents of S. spinescens (p < 0.05) (Figure 1). However, unlike TPC and flavonoids, the root bark contained significantly higher saponins concentration (112.58 mg EE/g) compared to the other plant constituents. Following the root bark, the leaf and stem bark had the highest concentrations of saponins (97.87 and 86.29 mg EE/g, respectively), while the whole stem, stem wood and root wood contained the lowest concentrations (42.97, 41.01 and 37.00 mg EE/g, respectively). Though the root bark contained the highest concentration of saponins, we found that the leaf had higher concentrations than the whole roots. These results are similar to those of Tava et al. (2020) that showed the leaves of Medicago marina L contained higher diversity and concentrations of saponins when compared to the roots of the plant. Asuk, Agiang, Dasofunjo, and Willie (2015) also found that the leaves, stem bark and roots of Jatropha curcas contain similar concentrations of saponins. However, while these studies compared the leaf with the roots of the plant, they did not analyse the root bark separately from the root wood for comparison. In this study we found that the root bark, distinct from the whole root and root wood, contains significantly higher saponins content than the leaves of the plant. Root bark has long been used in traditional medicine and has been the focus of previous studies investigating their phytochemistry for potential therapeutic applications (Liao et al., 2017; Wang et al., 2019). As saponins have been associated with various health benefits, such as strengthening immune systems, prevention of certain cancers and decreased risk of heart disease (MacDonald et al., 2005; Rajput et al., 2007), the ability to extract and isolate saponins from S. spinescens could have potential for medical applications. Our findings have shown that saponins are not only developed in the leaves, but also in the root bark of the plant. Therefore, both the leaf and root bark should be highly considered as starting material, if saponins are the target compounds for extraction, from S. spinescens.
3.4. The DPPH∗ radical scavenging, ABTS∗+ radical scavenging and ferric reducing antioxidant power in different constituents of S. spinescens
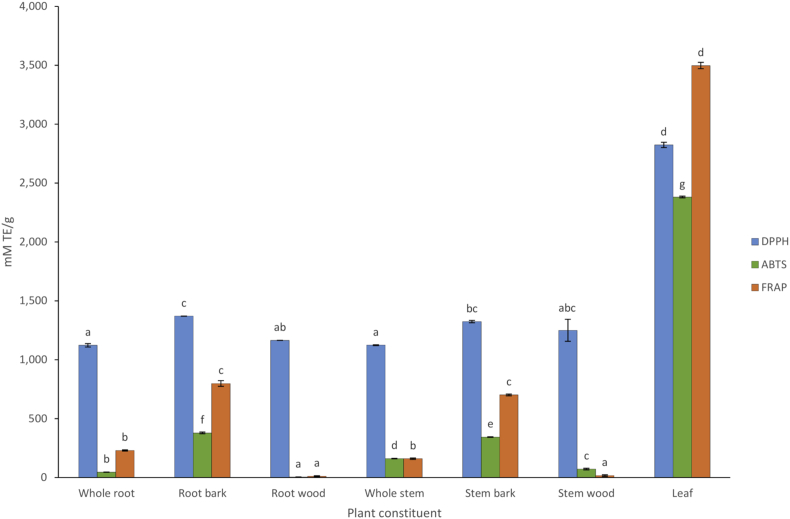
The results showed that the DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP varied significantly among the different constituents of S. spinescens (p < 0.05) (Figure 2), with the leaf extracts containing significantly higher DPPH, ABTS and FRAP capacity (2823.61, 2381.11 and 3497.46 mM TE/g, respectively), among all plant constituents. After the leaf extracts, DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP capacity was highest in the root bark and stem bark. Generally, the root wood and stem wood had the least DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP, which is particularly apparent within the FRAP and ABTS∗+ assays. In S. spinescens, the leaf extracts contained the highest DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP among all plant constituents. This might be because bioactive compounds, such as phenolics, flavonoids and saponins, can be responsible for the DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP properties in plant materials (Rathee et al., 2009; Visioli et al., 2011; Vuong, Zammit, et al., 2015). With the exception of saponins, TPC and flavonoids concentrations were highest in leaf extracts of S. spinescens, which may contribute to the high DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP found in the leaf.
Figure 2.
DPPH, ABTS and FRAP antioxidant capacity in S. spinescens whole root, root bark, root wood, whole stem, stem bark, stem wood, and leaf extracts. Values are means (n = 3) ± SE. Values in the same category (either DPPH, ABTS or FRAP) not sharing a letter at each column are significantly different from each other (p < 0.05).
3.5. Principle Component Analysis (PCA) exploring TPC, flavonoids, saponins, DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP among constituents of S. spinescens
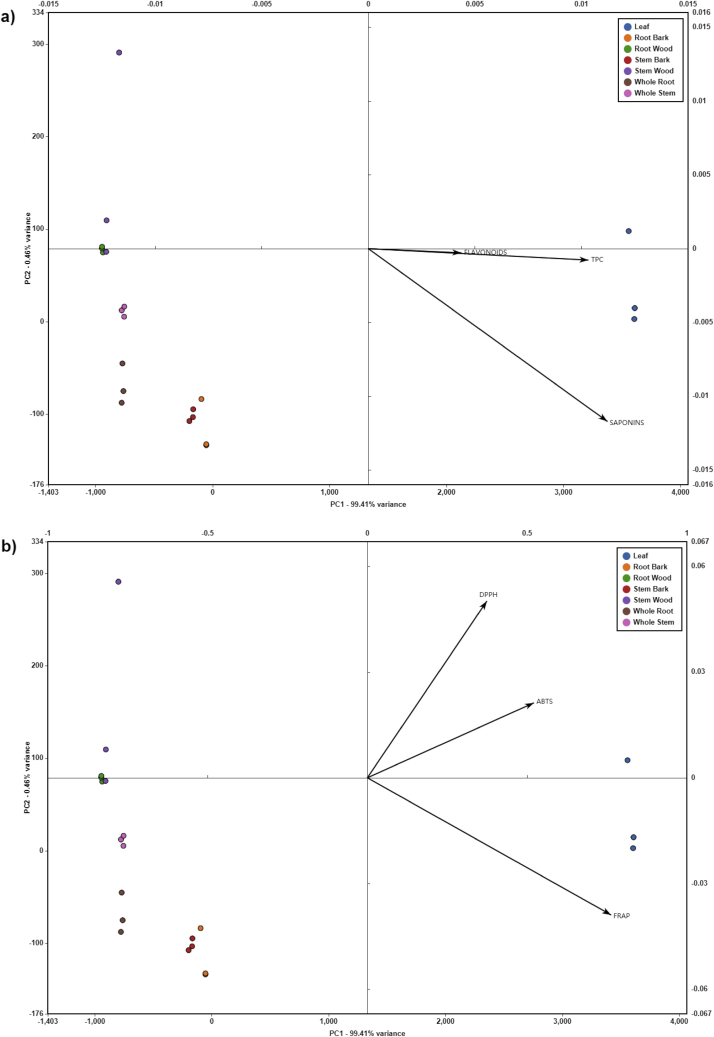
To examine the variation in bioactive compounds and antioxidant properties among the plant constituents, multivariate exploratory analysis was performed. An advantage of a multivariate ordination approach is obtaining graphical results, which can aid in visualization and interpretation of the sample data. PCA was used to identify the phytochemical attributes that contribute to the variation among the plant constituents. Figure 3a shows the PCA biplot with TPC, flavonoid and saponin loading vectors, and Figure 3b shows the PCA biplot with DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP loading vectors. Two principal components (PC) were able to explain 99.87% of the total variability. The first PC (PC1), representing 99.41% of the total variance, shows high positive values for the leaf extracts, intermediary values for root bark and stem bark, and negative values for whole root, whole stem, stem wood and root wood extracts (Figures 3a and 3b). PC2 only accounts for 0.46% of the total variance, displaying positive values for stem wood and root wood, intermediary values for whole stem, and negative values for whole root, stem bark, and root bark extracts (Figures 3a and 3b). The PCA biplot loading vectors show that TPC and flavonoids are the main contributors to PC1, while saponins have less influence on PC1 and more on PC2 (Figures 3a and 3b). All attribute vectors are in the positive direction indicating that they are correlated with each other. However, the small angle between TPC and flavonoids vectors indicates they are strongly correlated with one another.
Figure 3.
Principle Component Analysis (PCA) biplot of various plant constituents with TPC, flavonoids and saponins loading vectors (a), and DPPH, ABTS and FRAP loading vectors (b).
The PCA biplot shows that the leaf extracts are strongly associated with TPC, flavonoids and saponins, as well as DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP. These results support our previous findings (Figures 1 and 2) that the leaf extracts contain significantly higher bioactive compounds and antioxidant properties, with the exception of saponins, than all other plant constituents. Previous results showed that saponin concentrations were significantly highest in root bark (Figure 1), followed by leaf and stem bark extracts. Interestingly, the PCA biplot shows a similar pattern in that, as well as being positively associated to the leaf extract, saponins are also positively associated with root bark and stem bark extracts. Furthermore, the strong correlation between TPC and flavonoids shown in the PCA biplot supports our previous findings of similar patterns of TPC and flavonoids variations among the different plant constituents (Figure 1).
3.6. Identification of luteolin 7-glucoside in S. spinescens leaf extract
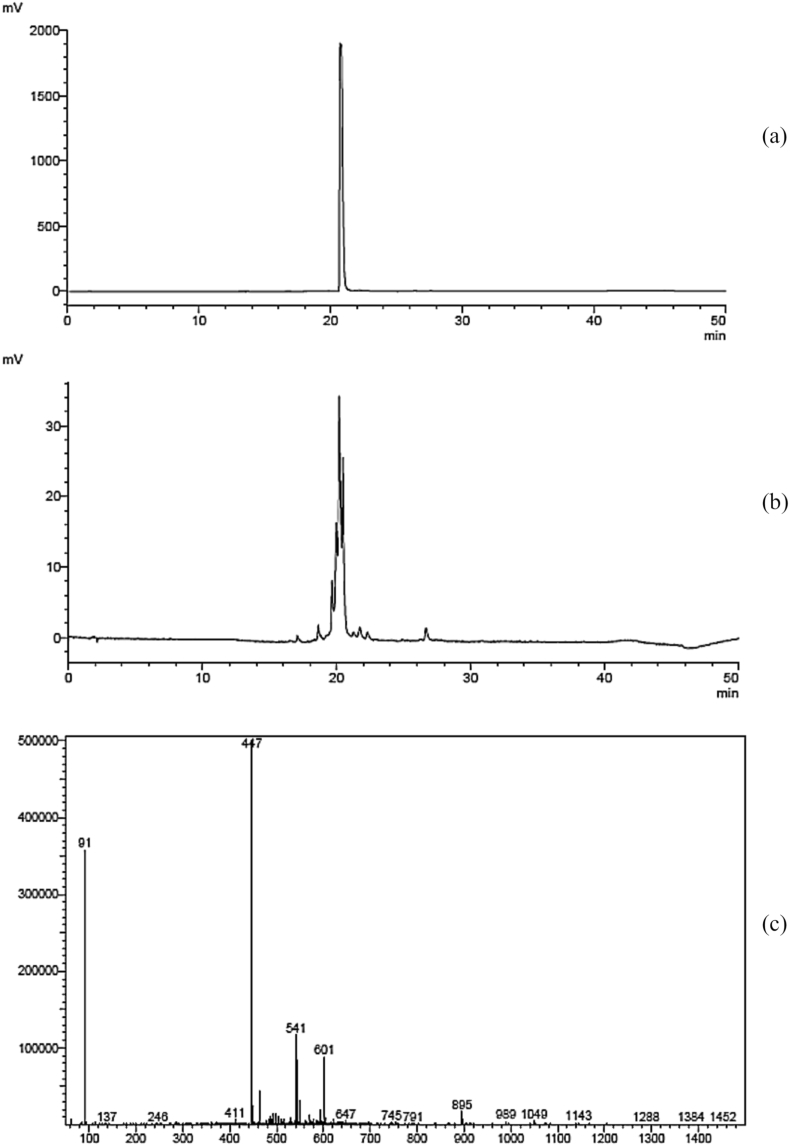
Figure 3 shows the HPLC chromatograms of luteolin 7-glucoside standard, isolated fraction and mass spectrum of corresponding fraction isolated from S. spinescens leaf extract. The results (Figure 4) shows that the fraction had a similar retention time to the luteolin 7-glucoside standard when running through HPLC with similar conditions. In addition, results from LC-MS analysis showed that the fraction detected at m/z 447 indicating a molecular mass of 448, further confirming the presence of luteolin 7-glucoside in S. spinescens. As luteolin 7-glucoside was identified in S. spinescens, this compound was further used as a standard for the HPLC chromatograms comparing major compounds among plant constituents.
Figure 4.
HPLC chromatogram of luteolin 7-glucoside standard (a) and corresponding fraction from S. spinescens leaf extract (b), and mass spectrum of corresponding fraction from S. spinescens leaf extract (c).
3.7. Major individual compounds in different constituents of S. spinescens
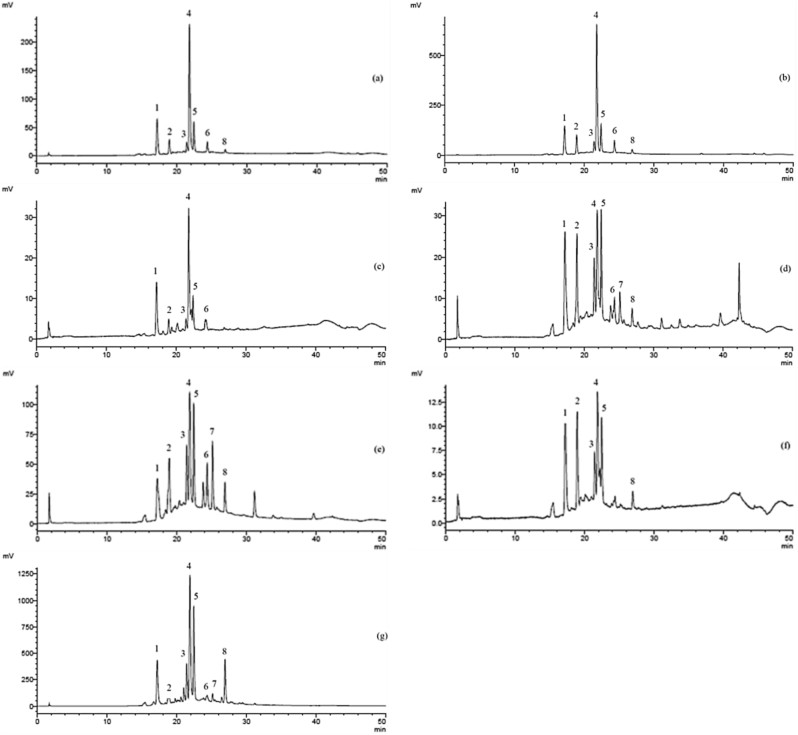
The HPLC chromatograms (Figure 5), revealed that there were 8 major compounds among all plant constituents of S. spinescens. Of these, the leaf, stem bark and whole stem contained the highest diversity (all 8 major compounds), followed by the root bark and whole root (7 major compounds), and the root wood and stem wood (6 major compounds) (Figure 5). In general, the leaf extracts contained significantly higher concentrations for the 8 major compounds (Table 1), and displayed the highest total content of the major compounds, followed by the root bark and stem bark. The root wood and stem wood contained the least amount of major compounds (Table 1). Notably, the leaf extract contained dramatically higher concentrations for all but one major compound analysed; compound number 2 which was also found in high concentrations in the root bark and stem bark extracts of S. spinescens (Table 1). Interestingly, we also found that the saponins content was highest in the root bark, leaf and stem bark (Table 1), suggesting that compound number 2 may be a major contributor to the saponins found in S. spinescens.
Figure 5.
Representative HPLC chromatograms detected at 320 nm displaying major compounds/peaks (numbered 1–8) for: the whole root (a), root bark (b), root wood (c), whole stem (d), stem bark (e), stem wood (f) and leaf (g).
Table 1.
Individual peaks/compounds in different plant constituents of S. spinescens.
| Peak/Compound | Quantity (mg LGE/g) |
||||||
|---|---|---|---|---|---|---|---|
| Whole root | Root bark | Root wood | Whole stem | Stem bark | Stem wood | Leaf | |
| 1 | 89.61 ± 0.13d | 116.49 ± 1.3e | 71.92 ± 0.26a | 78.34 ± 0.06b | 87.34 ± 0.22c | 72.49 ± 0.50a | 245.41 ± 0.38f |
| 2 | 75.83 ± 0.18b | 96.60 ± 0.33d | 68.50 ± 0.04a | 76.20 ± 0.17b | 89.26 ± 2.81c | 71.22 ± 0.51a | 99.94 ± 0.41e |
| 3 | 73.91 ± 0.15b | 86.36 ± 0.10c | 68.52 ± 0.07a | 74.03 ± 0.12b | 87.82 ± 1.24d | 69.70 ± 0.12a | 169.42 ± 0.21e |
| 4 | 131.04 ± 0.51d | 237.77 ± 5.57e | 75.48 ± 0.20ab | 79.54 ± 1.30b | 109.30 ± 0.18c | 71.57 ± 0.21a | 460.21 ± 0.59f |
| 5 | 84.50 ± 0.31c | 111.01 ± 1.41e | 69.99 ± 0.05a | 76.98 ± 0.26b | 98.56 ± 1.31d | 70.73 ± 0.36a | 405.61 ± 0.26f |
| 6 | 75.60 ± 0.47c | 91.90 ± 0.72e | 68.56 ± 0.42a | 71.50 ± 0.61b | 87.21 ± 0.54d | - | 130.39 ± 0.34f |
| 7 | - | - | - | 71.57 ± 0.19a | 89.78 ± 0.66b | - | 103.93 ± 0.54c |
| 8 | 72.28 ± 0.75b | 79.40 ± 1.39c | - | 70.56 ± 0.14b | 81.15 ± 0.84c | 68.40 ± 0.17a | 195.07 ± 0.39d |
Note: the values are expressed as mean ± SD (n = 3). Means with different superscript letters in the same row differ significantly (p < 0.05). LGE: Luteolin-7-glucoside equivalents.
It should be noted that this study provides a preliminary screening using PDA detection to find major compounds of interest. These preliminary findings will serve to aid in finding the major components of interest, and to identify which plant part could be used as starting material for future research and for the potential use of the plant in food and pharmaceutical application. Furthermore, these findings can have direct benefits for practitioners and end users using the plant for therapeutic applications. As well as illustrating the diversity and relative quantities of the major compounds present in the various plant parts, the HPLC results (Figure 5, Table 1) also show that the compounds are relatively well separated under the current HPLC conditions. Therefore, fractionating the individual major compounds could be easily achieved using a HPLC preparative system to isolate, identify and quantify these individual compounds. Future studies are recommended to isolate and identify these major compounds for further investigation.
4. Conclusions
This study further confirms that S. spinescens is a rich source of TPC, flavonoids and saponins, as well as DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP. However, these bioactive compounds and antioxidant properties, as well as major individual phytochemical compounds, differ among various parts of the plant. The leaf of S. spinescens displayed the highest concentrations of TPC and flavonoids, followed by the root bark and stem bark. For saponins, however, the root bark contained the highest concentrations, followed by the leaf and stem bark. The root wood and stem wood consistently displayed the lowest levels of bioactive compounds and DPPH∗ radical scavenging, ABTS∗+ radical scavenging and FRAP. The leaf extracts had the greatest diversity of major compounds, and in much higher concentrations, compared to the other plant constituents. The root bark also contained relatively high concentrations of compound number 2, and would be suitable to use as a starting material for a HPLC preparative system to isolate that specific compound. Therefore, as the leaf and root bark extracts contain the highest concentrations of phytochemicals, these plant parts are recommended as starting material for further investigation. Future studies are recommended to further isolate and identify the major bioactive compounds from S. spinescens and investigate their biological properties for use in pharmaceutical and food applications.
Declarations
Author contribution statement
Kien Q. Nguyen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Christopher J. Scarlett: Contributed reagents, materials, analysis tools or data.
Quan V. Vuong: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare the following conflict of interests: Quan V. Vuong; [is an advisory board member for Heliyon Food Science and Nutrition].
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to acknowledge and pay our respects to the traditional Aboriginal people of Cobar NSW and the surrounding lands. We pay our respect to elders past, present and future, and acknowledge the significance of their culture and knowledge and the role it plays in shaping research and future scientific understandings in Australia. We are grateful for the support from the Australian Postgraduate Award and thank Amanda Stokes and Jet Nguyen for their help in the field.
Contributor Information
Kien Q. Nguyen, Email: kien.nguyen@uon.edu.au.
Quan V. Vuong, Email: vanquan.vuong@newcastle.edu.au.
References
- Asuk A.A., Agiang M.A., Dasofunjo K., Willie A.J. The biomedical significance of the phytochemical, proximate and mineral compositions of the leaf, stem bark and root of Jatropha curcas. Asian Pac. J. Trop. Biomed. 2015;5(8):650–657. [Google Scholar]
- Bhat S.V., Bhat S.V., Nagasampagi B.A., Sivakumar M. Alpha Science Int'l Ltd; 2005. Chemistry of Natural Products. [Google Scholar]
- Cock I.E., Kukkonen L. An examination of the medicinal potential of Scaevola spinescens: toxicity, antibacterial, and antiviral activities. Pharmacogn. Res. 2011;3(2):85–94. doi: 10.4103/0974-8490.81955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmadasa R.M. Natural antidiabetic potential of Salacia chinensis L. (celastraceae) based on morphological, phytochemical, physico-chemical and bioactivity: a promising alternative for Salacia reticulata thw. World J. Agri. Res. 2016 [Google Scholar]
- Ghisalberti E.L. The Goodeniaceae. Fitoterapia. 2004;75(5):429–446. doi: 10.1016/j.fitote.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Hiai S., Oura H., Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976;29(2):116–122. doi: 10.1055/s-0028-1097639. [DOI] [PubMed] [Google Scholar]
- Larbat R., Paris C., Le Bot J., Adamowicz S. Phenolic characterization and variability in leaves, stems and roots of Micro-Tom and patio tomatoes, in response to nitrogen limitation. Plant Sci. 2014;224:62–73. doi: 10.1016/j.plantsci.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Lassak E.V., McCarthy T. New Holland Publishers; Australia: 2011. Australian Medicinal Plants: A Complete Guide to Identification and Usage. [Google Scholar]
- Liao Y.-R., Kuo P.-C., Tsai W.-J., Huang G.-J., Lee K.-H., Wu T.-S. Bioactive chemical constituents from the root bark of Morus australis. Bioorg. Med. Chem. Lett. 2017;27(2):309–313. doi: 10.1016/j.bmcl.2016.11.046. [DOI] [PubMed] [Google Scholar]
- MacDonald R.S., Guo J., Copeland J., Browning J.D., Jr., Sleper D., Rottinghaus G.E., Berhow M.A. Environmental influences on isoflavones and saponins in soybeans and their role in colon cancer. J. Nutr. 2005;135(5):1239–1242. doi: 10.1093/jn/135.5.1239. [DOI] [PubMed] [Google Scholar]
- Martin-Puzon J.J.R., Rivera W.L. Free-radical scavenging activity and bioactive secondary metabolites from various extracts of Glinus oppositifolius (L.) Aug. DC. (Molluginaceae) roots, stems and leaves. Asian Pac. J. Trop. Dis. 2015;5(9):711–715. [Google Scholar]
- Ngo Van T., Scarlett C., Bowyer M., Vuong Q. Phytochemical and antioxidant properties from different parts of Salacia chinensis L. J. Biol. Active Prod. Nature. 2017;7:401–410. [Google Scholar]
- Nguyen K.Q., Vuong Q.V., Nguyen M.H., Roach P.D. The effects of drying conditions on bioactive compounds and antioxidant activity of the Australian maroon bush Scaevola spinescens. J. Food Process. Preserv. 2018;42 [Google Scholar]
- Pham H.N.T., Vuong Q.V., Bowyer M.C., Scarlett C.J. Effect of extraction solvents and thermal drying methods on bioactive compounds and antioxidant properties of Catharanthus roseus (L.) G. Don (Patricia White cultivar) J. Food Process. Preserv. 2017;41(5):e13199. [Google Scholar]
- Rajan M., Rajkumar G., Farias Lima Guedes T.J., Chagas Barros R.G., Narain N. Performance of different solvents on extraction of bioactive compounds, antioxidant and cytotoxic activities in Phoenix loureiroi Kunth leaves. J. Appl. Res. Med. Aromatic Plants. 2020;17:100247. [Google Scholar]
- Rajput Z.I., Hu S.-h., Xiao C.-w., Arijo A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. - Sci. B. 2007;8(3):153–161. doi: 10.1631/jzus.2007.B0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee P., Chaudhary H., Rathee S., Rathee D., Kumar V., Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm. Allergy - Drug Targets. 2009;8(3):229–235. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- Semple S.J., Reynolds G.D., O'Leary M.C., Flower R.L.P. Screening of Australian medicinal plants for antiviral activity. J. Ethnopharmacol. 1998;60(2):163–172. doi: 10.1016/s0378-8741(97)00152-9. [DOI] [PubMed] [Google Scholar]
- Škerget M., Kotnik P., Hadolin M., Hraš A.R., Simonič M., Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89(2):191–198. [Google Scholar]
- Tava A., Biazzi E., Ronga D., Mella M., Doria F., Accogli R.…Avato P. Triterpenic saponins from Medicago marina L. Phytochemistry. 2020;174:112333. doi: 10.1016/j.phytochem.2020.112333. [DOI] [PubMed] [Google Scholar]
- Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19(6):669–675. [Google Scholar]
- Visioli F., De La Lastra C.A., Andres-Lacueva C., Aviram M., Calhau C., Cassano A.…Edeas M. Polyphenols and human health: a prospectus. Crit. Rev. Food Sci. Nutr. 2011;51(6):524–546. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- Vu H.T., Scarlett C.J., Vuong Q.V. Effects of drying conditions on physicochemical and antioxidant properties of banana (Musa cavendish) peels. Dry. Technol. 2017;35(9):1141–1151. [Google Scholar]
- Vuong Q., Hirun S., Roach P.D., Bowyer M.C., Phillips P.A., Scarlett C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013;3(3):104–111. [Google Scholar]
- Vuong Q., Sadeqzadeh E., Hirun S., Goldsmith C., Zammitt N., Bowyer M.…Scarlett C. Phenolic compounds, antioxidant and anti-cancer properties of the Australian maroon bush Scaevola spinescens (Goodeniaceae) J. Bioanal. Biomed. 2015;2014 S12:002. [Google Scholar]
- Vuong Q., Zammit N., Munro B.R., Murchie S., Bowyer M.C., Scarlett C.J. Effect of drying conditions on physicochemical and antioxidant properties of vitex agnus-castus leaves. J. Food Process. Preserv. 2015;39(6):2562–2571. [Google Scholar]
- Wang Z., Zhu C., Liu S., He C., Chen F., Xiao P. Comprehensive metabolic profile analysis of the root bark of different species of tree peonies (Paeonia Sect. Moutan) Phytochemistry. 2019;163:118–125. doi: 10.1016/j.phytochem.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.