Abstract
Specimens representing two new species of blood flukes (Digenea: Aporocotylidae), each representing a new genus, were collected from the banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979, in Borneo, Indonesia. Aetohemecus kirstenjensenae n. sp., n. gen. infected the heart of a banded eagle ray from Manggar, East Kalimantan, Borneo, Indonesia, and differs from its congeners by having an oviducal ampullae, an oötype posterior to all genitalia, and a uterus that extends anterior to the ovary. The new species resembles Selachohemecus spp., which infect requiem sharks (Carcharhinidae) in the Northwestern Atlantic Ocean and Gulf of Mexico, by having a single ventrolateral row of large C-shaped tegumental spines, X- or H-shaped intestine, and a post-caecal ovary. Specimens of Homestios janinecairae n. sp., n. gen. infected the heart of a banded eagle ray from Takisung, South Kalimantan, Borneo, Indonesia. The new species resembles other blood flukes that infect rays (Batoidea) by having a single, curving testis and an inverse U-shaped intestine as well as by lacking tegumental spines. It differs from all aporocotylids infecting batoids that lack spines by having a uterus that extends anteriad beyond the level of the seminal vesicle. The present study comprises the first record of an aporocotylid from Indonesia or from an eagle ray (Myliobatidae). To our knowledge, these are the first trematodes reported from a species of Aetomylaeus. The proposals of new genera and the description of two new species herein brings the total number of nominal chondrichthyan blood flukes to 13 species of 11 genera.
Keywords: Taxonomy, Systematics, Chondrichthyes, Elasmobranchii, Fish blood fluke, Batoidea
Graphical abstract

Highlights
-
•
Two new species of fish blood flukes assigned to two new genera infecting the heart of two banded eagle rays.
-
•
First record of an aporocotylid infection from Indonesia and from an eagle ray (Myliobatidae).
-
•
First record of a digenean infection for any species of Aetomylaeus.
-
•
A key is provided for all fish blood flukes that infect chondricthyans
1. Introduction
Currently, there are 633 nominal ray species (Chondrichthyes: Elasmobranchii: Batoidea) ranging in both marine and freshwater habitats (Last et al., 2016). More than 55 nominal batoid species of 12 families range in Indonesia, where the elasmobranch diversity and commercial fisheries landings (~110,000 tonnes) are high (Dharmadi et al., 2009). A large proportion of elasmobranch landings are bycatch and, because these sharks and rays typically are not identified to species at landing, this proportion is likely higher (Dharmadi et al., 2009, 2013). Given that locations with such high elasmobranch landings (tonnes of fish put on the dock) are rare, Indonesia is lucrative regarding opportune collections of parasites from high diversity of elasmobranchs (Dharmadi et al., 2009). No aporocotylid (Platyhelminthes: Digenea: Aporocotylidae Odhner, 1912) has been described from this region. Despite the fact that most of the major batoid lineages (Myliobatiformes, Rhinopristiformes, Torpediniformes) have been confirmed as aporocotylid hosts, only six batoid species (0.09% of the total batoids) have been reported as blood fluke hosts (Table 1). The only record from a skate (Rajiformes) is that of Bazikalova (1932), who reported an aporocotylid, which evidently remains innominate, infecting the lumen of the intestine of thorny skate, Amblyraja radiata (as Raja) (Donovan, 1808) Stehmann, 1973 (Rajiformes: Rajidae) (Bullard and Jensen, 2008). In comparison, of the 516 nominal shark species, only four (0.07%) have been reported as aporocotylid hosts (Table 1).
Table 1.
The blood flukes (Digenea: Aporocotylidae) infecting cartilaginous fishes (Chondrichthyes).
| Parasite | Host | Site of infection | Locality | Reference |
|---|---|---|---|---|
| Aetohemecus kirstenjensenae n. sp., n. gen. | banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 | heart | Makassar Strait, (01°12′55.20″S, 116°58′27.50″E), off Manggar, East Kalimantan, Borneo, Indonesia | present study |
| Achorovermis testisinuosus Warren and Bullard, 2020 | smalltooth sawfish, Pristis pectinata Latham, 1796 | heart | Eastern Gulf of Mexico, off Naples, Florida, USA | Warren et al. (2020) |
| Chimaerohemecus trondheimensis Van der land, 1967 | rabbit fish, Chimaera monstrosa Linnaeus, 1758 | dorsal aorta | NE Atlantic, off Bergen, Norway | Van der Land (1967); Lockyer et al., 2003b |
| spook fish, Hydrolagus mitsukurii (Jordan and Snyder, 1904) Nakaya, 1984 | dorsal aorta and postcardinal vein around kidney | Saruga Bay, Japan | Kamegai et al., 2002 | |
| Electrovermis zappum Warren and Bullard, 2019 | lesser electric ray, Narcine bancroftii (Griffith and Smith, 1834) Carvalho, 2001 | heart | Gulf of Mexico, off Fort Morgan, Alabama, USA | Warren and Bullard (2019) |
| Gymnurahemecus bulbosus Warren, Ruiz, Whelan, Kritsky, and Bullard, 2019 | smooth butterfly ray, Gymnura micrura (Bloch and Schneider, 1801) Uyeno, 1983 | heart | Gulf of Mexico, off Mobile, Alabama, USA | Warren et al. (2019) |
| Homestios janinecairae n. sp., n. gen. | banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 | heart | Java Sea, (03°52′28.00″S, 114°36′37.00″E), off Takisung, South Kalimantan, Borneo, Indonesia | present study |
| Hyperandrotrema cetorhini Maillard and Ktari, 1978 | basking shark, Cetorhinus maximus (Gunnerus, 1765) Springer, 1973 | circulatory system; heart | Mediterranean Sea, off Tunisia; Oslofjorden, Norway; North Sea, off Montrose, Scotland | Malliard and Ktari, 1978; Smith (1972) |
| Hyperandrotrema walterboegeri Orélis-Ribeiro and Bullard, 2013 | shortfin mako shark, Isurus oxyrinchus Rafinesque, 1810 | luminal surface (endocardium) of heart atrium and ventricle | Viosca Knoll, northern Gulf of Mexico, 123 km south/southwest of Dauphin Island, Alabama, USA | Orélis-Ribeiro et al. (2013) |
| Myliobaticola richardheardi Bullard and Jensen, 2008 | Atlantic stingray, Hypanus sabinus (Lesueur, 1824) Last, Manjaji-Matsumoto, Naylor, and White, 2016 | intertrabecular spaces of heart | Deer Island, Mississippi Sound, Northern Gulf of Mexico off Biloxi, Mississippi, USA | Bullard and Jensen (2008) |
| Ogawaia glaucostegi Cutmore, Cribb, and Yong, 2018 | giant shovelnose ray, Glaucostegus typus (Anonymous [Bennett], 1830) Compagno, Last, Stevens, and Alava, 2005 | valves of conus arteriosus; ventricle | Moreton Bay, Queensland, Australia | Cutmore et al. (2018) |
| Orchispirium heterovitellatum Madhavi and Hanumantha Rao, 1970 | Bengal whipray, Brevitrygon imbricata (Bloch and Schneider, 1801) Last, Manjaji-Matsumoto, Naylor, and White, 2016 | mesenteric blood vessels | Western Bay of Bengal, waters off Waltair, India | Madhavi and Hanumantha Rao, 1970 |
| Selachohemecus benzi Bullard, Overstreet, and Carlson, 2006 | blacktip shark, Carcharhinus limbatus (Valenciennes, 1839) Compagno, 1973 | heart | Apalachicola Bay, Florida, USA; Northern Gulf of Mexico, off Mississippi, USA | Bullard et al. (2006) |
| Selachohemecus olsoni Short, 1954 | Atlantic sharpnose shark, Rhizoprionodon terraenovae (Richardson, 1837) Springer, 1964 | heart | Alligator Harbor, Florida, USA; Apalachicola Bay, Florida, USA; Mississippi Sound, Mississippi, USA | Short (1954); Bullard et al. (2006) |
The aporocotylids that infect chondrichthyans comprise 11 spp. of nine genera (Table 1) and have large C-shaped tegumental spines (infecting sharks, a ray, and a chimaera) or lack spines (infecting batoids [except Gymnurahemecus bulbosus Warren, Ruiz, Whelan, Kritsky, and Bullard, 2019]) (Short, 1954; Van der Land, 1967; Madhavi and Hanumantha Rao, 1970; Maillard and Ktari, 1978; Bullard et al., 2006; Bullard and Jensen, 2008, Orélis-Ribeiro et al., 2013; Cutmore et al., 2018; Warren et al., 2019, 2020; Warren and Bullard, 2019). Further, aporocotylids infecting batoids (except G. bulbosus) have a curving testis (Madhavi and Hanumantha Rao, 1970; Bullard and Jensen, 2008; Cutmore et al., 2018; Warren and Bullard, 2019; Warren et al., 2020). The aporocotylids infecting bony fishes comprise ~155 spp. of 32 genera and differ by having transverse rows of tegumental spines or rosethorn-shaped spines (McIntosh, 1934; Bullard, 2013) or by lacking spines (Truong and Bullard, 2013; Orélis-Ribeiro and Bullard, 2015). Species of Plehniella infect the body cavity of catfishes, lack spines, and have a star-shaped intestine (Truong and Bullard, 2013; Orélis-Ribeiro and Bullard, 2015). The current separation of these two morphologically distinct lineages of fish blood flukes is further represented in nucleotide-based phylogenetic studies using the large subunit of ribosomal DNA (28S rDNA) (Warren et al., 2019; Warren and Bullard, 2019).
Herein, we describe two new species of fish blood flukes infecting the heart of two banded eagle rays, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 (Myliobatiformes: Myliobatidae) from Borneo, Indonesia and propose two new genera to accommodate each one. The present report comprises the first record of an aporocotylid infection from Indonesia and from an eagle ray (Myliobatidae) as well as the first record of a digenean infection for any species of Aetomylaeus. Further, we provide a key to all fish blood flukes that infect chondrichthyans (KEY).
2. Materials and methods
2.1. Specimen collection and preparation
One hundred and twenty specimens of 25 species of sharks and rays (21 rays, four sharks) were collected from 26 November 2006–4 August 2008 using gill nets and commercial trawls (Koch et al., 2012). Herein, we report the parasitological results for the banded eagle ray (Aetomylaeus nichofii) only. During collection, on 2 December 2006 and 3 August 2008, the hearts from two banded eagle rays (Aetomylaeus nichofii) in Takisung, South Kalimantan, Borneo (03°52′28.00″S, 114°36′37.00″E) and Manggar, East Kalimantan, Borneo (01°12′55.20″S, 116°58′27.50″E), Indonesia, respectively, were collected. At necropsy, the heart and spiral intestine were excised intact (heart bisected, spiral valve opened). Hearts and half of the spiral valve was placed in sampled bags and fixed with 10% neutral buffered formalin (nbf). From 2008 to 7 July 2020 the hearts (including the two hearts from the banded eagle rays) of all 120 shark and ray specimens were examined with the aid of a Meiji Techno RZ dissecting microscope to isolate fluke specimens for morphology. The hearts were teased apart with forceps to reveal adult aporocotylids, and sediment from the heart was examined using sedimentation method using a plastic cylinder.
Adult flukes (n = 5) were transferred to vials filled with 10% nbf, rinsed with distilled water, cleaned with fine brushes to remove any host tissue or debris, stained overnight in Van Cleave's hematoxylin with several additional drops of Ehrlich's hematoxylin, dehydrated using an ethanol series, cleared in clove oil, and permanently mounted in Canada balsam. Drawings were made with Leica DM 2500 and Leica DMR (Leica, Wetzler, Germany) microscopes each equipped with differential interference contrast (DIC), measured using an ocular micrometer, and illustrated using a drawing tube. Measurements are reported in micrometres (μm) as the range followed by the mean and sample size in parentheses. Scientific names, including taxonomic authorities and dates, for fishes follow Eschmeyer et al. (2016). Morphological terms and nomenclature for aporocotylids follows Bullard and Jensen (2008), Orélis-Ribeiro et al. (2013), Warren et al. (2019), Warren et al. (2020). Type and voucher materials of the new species were deposited in the National Museum of Natural History's Invertebrate Zoology Collection (USNM, Smithsonian Institution, Washington, D. C.).
3. Results
3.1. Aetohemecus n. gen (Figs. 1–2)
Fig. 1-2.
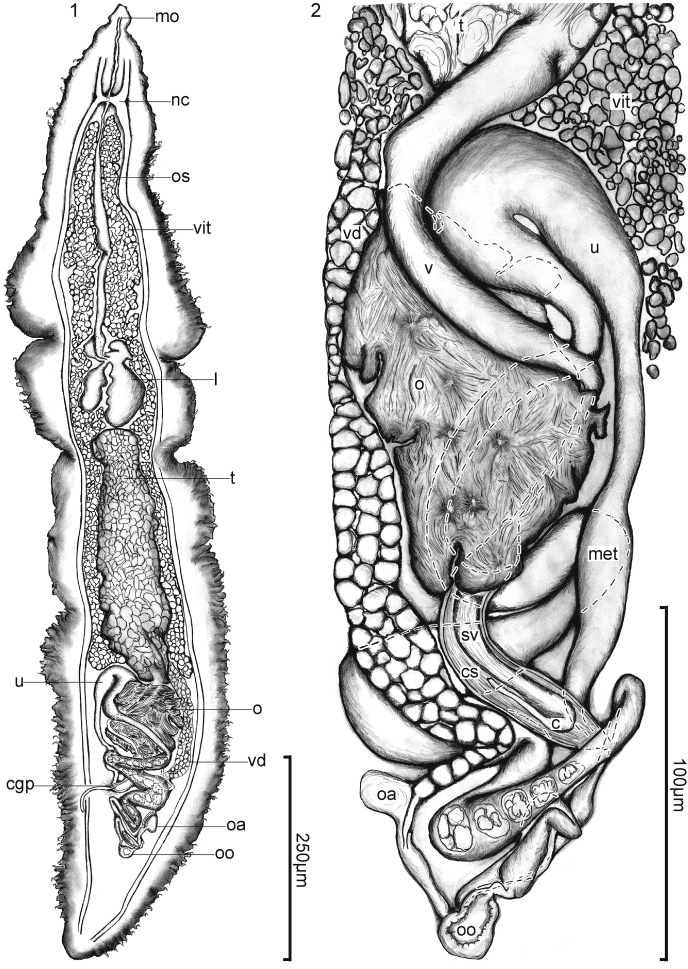
Aetohemecus kirstenjensenae Warren and Bullard n. gen., n. sp. (Digenea: Aporocotylidae) from the heart of the banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 (Myliobatiformes: Myliobatidae). (1) Body of holotype (USNM No. 1642775), dorsal view. Bar = 250 μm. (2) Genitalia, paratype (USNM No. 1642776), ventral view. Bar = 100 μm. Mouth (mo), nerve commissure (nc), oesophagus (os), vitellarium (vit), intestine (i), testis (t), uterus (u), metraterm (met), ovary (o), vas deferens (v), seminal vesicle (sv), cirrus sac (cs), cirrus (c), vitelline duct (vd), common genital pore (cgp), oviducal ampullae (oa), and oötype (oo).
3.1.1. Generic diagnosis
Body 4–6 × longer than wide, dorsoventrally flattened, ventrally concave, having anterior and posterior ends tapering equally, spinous; lateral tegumental spines C-shaped, directed ventrally, each on a muscular peduncle, distributing in a single ventrolateral column, not continuous anteriorly nor posteriorly. Rosethorn-shaped spines lacking. Nervous system comprising paired lateral nerve cords. Anterior sucker aspinous, lacking peduncle, diminutive, occupying space between anterior-most lateral tegumental spines. Mouth on mid-ventral surface of anterior sucker. Pharynx not evident. Oesophagus extending sinuously posteriad along mid-line for 1/3 of body length. Intestinal caeca X- or H-shaped, connecting to oesophagus ventrally, lacking diverticulae, posterior caeca terminating in anterior half of body. Testis single, medial, occupying middle 1/3 of body. Auxiliary external seminal vesicle lacking. Cirrus-sac present, enveloping internal seminal vesicle and cirrus. Ovary medial, ventral to ascending uterus, post-caecal, post-testicular; post-ovarian space comprising 1/6–1/4 of body length. Oviducal ampulla present. Laurer's canal absent. Oötype medial, posterior to genitalia, comprising an inconspicuous ovoid chamber. Uterus extending anterior to ovary; uterine eggs irregular, thin-shelled. Vitellarium follicular, symmetrical posteriorly, filling space between nerve commissure to ovary; common vitelline collecting duct extending from dextral branch of vitellarium. Common genital pore dorsal, post-gonadal, anterior to level of oötype.
3.1.2. Differential diagnosis
Body 4–6 × longer than wide; lateral tegumental spines C-shaped, directed ventrally, each on a muscular peduncle, distributing in a single ventrolateral column, not continuous anteriorly nor posteriorly. Intestinal caeca X- or H-shaped, posterior caeca terminating in anterior half of body. Testis single, medial, occupying middle 1/3 of body. Ovary medial, ventral to ascending uterus, post-caecal, post-testicular; post-ovarian space comprising 1/6–1/4 of body length. Oviducal ampulla present. Laurer's canal absent. Oötype medial, posterior to genitalia, comprising an inconspicuous ovoid chamber. Uterus extending anterior to ovary. Common vitelline collecting duct extending from dextral branch of vitellarium. Common genital pore post-gonadal, anterior to level of oötype.
3.1.3. Taxonomic summary
Type-species: Aetohemecus kirstenjensenae n. sp.
Type host: Banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 (Myliobatiformes: Myliobatidae).
Etymology: The Greek “Aeto” meaning eagle and “heme” meaning blood refers to the type species infecting the blood of an eagle ray.
3.2. Aetohemecus kirstenjensenae n. sp. (Figs. 1–2)
3.2.1. Diagnosis of adult specimens (based on four whole-mounted specimens; USNM coll. nos. 1642775-1642778)
Body 1000–1320 (1121 ± 152, 4) long, 235–245 (240 ± 4, 4) at greatest width, 4–6 × longer than wide (Fig. 1). Lateral tegumental spines 93–120 (106 ± 12, 3) per side of body or a total of 189–228 (210 ± 20, 3), ending 18–38 (27 ± 10, 4) or 1–3% (2 ± 0.09, 3) of body length from posterior end of body, base slightly bifurcate at posterior margin, tissue not associated with base on anterior-most lateral tegumental spines (Fig. 1), approximately equal in size throughout length of body; lateral tegumental spines in anterior region 7–8 (7.7 ± 0.5, 4) long, 1–2 (1.6 ± 0.5, 4) wide; mid-body 5–7 (6 ± 1, 4) long, 1–2 (1.2 ± 0.4, 4) wide, and posterior region 4–5 (4.5 ± 0.5, 4) long, 1 (1, 9) wide (Fig. 1); peduncles supporting lateral tegumental spines approximately equal in size; anterior peduncles 7–8 (7.7 ± 0.5, 4) long, 3–5 (4 ± 0.8, 4) wide; mid-body and posterior peduncles 5–7 (6 ± 0.5, 4) long, 5–6 (5.5 ± 0.5, 4) wide.
Ventrolateral nerve-cord 924–1120 (995 ± 109, 3) long, 10–15 (12 ± 2, 4) wide near mid-body at widest level, 50–58 (53 ± 4, 4) from body margin. Primary commissure perpendicular to mid-line of body, connecting ventrolateral nerve-cords, 95–115 (105 ± 8, 4) or 8%–10% (9% ± 1, 4) of body length from anterior end of body, 25–28 (26 ± 1.5, 4) across width of worm, 10–18 (12 ± 4, 4) in breadth; (Fig. 1); secondary commissure and nerve cords not evident in whole mounts.
Mouth 1–3 (2 ± 1.2, 3) in diameter, 1–8 (4 ± 4, 3) from terminal end of anterior sucker (Fig. 1). Oesophagus 325–425 (379 ± 43, 4) in total length or 30%–37% (34% ± 0.03, 4) of body length, 18–23 (21 ± 2, 4) in maximum width, ventral to primary nerve-commissure (Fig. 1); oesophageal wall thickening from 1 to 2 (1.5 ± 0.5, 4) near mouth to 5–10 (6.3 ± 2.5, 4) posteriorly. Caecal bifurcation 281–415 (355 ± 56, 4) or 21%–38% (32% ± 0.07, 4) of body length from anterior body end; anterior caeca 22–39 (31 ± 8, 4) in mean length or 2%–4% of body length, 21–27 (4) in mean width; posterior caeca 38–68 (57 ± 13, 4) in mean length or 4%–6% of body length, 19–34 (4) in mean width (Fig. 1).
Testis 230–270 (250 ± 20, 3) long or 23%–25% (24% ± 0.01, 3) of body length, 53–85 (74 ± 15, 4) wide or 22%–35% (31% ± 0.06, 4) of body width, 3–5 (4 ± 0.9, 3) × longer than wide, post-caecal (Fig. 1). Post-testicular space 335–380 (362 ± 20, 4) long or 29%–36% (33% ± 0.03, 4) of body length. Vasa efferentia comprising interconnecting meshwork of fine ducts entwined throughout testicular tissue, 8 (1) in diameter; vas deferens 118–210 (152 ± 40, 4) long, 8–18 (13 ± 4, 4) wide, emanating from postero-ventral portion of testis, curving dextrad ventral to anterior portion of ovary before curving mediad ventral to ovary to connect with cirrus-sac (Fig. 2). Cirrus-sac 53–88 (64 ± 16, 4) long, having extremely thin wall approximately 1–2 (2 ± 0.5, 4) thick, including seminal vesicle and cirrus; seminal vesicle 45–75 (55 ± 14, 4) long, 13–15 (14 ± 1, 4) wide, filling breadth of cirrus sac, curving dextrad, narrowing and opening dorsal (Fig. 2); everted cirrus long, 61 long or 1.8 × seminal vesicle length, 4 wide (Fig. 1); internal cirrus 27 long or 37% of seminal vesicle length, 3 wide (Fig. 2). Common genital pore 178–298 (227 ± 50, 4) or 18%–23% (20% ± 2, 4) of body length from posterior end of body, 50–58 (55 ± 3, 4) from sinistral body margin, 113–130 (123 ± 7, 4) from dextral body margin (Figs. 1 and 2).
Fig. 3-4.
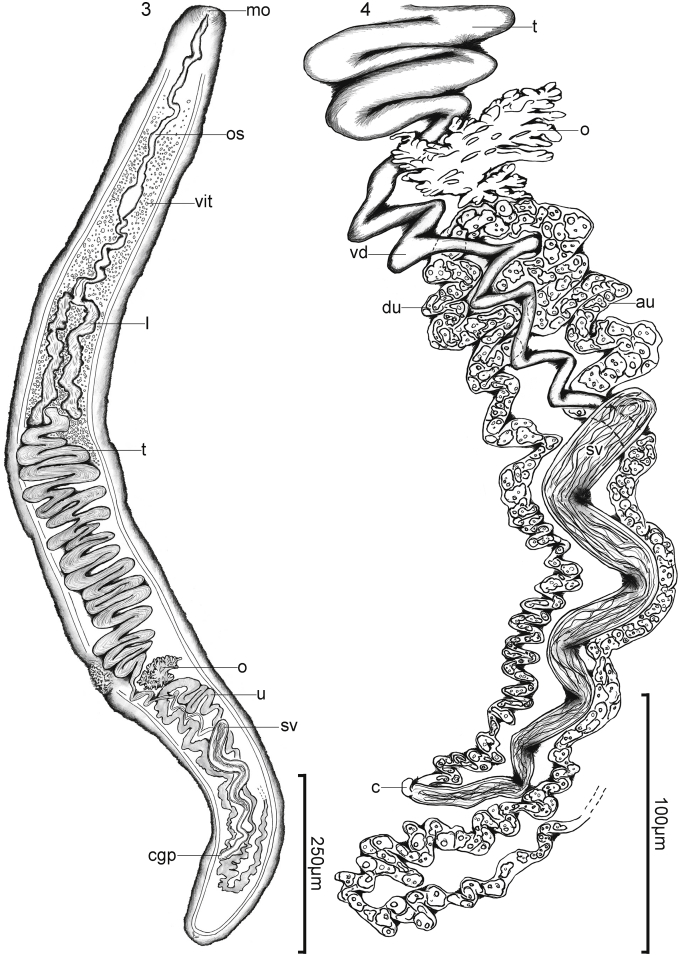
Homestios janinecairae Warren and Bullard n. gen., n. sp. (Digenea: Aporocotylidae) from the heart of the banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 (Myliobatiformes: Myliobatidae). (3) Body of holotype (USNM No. 1642774), dorsal view. Bar = 250 μm. (4) Genitalia of holotype (USNM No. 1642774), dorsal view. Bar = 100 μm. Mouth (mo), oesophagus (os), vitellarium (vit), intestine (in), testis (t), ovary (ov), vas deferens (vd), uterus, (u), ascending uterus (au), descending uterus (du), seminal vesicle (sv), cirrus (c), and common genital pore (cgp).
Ovary medial, lobed, 90–118 (102 ± 14, 4) long or 7%–11% (9% ± 0.01, 4) of body length, 63–100 (77 ± 16, 4) wide or 26%–42% (32% ± 0.07, 4) of body width, 0.9–1.6 (1.4 ± 0.3, 4) × longer than wide, post-caecal, post-testicular; post-ovarian space 205–253 (234 ± 20, 4) long or 18%–24% (21% ± 0.02, 4) of body length (Figs. 1 and 2). Oviduct and Laurer's canal not evident; oviducal ampulla 10–20 (15 ± 7, 2) long, 15 (2) wide (Fig. 2). Oötype 10–15 (13 ± 3, 3) in diameter, posterior to all genitalia (Figs. 1 and 2). Vitellarium having follicles compacted in dense lobules, occupying space dorsal and lateral to oesophagus, caeca, and testis; common collecting duct 163–223 (196 ± 25, 4) long, 13–20 (17 ± 3, 4) wide.
Uterus extending anteriad from oötype, 185–223 (207 ± 18, 4) long or 17–20 (19 ± 0.01, 4) of body length, 58–93 (74 ± 16, 4) wide, with wall 1 (4) thick; ascending portion extending sinuously anteriad and dorsal to seminal vesicle, common vitelline duct, and ovary before extending anterior to ovary and connecting to descending portion, containing eggs in all (4) specimens (Figs. 1 and 2); descending portion 80–105 (90 ± 11, 4) long or 36%–49% (44% ± 6, 4) of ascending uterus length, extending posteriad before connecting with metraterm; metraterm 30–58 (47 ± 14, 4) long or 11%–19% (16% ± 0.03, 4) of descending uterus, 8–15 (12 ± 3, 4) wide, comprising distal-most portion of female reproductive tract, demarcated from descending uterus by obvious constriction (Fig. 2). Uterine eggs 10–13 (12 ± 1, 4) in diameter or 40%–67% (54% ± 0.1, 4) of uterus width, containing a large spheroid body plus several smaller, dense lipid-like bodies, with thin shell (Fig. 2).
3.2.2. Taxonomic summary
Type and only reported host: Banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 (Myliobatiformes: Myliobatidae).
Site in host: Heart lumen.
Type locality: Off Manggar, Makassar Strait, (01°12′55.20″S, 116°58′27.50″E), East Kalimantan, Borneo, Indonesia.
Prevalence and intensity of infection: 1 (prevalence = 100%) banded eagle ray sampled on 3 August 2008 was infected by 4 specimens of A. kirstenjensenae.
Specimens deposited: Holotype (USNM 1642775), paratypes (USNM 1642776, 1642777, 1642778).
Etymology: The specific epithet “kirstenjensenae” honors Prof. Kirsten Jensen (Senior Curator of Invertebrate Zoology for the Biodiversity Institute and Natural History Museum; Associate Chair of Ecology and Evolutionary Biology at The University of Kansas, Lawrence, Kansas, USA) for her contributions to our knowledge of elasmobranch parasites.
3.2.3. Taxonomic remarks
Aetohemecus kirstenjensenae is most similar to Selachohemecus benzi Bullard, Overstreet, and Carlson, 2006, Selachohemecus olsoni Short, 1954, and G. bulbosus by the combination of having a single row of C-shaped lateral tegumental spines, posterior caeca terminating in the middle 1/3 of the body, and an ascending and descending uterus (Short, 1954; Bullard et al., 2006; Warren et al., 2019). Further, the new species is similar to G. bulbosus by having an oviducal ampulla and an oötype positioned posterior to the genitalia. Aetohemecus kirstenjensenae differs from S. olsoni and S. benzi by the combination of having an extruded cirrus that is as long as the seminal vesicle, an oviducal ampulla, a uterus that extends anterior to the ovary, and an oötype that is posterior to the genitalia. Aetohemecus kirstenjensenae differs from G. bulbosus by having a X- or H-shaped intestine, a uterus that extends anterior to the ovary, and an extruded cirrus that is as long as the seminal vesicle as well as by lacking an oesophogeal bulb (KEY) (Warren et al., 2019).
Aetohemecus kirstenjensenae further resembles several other fish blood flukes that infect chondrichthyans having large C-shaped spines (Chimaerohemecus trondheimensis Van der Land, 1967; Hyperandrotrema cetorhini Maillard and Ktari, 1978; Hyperandrotrema walterboegeri Orélis-Ribeiro and Bullard, 2013) (Table 1). The new species differs from C. trondheimensis and Hyperandrotrema spp. by the combination of having a single row of C-shaped lateral tegumental spines (vs. two rows or a field of spines), an X- or H-shaped intestine (vs. inverse U-shaped) that terminates in the middle 1/3 of the body (vs. the posterior end of the body), a post-caecal ovary (vs. intercaecal), and post-caecal common genital pore (vs. intercaecal) (KEY) (Van der Land, 1967; Maillard and Ktari, 1978; Orelis-Ribeiro et al., 2013).
Further, A. kirstenjensenae differs from other fish blood flukes that infect chondrichthyans in that those species lack lateral tegumental spines (Achorovermis testisinuosus Warren and Bullard, 2020; Electrovermis zappum Warren and Bullard, 2019; Myliobaticola richardheardi Bullard and Jensen, 2008; Ogawaia glaucostegi Cutmore, Cribb, and Yong, 2018; and Orchispirium heterovitellatum Madhavi and Hanumantha Rao, 1970) (Table 1). Until now, species of Selachohemecus were the only aporocotylids infecting chondrichthyans reported to have a X- or H-shaped intestine (KEY). Regarding the hosts for blood flukes, A. kirstenjensenae is the only nominal blood fluke reported from an eagle ray (Myliobatidae).
3.3. Homestios janinecairae n. gen (Figs. 3–4)
3.3.1. Generic diagnosis
Body 9 × longer than wide, dorsoventrally flattened, muscular, aspinous. Rosethorn-shaped spines absent. Nervous system comprising paired lateral nerve cords. Anterior sucker aspinous, lacking peduncle, diminutive. Mouth subterminal. Pharynx absent. Oesophagus extending sinuously posteriad along midline for ≤1/4 of body length; posterior oesophageal swelling present. Intestine inverse U-shaped, asymmetrical; posterior caeca shorter than oesophagus, connecting to oesophagus ventrally, lacking diverticulae, terminating in anterior half of body. Testis single, medial, curving, lacking lobed margins. Vas deferens long, > 70% of seminal vesicle length, extending posteriad from testis. Cirrus-sac present, enveloping internal seminal vesicle and cirrus. Internal seminal vesicle distinct, longer than vas deferens. Extruded cirrus short, < 5% of seminal vesicle length. Auxiliary external seminal vesicle absent. Common genital pore dorsal, post-gonadal. Ovary medial, post-caecal, wholly anterior to uterus; post-ovarian space comprising 1/3 of body length. Vitellarium follicular, diffuse, asymmetrical. Laurer's canal absent. Oötype indistinct. Uterus post-gonadal, not extensively convoluted, extending posteriad before curving anteriad extending anterior to posterior margin of ovary before crossing midline and extending posteriad; uterine eggs irregular. Uterine seminal receptacle absent. Excretory vesicle small, medial, with arms, visible in posterior most region of body.
3.3.2. Differential diagnosis
Body approx. 9 × longer than wide; aspinous, lacking lateral tubercles. Anterior sucker aspinous, lacking peduncle, diminutive. Pharynx absent. Medial and posterior oesophageal swelling present. Intestine inverse U-shaped, asymmetrical; posterior caeca terminating in anterior half of body, lacking diverticulae. Testis single, lacking lobed margins, curving <40 times. Vas deferens long, > 70% of seminal vesicle length. Internal seminal vesicle distinct, longer than vas deferens, enveloped by cirrus sac. Extruded cirrus short, < 5% of seminal vesicle length. Common genital pore post-caecal, post-gonadal. Ovary medial, post-caecal, dorsal to posterior portion of testis, wholly anterior to uterus. Laurer's canal absent. Uterus post-gonadal, uterus that extends anteriad beyond the level of the seminal vesicle, not extensively convoluted.
3.3.3. Taxonomic summary
Type-species: Homestios janinecairae n. sp. (Digenea: Aporocotylidae).
Type host: Banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 (Myliobatiformes: Myliobatidae).
Etymology: The Greek “Homestios” meaning ‘dwelling with’ refers to the same batoid species hosting the type species for both genera.
3.4. Homestios janinecairae n. sp. (Figs. 3–4)
3.4.1. Description of adult (based on a single whole-mounted specimen; USNM coll. no. 1642774)
Body 1305 long, 153 at greatest width, 9 × longer than wide (Fig. 3), muscular, tapering gradually until bluntly rounded. Nerve commissures not evident in whole-mount. Ventrolateral nerve-cords located 13 from lateral body margin, 5 in maximum width, becoming confluent posteriorly, 8 from posterior body margin. Anterior sucker, aspinous, centered on mouth. Mouth 3 in diameter, 3 from terminal end of body (Fig. 3). Oesophagus 408 in total length or 31% of body length, 18 in maximum width (Fig. 3). Caecal bifurcation 163 or 12% of body length from anterior body end; caeca extending posteriad in parallel, asymmetrical, dextral caecum 153 long or 11% of body length, sinistral caecum 178 long or 14% of body length, 22 in mean width or 14% of body width (Fig. 3); post-caecal space 765 long or 59% of body length (Fig. 3).
Testicular mass 363 long or 28% of body length, 103 wide, occupying 67% of body width, 4 × longer than wide, dorsal to caeca, curving 33 times (Fig. 3), narrowing and becoming confluent with vas deferens. Post-testicular space 418 long or 32% of body length. Vas deferens 181 long or 71% of seminal vesicle length, 4 wide, emanating from postero-ventral portion of testis, meandering sinistral to ovary and posteriad between ascending and descending uterine portions before connecting to the cirrus sac (Figs. 3 and 4). Cirrus-sac having thin wall 1 thick, including seminal vesicle and cirrus; seminal vesicle extending sinuously posteriad, 258 long or 20% of body length, 20 wide or 13% of body width, 13 × longer than wide, running between ascending and descending portions of the uterus, curving 6 times (Figs. 3 and 4), narrowing and curving sinistrally towards body margin (Figs. 3 and 4); cirrus short, 10 long or 3% of seminal vesicle length, 8 wide, cirrus pore 5 in diameter (Fig. 4). Common genital pore 120 or 9% of body length from posterior end of the body, 18 from sinistral body margin, 75 from dextral body margin (Figs. 3 and 4).
Ovary medial, small, irregular in shape, not extending lateral to nerve cords, 38 long or 2% of body length, 70 wide or 46% of body width, 2 × wider than long; post-ovarian space 380 long or 29% of body length (Figs. 3 and 4). Oviduct, vitelline duct, and oötype indistinct. Laurer's canal not observed. Ascending uterus 318 long, 15 in maximum width (Fig. 4), extending anteriad arching dorsally over medial portion of vas deferens before connecting to descending portion; descending uterus extends posteriorly 238 long or 75% of ascending uterus length, 13 in maximum width; post-uterine space 75 long or 6% of body length (Fig. 3). Uterine eggs13 long, 6 wide or 46% of uterus width, containing many small dense bodies, with thin shell (Fig. 4). Excretory vesicle 8 long, < 1 wide, with arms (Fig. 3).
3.4.2. Taxonomic summary
Type and only reported host: Banded eagle ray, Aetomylaeus nichofii (Bloch and Schneider, 1801) Capapé and Desoutter, 1979 (Myliobatiformes: Myliobatidae).
Site in host: Heart lumen.
Type locality: Off Takisung, Java Sea, (03°52′28.00″S, 114°36′37.00″E), South Kalimantan, Borneo, Indonesia.
Prevalence and intensity of infection: 1 (prevalence = 100%) banded eagle ray sampled on 2 December 2006 was infected by 1 specimen of H. janinecairae.
Specimens deposited: Holotype (USNM 1642774).
Etymology: The specific epithet “janinecairae” honors Prof. Janine N. Caira (University of Connecticut, Storrs, Connecticut USA) for her contributions to our knowledge of elasmobranch parasites.
3.4.3. Taxonomic remarks
Homestios janinecairae is most similar to M. richardheardi and all other nominal fish blood flukes infecting batoids (except G. bulbosus and A. kirstenjensenae) by the combination of having an aspinous, diminutive anterior sucker, an asymmetrical inverse U-shaped intestine, a curving testis, and a post-caecal common genital pore as well as by lacking any spine along the lateral tegument (Madhavi and Hanumantha Rao, 1970; Bullard and Jensen, 2008; Cutmore et al., 2018; Warren and Bullard, 2019; Warren et al., 2020). It differs from M. richardheardi by the combination of having >30 testicular curves (vs. 9–10), a vas deferens that is >70% of the seminal vesicle length, a seminal vesicle that curves 6 times (vs. 9–10), and a uterus that extends anteriad beyond the level of the seminal vesicle (KEY) (Bullard and Jensen, 2008). Homestios janinecairae differs from all other fish blood flukes infecting batoids (except G. bulbosus and A. kirstenjensenae) by having a body 9 × longer than wide, an oesophagus that is 1/3 of body length, >30 testicular curves, a vas deferens that is >70% of the seminal vesicle length, a sharply curved seminal vesicle that is 1/5 of body length, a cirrus that is <5% of seminal vesicle length, an ovary that is wider than long, and a uterus that extends anteriad beyond the level of the seminal vesicle (KEY). The only other species to have a uterus that extends anteriad beyond the level of the seminal vesicle is O. heterovitellatum and O. glaucostegi (Madhavi and Hanumantha Rao, 1970; Cutmore et al., 2018). Orchispirium heterovitellatum is unique by having lateral tubercles along the tegument, posterior caeca that extend into the posterior half of the body, and a testis that is intercecal and bearing lobes along the margin (Madhavi and Hanumantha Rao, 1970). The new species differs from O. glaucostegi by having a uterus that extends anteriad beyond the terminal margin of the testis (vs. remaining posterior to the ovary) before folding ventrally and continuing posterior to the common genital pore. Further, O. glaucostegi, E. zappum, and A. testisinuosus differ by having a body that is > 15 × longer than wide (vs. 9 in the new species and M. richardheardi) (KEY) (Cutmore et al., 2019; Warren and Bullard, 2019; Warren et al., 2020).
KEY. Key to the identification of fish blood flukes (Digenea: Aporocotylidae) infecting chondrichthyans
| 1a. Body spinous, lateral tegumental spines (LTSs) C-shaped | 2 |
| 1b. Body aspinous | 8 |
| 2a. Intestinal caeca X-or H-shaped | 3 |
| 2b. Intestinal caeca inverse U-shaped | 5 |
| 3a. Oviducal ampullae present; oötype posterior to all genitalia | Aetohemecus kirstenjensenae n. gen, n. sp. |
| 3b. Oviducal ampullae absent; oötype anterior to common genital pore | 4 |
| 4a. Body minute (<1.4 mm long), LTSs numbering > 170 per side of body | Selachohemecus olsoni |
| 4b. Body large (≥1.4 mm long), LTSs < 100 per side of body | Selachohemecus benzi |
| 5a. LTSs distributed in a single column, large oesophogeal bulb present | Gymnurahemecus bulbosus |
| 5b. LTSs distributed in multiple columns or lateral field | 6 |
| 6a. Caeca short, terminating at level of genital pores | Chimaerohemecus trondheimensis |
| 6b. Caeca elongate, terminating posterior to genitalia | 7 |
| 7a. Body 2 × longer than wide, mid-body LTSs <20 μm long | Hyperandrotrema cetorhini |
| 7b. Body 7–8 × longer than wide, mid-body LTSs ≥25 μm long | Hyperandrotrema walterboegeri |
| 8a. Body margin having lateral tubercles | Orchispirium heterovitellatum |
| 8b. Body margin lacking lateral tubercles | 9 |
| 9a. Body minute (<1 mm long), testis curving < 15 times | Myliobaticola richardheardi |
| 9b. Body elongated, testis curving >15 times | 11 |
| 11a. Testis curving <50 times | 12 |
| 11b. Testis curving >50 times | 13 |
| 12a. Seminal vesicle >40% of body width; uterus not extending anterior to seminal vesicle | Electrovermis zappum |
| 12b. Seminal vesicle <20% of body width; uterus extending anterior to seminal vesicle | Homestios janinecairae |
| 13a. Testis curving <80 times; uterus extending anterior to posterior margin of testis | Ogawaia glaucostegi |
| 13b. Testis curving >100 times; uterus wholly posterior to ovary | Achorovermis testisinuosus |
4. Discussion
4.1. Host-switching
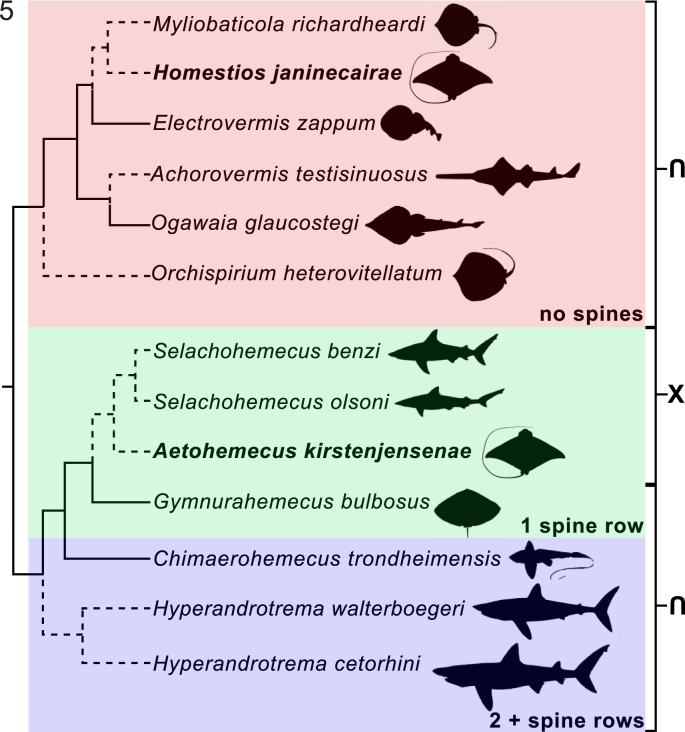
Host-switching is emerging as a key phenomenon to understanding the natural history of fish blood flukes. Aetomylaeus nichofii is infected by blood flukes of two species in two genera; one (A. kirstenjensenae) could be the result of a host-switching event. Schistosomes have been documented to switch intermediate hosts (Lockyer et al., 2002) but fish and turtle blood fluke host-switching are rarely discussed (Bullard et al., 2019; Warren et al., 2019). The 13 nominal species of chondrichthyan blood flukes can be split into two morphological groups: (i) those having C-shaped spines (H. cetorhini, H. walterboegeri, C. trondheimensis, G. bulbosus, A. kirstenjensenae, S. benzi, and S. olsoni) and (ii) aspinous species (O. heterovitellatum, O. glaucostegi, A. testisinuosus, H. janinecairae, M. richardheardi, and E. zappum) (Fig. 5). Not including the new taxa described herein, these two groups were recovered as monophyletic in a recent phylogenetic analysis (Warren and Bullard, 2019). We expected that batoids would host a fish blood fluke resembling H. janinecairae (aspinous, inverse U-shaped intestine, curving testis) but A. kirstenjensenae (with C-shaped spines) was unexpected (because it has a C-shaped tegumental spines, a X-shaped intestine, a single testis without curves, and an oötype posterior to the genitalia) and likely does not share a common ancestor with other batoid blood flukes, similar to G. bulbosus (Fig. 5).
Fig. 5.
Phylogenetic relationships of chondrichthyan blood flukes based on morphological characters (tegumental spines, shape of intestines). Host affiliations are included. Dashed lines indicate species with no nucleotide sequences. Boxes indicate spine rows: blue = 2 + spine rows, green = 1 spine row, and red = no spines. Shape of the intestine (⋂) inverse U-shaped and (X) X-shaped. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4.2. The intestinal morphology of chondrichthyan blood flukes
The intestinal morphology of fish blood flukes could inform ancestry. Aetohemecus kirstenjensenae is the only batoid blood fluke that has a X-shaped intestine, similar to S. olsoni and S. benzi. (Fig. 1-2, Fig. 5). Because of this, the new species likely shares a recent common ancestor with Selachohemecus spp. (Bullard et al., 2006). However, A. kirstenjensenae also has an oötype that is posterior to all genitalia, like that of G. bulbosus, which has an inverse U-shaped intestine (Warren et al., 2019). The most recent phylogenetic analysis available places G. bulbosus sister to C. trondheimensis, which are two clearly, morphologically different lineages of fish blood flukes. We predict that A. kirstenjensenae will clade with Selachohemecus spp. and that they share a recent common ancestor with G. bulbosus (Fig. 5). This prediction is based on the single row of C-shaped spines, which is a shared trait by all species of these 3 genera (Short, 1954; Bullard et al., 2006; Warren et al., 2019).
Other than Selachohemecus spp. and A. kirstenjensenae, fish blood flukes infecting chondrichthyans have inverse U-shaped intestines similar to that of several fish blood flukes that infect bony fishes (Acipensericola glacialis Warren and Bullard, 2017; Acipensericola petersoni Bullard, Snyder, Jensen, and Overstreet, 2008; Paracardicoloides yamaguti Martin, 1974) and turtle blood flukes (Spirorchis spp.) (Martin, 1974; Bullard et al., 2008, 2019; Warren et al., 2017). Orélis-Ribeiro et al. (2017) recovered P. yamaguti sister to species of Elopicola Bullard, 2014 forming a clade that shares a recent common ancestor with A. petersoni using the second internal transcribed spacer of ribosomal DNA (ITS-2) region (Orélis-Ribeiro et al., 2017). This is significant because in 28S rDNA phylogenies species of Elopicola are recovered as sister to all other fish blood flukes that infect actinopterygians, with exception to sequences sourced from cercariae shed from freshwater snails (Cribb et al., 2017; Warren et al., 2019). Further, no other fish blood fluke that infects a bony fish (except Acipensericola spp. and P. yamagutii) has an inverse U-shaped intestine (Martin, 1974; Bullard et al., 2008; Warren et al., 2017). Because of this, we predict that the inverse U-shaped (two posterior caeca) intestine is pleisiomorphic but has evolved independently in several lineages: Plehniella spp. Szidat, 1951; Nomasanguinicola canthoensis Truong and Bullard, 2013, Cardicola spp. Short, 1953 (Short, 1953; Truong and Bullard, 2013; Orélis-Ribeiro and Bullard, 2015).
Declaration of competing interest
The authors herein have no conflict of interest.
Acknowledgments
We thank Prof. Janine N. Caira (University of Connecticut) and Prof. Kirsten Jensen (The University of Kansas) for sending hearts examined in this study and the collection of elasmobranchs from Borneo, Indonesia; Anna Phillips, Katie Ahlfield, and William Moser (Department of Invertebrate Zoology at the National Museum of Natural History) for ensuring the safe deposition of our type materials. This study was supported by Auburn University Vice President for Research and Economic Development, Southeastern Cooperative Fish Parasite and Disease Project (Alabama Department of Conservation and Natural Resources), Alabama Agriculture Experiment Station, and US Department of Agriculture. Earlier exploratory aspects of this project were supported by grants from the National Science Foundation.
References
- Bazikalova A. Investigations into the Parasitologyg Ofo Murmansk fish.] Sbornik Nauchno-Promyslovikh Rabot Na Murman. In: Mittelman] S.Y., editor. Moskva; Leningrad: 1932. pp. 136–153. Narkomsnab SSR Tsentral’nya Institut Rybnogo Khozyaistva. [Russian] [Google Scholar]
- Bullard S.A. Cardicola langeli sp. n. (Digenea: Aporocotylidae) from heart of sheepshead, Archosargus probatocephalus (Actinopterygii: Sparidae) in the Gulf of Mexico, with an updated list of hosts, infection sites and localities for Cardicola spp. Folia Parasitol. 2013;60:17–27. doi: 10.14411/fp.2013.003. [DOI] [PubMed] [Google Scholar]
- Bullard S.A., Jensen K. Blood flukes (Digenea: Aporocotylidae) of stingrays (Myliobatiformes: Dasyatidae): Orchispirium heterovitellatum from Himantura imbricata in the Bay of Bengal and a new genus and species of Aporocotylidae from Dasyatis sabina in the northern Gulf of Mexico. J. Parasitol. 2008;94:1311–1321. doi: 10.1645/GE-1498.1. [DOI] [PubMed] [Google Scholar]
- Bullard S.A., Overstreet R.M., Carlson J.K. Selachohemecus benzi n. sp. (Digenea: Sanguinicolidae) from the blacktip shark Carcharhinus limbatus (Carcharhinidae) in the northern Gulf of Mexico. Syst. Parasitol. 2006;63:143–154. doi: 10.1007/s11230-005-9010-x. [DOI] [PubMed] [Google Scholar]
- Bullard S.A., Snyder S.D., Jensen K., Overstreet R.M. New genus and species of Aporocotylidae (Digenea) from a basal actinopterygian, the American paddle-fish, Polyodon spathula, (Acipenseriformes: Polyodontidae) from the Mississippi Delta. J. Parasitol. 2008;94:487–495. doi: 10.1645/GE-1323.1. [DOI] [PubMed] [Google Scholar]
- Bullard S.A., Roberts J.R., Warren M.B., Dutton H.R., Whelan N.V., Ruiz C.F., Platt T.R., Tkach V.V., Brant S.V., Halanych K.M. Neotropical turtle blood flukes: two new genera and species from the Amazon river basin with a key to genera and comments on a marine-derived parasite lineage in South America. J. Parasitol. 2019;105:497–523. [PubMed] [Google Scholar]
- Cribb T.H., Chick R.C., O'Conner W., O'Conner S., Johnson D., Sewell K.B., Cutmore S.C. Evidence that blood flukes (Trematoda: Aporocotylidae ) of chondrichthyans infect bivalves as intermediate hosts: indications of an ancient diversification of the Schistosomatoidea. Int. J. Parasitol. 2017;47:885–891. doi: 10.1016/j.ijpara.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Cutmore S.C., Cribb T.H., Yong R.Q.-Y. Aporocotylids from batoid and elopomorph fishes from Moreton Bay, Queensland, Australia, including a new genus and species of blood fluke infecting the Giant shovelnose ray, Glaucostegus typus (Rhinopristiformes: Glaucostegidae) Parasitol. Int. 2018;67:768–775. doi: 10.1016/j.parint.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Dharmadi D., Fahmi F., White W. Biodiversity of sharks and rays in South-Eastern Indonesia. Ind. Fish. Res. J. 2009;15:17–28. [Google Scholar]
- Dharmadi D., Faizah R., Saduyah L. Shark longline fishery in Tanjungluar-East Lombok. Ind. Fish. Res. J. 2013;19:39–46. [Google Scholar]
- Eschmeyer W.N., Fricke R., Van der Laan R. 2016. Catalog of Fishes: genera, Species, References.http://researcharchive.calacademy.org/research/ichthyology/fishcatmain.asp 08 October 2020. [Google Scholar]
- Koch K.R., Jensen K., Caira J.N. Three new genera and six new species of Lecanicephalideans (Cestoda) from eagle rays of the genus Aetomylaeus Myliobatiformes: Mayliobatidae) from Northern Australia and Borneo. J. Parasitol. 2012;98:175–198. doi: 10.1645/GE-2798.1. [DOI] [PubMed] [Google Scholar]
- Last P.R., White T.W., de Carvalho M.R., Séret S.B., Stehmann M.F.W., Naylor G.J.P. first ed. Cornell University Press; Ithaca: 2016. Rays of the World. 790 pp. [Google Scholar]
- Lockyer A.E., Olson P.D., Østergaard P., Rollinson D., Johnston D.A., Attwood S.W., Southgate V.R., Horak P., Snyder S.D., Le T.H., Agatsuma T. 2003. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitol. 126, p.203. [DOI] [PubMed] [Google Scholar]
- Madhavi R., Hanumantha Rao K. Orchispirium heterovitellatum gen. et sp. nov. (Trematoda: Sanguinicolidae) from the ray fish, Dasyatis imbricatus Day, from Bay of Bengal. J. Parasitol. 1970;56:41–43. [Google Scholar]
- Maillard C., Ktari M.H. Hyperandrotrema cetorhini n. g., n. sp. (Trematoda, Sanguinicolidae) parasite du système circulatoire de Cetorhinus maximus (Selacii) Ann. Parasit. Hum. Comp. 1978;53:359–365. doi: 10.1051/parasite/1978534359. [DOI] [PubMed] [Google Scholar]
- Martin W.E. Paracardicoloides yamagutii gen. et sp. n. from an Australian eel (Trematoda: Sanguinicolidae) Proc. Helm. Soc. Wash. 1974;41:22–25. [Google Scholar]
- McIntosh A. A new blood trematode, Paradeontacylix sanguinicoloides n.g., n.sp. from Seriola lalandi with a key to the species of the family Aporocotylidae. Parsitol. 1934;26:463–467. [Google Scholar]
- Orélis-Ribeiro R., Ruiz C.F., Curran S.S., Bullard S.A. Blood flukes (Digenea: Aporocotylidae) of epipelagic lamniforms: redescription of Hyperandrotrema cetorhini from basking shark (Cetorhinus maximus) and description of a new congener from shortfin mako shark (Isurus oxyrinchus) off Alabama. J. Parasitol. 2013;99:835–846. doi: 10.1645/12-125.1. [DOI] [PubMed] [Google Scholar]
- Orélis-Ribeiro R., Bullard S.A. Blood flukes (Digenea: Aporocotylidae) infecting body cavity of South American catfishes (Siluriformes: Pimelodidae): two new species from rivers in Bolivia, Guyana, and Peru with a re-assessment of Plehniella Szidat, 1951. Folia Parasitol. 2015;62:1. doi: 10.14411/fp.2015.050. [DOI] [PubMed] [Google Scholar]
- Short R.B. A new blood fluke, Selachohemecus olsoni, n. g., n. sp. (Aporocotylidae) from the sharp-nosed shark, Scoliodon terranovae. Proc. Helm. Soc. Wash. 1954;21:78–82. [Google Scholar]
- Smith J.W. The blood flukes (Digenea: Sanguinicolidae and Spirorchidae) of cold-blooded vertebrates and some comparison with the schistosomes. [Review article] Helminth. Abs. S. A. 1972;41:161–204. [Google Scholar]
- Truong T.N., Bullard S.A. Blood flukes (Digenea: Aporocotylidae) of walking catfishes (Siluriformes: Clariidae): new genus and species from the Mekong River (Vietnam) with comments on related catfish aporocotylids. Folia Parasitol. 2013;60:237. doi: 10.14411/fp.2013.027. [DOI] [PubMed] [Google Scholar]
- Van der Land J. A new blood fluke (Trematoda) from Chimaera monstrosa L. Proc. Koninklijke Akad. Wetenschappen Amster., S. C, Biol. Med. Sci. 1967;70:110–120. [Google Scholar]
- Warren M.B., Bakenhaster M.D., Scharer R.M., Poulakis G.R., Bullard S.A. A new genus and species of fish blood fluke. Achorovermis testisinuosus gen. 2020 doi: 10.14411/fp.2020.009. et. sp. n. (Digenea: Aporocotylidae), infecting critically endangered smalltooth sawfish, Pristis pectinata (Rhinopristiformes: Pristidae) in the Gulf of Mexico. Folia Parasitol. 67, 009. [DOI] [PubMed] [Google Scholar]
- Warren M.B., Bullard S.A. First elucidation of a blood fluke (Electrovermis zappum n. gen., n. sp.) life cycle including a chondrichthyan or bivalve. Int. J. Parasitol.: Parasit. Wild. 2019;10:170–183. doi: 10.1016/j.ijppaw.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M.B., Ruiz C.F., Whelan N.V., Bullard S.A. Gymnurahemecus bulbosus gen. et. sp. nov. (Digenea: Aporocotylidae) infecting smooth butterfly rays, Gymnura micrura (Myliobatiformes: Gymnuridae) in the northern Gulf of Mexico, with a taxonomic key and further evidence for monophyly of chondrichthyan blood flukes. Parasitol. Res. 2019;118:751–762. doi: 10.1007/s00436-018-06202-9. [DOI] [PubMed] [Google Scholar]
- Warren M.B., Roberts J.R., Arias C.R., Koenigs R.P., Bullard S.A. Acipensericola glacialis n. sp. (Digenea: Aporocotylidae) from the heart of lake sturgeon Acipenser fulvescens Rafinesque (Acipenseriformes: Acipenseridae) in the Great Lakes basin, lake Winnebago system, USA. Syst. Parasitol. 2017;94:875–889. doi: 10.1007/s11230-017-9751-3. [DOI] [PubMed] [Google Scholar]