Abstract
Long-term dietary management of Propionic acidemia (PA) includes natural protein restriction, and supplementation with medical formula enriched with leucine (Leu) and free of valine (Val), isoleucine (Ileu), methionine (Met), and threonine (Thr). As PA medical formulas have high leucine content, concerns started to arise regarding potential long-term health risks of unbalanced leucine intake. PA patients have chronically low plasma levels of Ile and Val, which led to the paradoxical need to supplement with propiogenic single amino acids (AAs). Our report takes a retrospective look at the long-term dietary management of four patients and its reflection on their plasma amino acids. The patients' total protein intake was above the recommended dietary allowance (RDA) and had a high Leu/Val and Leu/Ile intake ratios in diet. Despite adequate total protein intake, patients had chronically low plasma Ile and Val and a high plasma Leu/Val and Leu/Ile ratios, which could be attributed to high Leu intake. We conclude that the best approach to PA dietary management is to only use medical formula with patients not meeting their RDA through natural protein, and to monitor plasma amino acids levels closely.
Keywords: Propionic acidemia, Leucine intake, Dietary management
1. Introduction
Propionic acidemia (PA; OMIM # 606054) is an autosomal recessive disorder caused by a defect in the propionyl-CoA carboxylase enzyme, encoded by PCCA and PCCB genes. This enzyme catalyzes the conversion of propionate to methylmalonic acid, using biotin as a co-factor, which is a crucial step in the propionate metabolism, and directing metabolites resulting from the degradation of the “offending” amino acids (AAs) valine (Val), isoleucine (Ile), methionine (Met) and threonine (Thr), along with the odd-chain fatty acids and the side chain of cholesterol into the Krebs cycle.
Current PA management depends on long-term dietary adjustments that include natural (complete) protein restriction, supplementation with medical formula free of “offending” AAs (incomplete protein), levocarnitine supplementation, cyclic metronidazole use, and acute management of metabolic decompensation [1].
This current management concept has been evolving since the 1970's, when extensive natural protein restriction, even below the Dietary reference intake (DRI), was considered the mainstay of treatment. Yet, to meet the Recommended Daily Allowance (RDA) for protein, PA patients had to be supplemented with large amounts of medical formulas free of the “offending” AAs. Through a better understanding of the disease pathophysiology over time, and in recognizing that patients are mainly at risk when they are in catabolic stress, and that severe protein restriction can lead to decrease in the tolerance of the propiogenic AAs amount in diet, it became clear that PA patients can do better by limiting use of medical formulas and allowing more natural protein intake during periods of medical and biochemical stability; strong restriction in natural protein intake would be only necessary during illness, “sick day” [[2], [3], [4], [5], [6]].
Many metabolic centers use medical formula generously, i.e. 50% or higher of protein intake, with reports of improvement in growth centiles, plasma albumin, and transthyretin concentrations on higher amounts of medical formula [7,8], However, the benefits of this practice are now being questioned with the emergence of new reports portraying better growth outcomes with minimal or no medical formula usage [6,9].
Moreover, the unbalanced branched chain amino acids (BCAAs) diet with high leucine content in PA medical formulas has resulted in concerns regarding the potential long term health risks in this population [[10], [11], [12]].
Currently there is a preference to limit the use of medical formula to patients whose natural protein intake does not meet 100–120% of protein RDA [1,13]. In this paper we aim to provide a long- term detailed overview of the dietary intake, plasma amino acid profiles, and growth parameters of four of our PA patients.
2. Patients and methods
Our institution's ethics committee approved this study with REB application number H20–00548. Patients provided written informed consent for publication.
This is a longitudinal (10–17 years), retrospective, observational study of four PA patients (2 sibling pairs, 3 females and 1 male) followed in our clinic between 2000 and 2018.
The following data were collected retrospectively through review of the patients' health records:
-
•
Dietary intake:
Daily intake of protein (g/kg/day) from natural sources (complete), medical formula protein (incomplete), and total protein intake (all protein sources complete and incomplete). We compared the patients' protein intake with the current 2019 recommendations [13] to draw attention to the differences between past and current recommendations.
Total daily intake of amino acids i.e., Leu, Ile, Met, Thr. from diet and medical formula (mg/kg/day & mg/day), and total daily intake of valine (from diet, medical formula, and single amino acid supplements) were calculated and collected. Daily intake of all amino acids, except for leucine, was compared to the proposed nutrient intakes by Yannicelli 2006 [8]. Leucine intake (mg/kg/day) was compared to the Nutrition Board Medical Institute's suggested recommendations [14].
The data collected was of the actual intake and the dietary analysis performed was based on formula recipes calculations in addition to analysis of detailed dietary histories of 3-day food/intake records.
-
•
Laboratory:
Plasma amino acid (PAA) levels of leucine, valine, isoleucine, methionine, and threonine corresponding to the dietary data were collected. The PAAs were collected 2 to 3 h after feeds in the newborn and infancy period, while during childhood and adolescence the collection was done after 4 to 8 h fasting. The PAA was monitored on weekly basis until 6 months of age, and on monthly basis onwards. However, extra samples were collected if closer monitoring was deemed necessary based on the PAAs levels. The data collected was when the patients were well and on their regular diet. Data when patients were ill, on their sick day or admitted to the hospital were excluded. The PAAs were analyzed in BC children's Hospital Biochemical Genetics Laboratory and the observed levels were compared this lab's reference ranges for the corresponding age groups.
2.1. Statistical analysis
Data were collected in Microsoft Excel for Windows. Software used for the graphs and statistics were Microsoft Excel for windows, JASP 0.14.1.0, and R version 3.6.1. Data are presented as mean ± standard deviation (SD), median, Q1, Q3, maximum and minimum. One Sample t-Test was used to compare the PAAs ratios and the corresponding normal references with a significance value of P < 0.05.
3. Results
In this study we present 4 patients, two patients were diagnosed via targeted testing based on clinical presentation, while the other 2 patients (the younger siblings of the 2 probands) were diagnosed via selective newborn screening. The diagnosis was confirmed by molecular testing in all 4 patients and enzyme activity testing was available for one patient. During their management, the patients had an average of 3.16 ± 0.86/year unwell episodes, with an average of 2.06 ± 0.69 hospital admissions/year due to metabolic decompensation. Three of the four patients transitioned to the adult metabolic clinic at 18 years of age. One patient died due to cardiomyopathy and congestive heart failure at 9 years of age. More information about the clinical outcome and patient characteristics is described in (Table 1).
Table 1.
Patient clinical outcome and characteristics.
| Patient | 1† | 2† | 3ǂ | 4ǂ |
|---|---|---|---|---|
| Age at diagnosis | 38 days | 1 month. | 10 months | 1 month |
| Follow up duration | 16 yrs. and 4 months | 17 yrs. | 18 yrs. and 2 months | 9 yrs. and 2 months |
| Gender | Female | Male | Female | Female |
| Cardiac condition⁎ | Low systolic function with EF (45%–55%) and low normal diastolic function | Cardiomyopathy with EF of 49% | No | Cardiomyopathy with EF of 22% |
| Comorbidities | Sensory neural hearing loss (mild) | Generalized anxiety disorder | Intermittent Thrombocytopenia and neutropenia | Hypotonia |
| Episode of pancreatitis | Learning difficulties | Osteoporosis | Learning difficulties | |
| Osteoporosis - Learning difficulties | Hypotonia | |||
| Learning difficulties | ||||
| Concomitant medical conditions | Asthma | Asthma | Growth Hormone deficiency | N/A |
| Bernard Soulier | Bernard Souleir | |||
| Idiopathic Rt thoracic scoliosis symptomatic surgically corrected at 13 years of age | ||||
| G tube dependence with oral intake aversion | Yes | Yes | Yes | Yes |
| Height⁎⁎ | −1.33 SD | −1.15 SD | −0.8 SD | −1.37 SD |
| Weight⁎⁎ | +0.85 SD | −0.84 SD | −0.03 SD | +1.08 SD |
| BMI⁎⁎ | +1.25 SD | −0.28 SD | + 0.25 SD | +2.4 SD |
| Enzyme activity in fibroblasts | Propionyl CoA Carboxylase < 0.1§ | N/A | N/A | N/A |
| Molecular testing | Homozygous variant NM_000282.4(PCCA): c.134_135del (p. Leu45fs) | Compound heterozygous NM_000532.5(PCCB): c.337C>T (p. Arg113Ter) and c.1172T>C (P.Phe391ser.) | ||
| Final clinical outcome | Transfer to adult care metabolic service | Died due to cardiomyopathy and Congestive heart failure at 9 yrs. | ||
Patients 1 & 2 are siblings.
Patients 3 & 4 are sibling.
(nmol/h/mg), control range (134–254).
The EF was the last recorded measures for the 4 patients, done at 18 years of age for all except patient 4, whose measure was done at 8 years and 10 months of age.
Height, weight, and BMI were the last recorded prior to adult transition for all patients, except patient 4, whose last recorded measures were at 8 years and 10 months of age prior to patient's death.
All patients were on a long-term dietary treatment with restriction in natural protein intake and supplemented with medical formula. The Ross nutritional guidelines were used between the years 2001–2004 [15]. Starting in 2004 the Sass [16] recommendations was used. In addition, all patients received supplementation of l-carnitine (100 mg/kg/day) orally and were put on a cyclic metronidazole regime. Adjustments to dietary treatment were made based on patient age, weight, health status and biochemical monitoring.
3.1. Protein intake
Our Four patients received their daily requirements mainly through formula and had negligible oral intake due to severe food aversion that progressed with time. They received their prescribed formula as boluses during the daytime and continuous infusion overnight through a G-tube.
The mean natural (complete) protein intake (g/kg/day) was never less than 60% of RDA for all age groups but it never reached 100% RDA with average intake of 0.79 ± 0.15 g/kg/day (80% ± 13% of RDA). (Suppl. Fig. 1B).
The mean total protein intake, expressed as natural (complete) protein plus protein supplied from medical foods/formula (incomplete), exceeded 100% RDA and even surpassed 120% RDA between (0.5–8 years) with average intake of 1.81 ± 0.51 g/kg/day (182% ± 50% of RDA) (Suppl. Fig. 1A). The average protein intake from medical formula was 1.02 ± 0.49 g/kg/day (103% ± 49% of RDA) being higher in younger ages (Suppl. Fig. 1C), with the ratio of natural protein to protein from medical formula increasing gradually over the course of management (Suppl. Fig. 2).
3.2. Amino acids intake (Leu, Ile, Val, Met, Thr)
Regarding amino acid intake, all patients were supplemented with only valine 1 year after diagnosis due to persistent low valine levels in the plasma amino acids during monitoring. Supplements were prepared using a sterile solution containing 10 mg amino acid/ml (i.e., 1.0 g amino acid to make 100 ml total volume).
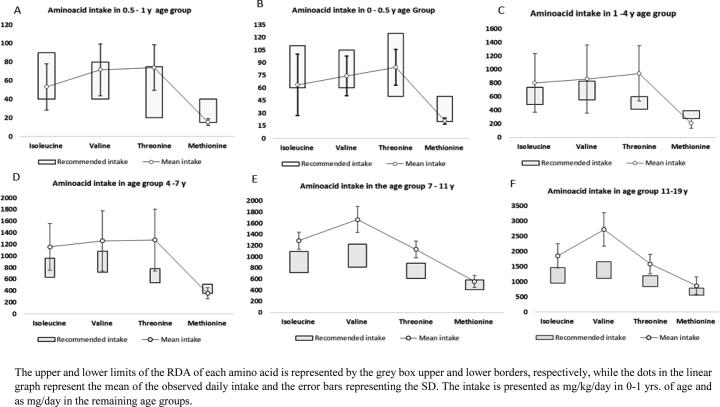
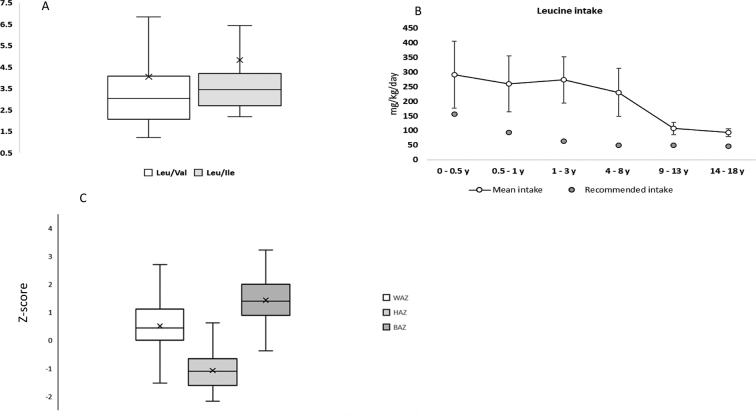
The median intake from the supplementation was 180 mg/day (10–1200 mg), which represented 17% ± 9% (2%–66%) of the total daily valine intake. The total intake of the two propiogenic amino acids valine and isoleucine was either within or even above the RDA, with an average intake of 61.02 ± 24.91, 52.11 ± 22.66 mg/kg/day across all age groups, respectively. The average methionine intake was just above the lower limit of RDA in the first year of life and was noted to be below the RDA in age groups 1–4 years and 4–7 years, with a mean of 204.34 ± 71.27, and 355.76 ± 94.18 mg/day, respectively. A description of the amino acid (Ile, Val, Met, Thr) intake compared to the RDA is shown in (Fig. 1). The average leucine intake was above the RDA in all age groups (Fig. 2B) with mean of 362% ± 165% of RDA which resulted in a high Leu/Val and Leu/Ile ratios in the diet (Fig. 2A).
Fig. 1.
Amino acids daily intake.
The upper and lower limits of the RDA of each amino acid is represented by the grey box upper and lower borders, respectively, while the dots in the linear graph represent the mean of the observed daily intake and the error bars representing the SD. The intake is presented as mg/kg/day in 0–1 yrs. of age and as mg/day in the remaining age groups.
Fig. 2.
(A) shows the ratio of Leu/Ile and Leu/Val in the daily intake in all four patients. The Box represents 25%–75% (i.e., Q1–Q3) with the transverse line as the median. The mean value is represented by the X mark, while the whiskers represent min and max.(B) Average leucine intake in diet (mg/kg/day) calculated as mean (white dot) ± St. deviation (error bars). The average intake of leucine in diet regimen exceeded the recommended daily intake (grey dots) as suggested by Food and Nutrition Board of the Institute of Medicine, Dietary reference intakes (2005) (C) shows the z score of the anthropometric measures of our case series WAZ = weight for age, HAZ = height for age, BAZ = BMI for age.
3.3. Anthropometric growth measure
Height-for-age (HAZ), weight-for-age (WAZ), and BMI-for-age (BAZ), expressed as z scores, are presented in (Fig. 2C) and in more detail in (Suppl. Table 1). The HAZ was the most affected parameter compared to the WAZ and BAZ with mean of −1.078 ± 0.957, (− 6.1–1.81). A more detailed description of the growth parameters' z score in relation to the WHO standard distribution curve is in (Suppl. Fig. 3).
3.4. Plasma amino acids level
Out of the five monitored plasma amino acids, valine (67.41 ± 33.05 μmol/l) and isoleucine (49.79 ± 25.67 μmol/l) were the two AAs most frequently found to be low, at rates of 91% and 42% respectively. This was reflected when calculating the Leu/Val and Leu/Ile ratios. The Leu/Val ratio was significantly higher than normal references, P = 0.01 for the 0–1 months group and p < 0.001 for the rest of the age groups. When comparing the plasma Leu/Ile ratio in our four patients with the normal references, we noted that it was also significantly higher, P = 0.017 for the 0–1 months group and p < 0.001 for the rest of the age groups. The plasma Leu/Thr ratio in our four patients was significantly higher in all age groups (p < 0.05), except for (3 months - 16 yrs.) age group (p = 0.114). On the other hand, the plasma Leu/Met ratio in our four patients was not significantly higher than the normal reference in all age groups (p > 0.05). A more detailed description of the PAAs ratios and the normal reference is in (Fig. 3 and suppl. Tables 2–5).
Fig. 3.
PAA ratios. These four graphs are a description of the PAA ratios in our 4 patients at different ages, represented as Box and whisker plot. The Box represents 25%–75% (i.e., Q1–Q3) with the transverse line as the median. The mean value is represented by the X mark, while the whiskers represent min and max. The corresponding normal reference of the PAA ratio is represented by the white dots in the liner graph.
4. Discussion
This retrospective study aimed to provide a detailed description of the long-term dietary management of four patients with PA, and the reflection of this management on the plasma amino acid levels while on their regular daily diet during periods of metabolic stability. For the majority of the follow up (2000–2018), dietary management followed guidelines available at the time (The Ross followed by the Sass. et al., recommendations) [15,16] and not the latest 2019 PA dietary guidelines [13]. The Ross Nutrition support protocol advised to prescribe protein in an amount that exceeds the RDA especially if the patients are dependant on L-amino acids (medical formula) for most of the protein supply. The initial prescription should be the highest RDA and 50% of the total prescription should be of natural protein, with a possibility of increasing the natural protein if PAA levels were low on the monitoring blood work [15].
This generous protein prescription was because protein requirements can be higher when using incomplete protein source from medical formula since it has a faster absorption and catabolism rates, therefore, the plasma levels of total and essential amino acids can be higher, peak faster and decrease more rapidly after consuming large portions of protein supplied by medical formula [17,18].
The Sass et al. recommendations was published in 2004 [16], it suggested a lower total protein intake when compared to the Ross protocols, and it allowed a more liberal approach when it came to the natural protein intake (up to 75% of RDA). Despite the emergence of the European guidelines in 2014, our center opted to continue with the Sass recommendations, as it allowed a more unified approach and limited variable individual therapeutic approaches within the same center by having a clear description of the complete, incomplete, and total protein RDA. On the other hand, the European guidelines used the FAO/WHO/UNU (2007) recommendations for protein intake (safe levels of protein intake titrated as an age adjusted mean + 2 SD) which was meant for healthy population, and did not provide a clear description of different protein intakes (complete, incomplete, and total protein), however it did recommend that medical formulas should only be used if the patient did not tolerate the minimal RDA from natural protein [1].
4.1. Protein intake
The prescription of protein intake in PA can be quite challenging, it requires a fine balance between maintaining good metabolic control and providing nutritional support. This can be a very difficult task as the tolerance to natural protein as well as total protein requirement vary for the same patient depending on the age, state of health, rate of growth and metabolic monitoring. It can also vary between patients depending on the genotype i.e., enzyme activity.
Deciding on the portion of the protein supplied by medical formula can be highly variable even within the same center [2,16,19], and depends on the physician's experience, or the specific protocols of each metabolic centre; currently there is no consensus to specify a safe percentage of medical formula intake.
Generous medical formula supplementation was a common practice in many centers [7,8,19], and average protein intake from medical formula reported in most of the previous studies was 15%–50% of the total protein prescription [19,20]. Yet, others opted to limit [6,9,11], or completely avoid the use of medical formula [9] and were able to report a well managed cohort of patients with a good growth outcome [9].
Although the natural protein intake in our four patients was within the range recommended by current 2019 guidelines [13], it never reached 100% RDA and represented 47% ± 15% of the total protein, while total protein intake exceeded RDA due to generous usage of medical formula. Given the restriction of natural protein, we tended to meticulously follow the PAA levels on regular bases. Once weekly until 6 months of age and once monthly there after. Once a deficiency was noted we made an increase of 10% in the natural protein and remeasured the PAA in 3 days time.
Assigning an adequate RDA for protein in this population is complex task, as most of the proposed guidelines use RDA made for healthy population [1]. While patients with PA appear to be having a total protein intake (complete and incomplete) that is more than adequate (above RDA), failure to adjust for protein quality, digestibly, absorption as well as rate of catabolism put them at risk of insufficiency. In addition, current guidelines [1,13] do not adjust for conditions of energy deficit and increased energy demand i.e., infections.
4.2. Leucine intake
There are no leucine intake guidelines specific to PA [1,13]. Therefore, we compared leucine intake to the dietary reference intakes suggested by the Food and Nutrition Board (2005) [10]. Leucine intake was consistently higher than RDA in all age groups as described in (Fig. 2B).
This can be attributed to the fact that medical formula has a high leucine content, 141 mg of Leu per 1 g of protein, compared to approximately 96 mg of Leu per 1 g of protein in infant formula, and 86 mg of Leu per 1 g of protein in human milk [10,22]. For our patients, an average of 71% ± 13% (148.16 ± 70 mg/kg/day) of total leucine intake was from medical formula compared to only 28% ± 13% (47.7 ± 9.4 mg/kg/day) from natural protein.
The high leucine intake along with restriction in Val., and Ile., in our patients, contributed to high Leu/Val and Leu/Ile ratios in the diet with a median of 3.03: 1 and 3.4: 1, respectively. This observation is not new and has been described in previous reports [5,11] in methylmalonic aciduria (MMA), a disease that has the same nutritional management as PA [1]. Although leucine is an important amino acid that plays a role in protein synthesis in muscle and glucose regulation [23], concerns are emerging regarding the potential long term effects of high leucine intake in PA patients [5,6,10,11] as high leu diet seems not to be without adverse effects [10,24].
It can supress appetite by signalling to the hypothalamus [25], and whether this plays a major part in the food aversion that is observed in many PA patients - including our four patients who struggled with severe food aversion that did not resolve over time - is yet to be determined. It was found to induce hyperammonemia in adult males when the intake was at 550 mg/kg/day [21], but the cut-off of leucine intake that can induce hyperammonaemia in children has not been described, thus we can not assign a safe upper level of intake. This effect can be detrimental in populations vulnerable to hyperammonemia like PA patients especially that most of the published studies, including ours, reported a high intake of leucine [5,11].
High leucine intake also disturbs the neurotransmitter balance in the central nervous system (CNS); as it competes with other large neutral amino acids (LNAA) for the LNAA transporter (LAT1) at the blood brain barrier, impacting other LNAA levels in the CNS [26,27].
4.3. Valine and isoleucine plasma levels
Of the five amino acids recorded in our patients, valine and isoleucine were most frequently below the normal range. This was observed despite the fact that our patients' total protein intake exceeded the RDA, and their total amino acid intake frequently surpassed proposed recommendations (Fig. 1), which aligns with previous reports [6,11,12,20,28]. Hence, it can not be hypothesized that these low levels are merely due to dietary restriction per se [27], and one can assume that the high leucine intake played a role.
In our patients, leucine intake was higher than the amount of leucine that led to a significant decrease in the plasma concentrations of isoleucine and valine when given as a bolus to young healthy men [29]. It has been found that both the oral and IV administration of leucine leads to a decrease in Ile and Val plasma levels by increasing the influx of these amino acids across the cell membrane and increasing the rate of their oxidation through inhibiting the deactivation of the branched chain ketoacid dehydrogenase [29,30]. This increase in the catabolism of Val and Ile can actually lead to higher Val and Ile demands [24] to meet the requirements and to maintain normal levels, which is paradoxical to the main goal of PA dietary management.
The high Leu/Val and Leu/Ile intake ratios in our patients were reflected in the corresponding plasma AAs ratio, as the Leu/Val and Leu/Ile ratios in the plasma was persistently higher than the normal level. It was previously reported that patients with high intake of medical formula has BCAA ratios that were more distorted than those not on medical formula, in addition the cessation of medical formula led to restoration of normal ratios [11].
Although HAZ (−1.078 ± 0.957) of our four patients was not different than previous reports [3,5] and may be better [16], it did not reflect an ideal linear growth outcome. PA still does not have an ideal growth outcome, and those patients are liable to growth retardation and short stature due to many contributing factors [9], including, but not limited to excessive natural protein restriction, acute decompensation episodes, metabolic acidosis and food aversion [16].
Patients on a higher medical formula intake who had low plasma valine and isoleucine levels, like our patients, had HAZ and WAZ that correlated negatively with the Leu/Val intake ratio even though their natural protein prescription was at or above the RDA [12].
Essential amino acid deficiency has been linked to childhood stunting [31,32], and Ile and Val are essential AAs that support normal growth and development. Chronically low levels can potentially be harmful, inducing catabolism, hyperammonemia, aggravating metabolic decompensations, and contributing to stunted growth [3,12,20,27]. But to what extent long term low plasma BCCAs levels Val, and Ile affected the growth outcome in our patients can not be determined by this report.
4.4. Valine supplementation
Given the persistently low plasma valine in all of our patients that was not adequately corrected despite increments in the natural protein intake, all four patients received single AA valine supplementation, [11] without any observed normalization in plasma valine levels long term.
This observation can be explained by the fact that high leucine intake can enhances the oxidation of the BCCAs, and that PA patients' diet is mainly composed of sources with low biological value and digestibility [10,12], it can be proposed that the Ile and Val requirements in PA patients can be higher [10,30] as discussed above.
5. Conclusions
Despite high total protein and amino acid intake, our patients showed an imbalance of plasma BCAAs ratios with a tendency towards low valine and isoleucine, despite Val supplementation, which can be a reflection of the high Leu/Val, and Leu/Ile intake ratios. As leucine content is higher in medical formulas, and high leucine intake seems to be not without a side effect, the most recent guidelines states that medical formula free of offending AAs should be prescribed only to patients who are not able to tolerate their RDA of total protein from natural sources.
Due to the persistently low Val and Ile plasma AAs levels observed in our patients, we advise that the BCAAs levels should be monitored closely particularly for those patients who do not tolerate sufficient amounts of natural protein and needing medical formulas free of offending AA, in order to keep them within normal ranges until we have more evidence about a safe level and more studies that explore the effect of the BCCAs ratio imbalance on the outcome.
The extent this imbalance exerted on the final long-term outcomes in our patients cannot be answered by this study; its small sample size and retrospective data collection meant that time- controlled monitoring and exclusion of other confounding factors was not possible. Given that this is a retrospective study, some biochemical markers were not collected due to lack of availability. In addition, other nutritional and biochemical markers and energy intake were not collected or reviewed, as this study's focus was on the PAA intake and the corresponding changes in plasma BCCA ratio.
Disclosure
The authors of the manuscript have nothing to disclose
Acknowledgments
Acknowledgement
The authors of this manuscript would like to show their appreciation to K. Ueda, C. Hartnett for their contribution to the dietary management of these patients, and to Gloria Ho, and A. Giezen for their advice and input during the writing of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2021.100757.
Appendix A. Supplementary data
Supplementary material
References
- 1.Baumgartner M.R., Hörster F., Dionisi-Vici C., Haliloglu G., Karall D., Chapman K.A., Huemer M., Hochuli M., Assoun M., Ballhausen D., Burlina A., Fowler B., Grünert S.C., Grünewald S., Honzik T., Merinero B., Pérez-Cerdá C., Scholl-Bürgi S., Skovby F., Wijburg F., MacDonald A., Martinelli D., Sass J.O., Valayannopoulos V., Chakrapani A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser N.S., Manoli I., Graf J.C., Sloan J., Venditti C.P. Variable dietary management of methylmalonic acidemia: metabolic and energetic correlations. Am. J. Clin. Nutr. 2011;93:47–56. doi: 10.3945/ajcn.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molema F., Haijes H.A., Janssen M.C., Bosch A.M., van Spronsen F.J., Mulder M.F., Verhoeven-Duif N.M., Jans J. Clinical nutrition (Edinburgh, Scotland) Advance online publication; 2020. High protein prescription in methylmalonic and propionic acidemia patients and its negative association with long-term outcome. (S0261-5614(20)30702-0) [DOI] [PubMed] [Google Scholar]
- 4.de Baulny H.O., Benoist J.F., Rigal O., Touati G., Rabier D., Saudubray J.M. Methylmalonic and propionic acidaemias: management and outcome. J. Inherit. Metab. Dis. 2005;28(3):415–423. doi: 10.1007/s10545-005-7056-1. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein L.E., Burns C., Drumm M., Gaughan S., Sailer M., Baker P.R., II Impact on isoleucine and valine supplementation when decreasing use of medical food in the nutritional management of methylmalonic acidemia. Nutrients. 2020;12:473. doi: 10.3390/nu12020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touati G., Valayannopoulos V., Mention K., de Lonlay P., Jouvet P., Depondt E., Assoun M., Souberbielle J.C., Rabier D., Ogier de Baulny H., Saudubray J.-M. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J. Inherit. Metab. Dis. 2006;29:288–298. doi: 10.1007/s10545-006-0351-7. [DOI] [PubMed] [Google Scholar]
- 7.Yannicelli S., Acosta P.B., Velazquez A., Bock H.G., Marriage B., Kurczynski T.W., Miller M., Korson M., Steiner R.D., Rutledge L., Bernstein L., Chinsky J., Galvin-Parton P., Arnold G.L. Improved growth and nutrition status in children with methylmalonic or propionic acidemia fed an elemental medical food. Mol. Genet. Metab. 2003;80:181–188. doi: 10.1016/j.ymgme.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Yannicelli S. Nutrition therapy of organic acidaemias with amino acid-based formulas: emphasis on methylmalonic and propionic acidaemia. J. Inherit. Metab. Dis. 2006;29:281–287. doi: 10.1007/s10545-006-0267-2. [DOI] [PubMed] [Google Scholar]
- 9.Evans M., Truby H., Boneh A. The relationship between dietary intake, growth, and body composition in inborn errors of intermediary protein metabolism. J. Pediatr. 2017;188:163–172. doi: 10.1016/j.jpeds.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Myles J.G., Manoli I., Venditti C.P. Effects of medical food leucine content in the MANA$gement of methylmalonic and propionic acidemias. Curr. Opin. Clin. Nutr. Metab. Care. 2018;21:42. doi: 10.1097/MCO.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manoli I., Myles J.G., Sloan J.L., Shchelochkov O.A., Venditti C.P. 2016. A critical reappraisal of dietary practices in methylmalonic acidemia raises concerns about the safety of medical foods. Part 1: isolated methylmalonic acidemias. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molema F., Gleich F., Burgard P., Ploeg A.T., Summar M.L., Chapman K.A., Barić I., Lund A.M., Kölker S., Williams M., Hörster F., Jelsig A.M., Lonlay P., Wijburg F.A., Bosch A., Freisinger P., Posset R., Augoustides-Savvopoulou P., Avram P., Deleanu C., Baumgartner M.R., Häberle J., Blasco-Alonso J., Burlina A.B., Rubert L., Cazorla A.G., Saladelafont E.C.I., Dionisi-Vici C., Martinelli D., Dobbelaere D., Mention K., Grünewald S., Chakrapani A., Hwu W., Chien Y., Lee N., Karall D., Scholl-Bürgi S., Lachmann R., De Laet C., Matsumoto S., Meirleir L., Mühlhausen C., Schiff M., Peña-Quintana L., Djordjevic M., Sarajlija A., Sykut-Cegielska J., Wisniewska A., Leao-Teles E., Alves S., Vara R., Vives-Pinera I., Ortega D.G., Morris A., Zeman J., Honzik T., Chabrol B., Arnaudo F., Cano A., Thompson N., Eyskens F., Lindner M., Lüsebrink N., Jalan A., Sokal E., Legros V., Nassogne M.C. Evaluation of dietary treatment and amino acid supplementation in organic acidurias and urea-cycle disorders: on the basis of information from a European multicenter registry. J. Inherit. Metab. Dis. 2019;42:1162–1175. doi: 10.1002/jimd.12066. [DOI] [PubMed] [Google Scholar]
- 13.Jurecki E., Ueda K., Frazier D., Rohr F., Thompson A., Hussa C., Obernolte L., Reineking B., Roberts A.M., Yannicelli S., Osara Y., Stembridge A., Splett P., Singh R.H. Nutrition management guideline for propionic acidemia: an evidence- and consensus-based approach. Mol. Genet. Metab. 2019;126:341–354. doi: 10.1016/j.ymgme.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine . The National Academies Press; Washington, DC, USA: 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [Google Scholar]
- 15.Acosta P. 4th ed. Ross Products Division Abbot Laboratories; Columbus Ohio: 2001. Nutrition Support Protocols : The Ross Metabolic Formula System. [Google Scholar]
- 16.Sass J.O., Hofmann M., Skladal D., Mayatepek E., Schwahn B., Sperl W. Propionic acidemia revisited: a workshop report. Clin. Pediatr. (Phila) 2004;43:837–843. doi: 10.1177/000992280404300908. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.L., Arteaga C., Heymsfield S.B. Increased ureagenesis and impaired nitrogen use during infusion of a synthetic amino acid formula. N. Engl. J. Med. 1982;306:1013–1018. doi: 10.1056/nejm198204293061702. [DOI] [PubMed] [Google Scholar]
- 18.Gropper S.S., Acosta P.B. Effect of simultaneous ingestion of l-amino acids and whole protein on plasma amino acid and urea nitrogen concentrations in humans. J. Parenter. Enter. Nutr. 1991;15:48–53. doi: 10.1177/014860719101500148. [DOI] [PubMed] [Google Scholar]
- 19.Daly A., Pinto A., Evans S., Almeida M.F., Assoun M., Belanger-Quintana A., Bernabei S.M., Bollhalder S., Cassiman D., Champion H., Chan H., Dalmau J., de Boer F., de Laet C., de Meyer A., Desloovere A., Dianin A., Dixon M., Dokoupil K., Dubois S., Eyskens F., Faria A., Fasan I., Favre E., Feillet F., Fekete A., Gallo G., Gingell C., Gribben J., Hansen K. Kaalund, Ter Horst N.M., Jankowski C., Janssen-Regelink R., Jones I., Jouault C., Kahrs G.E., Kok I.L., Kowalik A., Laguerre C., Le Verge S., Lilje R., Maddalon C., Mayr D., Meyer U., Micciche A., Och U., Robert M., Rocha J.C., Rogozinski H., Rohde C., Ross K., Saruggia I., Schlune A., Singleton K., Sjoqvist E., Skeath R., Stolen L.H., Terry A., Timmer C., Tomlinson L., Tooke A., Kerckhove K. Vande, van Dam E., van den Hurk T., van der Ploeg L., van Driessche M., van Rijn M., van Wegberg A., Vasconcelos C., Vestergaard H., Vitoria I., Webster D., White F.J., White L., Zweers H., MacDonald A. Dietary practices in propionic acidemia: a European survey. Mol. Genet. Metab. Reports. 2017;13:83–89. doi: 10.1016/j.ymgmr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly A., Gerrard S.E.A. 2015. The Nutritional Intake of Patients with Organic Acidaemias on Enteral Tube Feeding: Can We Do Better ? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elango R., Rasmussen B., Madden K. Safety and tolerability of leucine supplementation in elderly men. J. Nutr. 2016;146:2630S–2634S. doi: 10.3945/jn.116.234930. [DOI] [PubMed] [Google Scholar]
- 22.Melnik B.C. Excessive leucine-mTORC1-signalling of cow milk-based infant formula: the missing link to understand early childhood obesity. J. Obes. 2012;2012 doi: 10.1155/2012/197653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Sakamoto O., Matsubara Y., Kure S., Suzuki Y., Aoki Y., Suzuki Y., Sakura N., Takayanagi M., Iinuma K., Ohura T. Mutation analysis of the MMAA and MMAB genes in Japanese patients with vitamin B12-responsive methylmalonic acidemia: identification of a prevalent MMAA mutation. Mol. Genet. Metab. 2004;82:329–333. doi: 10.1016/j.ymgme.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Wiltafsky M.K., Pfaffl M.W., Roth F.X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 2010;103:964–976. doi: 10.1017/S0007114509992212. [DOI] [PubMed] [Google Scholar]
- 25.Li F., Yin Y., Tan B., Kong X., Wu G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41 doi: 10.1007/s00726-011-0983-2. [DOI] [PubMed] [Google Scholar]
- 26.Strauss K.A., Wardley B., Robinson D., Hendrickson C., Rider N.L., Puffenberger E.G., Shelmer D., Moser A.B., Morton D.H. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol. Genet. Metab. 2010;99:333–345. doi: 10.1016/j.ymgme.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholl-Bürgi S., Sass J.O., Zschocke J., Karall D. Amino acid metabolism in patients with propionic acidaemia. J. Inherit. Metab. Dis. 2012;35:65–70. doi: 10.1007/s10545-010-9245-9. [DOI] [PubMed] [Google Scholar]
- 28.Scholl-Bürgi S., Sass J.O., Heinz-Erian P., Amann E., Haberlandt E., Albrecht U., Ertl C., Sigl S.B., Lagler F., Rostasy K., Karall D. Changes in plasma amino acid concentrations with increasing age in patients with propionic acidemia. Amino Acids. 2010;38:1473–1481. doi: 10.1007/s00726-009-0356-2. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto T., Nakamura K., Matsumoto H., Sakai R., Kuwahara T., Kadota Y., Kitaura Y., Sato J., Shimomura Y. Bolus ingestion of individual branched-chain amino acids alters plasma amino acid profiles in young healthy men. Springerplus. 2014;3:35. doi: 10.1186/2193-1801-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harper A.E., Miller R.H., Block K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 31.Uauy R., Suri D.J., Ghosh S., Kurpad A., Rosenberg I.H. Low circulating amino acids and protein quality: an interesting piece in the puzzle of early childhood stunting. EBioMedicine. 2016;8 doi: 10.1016/j.ebiom.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semba R.D., Shardell M., Sakr Ashour F.A., Moaddel R., Trehan I., Maleta K.M., Ordiz M.I., Kraemer K., Khadeer M.A., Ferrucci L., Manary M.J. Child stunting is associated with low circulating essential amino acids. EBioMedicine. 2016;6 doi: 10.1016/j.ebiom.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material