Abstract
ANGIOGENESIS IS A PROCESS CRITICAL TO both tumour growth and metastasis. It is a dynamic integrated process involving basement membrane degradation, endothelial cell proliferation and migration, and capillary tubule formation. Under normal circumstances, the microvasculature is maintained in a quiescent state. The acquisition of the angiogenic phenotype depends on the outcome of stimulatory and inhibitory regulation by the tumour and its microenvironment. There are markers of angiogenesis that potentially could provide prognostic information in addition to that gained from conventional clinicopathologic data, and antiangiogenic therapy for urologic cancers has potential advantages over current therapeutic strategies. Promising preclinical studies have led to the initiation of phase I studies of antiangiogenic therapy in combination with chemotherapy, which may lead to novel treatments for urologic malignant tumours and may identify new intermediate markers for the response to therapy.
Prostate adenocarcinoma (PCa) is estimated to be the most commonly diagnosed noncutaneous malignant disease and the second leading cause of cancer deaths in men, whereas bladder transitional cell carcinoma (TCC) and renal cell carcinoma (RCC) are estimated to be the sixth and ninth most commonly diagnosed malignant diseases respectively.1 Despite significant improvements in local and systemic therapies, most deaths from cancers are due to metastases that resist conventional therapies.2,3,4 A major barrier to effective treatment is the biologic heterogeneity of cancer cells exhibited by genetic, biochemical, immunologic and biologic characteristics, such as cell-surface receptors, enzymes, karyotypes, cell morphology, growth parameters and drug resistance. Because of this heterogeneity, continued empiricism in the treatment of urologic cancers is unlikely to produce significant clinical improvements in patient outcomes.4 Rather, treatments directed toward critical processes that are involved in tumorigenesis and metastasis are more likely to result in significant advances in the treatment of urologic cancers. There are several lines of evidence that carcinogenesis is a multistep process reflecting genetic alterations that occur in the transformation of normal cells into highly malignant derivatives.5 Similarly, the process of angiogenesis appears to be acquired in discrete steps during tumour development and is critical to tumorigenicity and metastasis.5 In this article, we review the compelling evidence that supports the importance of angiogenesis and its clinical relevance in urologic cancers.
Angiogenesis
Tumour growth and metastasis depend upon the development of a neovasculature in and around the tumour.6,7,8,9,10 This process, called angiogenesis, is regulated by the balance between stimulatory and inhibitory factors released by the tumour and its microenvironment (Table 1).8,11,12,13,14,15 Angiogenesis facilitates progressive tumour growth by providing adequate oxygenation to the tumour through a series of interrelated steps, including endothelial cell proliferation, motility of endothelial cells through the extracellular matrix toward angiogenic stimuli, and capillary differentiation (Fig. 1).
Table 1
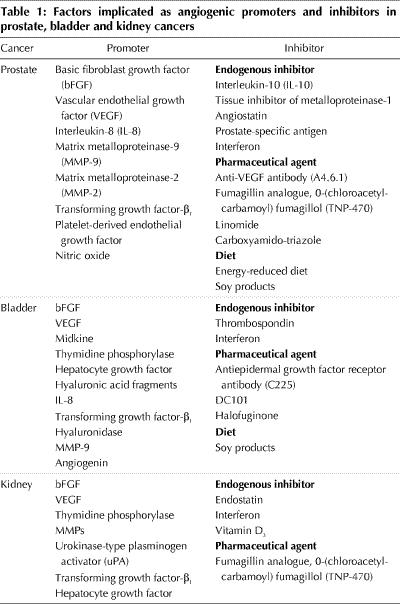
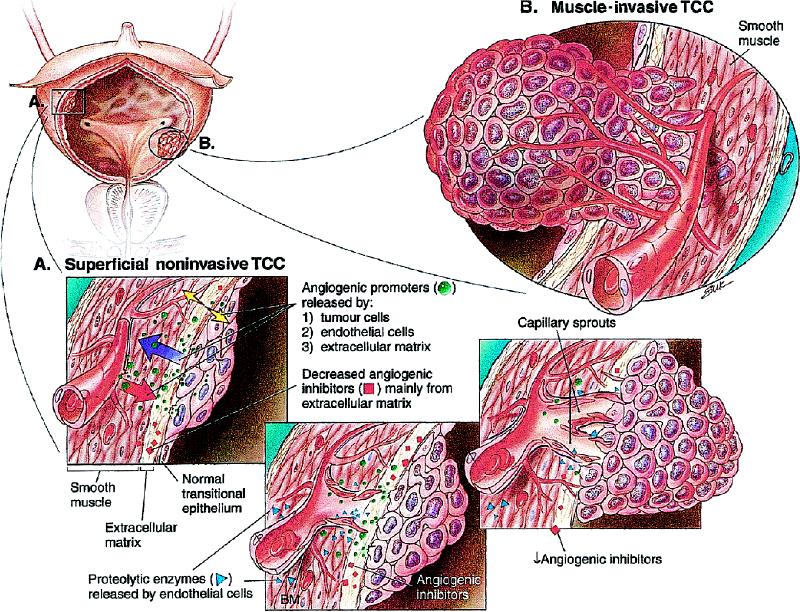
Fig. 1: Angiogenesis in (A) superficial and (B) muscle-invasive transitional cell carcinoma of the bladder is, in general, illustrative of the process of tumour neovascularization in other cancers. The multiple, sequential and interrelated steps by which angiogenesis is hypothesized to occur involve angiogenic stimuli or lack of endogenous angiogenic inhibitors, or both; the inciting by endothelial cells within venules of local proteolysis to degrade the basement membrane; the protrusion of endothelial cells through the wall of the venule; degradation of the interstitial matrix; continuing movement of endothelial cells toward the angiogenic stimulus; formation by endothelial cells of capillary sprouts that form a lumen; proliferation within sprouts; joining of tips of sprouts; blood flow; and formation of new basement membrane and incorporation of microvascular pericytes. TCC = transitional cell carcinoma, BM = basement membrane.
Much of our knowledge of the biology of PCa, TCC and RCC arises from animal experiments in which human tumour cells have been implanted into immune-deficient nude mice. The angiogenic, tumorigenic and metastatic potential of these human xenografts grown in nude mice is enhanced by implanting these tumour cells and growing them within the orthotopic site rather than a heterotopic site.16,17,18,19,20 Thus, mechanisms regulating angiogenesis are tissue specific,21 and the angiogenic phenotype is regulated by the differential expression of cytokines and growth factors within the microenvironment of the organ.22 This concept of organ-specific angiogenesis is important for the interpretation of preclinical studies evaluating angiogenesis and antiangiogenic therapies for human cancers, because heterotopic tumour models will not reflect accurately the interactions between the microenvironment of the organ and the tumour.
Prognostic markers
A biomarker is biologic material that can be used to enhance the detection of disease or provide prognostic information. The ideal prognostic biomarker would be noninvasive and would provide predictive information regarding the natural history of the disease or the response to treatment in addition to that gained from conventional clinicopathologic parameters. Markers of angiogenesis, including angiogenesis factor expression and microvessel density (MVD), have been evaluated as prognostic markers for urologic cancers.23
Antiangiogenic therapies
Because tumour growth and metastasis depend on angiogenesis, a great deal of attention has been focused on therapy that can interrupt this process. Antiangiogenic therapy can target endothelial cells directly; inhibit the production or action, or both, of proangiogenic peptides by the tumour cells or host; or enhance the expression of angiogenesis inhibitors within the tumour.24 Antiangiogenic therapy that targets endothelial cells, rather than tumour cells directly, has been evaluated as a novel therapeutic strategy for malignant diseases.25,26 The theoretical advantage to this therapy is that endothelial cells are unlikely to acquire mutations that lead to drug resistance.27 Endothelial cells are also readily exposed to bloodborne agents, circumventing the problem of drug delivery. Although it was widely assumed that antiangiogenic therapy was antiproliferative, it has recently been found that antiangiogenic therapy can induce apoptosis and tumour regression.28
Prostate cancer
Angiogenesis
Early studies reported that conditioned medium from cultures of human PCa cells stimulated endothelial cells, suggesting that these cells produce proangiogenic factors,29 and both basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) proteins have been measured in the urine of patients with PCa.30 Subsequently, it was reported that human PCa produces VEGF and interleukin-8 (IL-8) protein.31 These and other proangiogenic factors are believed to enhance the tumorigenicity and metastasis of PCa, because xenografts of the highly metastatic PC-3M-LN4 PCa cell line overexpressed bFGF, IL-8 and matrix metalloproteinase-9 (MMP-9) mRNA compared with the poorly metastatic parental PC-3M cell line from which it was selected.32 Similarly, the highly metastatic LNCaP-LN3 cell line overexpressed VEGF compared with either the parental LNCaP cell line or LNCaP-Pro5, which was selected for its tumorigenicity in the prostate.33 LNCaP-LN3 was also more metastatic and overexpressed VEGF mRNA and protein and VEGF receptor protein, with enhanced tumour-induced angiogenesis, compared with either LNCaP or LNCaP-Pro5, after implantation into the prostate of athymic nude mice.
The importance of IL-8 as a mediator of PCa angiogenesis was shown by experiments in which PC-3P cells with lower metastatic potential were transfected with sense IL-8, whereas highly metastatic PC-3M-LN4 cells were transfected with antisense IL-8 constructs.34 Following orthotopic implantation, the sense-transfected PC-3P cells overexpressed IL-8 and MMP-9 and were highly angiogenic, tumorigenic and metastatic compared with parental PC-3P cells or controls. Antisense transfection of the PC-3M-LN4 cells reduced the expression of IL-8 and MMP-9 and tumour-induced angiogenesis, resulting in inhibited tumorigenicity and metastasis. Therefore, IL-8 also seems to be involved in the regulation of angiogenesis and tumour growth in PCa, in part by induction of MMP expression.
The angiogenic and metastatic phenotype of PCa was also shown to be directly related to the expression of transforming growth factor-β1 and MMP-2 and inversely related to the expression of IL-10, which upregulated a natural tissue inhibitor of metalloproteinase-1.35,36
Androgens seem to regulate VEGF expression in PCa cells and prostatic fibroblasts.37,38 Hypoxia also induces VEGF transcription through the mediator, hypoxia-inducible factor-1,39 whose promoter activity is inhibited by protein kinase C inhibitors.40 Therefore, hypoxia, which can occur when tumours outgrow their blood supply, is a likely explanation for the induction of angiogenesis in PCa.
Several human PCa cell lines express enzymes that convert plasminogen or plasmin to the potent antiangiogenic product angiostatin.41,42 Furthermore, Fortier and colleagues found that prostate-specific antigen has dose-dependent antiangiogenic activity and inhibits endothelial cell response to both bFGF and VEGF.43 This antiangiogenic activity may explain the slow progression of certain PCas; however, levels of prostate-specific antigen studied by Fortier and coworkers were, for the most part, much higher than those observed in the serum of patients with PCa. Aberrant nitric oxide production by prostate tumour or host cells may also be a factor in tumour progression because of stimulation mediated by nitric oxide of cancer cell migration and invasiveness.44,45,46
Although the biology of PCa has been further characterized by the identification of relevant angiogenic factors, additional studies are needed to fully elucidate the multiple mechanisms involved in PCa angiogenesis.
Prognostic markers
Both MVD and the expression of various angiogenic factors have been evaluated as prognostic factors for patients with PCa. MVD is a potential prognostic factor that has been correlated with clinical stage, pathologic stage, metastasis, and histopathologic grade and is a significant predictor of disease-specific survival and progression after therapy.23,47,48,49,50,51 However, MVD has not been consistently shown to correlate independently with outcome after radical prostatectomy52,53,54,55,56 and, therefore, the prognostic value of MVD needs to be confirmed in prospective studies prior to widespread clinical use.
Both VEGF and bFGF have also been evaluated as prognostic factors in PCa, however, neither is prognostic and their detection may merely reflect the presence of benign prostatic hyperplasia, which is usually present in most patients with PCa.30,57,58,59 In preliminary studies, transforming growth factor-β1 correlated with tumour grade, MVD, bone metastases and a shorter median cancer-specific survival,60 whereas platelet-derived endothelial growth factor correlated with increased MVD.61
Antiangiogenic therapies
Most studies that have evaluated antiangiogenic therapy for PCa have been in the preclinical setting, and the efficacy demonstrated suggests that these agents may be useful clinically. Systemic therapy with the anti-VEGF antibody A4.6.1 inhibited neovascularization, the local growth of human PCa xenografts62 and lung metastasis.63 Treatment of the PC-3 and Dunning R-3327 cell lines growing subcutaneously in athymic mice with the synthetic fumagillin analogue 0-(chloroacetyl-carbamoyl) fumagillol (TNP-470) inhibited tumour growth and metastasis in a dose-dependent manner.63,64 Another antiangiogenic agent, linomide, inhibited tumour-induced angiogenesis and growth of PC-3 tumours implanted subcutaneously in nude mice,65,66 and it appeared to act directly on tumours with no significant effect on VEGF or bFGF expression relative to controls.67
Interferon-α (IFN-α&!!ERROR!! No Symbol Entity Found% and IFN-β are cytokines with antiangiogenic activity. Transfected PCa cells with the murine IFN-β gene may have decreased tumorigenesis and metastasis resulting from bystander effects (the effect of interferon on murine inflammatory cells and stroma), resulting in decreased tumour angiogenesis.68 Although interferon has been relatively ineffective in the treatment of most solid tumours clinically, the delivery of optimal doses using effective schedules69 and local delivery via gene therapy68 may significantly improve the results of interferon therapy.
In a phase II clinical trial, the antiangiogenic agent carboxyamido-triazole was administered to 15 patients with androgen-independent PCa, but no significant clinical response was seen.70 PCa angiogenesis is thought to be androgen dependent; however, in a study evaluating neoadjuvant androgen deprivation, no effect on MVD was observed in 80 patients who had undergone radical prostatectomy.71
Consumption of energy-reduced diets by mice with human PCa xenographs resulted in antiangiogenic activity and decreased VEGF expression,72 whereas the consumption of soy products enhanced apoptosis and decreased tumour cell proliferation, MVD and tumour volume.73
Thus far, no reported antiangiogenic therapies have demonstrated significant efficacy in clinical studies. Promising novel and more specific molecules such as the anti-VEGF receptor monoclonal antibody DC101, used as monotherapy or adjuvantly with chemotherapy with or without androgen deprivation, are undergoing preclinical studies.
Bladder cancer
Angiogenesis
The role of angiogenesis in TCC is well established.74,75,76,77,78,79,80,81,82,83,84,85 Although the expression of bFGF is variable, it appears to be an important mediator of angiogenesis in TCC. Highly metastatic variants of the human 253J TCC cell line expressed high levels of bFGF mRNA compared with the parental cell line and were highly angiogenic and metastatic.86 However, the balance between proangiogenic peptides and inhibitors ultimately determines the angiogenic phenotype of the tumour cell, and downregulation of the endogenous angiogenic inhibitor thrombospondin seems to be another key event in bladder carcinogenesis.87
Thymidine phosphorylase has angiogenic activity and may also play a role in the pathogenesis of TCC.88 Hyaluronidase degrades hyaluronic acid into small fragments that stimulate angiogenesis, and urinary levels of hyaluronic acid, hyaluronidase and hyaluronic acid fragments are elevated in patients with TCC.89,90,91
Prognostic markers
MVD has been extensively evaluated as a prognostic factor for TCC and correlated with lymph node metastasis, disease recurrence and survival, and it stratified the risk of recurrence attributed to p53 expression in muscle-invasive TCC.92,93,94,95,96 However, MVD was not predictive of disease progression in patients with superficial lamina propria–invasive tumours.97 It appears that MVD can be used to identify patients with locally advanced TCC who are at risk of developing metastatic disease after definitive therapy.
VEGF and bFGF expression by human TCC has also been evaluated, and thus far no definitive conclusions can be reached regarding their prognostic value.77,98,99,100,101,102,103 However, bFGF may be a marker of chemoresistance, because the HT1376 TCC cell line, following transfection with the bFGF sense cDNA, demonstrated cisplatin resistance.104 Overexpression of bFGF mRNA in radical cystectomy specimens also independently predicted poor outcome for patients who received neoadjuvant chemotherapy.95
Angiogenin is another proangiogenic factor that is overexpressed in muscle-invasive TCC relative to superficial TCC, and increased serum angiogenin levels were associated with a lower overall survival rate.105 The loss of expression of thrombospondin protein has also been suggested to be an independent predictor of disease recurrence and overall survival in patients undergoing radical cystectomy.85 Invasive tumours have also been found to overexpress the heparin-binding growth factor midkine80 and hepatocyte growth factor.81,82 Expressions of several angiogenic factors and MVD appear to be potential prognostic markers for TCC, but again prospective studies are necessary before their widespread clinical use.
Antiangiogenic therapies
Several antiangiogenic therapies have demonstrated efficacy for TCC in preclinical studies. Subcutaneous IFN-α therapy inhibited tumour growth and decreased MVD and the expressions of bFGF and VEGF in human TCC growing orthotopically in nude mice.106 This effect was observed in tumours that were resistant to the antiproliferative effect of interferon, indicating that the inhibition of tumour growth was dependent upon the antiangiogenic effect of interferon. Subsequently, it has been demonstrated that the optimal antiangiogenic effect and efficacy of IFN-α is highly dependent upon the dose and schedule of administration.69
Antiangiogenic activity has been observed following therapy for human bladder cancer xenografts with the antiepidermal growth factor receptor monoclonal antibody C225. Therapy with C225 caused the regression of established human xenografts by downregulating the expression of VEGF, bFGF and IL-8 with concomitant inhibition of growth and metastasis.107
Similarily, therapy with DC101, in combination with paclitaxel, inhibited tumour growth and metastasis and improved the survival of mice with human bladder cancer xenografts growing orthotopically within the bladder of athymic nude mice.108 In a heterotopic murine model for bladder TCC, both the pharmaceutical agent halofuginone and dietary soy products have demonstrated antiangiogenic effects.109,110
Renal cell carcinoma
Angiogenesis
RCC is among the most vascular of solid tumours, which suggests a prominent role for neovascularization in its pathogenesis, and experiments have demonstrated that human RCC cells induced angiogenesis.111 VEGF may play a prominent role in RCC angiogenesis, because several studies have demonstrated higher expression levels of VEGF mRNA and protein in tumours relative to normal renal tubules112,113,114 and benign oncocytomas,114 and VEGF appears to act on RCC endothelial cells in a paracrine fashion.112,115 VEGF has 5 isoforms and expresses variable levels of each, and the increased expression of VEGF-189 in RCC has been associated with higher stage tumours.116 The role of bFGF in RCC angiogenesis and tumorigenesis is less clear.113,117
The activity of MMPs is regulated by the balance of expression with tissue inhibitors of metalloproteinase, whose inactivation by methylation has been observed in RCC.118 Urokinase-type plasminogen activator (uPA) is a serine protease that activates plasminogen to form plasmin, resulting in alterations of the extracellular matrix degradation, thereby facilitating endothelial cell migration during angiogenesis. Overexpression of the uPA receptor has been observed in RCC.119 Levels of uPA correlate with inactivation of the von Hippel-Lindau gene, a tumour suppressor gene inactivated in the majority (75%) of sporadic RCCs.120,121 This suggests that the uPA system plays an important role in angiogenesis, tumorigenicity and metastasis in RCC. The von Hippel-Lindau protein may also be involved in several alternative pathways affecting angiogenesis in RCC by regulating VEGF production and causing regression of the angiogenic mediator transforming growth factor-β1.122,123,124
Prognostic markers
MVD and the expression of angiogenic factors have been evaluated as prognostic markers for RCC, but it is unclear whether either contributes any additional prognostic information in addition to that obtained from current clinicopathologic parameters.125,126,127,128,129,130,131 Thymidine phosphorylase overexpression correlated with MVD and was an independent prognostic factor for survival in RCC.125
Angiogenesis factors have also been identified in the serum of patients with RCC. VEGF levels were significantly higher in patients with RCC relative to control patients, but they did not remain a prognostic factor upon multivariate analysis, which included tumour grade and stage.132 Associations between bFGF or VEGF levels and tumour grade, stage or overall survival have differed among studies.132,133 The VEGF receptor (FLT-1) has also been evaluated as a prognostic factor in RCC, and increased FLT-1 mRNA expression was observed in RCC compared with normal renal tissue.134 No angiogenic factor appears to be a candidate prognostic marker for RCC.
Antiangiogenic therapies
Several antiangiogenic therapies for RCC have shown promise in preclinical studies and are currently being evaluated in clinical trials. Systemic administration of the endogenous angiogenic inhibitor endostatin suppressed tumour growth in RCC in a murine model,135 and endostatin is now undergoing evaluation in a multi-institutional phase I clinical trial for patients with solid malignant tumours. TNP-470 demonstrated promising results in preclinical studies, however, a phase II clinical study of 33 patients with metastatic RCC found that TNP-470 had manageable toxic effects, but led to only one partial response of short duration.136,137,138
The results of interferon therapy for locally advanced or metastatic RCC have been disappointing. In a phase II clinical study, subcutaneous IFN-α-2b was used to treat 12 patients with progressive metastatic RCC.139 Its efficacy was minimal, although higher responses were observed in patients with higher bFGF serum concentrations. Two well-designed, randomized prospective studies of patients with metastatic RCC did not demonstrate a survival advantage with IFN-α-2a or IFN-γ-1b.140,141 Several other phase II or III clinical trials, with less rigorous study designs, have also demonstrated modest responses or no efficacy for interferon therapy with or without chemotherapeutic agents or other immunotherapies in the treatment of metastatic RCC.142,143,144,145,146,147,148,149,150,151 Significant toxicity was experienced in these trials. Only 2 trials have shown that interferon provided a modest survival advantage compared with chemotherapy or hormonal therapy alone.152,153 Optimal doses, scheduling and the type of systemic interferon administered may improve the antiangiogenic effect and clinical efficacy.68 Vitamin D3 agents also inhibited angiogenesis, tumorigenicity and metastases in animal xenografts of RCC.154 Currently, clinical trials are evaluating the efficacy of the angiogenesis inhibitor thalidomide in patients with advanced RCC.
Conclusion
The mechanisms regulating the acquisition of the angiogenic phenotype of urologic cancers are undergoing vigorous study but have not, as yet, been fully elucidated. Angiogenesis plays a critical role in tumorigenesis and metastasis in prostate, bladder and renal cancers. Several angiogenic biomarkers have been implicated as prognostic markers for urologic malignant diseases in preliminary reports, but these results need to be confirmed in prospective studies. Antiangiogenic therapy targets pathways regulating tumour growth and metastasis, and promising preclinical studies have led to the initiation of phase I trials for patients with muscle-invasive TCC using the antiangiogenic agents C225 with chemotherapy and interferon with chemotherapy in a neoadjuvant setting. These antiangiogenic strategies, especially in combination with cytoreductive chemotherapy, may lead to novel treatments for urologic cancers and may identify new intermediate markers for the response to therapy.
Footnotes
This article has been peer reviewed.
Acknowledgement: This review was supported in part by the Royal College of Physicians and Surgeons of Canada Detweiler Traveling Fellowship awarded to Dr. Izawa.
Competing interests: None declared.
Reprint requests to: Dr. Colin P.N. Dinney, Associate Professor of Urology and Cancer Biology, Department of Urology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., PO Box 110, Houston TX 77030; fax 713 794-4824; cdinney@notes.mdacc.tmc.edu
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin 2000;50:7-33. [DOI] [PubMed]
- 2.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature 1979;283: 139-46. [DOI] [PubMed]
- 3.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science 1982;217:998-1003. [DOI] [PubMed]
- 4.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes Memorial Award Lecture. Cancer Res 1990;50:6130-8. [PubMed]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [DOI] [PubMed]
- 6.Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes Memorial Award Lecture. Cancer Res 1986;46:467-73. [PubMed]
- 7.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989;339:58-61. [DOI] [PubMed]
- 8.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991;64:327-36. [DOI] [PubMed]
- 9.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 1994;79:185-8. [DOI] [PubMed]
- 10.Folkman J. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757-63. [DOI] [PubMed]
- 11.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg 1972;175:409-16. [DOI] [PMC free article] [PubMed]
- 12.Gimbrone MA Jr, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst 1974;52:413-27. [DOI] [PubMed]
- 13.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353-64. [DOI] [PubMed]
- 14.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27-31. [DOI] [PubMed]
- 15.Singh RK, Llansa N, Bucana CD, Sanchez R, Koura A, Fidler IJ. Cell density-dependent regulation of basic fibroblast growth factor expression in human renal cell carcinoma cells. Cell Growth Differ 1996;7:397-404. [PubMed]
- 16.Naito S, von Eschenbach AC, Giavazzi R, Fidler IJ. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res 1986;46:4109-15. [PubMed]
- 17.Fidler IJ, Naito S, Pathak S. Orthotopic implantation is essential for the selection, growth and metastasis of human renal cell cancer in nude mice. Cancer Metastasis Rev 1990;9:149-65. [DOI] [PubMed]
- 18.Fidler IJ, Wilmanns C, Staroselsky A, Radinsky R, Dong Z, Fan D. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev 1994;13:201-22. [DOI] [PubMed]
- 19.Perrotte PJR, Bielenberg DR, Eve BY, Dinney CPN. Organ-specific angiogenesis and metastases of human bladder carcinoma growing in athymic nude mice. Mol Urol 1997;1:299-307.
- 20.Staroselsky AN, Radinsky R, Fidler IJ, Pathak S, Chernajovsky Y, Frost P. The use of molecular genetic markers to demonstrate the effect of organ environment on clonal dominance in a human renal-cell carcinoma grown in nude mice. Int J Cancer 1992;51:130-8. [DOI] [PubMed]
- 21.Hanahan D, Christofori G, Naik P, Arbeit J. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer 1996;32A:2386-93. [DOI] [PubMed]
- 22.Kumar R, Fidler IJ. Angiogenic molecules and cancer metastasis. In Vivo 1998;18:27-34. [PubMed]
- 23.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993;143:401-9. [PMC free article] [PubMed]
- 24.Hayes AJ, Li LY, Lippman ME. Science, medicine, and the future. Antivascular therapy: a new approach to cancer treatment. BMJ 1999;318:853-6. [DOI] [PMC free article] [PubMed]
- 25.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994;79:1157-64. [DOI] [PubMed]
- 26.Huang X, Molema G, King S, Watkins L, Edgington TS, Thorpe PE. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997;275:547-50. [DOI] [PubMed]
- 27.Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997; 390:404-7. [DOI] [PubMed]
- 28.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995;1:149-53. [DOI] [PubMed]
- 29.Hepburn PJ, Griffiths K, Harper ME. Angiogenic factors expressed by human prostatic cell lines: effect on endothelial cell growth in vitro. Prostate 1997;33:123-32. [DOI] [PubMed]
- 30.Weingartner K, Ben-Sasson SA, Stewart R, Richie JP, Riedmiller H, Folkman J. Endothelial cell proliferation activity in benign prostatic hyperplasia and prostate cancer: an in vitro model for assessment. J Urol 1998;159:465-70. [DOI] [PubMed]
- 31.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertson PC, Laudone VP, et al. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology 1998;51:161-7. [DOI] [PubMed]
- 32.Greene GF, Kitadai Y, Pettaway CA, von Eschenbach AC, Bucana CD, Fidler IJ. Correlation of metastasis-related gene expression with metastatic potential in human prostate carcinoma cells implanted in nude mice using an in situ messenger RNA hybridization technique. Am J Pathol 1997;150:1571-82. [PMC free article] [PubMed]
- 33.Balbay MD, Pettaway CA, Kuniyasu H, Inoue K, Ramirez E, Li E, et al. Highly metastatic human prostate cancer growing within the prostate of athymic mice overexpresses vascular endothelial growth factor. Clin Cancer Res 1999;5:783-9. [PubMed]
- 34.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay D, et al. Interleukin-8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res 2000;6:2104-19. [PubMed]
- 35.Stearns ME, Garcia FU, Fudge K, Rhim J, Wang M. Role of interleukin 10 and transforming growth factor beta1 in the angiogenesis and metastasis of human prostate primary tumor lines from orthotopic implants in severe combined immunodeficiency mice. Clin Cancer Res 1999;5:711-20. [PubMed]
- 36.Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res 1999;5:189-96. [PubMed]
- 37.Joseph IB, Nelson JB, Denmeade SR, Isaacs JT. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res 1997;3:2507-11. [PubMed]
- 38.Levine AC, Liu XH, Greenberg PD, Eliashvili M, Schiff JD, Aaronson SA, et al. Androgens induce the expression of vascular endothelial growth factor in human fetal prostatic fibroblasts. Endocrinology 1998;139:4672-8. [DOI] [PubMed]
- 39.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999;59:5830-5. [PubMed]
- 40.Kruger EA, Blagosklonny MV, Dixon SC, Figg WD. UCN-01, a protein kinase C inhibitor, inhibits endothelial cell proliferation and angiogenic hypoxic response. Invasion Metastasis 2000;18:209-18. [DOI] [PubMed]
- 41.Gately S, Twardowski P, Stack MS, Patrick M, Boggio L, Cundiff DL, et al. Human prostate carcinoma cells express enzymatic activity that converts human plaminogen to the angiogenesis inhibitor, angiostatin. Cancer Res 1996;56:4887-90. [PubMed]
- 42.Heidtmann HH, Nettelbeck DM, Mingels A, Jager R, Welker HG, Kontermann RE. Generation of angiostatin-like fragments from plasminogen by prostate-specific antigen. Br J Cancer 1999;81:1269-73. [DOI] [PMC free article] [PubMed]
- 43.Fortier AH, Nelson BJ, Grella DK, Holaday JW. Antiangiogenic activity of prostate-specific antigen. J Natl Cancer Inst 1999;91:1635-40. [DOI] [PubMed]
- 44.Thomsen LL, Miles DW. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev 1998;17:107-18. [DOI] [PubMed]
- 45.Jadeski LC, Lala PK. Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol 1999;155:1381-90. [DOI] [PMC free article] [PubMed]
- 46.Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer 1998;82:1897-1903. [PubMed]
- 47.Fregene TA, Khanuja PS, Noto AC, Gehani SK, van Egmont EM, Luz DA, et al. Tumor-associated angiogenesis in prostate cancer. Anticancer Res 1993; 13:2377-82. [PubMed]
- 48.Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer 1998;78:940-4. [DOI] [PMC free article] [PubMed]
- 49.Brauer MK, Deering RE, Brown M, Preston SD, Bigler SA. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer 1994;73:678-87. [DOI] [PubMed]
- 50.Silberman MA, Partin AW, Veltri RW, Epstein JI. Tumor angiogenesis correlates with progression after radical prostatectomy but not with pathologic stage in Gleason sum 5 to 7 adenocarcinoma of the prostate. Cancer 1997; 79:772-9. [DOI] [PubMed]
- 51.Strohmeyer D, Rossing C, Strauss F, Bauerfeind A, Kaufmann O, Loening S. Tumor angiogenesis is associated with progression after radical prostatectomy in pT2/pT3 prostate cancer. Prostate 2000;42:26-33. [DOI] [PubMed]
- 52.Hall MC, Troncoso P, Pollack A, Zhau HYE, Zagars GK, Chung LWK, et al. Significance of tumor angiogenesis in clinically localized prostatic carcinoma treated with external beam radiotherapy. Urology 1994;44:869-75. [DOI] [PubMed]
- 53.Bettencourt MC, Bauer JJ, Sesterhenn IA, Connelly RR, Moul JW. CD34 immunohistochemistry assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J Urol 1998;160:459-65. [PubMed]
- 54.Moul JW. Angiogenesis, p53, bcl-2 and Ki-67 in the progression of prostate cancer after radical prostatectomy. Eur Urol 1999;35:399-407. [DOI] [PubMed]
- 55.Rubin MA, Buyyounouski M, Bagiella E, Sharir S, Neugut A, Benson M, et al. Microvessel density in prostate cancer: lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology 1999;53:542-7. [DOI] [PubMed]
- 56.Gettman MT, Parelli A, Slezak J, Bergstralh EJ, Blute M, Zincke H, et al. Role of microvessel density in predicting recurrence in pathologic stage T3 prostatic adenocarcinoma. Urology 1999;54:479-85. [DOI] [PubMed]
- 57.Duque JL, Loughlin KR, Adam RM, Kantoff PW, Zurakowski D, Freeman MR. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology 1999;54:523-7. [DOI] [PubMed]
- 58.Walsh K, Sherwood RA, Dew TK, Mulvin D. Angiogenic peptides in prostatic disease. Br J Urol 1999;84:1081-3. [DOI] [PubMed]
- 59.Meyer GE, Yu E, Siegal JA, Petteway JC, Blumenstein BA, Brawer MK. Serum basic fibroblast growth factor in men with and without prostate carcinoma. Cancer 1995;76:2304-11. [DOI] [PubMed]
- 60.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 1998;37:19-29. [DOI] [PubMed]
- 61.Sugamoto T, Tanji N, Nishio S, Yokoyama M. Expression of platelet-derived endothelial cell growth factor in prostatic adenocarcinoma. Oncol Rep 1999;6:519-22. [DOI] [PubMed]
- 62.Borgstrom P, Bourdon MA, Hillan KJ, Sriramarao P, Ferrara N. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate 1998;35:1-10. [DOI] [PubMed]
- 63.Miki T, Nonomura N, Nozawa M, Harada Y, Nishimura K, Kojima Y, et al. Angiogenesis inhibitor TNP-470 inhibits growth and metastasis of a hormone-independent rat prostatic carcinoma cell line. J Urol 1998;160:210-3. [PubMed]
- 64.Yamaoka M, Yamamoto T, Ikeyama S, Sudo K, Fujita T. Angiogenesis inhibitor TNP-470 (AGM-1470) potently inhibits the tumor growth of hormone-independent human breast and prostate carcinoma cell lines. Cancer Res 1993;53:5233-6. [PubMed]
- 65.Vukanovic J, Isaacs JT. Human prostatic cancer cells are sensitive to programmed (apoptotic) death induced by the antiangiogenic agent linomide. Cancer Res 1995;55:3517-20. [PubMed]
- 66.Joseph IB, Isaacs JT. Potentiation of the antiangiogenic ability of linomide by androgen ablation involves down-regulation of vascular endothelial growth factor in human androgen-responsive prostatic carcinomas. Cancer Res 1997;57:1054-7. [PubMed]
- 67.Joseph IB, Isaacs JT. Macrophage role in the anti-prostate cancer response to one class of antiangiogenic agents. J Natl Cancer Inst 1998;90:1648-53 [DOI] [PubMed]
- 68.Dong Z, Greene G, Pettaway C, Dinney CPN, Eue I, Lu W, et al. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-β. Cancer Res 1999;59:872-9. [PubMed]
- 69.Slaton JW, Perrotte P, Inoue K, Dinney CPN, Fidler IJ. Interferon-α-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res 1999;5:2726-34. [PubMed]
- 70.Bauer KS, Figg WD, Hamilton JM, Jones EC, Premkumar A, Steinberg SM, et al. A pharmacokinetically guided phase II study of caroboxyamido-triazole in androgen-independent prostate cancer. Clin Cancer Res 1999;5:2324-9. [PubMed]
- 71.Matsushima H, Goto T, Hosaka Y, Kitamura T, Kawabe K. Correlation between proliferation, apoptosis, and angiogenesis in prostate carcinoma and their relation to androgen ablation. Cancer 1999;85:1822-7. [DOI] [PubMed]
- 72.Mukherjee P, Sotnikov AV, Mangian HJ, Zhou J, Visek WJ, Clinton SK. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J Natl Cancer Inst 1999;91:512-23. [DOI] [PubMed]
- 73.Zhou J, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr 1999;129:1628-35. [DOI] [PubMed]
- 74.Chodak GW, Scheiner CJ, Zetter BR. Urine from patients with transitional-cell carcinoma stimulates migration of capillary endothelial cells. N Engl J Med 1981;305:869-74. [DOI] [PubMed]
- 75.Chodak GW, Summerhayes I. Detection of angiogenesis activity in malignant bladder tissue and cells. J Urol 1984;132:1032-5. [DOI] [PubMed]
- 76.Chopin DK, Caruelle JP, Columbel M, Palcy S, Ravery V, Caruelle D, et al. Increased immunodetection of acidic fibroblast growth factor in bladder cancer, detectable in urine. J Urol 1993;150:1126-30. [DOI] [PubMed]
- 77.Nguyen M, Watanabe H, Budson AE, Richie JP, Folkman J. Elevated levels of the angiogenic peptide basic fibroblast growth factor in urine of bladder cancer patients. J Natl Cancer Inst 1993;85:241-2. [DOI] [PubMed]
- 78.O'Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL. Two mechanisms of basic fibroblast growth factor-induced angiogenesis in bladder cancer. Cancer Res 1997;57:136-40 [PubMed]
- 79.O'Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL. Different angiogenic pathways characterize superficial and invasive bladder cancer. Cancer Res 1995;55:510-3. [PubMed]
- 80.O'Brien TS, Cranston D, Fuggle S, Bicknell R, Harris AL. The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res 1996; 56:2515-8. [PubMed]
- 81.Joseph A, Weiss GH, Jin L, Fuchs A, Chowdhury S, O'Shaugnessy P, et al. Expression of scatter factor in human bladder carcinoma. J Natl Cancer Inst 1995;87:372-7. [DOI] [PubMed]
- 82.Rosen EM, Joseph A, Jin L, Yao Y, Chau MH, Fuchs A, et al. Urinary and tissue levels of scatter factor in transitional cell carcinoma of bladder. J Urol 1997;157:72-8. [PubMed]
- 83.Tachibana M, Miyakawa A, Nakashima J, Murai M, Nakamura K, Kubo A, et al. Constitutive production of multiple cytokines and a human chorionic gonadotropin beta-subunit by a human bladder cancer cell line (KU-19-19): possible demonstration of totipotential differentiation. Br J Cancer 1997; 76:163-74. [DOI] [PMC free article] [PubMed]
- 84.Eder IE, Stenzl A, Hobisch A, Cronauer MV, Bartsch G, Klocker H. Expression of TGF's beta-1, beta 2 and beta 3 in human bladder carcinomas. Br J Cancer 1997;75:1753-60. [DOI] [PMC free article] [PubMed]
- 85.Grossfeld GD, Ginsberg DA, Stein JP, Bochner BH, Esrig D, Groshen S, et al. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst 1997;89:219-27. [DOI] [PubMed]
- 86.Dinney CP, Fishbeck R, Singh RK, Eve B, Pathak S, Brown N, et al. Isolation and characterization of metastatic variants from human transitional cell carcinoma passaged by orthotopic implantation in athymic nude mice. J Urol 1995;154:1532-8. [PubMed]
- 87.Campbell SC, Volpert OV, Ivanovich M, Bouck NP. Molecular mediators of angiogenesis in bladder cancer. Cancer Res 1998;58:1298-1304. [PubMed]
- 88.Kubota Y, Miura T, Moriyama M, Noguchi S, Matsuzaki J, Takebayashi S, et al. Thymidine phosphorylase activity in human bladder cancer: difference between superficial and invasive cancer. Clin Cancer Res 1997;3:973-6. [PubMed]
- 89.Lokeshwar VB, Obek C, Soloway MS, Block NL. Tumor-associated hyaluronic acid: a new sensitive and specific urine marker for bladder cancer. Cancer Res 1997;57:773-7. [PubMed]
- 90.Lokeshwar VB, Obek C, Pham HT, Wei D, Young MJ, Duncan RC, et al. Urinary hyaluronic acid and hyaluronidase: markers for bladder cancer detection and evaluation of grade. J Urol 2000;163:348-56. [DOI] [PubMed]
- 91.Lokeshwar VB, Young MJ, Goudarzi G, Iida N, Yudin AI, Cherr GN, et al. Identification of bladder tumor-derived hyaluronidase: its similarity to HYAL1. Cancer Res 1999;59:4464-70. [PubMed]
- 92.Dickenson AJ, Fox SB, Persad RA, Hollyer J, Sibley GNA, Harris AL. Quantification of angiogenesis as an independent predictor of prognosis in invasive bladder carcinomas. Br J Urol 1994;74:762-6. [DOI] [PubMed]
- 93.Bochner BH, Cote RJ, Weidner N, Groshen S, Chen S, Skinner DG, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst 1995:87:1603-12. [DOI] [PubMed]
- 94.Jaeger TM, Weidner N, Chew K, Moore DH, Kerschmann RL, Waldman FM, et al. Tumor angiogenesis correlates with lymph node metastases in invasive bladder cancer. J Urol 1995;154:69-71. [PubMed]
- 95.Chaudhary R, Bromley M, Clarke NW, Betts CD, Barnard RJ, Ryder WD, et al. Prognostic relevance of micro-vessel density in cancer of the urinary bladder. Anticancer Res 1999;19:3479-84. [PubMed]
- 96.Bochner BH, Esrig D, Groshen S, Dickinsin M, Weidner N, Nichols PW, et al. Relationship of tumor angiogenesis and nuclear p53 accumulation in invasive bladder cancer. Clin Cancer Res 1997;3:1615-22. [PubMed]
- 97.Dinney CP, Babkowski RC, Antelo M, Perrotte P, Liebert M, Zhang HZ, et al. Relationship among cystectomy, microvessel density and prognosis in stage T1 transitional cell carcinoma of the bladder. J Urol 1998;160:1285-90. [PubMed]
- 98.Gazzaniga P, Gandini O, Gradilone A, Silvestri I, Giuliani L, Magnanti M, et al. Detection of basic fibroblast growth factor mRNA in urinary bladder cancer: correlation with local relapses. Int J Oncol 1999;14:1123-7. [DOI] [PubMed]
- 99.Crew JP, O'Brien T, Bicknell R, Fuggle S, Cranston D, Harris AL. Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. J Urol 1999;161:799-804. [PubMed]
- 100.Edgren M, Lennernas B, Larsson A, Nilsson S. Serum concentrations of VEGF and b-FGF in renal cell, prostate and urinary bladder carcinomas. Anticancer Res 1999;19:869-74. [PubMed]
- 101.Crew JP, O'Brien T, Bradburn M, Fuggle S, Bicknell R, Cranston D, et al. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res 1997;57:5281-5. [PubMed]
- 102.Crew JP, Fuggle S, Bicknell R, Cranston DW, de Benedetti A, Harris AL. Eukaryotic initiation factor-4E in superficial and muscle invasive bladder cancer and its correlation with vascular endothelial growth factor expression and tumour progression. Br J Cancer 2000;82:161-6. [DOI] [PMC free article] [PubMed]
- 103.Chow NH, Liu HS, Chan SH, Cheng HL, Tzai TS. Expression of vascular endothelial growth factor in primary superficial bladder cancer. Anticancer Res 1999;19:4593-7. [PubMed]
- 104.Miyake H, Hara I, Gohji K, Yoshimura K, Arakawa S, Kamidono S. Expression of basic fibroblast growth factor is associated with resistance to cisplatin in a human bladder cancer cell line. Cancer Lett 1998;123:121-6. [DOI] [PubMed]
- 105.Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S. Increased angiogenin expression in the tumor tissue and serum of urothelial carcinoma patients is related to disease progression and recurrence. Cancer 1999;86:316-24. [PubMed]
- 106.Dinney CPN, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, et al. Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-α admininstration. Cancer Res 1998;58:808-14. [PubMed]
- 107.Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res 1999;5:257-64. [PubMed]
- 108.Inoue K, Slaton JW, Davis DW, Hicklin DJ, McConkey DJ, et al. Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res. 2000;6(7):2635-43. [PubMed]
- 109.Elkin M, Ariel I, Miao H, Nagler A, Pines M, de-Groot N, et al. Inhibition of bladder carcinoma angiogenesis, stromal support, and tumor growth by halofuginone. Cancer Res 1999;59:4111-8. [PubMed]
- 110.Zhou J, Mukherjee P, Gugger ET, Tanaka T, Blackburn GL, Clinton SK. Inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res 1998;58:5231-8. [PubMed]
- 111.Bard RH, Mydlo JH, Freed SZ. Detection of tumor angiogenesis factor in adenocarcinoma of kidney. Urology 1986;27:447-50. [DOI] [PubMed]
- 112.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol 1993;143:1255-62. [PMC free article] [PubMed]
- 113.Takahashi A, Sasaki H, Kim SJ, Tobisu K, Kakizoe T, Tsukamoto T, et al. Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res 1994;54:4233-7. [PubMed]
- 114.Nicol D, Hii S, Walsh M, The B, Thompson L, Kennett C, et al. Vascular endothelial growth factor expression is increased in renal cell carcinoma. J Urol 1997;157:1482-6. [PubMed]
- 115.Nakagawa M, Emoto A, Hanada T, Nasu N, Nomura Y. Tubulogenesis by microvascular endothelial cells is mediated by vascular endothelial growth factor (VEGF) in renal cell carcinoma. Br J Urol 1997;79:681-7. [DOI] [PubMed]
- 116.Tomisawa M, Tokunaga T, Oshika Y, Tsuchida T, Fukushima Y, Sato H, et al. Expression pattern of vascular endothelial growth factor isoform is closely correlated with tumour stage and vascularisation in renal cell carcinoma. Eur J Cancer 1999;35:133-7. [DOI] [PubMed]
- 117.Emoto A, Nakagawa M, Wakabayashi Y, Hanada T, Naito S, Nomura Y. Induction of tubulogenesis of microvascular endothelial cells by basic fibroblast growth factor from human SN12C renal cancer cells. J Urol 1997;157:699-703. [PubMed]
- 118.Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, et al. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggests a suppressor role in kidney, brain, and other human cancers. Cancer Res 1999;59:798-802. [PubMed]
- 119.Xu Y, Hagege J, Doublet J, Callard P, Sraer J, Ronne E, et al. Endothelial and macrophage upregulation of urokinase receptor expression in human renal cell carcinoma. Hum Pathol 1997;28:206-13. [DOI] [PubMed]
- 120.Foster K, Prowse A, van den Berg A, Fleming S, Hulsbeek MM, Crossey PA, et al. Somatic mutations of the von-Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet 1994;3:2169-73. [DOI] [PubMed]
- 121.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 1994;7:85-90. [DOI] [PubMed]
- 122.Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Reversion of deregulated expression of vascular endothelial growth factor in human renal cell carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res 1996;56:2299-301. [PubMed]
- 123.Pal S, Claffey KP, Dvorak HF, Mukhopadhyay D. The von Hippel-Lindau gene product inhibits vascular permeability factor/vascular endothelial growth factor expression in renal cell carcinoma by blocking protein kinase C pathways. J Biol Chem 1997;272:27509-12. [DOI] [PubMed]
- 124.Ananth S, Knebelmann B, Gruning W, Dhanabal M, Walz G, Stillman IE, et al. Transforming growth factor β1 is a target for the von Hippel-Lindau tumor suppressor and a critical growth factor for clear cell renal carcinoma. Cancer Res 1999;59:2210-6. [PubMed]
- 125.Imazano Y, Takebayashi Y, Nishiyama K, Akiba S, Miyadera K, Yamada Y, et al. Correlation between thymidine phosphorylase expression and prognosis in human renal cell carcinoma. J Clin Oncol 1997;15:2570-8. [DOI] [PubMed]
- 126.MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic signigicance. Urology 1995;46:27-30. [DOI] [PubMed]
- 127.Gelb AB, Sudilovsky D, Wu CD, Weiss LM, Medeiros LJ. Appraisal of intratumoral microvessel density, MIB-1 score, DNA content, and p53 protein expression as prognostic indicators in patients with locally confined renal cell carcinoma. Cancer 1997;80:1768-75. [DOI] [PubMed]
- 128.Herbst C, Kosmehl H, Stiller KJ, Berndt A, Eiselt M, Schubert J, et al. Evaluation of microvessel density by computerized image analysis in human renal cell carcinoma. Correlation to pT category, nuclear grade, proliferative activity and occurrence of metastasis. J Cancer Res Clin Oncol 1998;124:141-7. [DOI] [PMC free article] [PubMed]
- 129.Yoshino S, Kato M, Okada K. Prognostic significance of microvessel count in low stage renal cell carcinoma. Int J Urol 1995;2:156-60. [DOI] [PubMed]
- 130.Nativ O, Sabo E, Reiss A, Wald M, Madjar S, Moskovitz B. Clinical significance of tumor angiogenesis in patients with localized renal cell carcinoma. Urology 1998;51:693-6. [DOI] [PubMed]
- 131.Delahunt B, Bethwaite PB, Thornton A. Prognostic significance of microscopic vascularity for clear cell renal cell carcinoma. Br J Urol 1997;80:401-4. [DOI] [PubMed]
- 132.Dosquet C, Coudert M, Lepage E, Cabane J, Richard F. Are angiogenic factors, cytokines, and soluble adhesion molecules prognostic factors in patients with renal cell carcinoma? Clin Cancer Res 1997;3:2451-8. [PubMed]
- 133.Wechsel HW, Bichler K, Feil G, Loeser W, Lahme S, Petri E. Renal cell carcinoma: relevance of angiogenic factors. Anticancer Res 1999;19:1537-40. [PubMed]
- 134.Takahashi A, Sasaki H, Kim SJ, Kakizoe T, Miyao N, Sugimura T, et al. Identification of receptor genes in renal cell carcinoma associated with angiogenesis by differential hybridization technique. Biochem Biophys Res Commun 1999;257:855-9. [DOI] [PubMed]
- 135.Dhanabal M, Ramchandran R, Volk R, Stillman IE, Lombardo M, Iruela-Arispe ML, et al. Endostatin: yeast production, mutants, and antitumor effect in renal cell carcinoma. Cancer Res 1999;59:189-97. [PubMed]
- 136.Morita T, Shinohara N, Tokue A. Antitumour effect of a synthetic analogue of fumagillin on murine renal carcinoma. Br J Urol 1994;74:416-21. [DOI] [PubMed]
- 137.Fujioka T, Hasegawa M, Ogiu K, Matsushita Y, Sato M, Kubo T. Antitumor effects of angiogenesis inhibitor 0-(choroacetyl-carbamoyl) fumagillol (TNP-470) against murine renal cell carcinoma. J Urol 1996;155:1775-8. [PubMed]
- 138.Stadler WM, Kuzel T, Shapiro C, Sosman J, Clark J, Vogelzang NJ. Multi-insitutional study of the angiogenesis inhibitor TNP-470 in metastatic renal carcinoma. J Clin Oncol 1999;17:2541-5. [DOI] [PubMed]
- 139.Vermeulen PB, Dirix LY, Martin M, Lemmens J, Van Oosterom AT. Serum basic fibroblast growth factor and vascular endothelial growth factor in metastatic renal cell carcinoma treated with interferon alfa-2b. J Natl Cancer Inst 1997;89:1316-7. [DOI] [PubMed]
- 140.Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N Engl J Med 1998;338:1272-8. [DOI] [PubMed]
- 141.Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian Urologic Oncology Group. N Engl J Med 1998;338:1265-71. [DOI] [PubMed]
- 142.Henriksson R, Nilsson S, Colleen S, Wersall P, Helsing M, Zimmerman R, et al. Survival in renal cell carcinoma-a randomized evaluation of tamoxifen vs interleukin 2, alpha-interferon (leucocyte) and tamoxifen. Br J Cancer 1998; 77:1311-7. [DOI] [PMC free article] [PubMed]
- 143.Stadler WM, Kuzel T, Dumas M, Vogelzang NJ. Multicenter phase II trial of interleukin-2, interferon-alpha, and 13-cis-retinoic acid in patients with metastatic renal-cell carcinoma. J Clin Oncol 1998;16:1820-5. [DOI] [PubMed]
- 144.Tourani JM, Pfister C, Berdah JF, Benhammouda A, Salze P, Monnier A, et al. Outpatient treatment with subcutaneous interleukin-2 and interferon alfa administration in combination with fluorouracil in patients with metastatic renal cell carcinoma: results of a sequential nonrandomized phase II study. Subcutaneous Administration Propeukin Program Cooperative Group. J Clin Oncol 1998;16:2505-13. [DOI] [PubMed]
- 145.Jayson GC, Middleton M, Lee SM, Ashcroft L, Thatcher N. A randomized phase II trial of interleukin 2 and interleukin 2-interferon alpha in advanced renal cancer. Br J Cancer 1998;78:366-9. [DOI] [PMC free article] [PubMed]
- 146.Ravaud A, Audhuy B, Gomez F, Escudier B, Lesimple T, Chevreau C, et al. Subcutaneous interleukin-2, interferon alfa-2a, and continuous infusion of fluorouracil in metastatic renal cell carcinoma: a multicenter phase II trial. Groupe Francais d'Immunotherapie. J Clin Oncol 1998;16:2728-32. [DOI] [PubMed]
- 147.Igarashi T, Marumo K, Onishi T, Kobayashi M, Aiba K, Tsushima T, et al. Interferon-alpha and 5-fluorouracil therapy in patients with metastatic renal cell cancer: an open multicenter trial. The Japanese Study Group Against Renal Cancer. Urology 1999;53:53-9. [DOI] [PubMed]
- 148.Clark JI, Gaynor ER, Martone B, Budds SC, Manjunath R, Flanigan RC, et al. Daily subcutaneous ultra-low-dose interferon-alpha in patients with advanced renal cell carcinoma. Clin Cancer Res 1999;5:2374-80. [PubMed]
- 149.Soori GS, Schulof RS, Stark JJ, Wiemann MC, Honeycutt PJ, Church CK, et al. Continuous-infusion floxuridine and alpha interferon in metastatic renal cancer: a national biotherapy study group phase II study. Cancer Invest 1999;17:379-84. [DOI] [PubMed]
- 150.Rohde D, Thiemann D, Wildberger J, Wolff J, Jakse G. Treatment of renal cancer patients with gemcitabine (2́,2́-difluorodeoxycytidine) and interferons: antitumor activity and toxicity. Oncol Rep 1998;5:1555-1560. [DOI] [PubMed]
- 151.Fujii A, Yui-En K, Ono Y, Yamamoto H, Gohji K, Takenaka A. Preliminary results of the alternating administration of natural interferon-alpha and recombinant interferon-gamma for metastatic renal cell carcinoma. Br J Urol 1999;84:399-404. [DOI] [PubMed]
- 152.Pyrhonen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela T, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 1999;17:2859-67. [DOI] [PubMed]
- 153.Medical Research Council Renal Cancer Collaborators. Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Lancet 1999;353:14-7. [PubMed]
- 154.Fujioka T, Hasegawa M, Ishikura K, Matsushita Y, Sato M, Tanji S. Inhibition of tumor growth and angiogenesis by vitamin D3 agents in murine renal cell carcinoma. J Urol 1998;160:247-51. [PubMed]


