Abstract
Acute phase response (APR) is an innate stress reaction to tissue trauma or injury, infection, and environmental insults like ozone (O3). Regardless of the location of stress, the liver has been considered the primary contributor to circulating acute phase proteins (APPs); however, the mechanisms underlying APR induction are unknown. Male Wistar-Kyoto rats were exposed to air or O3 (1 ppm, 6-hr/day, 1 or 2 days) and examined immediately after each exposure and after 18-hr recovery for APR proteins and gene expression. To assess the contribution of adrenal-derived stress hormones, lung and liver global gene expression data from sham and adrenalectomized rats exposed to air or O3 were compared for APR transcriptional changes. Data demonstrated serum protein alterations for selected circulating positive and negative APPs following 2 days of O3 exposure and during recovery. At baseline, APP gene expression was several fold higher in liver relative to lung. O3-induced increases were significant for lung but not liver for some genes including orosomucoid-1. Further, comparative assessment of mRNAseq data for known APPs in sham rats exhibited marked elevation in lung but not liver, and a near complete abolishment of APP mRNA levels in lung tissue of adrenalectomized rats. Thus, the lung appears to play a critical role in O3-induced APP synthesis and requires the presence of circulating adrenal-derived stress hormones. The relative contribution of lung versus liver and role of neuroendocrine stress hormones needs to be considered in future APR studies involving inhaled pollutants.
Keywords: acute phase response, gene expression, lung, liver, adrenalectomy, stress hormones
INTRODUCTION
The acute phase response (APR) is a rapid host defense system characterized by synthesis of specialized proteins that drive physiological changes to counteract stressful events (Gruys et al., 2005). Immediately following the encounter of common stressors like tissue injury or infection, pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor-α (TNF-α), are proposed to trigger the production of acute phase proteins (APPs) (Gulhar et al., 2020) to augment tissue repair processes and reestablish homeostasis (Cray et al., 2009). The APR may also be induced by homeostatic imbalances in response to inhaled environmental pollutant exposures (Shannahan et al., 2012; Elder et al., 2004). In addition, acute psychosocial pressures which activate the hypothalamus-pituitary-adrenal (HPA) stress axis act through neuroendocrine mechanisms to induce an acute phase event (Marsland et al., 2017). Previous reviews of APR identified the liver as the primary target of cytokine signaling and subsequent APP synthesis and regulation (Kuscuoglu et al., 2018; Cray et al., 2009).
A number of air pollutants, including particulate matter (PM) (Elder et al., 2004) and nanoparticles (Saber et al., 2013; Hadrup et al., 2019), have been linked to APR activation and adverse pulmonary and systemic health effects in rodents. Acute Libby (Montana) asbestos exposures in rats was associated with increased circulating APPs and lung inflammation (Shannahan et al., 2012). O3 inhalation was also shown to increase serum APPs, which coincides with pulmonary injury and inflammation (Bass et al., 2013). Although hepatic APP production is generally believed to be the major contributor to circulating APPs (Gruys et al., 2005), in the case of air pollutants, the relative contribution of liver versus lung has not been examined. Exposure to nanoparticles was reported to induce APR genes in the lung (Saber et al., 2013) but not liver (Saber et al., 2009). Induction of APR by O3 in liver versus lung, as well as tissue specific synthesis and release of APPs, is not well established. Moreover, mediators of APR following air pollutant inhalation are unknown.
Previously, Snow et al (2018) and Kodavanti (2019) reviewed that O3-induced lung injury and inflammation are mediated through activation of HPA and sympathetic-adrenal-medullary (SAM) axes that respond to stress signals in the brain. This activation of the stress response after O3 exposure is associated with release of epinephrine and glucocorticoids into circulation, which mediates a variety of homeostatic processes through glucocorticoid and adrenergic receptors (Henriquez et al., 2017; 2018). Adrenal-derived catecholamines and glucocorticoids drive stress pathway activation, which results in changes in immune mediator production and lung inflammation (Henriquez et al., 2017). Further, lung injury and inflammation induced by O3 exposure are diminished in adrenalectomized rats having severely depleted circulating epinephrine and corticosterone (Miller et al., 2016; Henriquez et al., 2019). However, the potential contribution of these stress hormones in mediating APR remains to be examined.
The goal of this study was to determine the relative contribution of the liver and lung in mediating APR for major APPs that are abundantly present in circulation and to assess the contribution of adrenal-derived stress hormones following O3 exposure. It was postulated that O3-induced elevation in serum levels of APPs might occur through their enhanced transcriptional expression in lung as well as liver. Further, since O3-induced lung injury and inflammation are mediated through activation of neuroendocrine stress pathways (Kodavanti, 2019), it was postulated that adrenal-derived stress hormones contribute to APR gene induction in the lung as well as liver. To address these hypotheses, the temporality of an O3-induced APR in lung versus liver tissue was initially examined in a Wistar Kyoto rat model (Study 1). Secondly, using mRNA sequencing data from liver and lung tissues obtained from air or O3 exposed sham and adrenalectomized rats, the role of circulating adrenal-derived hormones in activating APR genes was assessed (Study 2).
MATERIALS AND METHODS
Animals
Male Wistar Kyoto (WKY) rats (11-12 weeks old, weighing 250 – 300 g) purchased from Charles River Laboratories Inc. (Raleigh, NC) were housed (2/cage) in polycarbonate cages with hardwood chip bedding and maintained in an animal room at 21 ± 1 °C, 50-65% relative humidity under a 12 hr light/dark cycle. Purina (5001) rat chow (Brentwood, MO) and water were provided ad libitum. All animal procedures received approval from the U.S. Environmental Protection Agency (U.S. EPA) Center for Public Health and Environmental Assessment’s Animal Care and Use Committee. Animal facilities are approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
Experimental Protocol
Samples were collected from two different studies from our lab to assess markers of the APR. In Study 1) the relative contribution of O3 inhalation on APR induction was determined in lung and liver tissue and in Study 2) the contribution of adrenal-derived stress hormones was examined in mediating lung and liver APR gene expression. Study 1 rats (n=6-8 animals/group for serum protein and n=4-5 animals/group for qRT-PCR analysis) were exposed to air or 1 ppm O3 for 1 or 2 consecutive days (6 hr/day) and euthanized within 1-2 hr after treatment (Figure 1). An additional subset of rats exposed to air or O3 for 2 days was euthanized following an 18 hr recovery period. In Study 2, mRNA sequencing data (n=4-5 animals/group) from lung tissues in which rats underwent sham (SHAM) or total bilateral adrenalectomy (ADREX) surgery and then exposed to air or O3 (1 ppm, 4 hr) were examined for APP gene markers (Miller et al., 2016; Henriquez et al., 2017). For liver, expressions of APP genes were assessed from mRNA sequencing data in which tissues were obtained from SHAM and ADREX rats exposed to air or 0.8 ppm O3 for 4 hr (Henriquez et al., 2018). In each case, lung and liver tissues were obtained within 2 hr following a single air or O3 exposure.
Figure 1.
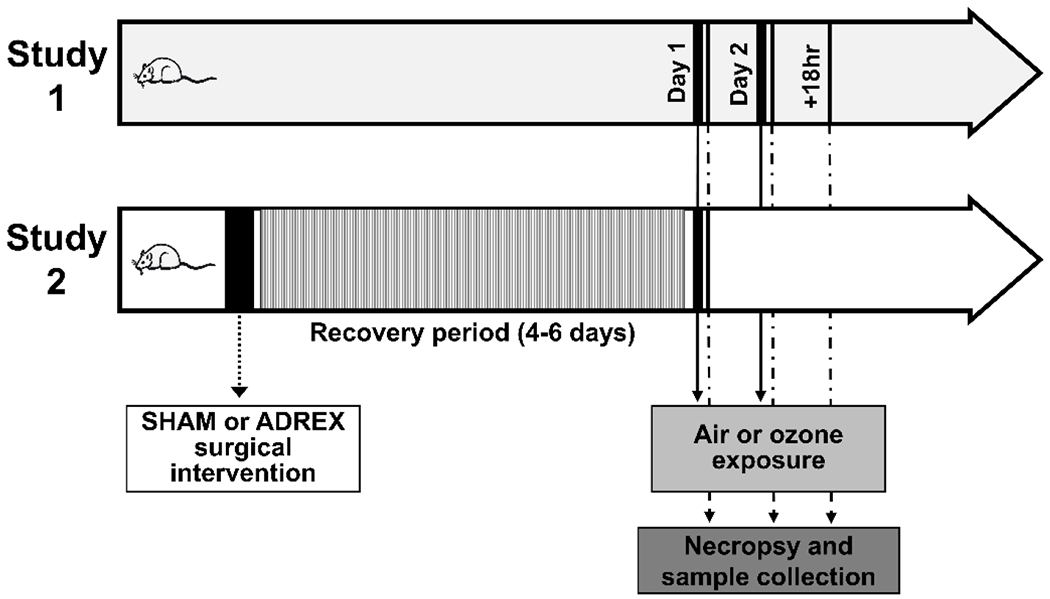
Experimental design and timeline for studies 1 and 2. Study 1 animals were exposed to air or ozone (O3) (1 ppm, 6hr) for 1 (Day 1) or 2 (Day 2) consecutive days. Groups were necropsied within 2 hr, with the exception of a third group being necropsied after an 18 hr recovery period following the second exposure day (+18 hr). Study 2 animals underwent SHAM or bilateral adrenalectomy (ADREX) surgery. Then, following a 4-6-day recovery period, animals were exposed to air or O3 (1 ppm, lung; 0.8 ppm, liver) for 4 hr and necropsied within 2 hr after exposure.
Animal Surgeries
For Study 2, as reported previously by Miller et al (2016) and Henriquez et al (2017; 2018), male WKY rats weighing 250 – 300 g underwent either SHAM or ADREX surgery at 12-13 weeks of age. Briefly, rats were anesthetized with ketamine and xylazine before being administered the analgesic buprenorphine (0.02 mg/kg/ml in saline, s.c.). If needed, aerosolized isoflurane (≈ 3%) was also provided via nose-cone during surgery for additional anesthesia. Aseptic sterile technique was used and bilateral ADREX was performed (Miller et al., 2016). For SHAM surgery, the adrenals remained in place while all other surgical aspects were identical to ADREX. After surgery, ADREX rats received water containing 0.9% sodium chloride since ADREX will lead to inhibition of salt reabsorption and dysregulation of salt-water balance due to diminution of adrenal-derived mineralocorticoids. A recovery period of 4-6 days was allowed before air or O3 exposure occurred.
O3 Exposures
O3 generated from a silent arc discharge generator (OREC, Phoenix, AZ) and controlled by mass flow controllers (Coastal Instruments Inc., Burgaw, NC) was transported into Rochester-style Hinners chambers as reported (Miller et al., 2016; Henriquez et al., 2018). Chamber O3 concentrations were continuously recorded by photometric O3 analyzers (API Model 400, Teledyne Instruments, San Diego, CA), and mean chamber temperature and relative humidity were monitored hourly as reported previously (Miller et al., 2016; Henriquez et al., 2018).
Necropsy and Sample Collection
For studies 1 and 2, necropsy and sample collection occurred within 2 hr after exposure, except for Study 1 animals assigned to 18 hr recovery time being necropsied following the recovery period as detailed in previous publication (Miller et al., 2015). Animals were weighed and euthanized using sodium pentobarbital (Virbac AH, Inc., Fort Worth, TX; >200 mg/kg, i.p.). For both studies, blood samples were collected from the abdominal aorta in serum separator vacutainer tubes and centrifuged at 3500 × g for 10 min. Serum was stored in aliquots at −80 °C for later analysis. Liver and lavaged right caudal lung lobe tissues were collected, frozen in liquid nitrogen, and stored at −80 °C for RNA analysis (Miller et al., 2016; Henriquez et al., 2017; 2018).
Serum Protein Analysis
For Study 1, serum concentrations were analyzed for APR proteins C-reactive protein (CRP), α2-macroglobulin (A2M), orosomucoid-1 (ORM-1), transferrin (TRF), and haptoglobin (HP). Serum samples were analyzed for CRP and TRF using kits for Sekisui Inc. (Renton, WA), while HP levels were analyzed using a kit obtained from Diasorin Inc. (Stillwater, MN). These assays were adapted for use on the Konelab Arena 30 system (Thermo LabSystems, Espoo, Finland). For A2M and ORM-1, a duplex, rat-specific electrochemiluminescence (ECL) assay kit was used (Meso Scale Discovery, Gaithersburg, MD).
Lung and Liver Tissue RNA Isolation
RNA was isolated from 10-20 mg lung tissue and ≈ 10 mg liver tissue from consistent regions. RNeasy mini kits (Qiagen, Valencia, CA) were used to extract total RNA following the directions provided by the manufacturer. Total RNA was quantified spectrophotometrically using NanoDrop 1000 (Thermo Fisher Scientific Inc., Wilmington, DE).
qRT-PCR
For Study 1, total RNA samples were diluted to 10 ng/μl. Using 50 ng RNA, one-step qPCR was performed using Superscript III kit (Invitrogen, Waltham, MA) and DNA amplification was assessed using an ABI Prism 7900 HT sequence detection system (Applied Biosystems, Foster City, CA). Primers purchased from Applied Biosystems (Foster City, CA) containing a 6-carboxy-fluorescein (FAM dye) 5’ label were employed for the following genes: β-actin (Actb, Rn00667869_m1), α2-macroglobulin (A2m, Rn00560589_m1), orosomucoid-1 (Orm1, Rn00583913_m1), serpin peptidase inhibitor, clade A, member 1 (Serpina1, Rn00574670_m1), C-reactive protein (Crp, Rn00567307_g1), haptoglobin (Hp, Rn00561393_m1), ceruloplasmin (Cp, Rn00561049_m1), and hepcidin (Hamp, Rn00584987_m1). Using Actb as an endogenous control, data were analyzed via ABI software, version 2.2. Relative expression for Day 1 and Day 2 rats was calculated separately in relation to fold change from respective air exposed rats of each day for a given tissue using the 2−ΔΔCT method.
RNA Sequencing
Lung mRNA data are published in Henriquez et al (2017) and liver RNA from a follow up study (Henriquez et al., 2018) was processed in an identical manner to lung. Briefly, total liver RNA was analyzed for quality using Agilent bioanalyzer (Agilent Technologies, Santa Clara, CA). To isolate 500 ng mRNA from total RNA of each sample, a prepX polyA mRNA Isolation Kit (Wafergen Biosystems, Fremont, CA) was used. Manufacturer’s protocol on sample preparation was followed on the Apollo324 sample processing system for mRNA selection before RNAseq library prep using Wafergen’s PrepX mRNA 48 protocol. cDNA obtained from this process underwent PCR thermocycling for 35 cycles with indexing primers as directed by Wafergen’s protocol. Using 1 μl amplification mix, each library was quantified via Qubit dsDNA HS Assay kit (Molecular Probes, Eugene, OR) and sample quality was checked by Agilent Bioanalyzer. Molecular concentration indicated by Bioanalyzer results and Qubit measurement was utilized to estimate average molecular size. Each library was then diluted to 4 nM and again checked by Qubit to confirm working concentrations. Following Illumina NetSeq 500 protocol (Illumina Inc., San Diego, CA), samples were denatured and diluted to a final concentration of 1.8 pM + 5% PhiX for Illumina sequencing. Sequencing data were stored in Illumina’s BaseSpace-cloud. Partek® Flow suite was used to map sequenced mRNA reads to the rat genome (rn6). DESeq2 package in R (v. 1.10.1) was employed to assess differential gene expression (Love et al., 2014) and normalized reads were assessed for group differences. These mRNA sequencing data were used to separate selected APR genes for each tissue to determine O3 effects in SHAM and ADREX rats.
Sequencing APR Gene Analysis
Genes associated with the APR were selected from normalized mRNA seq data set for lung (Henriquez et al., 2017) and liver. Average centered Row z scores were used to construct heat maps using normalized counts for each gene in lung or liver to show air vs. O3 and SHAM vs. ADREX response. Genes up-regulated after O3 exposure are shown as red, and down-regulated as green. Changes in expression in each tissue due to O3 or ADREX were assessed based upon the adjusted p-value-cut-off of 0.05. Kruskal–Wallis one-way analysis of variance and Dunn’s multiple comparison tests were used to determine significant differences in gene expression induced by O3 exposure or ADREX surgery for each tissue. Average linkage clustering and Euclidean distance measurement methods were used for lung tissue heat map, and then liver genes were arranged in the same sequence as lung for ease of comparison. The analysis was performed using the online tool provided by http://heatmapper.ca/.
Statistics for Serum APP and PCR Data
Analysis of serum APPs and qRT-PCR gene expression data were performed using a non-parametric t-test analysis (two-tailed Mann-Whitney test). The criterion for significance was set at p ≤ 0.05. Prior to analysis, outliers were identified and discarded (≤ 2 outliers/group) from tests using ROUT (Q = 1.0%).
RESULTS
O3 Exposure Influence on Serum APPs
At Day 2 immediately following O3 exposure, CRP levels increased relative to air controls, but this rise did not persist into Day 2 +18 hr recovery (Figure 2A). Effects of O3 exposure on serum A2M and ORM-1 protein levels were not detected when determined immediately following Day 1 or Day 2 exposure; however, both A2M and ORM-1 were elevated when measured +18 hr after Day 2 exposure (Figure 2B and C). TRF, a negative APP (Gulhar et al., 2020), was decreased by O3 when measured at Day 2 +0 hr (Figure 2D). Serum HP levels tended to increase numerically in O3 exposed animals relative to air controls at recovery time point, but this change was not significant (Figure 2E).
Figure 2.
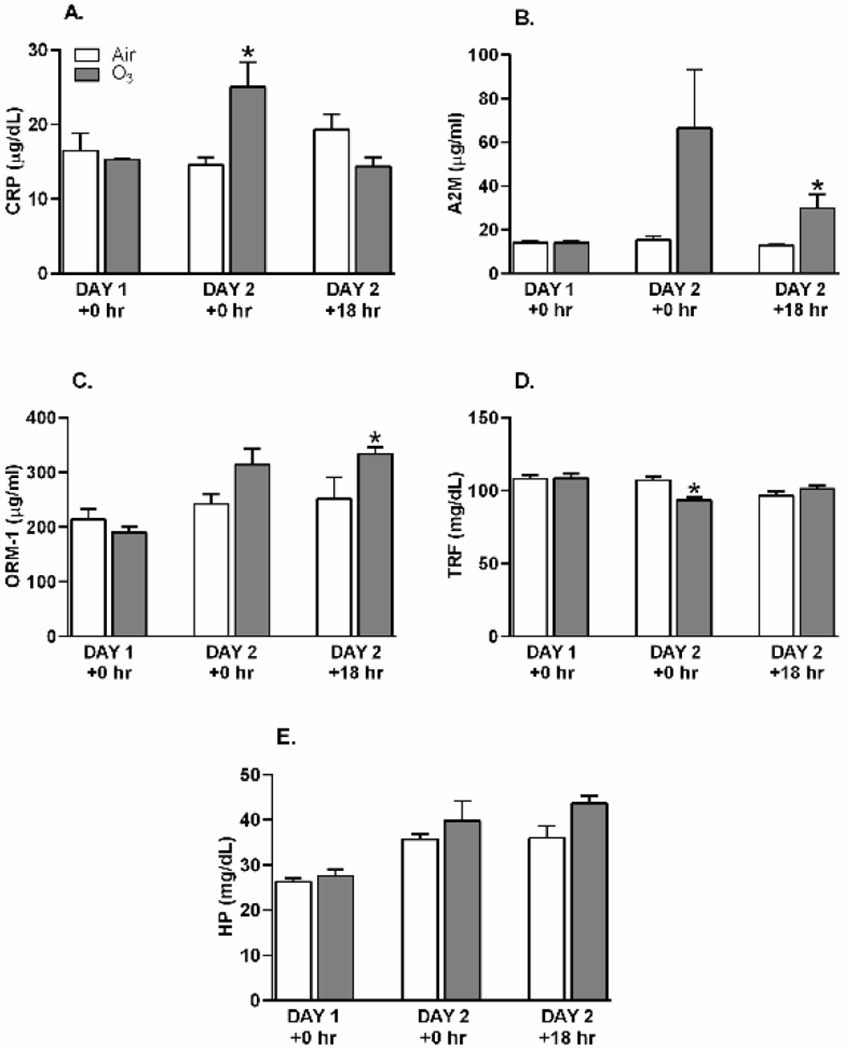
Alterations in serum APP concentrations after O3 exposure. Rats were exposed to air or ozone (O3) (1 ppm, 6 hr/day) for 1 (Day 1 +0 hr) or 2 (Day 2 +0 hr) consecutive days and samples collected within 2 hr post exposure. An additional group of rats was exposed to air or O3 for 2 days and allowed an 18 hr recovery before collecting samples (Day 2 +18 hr). A. C-reactive protein (CRP), B. α2-macroglobulin (A2M), C. orosomucoid-1 (ORM-1), D. transferrin (TRF), and E. haptoglobin (HP). Bar graphs show mean ± SEM of n=6-8/group, with * indicating a significant (p ≤ 0.05) O3 effect.
O3 Effect on Expression of Positive APP Genes in Lung and Liver
To understand differential tissue induction of an APR, O3-induced changes in expression of genes encoding APPs relative to air controls were determined for both lung and liver following Day 1 and Day 2 exposures. The expression of Actb, at baseline and after O3 inhalation, was also analyzed to assess potential differences in lung versus liver at a given RNA concentration. All lung and liver RNA samples for a given gene were accommodated in the same 96-well PCR plate to directly compare relative degree of gene expression in liver versus lung at baseline and after O3 exposure. Figure 3A illustrates O3 exposure-induced relative fold change in lung and liver A2m expression normalized to air controls of each tissue. Day 1 and Day 2 measures in both tissues showed O3-induced increased expression of A2m, with a significant rise in liver at Day 1 and in lung at Day 2. The PCR amplification plot of A2m, for ΔRn vs. amplification cycles, displays relative expression in lung and liver samples. Here, it may be noted that A2m gene amplification occurred at fewer cycles in liver than lung. This is despite no significant difference in amplification cycles for Actb between lung and liver at same RNA concentration (data not shown).
Figure 3.
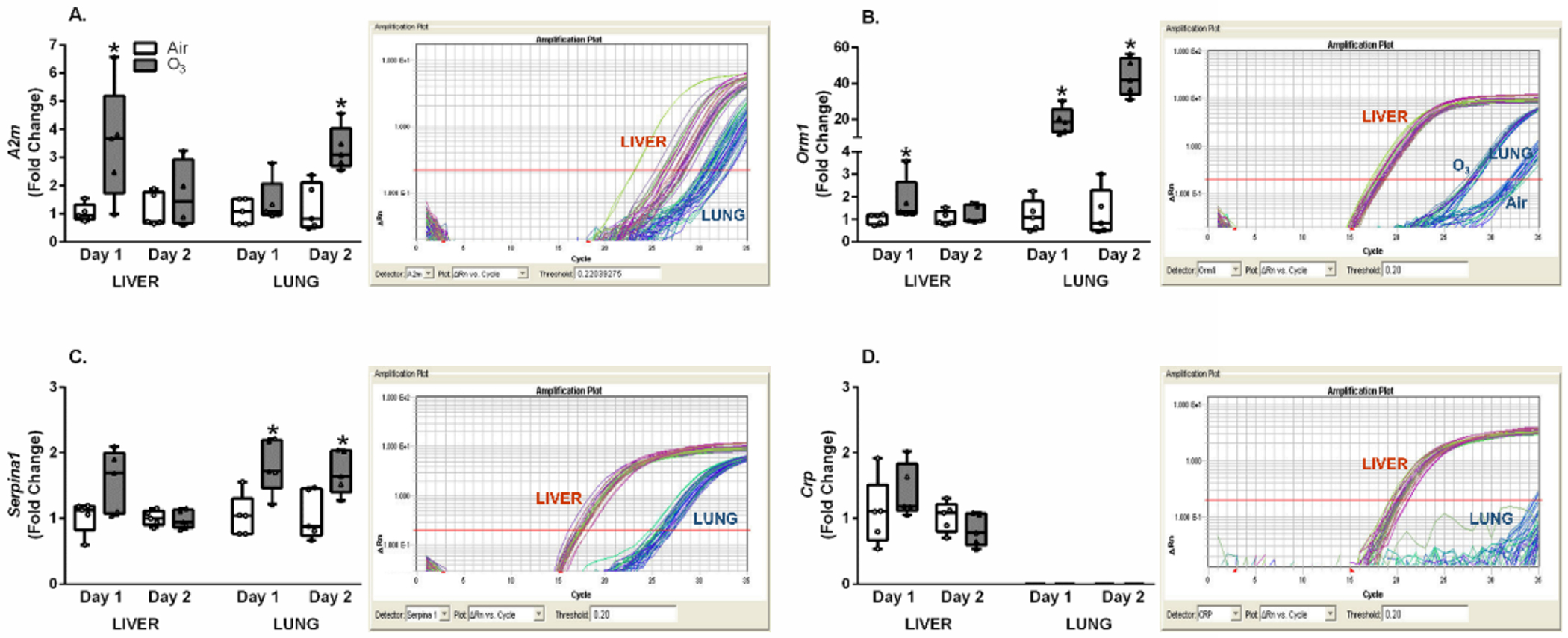
O3-induced alterations in inflammation and injury associated APPs in lung and liver. Lung and liver mRNA expression was assessed using qRT-PCR for rats exposed to air or ozone (O3) (1 ppm, 6 hr/day) for 1 (Day 1) or 2 (Day 2) consecutive days. Relative fold changes from air controls are indicated in box and whisker plots, along with captured PCR amplification plots to compare relative abundance of given genes in liver versus lung (note that Actb was similarly expressed at a given RNA concentration in both tissues); A. α2-macroglobulin (A2m), B. orosomucoid-1 (Orm1), C. serpin peptidase inhibitor, clade A, member 1 (Serpina1), and D. C-reactive protein (Crp). Box and whisker plots show all points (n=4-5/group). A significant (p ≤ 0.05) O3 effect is indicated by an *.
Orm1 was also significantly elevated after O3 exposure at Days 1 (nearly 20-fold) and 2 (over 40-fold) in lung, and to a smaller degree at Day 1 (≈ 1.8-fold) in liver (Figure 3B). Orm1 amplification plot shown in Figure 3B demonstrates clear differential amplification between lung and liver tissue and exposure condition for lung samples. Early amplification of Orm1 in liver samples point to high levels of liver baseline Orm1 expression relative to lung regardless of exposure condition. In addition, the O3-induced response of increased lung Orm1 expression occurring prior to air exposed Orm1 amplification (Figure 3B) demonstrates robust up-regulation in the lung by O3. While Actb expression for lung and liver at a given RNA concentration was similar at baseline (data not shown), PCR plots (Figure 3A and B) illustrated liver expression to be several fold higher than lung, suggesting tissue differences in Orm1.
In liver, Serpina1 expression was up-regulated after O3 inhalation in rats at Day 1, but at Day 2 this change was not significant when compared to the air group. In lung, there was a significant increase in Serpina1 expression after O3 exposure for both Days 1 and 2 (Figure 3C). Figure 3C illustrates the Serpina1 amplification plot of lung and liver for air and O3 treated animals. Actb expression remained similar at baseline for liver and lung while Serpina1 liver amplification occurred earlier relative to lung, indicating greater expression in liver than lung. The expression of Crp, a major complement factor and inflammatory APP (Kuscuoglu et al., 2018), was also analyzed (Figure 3D). Fold changes in Crp expression between air and O3 groups of liver were not significantly different. Expression of Crp in lung was not quantified because of the lack of amplification during the PCR process, and thus, was not represented in the bar graph. These results indicate minimal lung expression of Crp regardless of exposure condition.
Expression of Hp, Cp, and Hamp in Lung and Liver Following O3 Exposure
Expression of Hp, Cp, and Hamp were also measured in lung and liver of air and O3 exposed rats. Hp and Cp are positive APPs that bind hemoglobin and iron respectively following a stress response (Gruys et al., 2005). Hp expression increased after Day 1 O3 exposure in liver, however, the change in lung Hp expression at Day 1 was not significant (Figure 4A). Cp expression in lung or liver was not significantly altered after O3 exposure at any timepoints (Figure 4B). Further, liver expression of Hamp, involved in iron homeostasis (Nemeth & Ganz, 2009), was not markedly affected by O3 at Day 1, but significantly increased at Day 2 (Figure 4C). Lung expression of Hamp was highly variable for Day 2 air group, making it difficult to use these data for statistical analysis. Nevertheless, it was apparent that O3 inhalation led to marked up-regulation in expression at Day 1. Hp (Figure 4A) and Cp (Figure 4B) PCR amplification plots showed liver gene amplification occurring prior to lung. This is despite baseline Actb expression measured in liver and lung remaining similar, indicating liver Hp and Cp expression as several fold higher than lung (Figure 4A and B).
Figure 4.
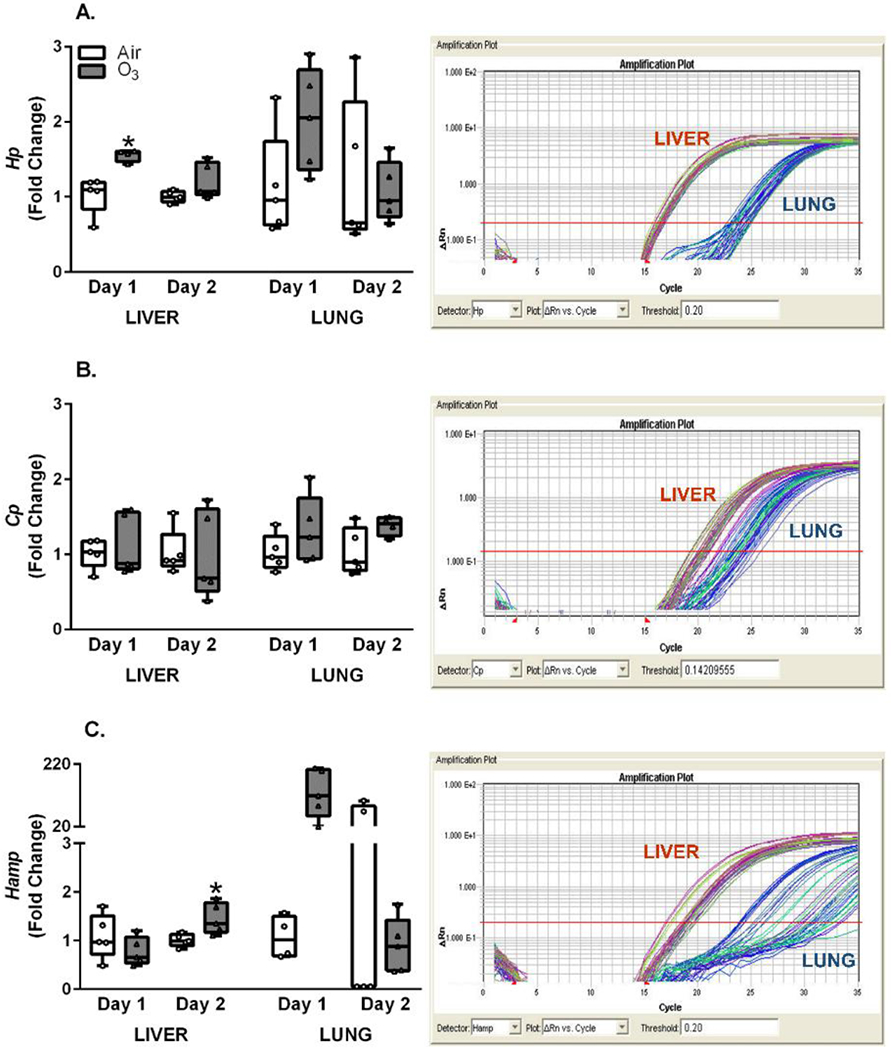
O3-induced expression alterations in inflammation and pathogenic infection associated APP genes. Lung and liver mRNA expression was assessed using qRT-PCR after exposure of rats to air or ozone (O3) (1 ppm, 6 hr/day) for 1 (Day 1) or 2 (Day 2) consecutive days. Relative fold changes from air controls measured in lung and liver tissue samples are indicated in box and whisker plots, along with captured PCR amplification plots (note that Actb was similarly expressed at a given RNA concentration in both tissues); A. haptoglobin (Hp), B. ceruloplasmin (Cp), and C. hepcidin (Hamp). PCR plots are included to compare relative abundance of mRNA in liver versus lung. Box and whisker plots show all points (n=4-5/group). A significant (p ≤ 0.05) O3 effect is indicated by an *.
Transcriptomic Data of APR Genes in Lung and Liver: Mitigation by ADREX
Snow et al (2018) reviewed that O3-induced acute effects are mediated by the activation of HPA and SAM axes. Therefore, it was postulated that induction of APR genes after O3 exposure might be mediated by circulating adrenal-derived stress hormones. Lung mRNA data were re-analyzed from a previous study (Henriquez et al., 2017) and liver mRNA sequencing was performed from a follow up study involving ADREX (Henriquez et al., 2018) to determine the contribution of circulating adrenal-derived epinephrine and corticosterone, two major stress hormones in mediating the APR. Genes involved in the APR were selected from the literature and pulled from lung and liver mRNA sequencing dataset that involved air or O3 exposure for SHAM and ADREX rats. For lung tissue, O3 exposure in SHAM rats led to significant increases in mRNA expression of multiple genes involved in APR such as coagulation factors, major serum acute-phase reactants, and inflammatory mediators (Figure 5). Surprisingly, O3 inhalation did not induce these genes in livers of SHAM rats except for a numerical non-significant change in Orm1 consistent with that noted using PCR in Study 1. Crp expression was decreased in liver of air-exposed ADREX relative to air-exposed SHAM rats. Unlike O3-induced elevation in lung APR genes of SHAM rats, these increases in lungs of ADREX rats were markedly diminished. Serpine1 (plasminogen activator inhibitor-1) expression in lung was increased in air-exposed ADREX rats relative to air-exposed SHAM. ADREX in air exposed rats changed some liver APP genes. ADREX elevated interleukin-1β (IL-1β, Hamp, and complement factor I (Cfi) and reduced Orm1 in liver of O3 exposed rats when compared to O3-exposed SHAM rats. O3-induced decrease in coagulation factor V (F5) was observed in liver of ADREX rats relative to air-exposed ADREX rats. These data demonstrated that O3 induces an APR to a greater extent in lung than liver, and this lung response is inhibited by ADREX. In the livers of O3-exposed ADREX rats, some APR genes were over-expressed relative to O3-exposed SHAM rats.
Figure 5.
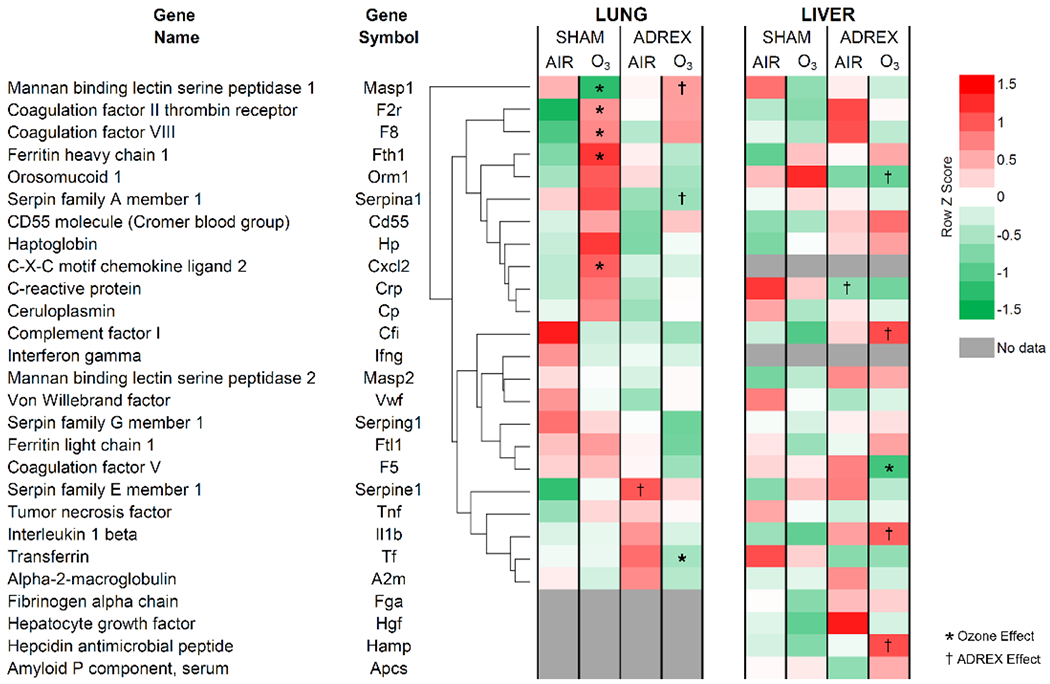
Altered expression of APP genes in the lung and liver after 1 day of O3 exposure in SHAM and ADREX rats (Study 2). Selected APR genes were identified from RNAseq Illumina data set for lung and liver from air or O3 (1 ppm for lung and 0.8 ppm for liver; each for 4 hr) exposed SHAM and ADREX rats (n=4-5/group). Normalized gene expression values for selected APP genes from each tissue were separated from RNAseq data set, and heat maps of Row z scores were constructed. For lung tissue, average linkage clustering and Euclidean distance measurement was employed, and then liver genes were arranged in the same sequence as lung without clustering for ease of comparison. Red and green indicate increased and decreased gene expression, respectively. Significant changes in expression due to O3 exposure is indicated by an *, and due to surgical treatment is indicated by a † (adjusted-p-value-cut-off of 0.05).
DISCUSSION
A variety of environmental stressors induce an APR, including air pollutants. The APR, comprised of an array of protein signaling and biochemical reactions that prepare the host for defense against acute inflammation, trauma, or infection, has been studied for decades. However, the mechanisms initiating this response are not well understood. It is presumed that regardless of the location of stressor-induced injury in the body, major APPs are synthesized in the liver (Gruys et al., 2005; Cray et al., 2009). Further, it is also known that adrenal-derived stress hormones, catecholamines and glucocorticoids, are involved in APP induction (Gool et al., 1984). Henriquez et al (2018) and Miller et al (2016) showed that pulmonary and systemic effects of inhaled O3 are mediated through neuroendocrine activation and adrenal-derived stress hormones, however, their contribution in mediating an O3-induced APR has not been examined. Further, since the lung is the target organ for air-pollution-induced injuries, contribution of liver versus lung in APR regulation remains to be explored. Our findings demonstrated that O3 exposure is associated with increases in circulating positive APPs. Although APP genes at baseline were more abundantly expressed in liver relative to lung, O3 exposure initiated enhanced production of APP mRNAs in lung rather than liver. Importantly, ADREX nearly abolished O3-induced changes in lung expression of APR genes, suggesting the contribution of adrenal-derived stress hormones in mediating the lung APR.
Major APPs display exponential changes following induction of a stress or injury/infection response and are often used in pathology diagnostics (Blackburn, 1994; Jain et al., 2011). In this study, proteins were selected based upon their vital functional roles and known alteration during traditional acute phase events. It is noteworthy that primary and abundant APPs such as A2M and ORM-1 were increased following O3-induced activation of the neuroendocrine response, concurrent to lung inflammatory response without marked changes in circulating cytokines (Bass et al., 2013; Erickson et al., 2017), suggesting that circulating cytokines might not be involved in APR induction in the lung. The question remains as to if the lack of significant systemic cytokine release after O3 exposure (Bass et al., 2013) relates to the lack of APR induction in the liver.
For infection-induced APR, it is known that, through paracrine and endocrine signaling, cytokines act on hepatocytes to induce APP synthesis and release (Carrillo et al., 2017) and eventual clearance of infectious pathogens through various molecular pathways. CRP, which increases by up to 1000-fold following pathogen detection, is a pro-inflammatory APP that aids in complement activation and promotes phagocytosis (Gulhar et al., 2020). Serum CRP assessment immediately following Day 2 of exposure exhibited a significant O3-induced rise. However, it is noteworthy that, in the case of inhalant-induced injury, there was no induction of lung or liver Crp gene expression, suggesting that the mechanism and characteristics of an O3-induced APR are likely different than that of infection. In addition, where A2M and ORM-1 are strongly reactive APPs in rodents, CRP is a major human but not rat APP (Schreiber et al., 1989). During bacterial infection, ceruloplasmin (CP) and HP bind copper and hemoglobin, respectively, to inhibit utilization of host resources for pathogenic growth (Jain et al., 2011; Gulhar et al., 2020). Similarly, since iron is a micronutrient for bacterial growth, hepcidin (HAMP) and TRF modulate ferroportin internalization to inhibit pathogenic iron scavenging (Kuscuoglu et al., 2018). With O3 exposure, only modest decreases in TRF were noted. As TRF is highly abundant in the circulation, it is likely that O3-induced lung injury, not involving pathogen-mediated signaling, might not result in significant changes of these proteins.
Reactants ORM-1 and A2M interact with cytokines, namely TNF-α, to disrupt pro-inflammatory signaling cascades and mitigate stress-induced injury (Ligresti et al., 2012; Kuscuoglu et al., 2018). In this study, a delayed release of these APPs into the circulation was observed following O3 inhalation. Serum measurements of A2M and ORM-1 displayed a time-dependent increase, with maximum levels noted after Day 2 of O3 exposure and persisting +18 hr post-treatment. These increases in APPs may play a role in recovery from lung injury and inflammation after O3 exposure; however, the specific roles of each protein in reversing the course of lung injury and inflammation remains unknown.
Aside from hepatic contribution to the APR, non-hepatic APP sources seem less elucidated. It is presumed that pro-inflammatory molecules, including TNF-α, IL-6, and interferon-γ, are secreted into the circulation by phagocytes in response to stress and activate hepatocytes to induce APP genes (Heinrich et al., 1990; Geiger et al., 1988). However, appreciable increases were not detected in circulating cytokines after O3 exposure (Bass et al., 2013; Erickson et al., 2017). Our results demonstrated that the liver, with abundant metabolically active cellular mass, highly expresses APP genes at baseline relative to lung. However, O3 inhalation led to greater relative elevation in APP gene expressions in lung compared to liver as demonstrated by gene specific PCR for selected APPs. In the case of PM, Hadrup et al (2019) showed that APR genes might be locally induced along with lung injury and inflammation. Although marked induction of few APP genes was noted in lung but not liver, it is difficult to determine the relative contribution of each organ in observed circulating APP increases. Since liver tissue mass is more than ten-folds bigger than that of lung, and it expresses these genes abundantly, a modest rise in liver protein synthesis might contribute significantly to the circulating pool of APPs. Moreover, since some APPs are highly enriched in the serum relative to other proteins, such as A2M and ORM-1 (Cray et al., 2009), the relative contribution of liver versus lung for inhaled pollutant-induced elevation in each circulating APP needs to be individually considered.
APP gene expression assessed using PCR indicated tissue and target-dependent alterations in both lung and liver. Notably, O3 increased selected positive APPs in both tissues, suggesting biomarkers of APR are not solely produced in the liver following O3 exposure. O3-induced early up-regulation of Orm1 suggests the involvement of both tissues in protein production. O3 induced a 40-fold rise in Orm1 lung expression compared with a 2-fold elevation in liver, suggesting the lung is an important source of APPs. Orm1 is regulated by glucocorticoids and cytokines (Sai et al., 2014). It is noteworthy that glucocorticoids increased systemically while cytokines primarily increased in the lung after O3 exposure (Henriquez et al., 2018), suggesting that glucocorticoids, together with target organ injury and cytokine production, may play a significant role in mediating Orm1 expression.
Macrophages were previously found to express Crp (Dong & Wright, 1996), however, pulmonary Crp expression was minimal in our study. Similarly, Hamp lung expression at baseline was markedly lower than liver and exhibited high variability. This pattern of APP expression between lung and liver displays gene-specific baseline tissue differences. Further, from our data, it may be inferred that the lung plays an important role in O3-induced APP synthesis and regulation for selected APPs and that the contribution of lung versus liver might be APP-specific. The induction of an APR may also depend on the degree and characteristics of stress.
Although numerous reviews have detailed characteristics of an APR in response to a variety of stressors (Cray et al., 2009; Gruys et al., 2005), induction mechanisms remain unclear, especially in the case of inhaled toxicants. Recently, as reviewed by Snow et al (2018) and Kodavanti (2019), O3-induced lung injury and inflammation, as well as systemic metabolic effects, are mediated through the activation of HPA and SAM axes, and adrenal-derived epinephrine and corticosterone are crucial mediators of lung injury and inflammation (Miller et al., 2016; Henriquez et al., 2018). Previously, Laskin et al (1994) reported O3 inhalation coinciding with hepatocyte protein production, a response they attributed to release of inflammatory cytokines TNF-α and IL-1 from activated macrophages in the lung. However, the exact relationship between inhaled toxicants and enhanced hepatic protein synthesis remains unknown. In this study, whether stress hormones are necessary for mediating the APR was examined in lung and liver after an acute O3 exposure.
To determine the contribution of circulating stress hormones, global transcriptomic data of lung and liver samples for expression of selected APPs in which SHAM and ADREX rats exposed to air or O3 was determined. Previous reviews have indicated both catecholamines and glucocorticoids as modulators of certain immune functions (Sternberg, 2006; Cray et al., 2009). Known major APP gene signatures following O3 exposure in SHAM rats indicated generalized increases in expression in lung but not liver, confirming our PCR outcome. Importantly, it was noted that ADREX largely abolished O3-induced elevation in APP transcription in the lung, suggesting APP gene induction might be regulated by circulating stress hormones. However, it should be noted that while stress hormones are increased systemically, increase in cytokines occurred primarily in the lung at sites of O3-induced injury (Henriquez et al., 2018), suggesting that some APP genes might be responsive to both circulating stress hormones and local lung irritation-related changes. Previously, investigators confirmed the depletion of circulating stress hormones after ADREX (Miller et al., 2016; Henriquez et al., 2018), therefore, these data show that removing catecholamines and glucocorticoids from circulation dampens the ability of lung and liver tissues to induce APP transcription. Depletion of circulating stress hormones after ADREX was associated with diminution of lung injury and inflammation (Miller et al., 2016; Henriquez et al., 2018). Thus, it is conceivable that pulmonary-derived circulating bioactive components might not be present to induce APP genes in ADREX animals. However, the identity of other byproducts has not been confirmed. Further, circulating cytokines are not significantly increased after O3 exposure in healthy rats (Bass et al., 2013; Erickson et al., 2017).
A number of limitations for this study can be identified. Only major APPs were selected for analysis and a comprehensive APP investigation was not performed. In addition, our endpoints only assessed positive APPs, and did not address potentially impacted negative APPs, except for TRF. Although lung and liver mRNAseq data for SHAM and ADREX rats were derived from studies that involved slightly different O3 concentrations (1 ppm for lung versus 0.8 ppm for liver), it is conceivable that responses are comparable based upon our many prior studies using these two concentrations (Miller et al., 2015; 2016; Henriquez et al., 2018). Though used in numerous previous studies, the O3 concentrations employed are not environmentally relevant, but are comparable to human clinical studies performed at 1/4th the concentrations during intermittent exercise (Hatch et al., 2013). The concentrations used produced a significant APR where it was possible to demonstrate alleviation by ADREX.
In conclusion, data demonstrated that an O3-induced APR is associated with increases in circulating APPs. Although baseline liver APP gene expression relative to Actb is several fold higher than expression in lung, and on a mass basis liver is nearly 10-fold greater than lung, gene expression of selected APPs is primarily enhanced in lung after acute O3 exposure rather than liver. Further, our findings showed that APP increases in lung tissue are largely abolished by ADREX, suggesting a causal role of adrenal-derived stress hormones in mediating an APR. These data emphasize the caution in generalizing the liver as the sole contributor of APPs, especially after inhalation of air pollutants, and demonstrate that the location of APR-mediated APP synthesis may depend upon the type of APP being examined. Adrenal-derived stress hormones might mediate the O3-induced APR and any perturbation in stress hormone release may hamper the protective mechanisms involved in homeostasis and tissue repair.
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Ian Gilmour and Stephen Gavett of the U.S. EPA and Dr. Jonathan Shannahan of Purdue University (Lafayette, IN) for their critical review of the manuscript. We thank Dr. Mark Higuchi, Mr. Allen Ledbetter (retired), and Mr. Malek Khan of the U.S. EPA for their help in conducting ozone exposures and Ms. Judy Richards of the U.S. EPA (retired) for assessing select serum proteins.
FUNDING INFORMATION
This work was supported by the U.S. EPA Intramural Research funds. ARH was recipient of the Fulbright (CONICYT) and a trainee under EPA-UNC Cooperative Agreement (CR-83515201). DIA was supported in part by an appointment to the Research Participation Program at the Center for Public Health and Environmental Assessment at the U.S. EPA, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and U.S. EPA.
Abbreviations:
- APR
Acute phase response
- APPs
Acute phase proteins
- O3
Ozone
- SHAM
Sham surgery
- ADREX
Adrenalectomy surgery
- HPA
Hypothalamus-pituitary-adrenal
- SAM
Sympathetic-adrenal-medullary
- PM
Particulate matter
- CRP
C-reactive protein (protein)
- A2M
α2-macroglobulin (protein)
- ORM-1
Orosomucoid-1 (protein)
- TRF
Transferrin (protein)
- HP
Haptoglobin (protein)
- CP
Ceruloplasmin (protein)
- HAMP
Hepcidin (protein)
- Actb
β-actin (gene)
- A2m
α2-macroglobulin (gene)
- Orm1
Orosomucoid-1 (gene)
- Serpina1
Serpin peptidase inhibitor, clade A, member 1 (gene)
- Crp
C-reactive protein (gene)
- Hp
Haptoglobin (gene)
- Cp
Ceruloplasmin (gene)
- Hamp
Hepcidin (gene)
Footnotes
Declaration
Authors declare no competing financial interests.
DISCLOSURES
The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, U.S. EPA and approved for publication. Approval does not signify that the contents necessarily reflect the views and the policies of the Agency, nor does mention of trade names of commercial products constitute endorsement or recommendation for use.
REFERENCES
- Bass V, Gordon CJ, Jarema KA, MacPhail RC, Cascio WE, Phillips PM, Ledbetter AD, Schladweiler MC, Andrews D, Miller D, Doerfler DL, Kodavanti UP 2013. Ozone induces glucose intolerance and systemic metabolic effects in young and aged Brown Norway rats. Toxicology and Applied Pharmacology 273:551–560. 10.1016/j.taap.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn WD Jr. 1994. Validity of acute phase proteins as markers of disease activity. The Journal of Rheumatology Supplement 42:9–13. [PubMed] [Google Scholar]
- Carrillo JLM, García FPC, Coronado OG, García MAM, Cordero JFC 2017. Physiology and pathology of innate immune response against pathogens. Physiology and Pathology of Immunology. 10.5772/intechopen.70556. [DOI] [Google Scholar]
- Cray C, Zaias J, Altman NH 2009. Acute phase response in animals: A review. Comparative Medicine 59:517–526. [PMC free article] [PubMed] [Google Scholar]
- Dong Q and Wright JR 1996. Expression of C-reactive protein by alveolar macrophages. Journal of Immunology 156:4815–4820. [PubMed] [Google Scholar]
- Elder A, Gelein R, Finkelstein J, Phipps R, Frampton M, Utell M, Kittelson DB, Watts WF, Hopke P, Jeong CH, Kim E, Liu W, Zhao W, Zhuo L, Vincent R, Kumarathasan P, Oberdörster G 2004. On-road exposure to highway aerosols. 2. Exposures of aged, compromised rats. Inhalation Toxicology 16 Suppl 1:41–53. 10.1080/08958370490443222. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Jude J, Zhao H, Rhea EM, Salameh TS, Jester W, Pu S, Harrowitz J, Nguyen N, Banks WA, Panettieri RA Jr, Jordan-Sciutto KL 2017. Serum amyloid A: an ozone-induced circulating factor with potentially important functions in the lung-brain axis. FASEB J. 31(9):3950–3965, Erratum in: FASEB J 2018 31(1):535. 10.1096/fj.201600857RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T, Andus T, Klapproth J, Hirano T, Kishimoto T, Heinrich PC 1988. Induction of rat acute-phase proteins by interleukin 6 in vivo. European Journal of Immunology 18:717–721. 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- Gool JV, Boers W, Sala M, Ladiges NC 1984. Glucocorticoids and catecholamines as mediators of acute-phase proteins, especially rat alpha-macrofoetoprotein. Biochemical Journal 220:125–132. 10.1042/bj2200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ 2005. Acute phase reaction and acute phase proteins. Journal of Zhejiang University Science B 6:1045–1056. 10.1631/jzus.2005.B104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulhar R, Ashraf MA, Jialal I 2020. Physiology, acute phase reactants. StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK519570/. [PubMed] [Google Scholar]
- Hadrup N, Rahmani F, Jacobsen NR, Saber AT, Jackson P, Bengtson S, Williams A, Wallin H, Halappanavar S, Vogel U 2019. Acute phase response and inflammation following pulmonary exposure to low doses of zinc oxide nanoparticles in mice. Nanotoxicology 13:1275–1292. 10.1080/17435390.2019.1654004. [DOI] [PubMed] [Google Scholar]
- Hatch GE, McKee J, Brown J, McDonnell W, Seal E, Soukup J, Slade R, Crissman K, Devlin R 2013. Biomarkers of dose and effect of inhaled ozone in resting versus exercising human subjects: comparison with resting rats. Biomark Insights 8:53–67. 10.4137/BMI.S11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T 1990. Interleukin-6 and the acute phase response. Biochemical Journal 265:621–636. 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez A, House J, Miller DB, Snow SJ, Fisher A, Ren H, Schladweiler MC, Ledbetter AD, Wright F, Kodavanti UP 2017. Adrenal-derived stress hormones modulate ozone-induced lung injury and inflammation. Toxicology and Applied Pharmacology 329:249–258. 10.1016/j.taap.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez AR, House JS, Snow SJ, Miller CN, Schladweiler MC, Fisher A, Ren H, Valdez M, Kodavanti PR, Kodavanti UP 2019. Ozone-induced dysregulation of neuroendocrine axes requires adrenal-derived stress hormones. Toxicological Sciences 172:38–50. 10.1093/toxsci/kfz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez AR, Snow SJ, Schladweiler MC, Miller CN, Dye JA, Ledbetter AD, Richards JE, Hargrove MM, Williams WC, Kodavanti UP 2018. Beta-2 adrenergic and glucocorticoid receptor agonists modulate ozone-induced pulmonary protein leakage and inflammation in healthy and adrenalectomized rats. Toxicological Sciences 166:288–305. 10.1093/toxsci/kfy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Gautam V, Naseem S 2011. Acute-phase proteins: as diagnostic tool. Journal of Pharmacy and Bioallied Sciences 3:118–127. 10.4103/0975-7406.76489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti UP 2019. Susceptibility variations in air pollution health effects: Incorporating neuroendocrine activation. Toxicology Pathology 47:962–975. 10.1177/0192623319878402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuscuoglu D, Janciauskiene S, Hamesch K, Haybaeck J, Trautwein C, Strnad P 2018. Liver master and servant of serum proteome. Journal of Hepatology 69:512–524. 10.1016/j.jhep.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Pendino KJ, Punjabi CJ, Rodriguez del Valle M, Laskin JD 1994. Pulmonary and hepatic effects of inhaled ozone in rats. Environmental Health Perspectives 102 Suppl 10(Suppl 10):61–64. 10.1289/ehp.94102s1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti G, Aplin AC, Dunn BE, Morishita A, Nicosia RF 2012. The acute phase reactant orosomucoid-1 is a bimodal regulator of angiogenesis with time-and context-dependent inhibitory and stimulatory properties. Public Library of Science One 7: e41387. 10.1371/journal.pone.0041387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain, Behavior, and Immunity 64:208–219. 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Karoly ED, Jones JC, Ward WO, Vallanat BD, Andrews DL, Schladweiler MC, Snow SJ, Bass VL, Richards JE, Ghio AJ, Cascio WE, Ledbetter AD, Kodavanti UP 2015. Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicology and Applied Pharmacology 286:65–79. 10.1016/j.taap.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Snow SJ, Schladweiler MC, Richards JE, Ghio AJ, Ledbetter AD, Kodavanti UP 2016. Acute ozone-induced pulmonary and systemic metabolic effects are diminished in adrenalectomized rats. Toxicological Sciences 150: 312–322. 10.1093/toxsci/kfv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E and Ganz T 2009. The role of hepcidin in iron metabolism. Acta Haematologica 122:78–86. 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber AT, Halappanavar S, Folkmann JK, Bornholdt J, Boisen AMZZ, Møller P, Williams A, Yauk C, Vogel U, Loft S, Wallin H 2009. Lack of acute phase response in the livers of mice exposed to diesel exhaust particles or carbon black by inhalation. Particle and Fibre Toxicology 6:12. 10.1186/1743-8977-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber AT, Lamson JS, Jacobsen NR, Ravn-Haren G, Hougaard KS, Nyendi AN, Wahlberg P, Madsen AM, Jackson P, Wallin H, Vogel U 2013. Particle-induced pulmonary acute phase response correlates with neutrophil influx linking inhaled particles and cardiovascular risk. Public Library of Science One 8:e69020. 10.1371/journal.pone.0069020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai K, Kurose K, Koizumi T, Katori N, Sawada J, Matsumura Y, Saijo N, Yamamoto N, Tamura T, Okuda H, Saito Y 2014. Distal promoter regions are responsible for differential regulation of human orosomucoid-1 and -2 gene expression and acute phase responses. Biological Pharmaceutical Bulletin 37(1):164–8. 10.1248/bpb.b13-00551. [DOI] [PubMed] [Google Scholar]
- Schreiber G, Tsykin A, Aldred AR, Thomas T, Fung WP, Dickson PW, Cole T, Birch H, De Jong FA, Milland J 1989. The acute phase response in the rodent. Annals of the New York Academy of Sciences 557:61–86. 10.1111/j.1749-6632.1989.tb24000.x. [DOI] [PubMed] [Google Scholar]
- Shannahan JH, Alzate O, Winnik WM, Andrews D, Schladweiler MC, Ghio AJ, Gavett SH, Kodavanti UP 2012. Acute phase response, inflammation and metabolic syndrome biomarkers of libby asbestos exposure. Toxicology and Applied Pharmacology 260:105–114. 10.1016/j.taap.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Snow SJ, Henriquez AR, Costa DL, Kodavanti UP 2018. Neuroendocrine regulation of air pollution health effects: emerging insights. Toxicological Sciences 164:9–20. 10.1093/toxsci/kfy129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM 2006. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nature Reviews Immunology 6:318–328. 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]


