Abstract
Background
Little is known about health-related quality of life (HRQOL) following treatment for bladder cancer (BC).
Objective
To determine this, we undertook a cross-sectional survey covering 10% of the English population.
Design, setting, and participants
Participants 1–10 yr from diagnosis were identified through national cancer registration data.
Intervention
A postal survey was administered containing generic HRQOL and BC-specific outcome measures. Findings were compared with those of the general population and other pelvic cancer patients.
Outcome measurements and statistical analysis
Generic HRQOL was measured using five-level EQ-5D (EQ-5D-5L) and European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ)-C30. BC-specific outcomes were derived from EORTC QLQ-BLM30 and EORTC QLQ-NMIBC24.
Results and limitations
A total of 1796 surveys were completed (response rate 55%), including 868 (48%) patients with non–muscle-invasive BC, 893 (50%) patients who received radiotherapy or radical cystectomy, and 35 (1.9%) patients for whom treatment was unknown. Most (69%) of the participants reported at least one problem in any EQ-5D dimension. Age/sex-adjusted generic HRQOL outcomes were similar across all stages and treatment groups, whilst problems increased with age (problems in one or more EQ-5D dimensions: <65 yr [67% {95% confidence interval or CI: 61–74}] vs 85+ yr [84% {95% CI: 81–89}], p = 0.016) and long-term conditions (no conditions [53% {95% CI: 48–58}] vs more than four conditions [94% {95% CI: 90–97}], p < 0.001). Sexual problems were reported commonly in men, increasing with younger age and radical treatment. Younger participants (under 65 yr) reported more financial difficulties (mean score 20 [95% CI: 16–25]) than those aged 85+ yr (6.8 [4.5–9.2], p < 0.001). HRQOL for BC patients (for comparison, males with problems in one or more EQ-5D dimensions 69% [95% CI: 66–72]) was significantly worse than what has been found after colorectal and prostate cancers and in the general population (51% [95% CI: 48–53], all p < 0.05).
Conclusions
HRQOL following BC appears to be relatively independent of disease stage, treatment, and multimodal care. Issues are reported with sexual function and financial toxicity. HRQOL after BC is worse than that after other pelvic cancers.
Patient summary
Patients living with bladder cancer often have reduced quality of life, which may be worse than that for other common pelvic cancer patients. Age and other illnesses appear to be more important in determining this quality of life than the treatments received. Many men complain of sexual problems. Younger patients have financial worries.
Keywords: Bladder cancer, Bacillus Calmette-Guerin, Radical cystectomy, Quality of life, Health-related quality of life, Patient-reported outcomes, EQ-5D
Take Home Message
Patients living with bladder cancer often have reduced quality of life, which may be worse than that in patients with other common pelvic cancers. Age and other illnesses appear to be more important in determining this quality of life than the treatments received. Many men complain of sexual problems. Younger patients have financial worries.
1. Introduction
In 2018, 500 000 new cases of bladder cancer (BC) were diagnosed worldwide [1]. BC encompasses a spectrum of disease, from indolent non–muscle-invasive BC (NMIBC) with a long natural history [2] to aggressive muscle-invasive BC (MIBC) requiring radical surgery, radiotherapy (RT), and chemotherapy [3], [4]. Patients have variable life expectancies due to competing comorbidities [5]. Of the three key questions that matter to cancer patients (survival, experience of care [6], [7], and future quality of life [8]), there are significant gaps in our understanding of health-related quality of life (HRQOL). Most HRQOL reports have focussed on survivors of MIBC [9], [10], in whom radical cystectomy (RC) or radical RT impact urinary [11], bowel [12], and sexual function [13], [14] and body image [15], [16]. Deficits in social interactions, physical activity, and emotional function have been described [17]. Little is known about HRQOL following diagnosis of NMIBC [18], [19], longer-term BC outcomes, and how these patients compare with other cancer patients.
Robust, large-scale, patient-centred studies are vital to fully understand outcomes, inform treatment options, and deliver services to support unmet needs [20]. Most studies on patient-reported outcome measures (PROMs) for BC have been small, with limited follow-up [21]. In a pilot study of patients 1–5 yr after diagnosis [17], we identified better HRQOL in patients with NMIBC who received adjuvant treatments rather than just endoscopic surgery. Lowest HRQOL was found for patients receiving RT for advanced BC (problems with mobility, self-care, performing usual activities, and urinary frequency; and more likely to be socially distressed, lack energy, and be unable to work compared with others). However, this work collected limited treatment information, used generic PROM tools, and did not allow comparison with other cancer groups.
Our primary objective was to define, at a population level, the HRQOL of individuals living with and beyond BC diagnosed within the previous 10 yr. Our secondary objective was to compare this HRQOL with that in other pelvic cancer patients and the general population.
2. Patients and methods
2.1. Study design and setting
A cross-sectional survey of individuals 1–10 yr after BC diagnosis was performed during January 2007–December 2016 (as detailed by Mason et al [9]). Eligible patients were diagnosed in National Health Service (NHS) hospitals within Yorkshire and Humber, North Derbyshire, or South Tees regions of England (area covering approximately 5.9 million persons [11% of the total English population]), with 22 hospitals providing urological services (Supplementary Fig. 1). Individuals were identified through the National Cancer Registration and Analysis Service (NCRAS) and excluded if under 18 yr of age, serving a custodial sentence (in Her Majesty’s Prison Service), or had registered objection to participating in research (type 2 with NHS Digital) [22]. Survey administration was coordinated by an NHS-approved independent survey provider (Quality Health Ltd., Unit 1, Holmewood Business Park, Chesterfield Road, Holmewood, Chesterfield S42 5US, UK). Participants consented by returning a completed questionnaire or declined by not responding, returning an unanswered survey, or opting out via a Freephone helpline. Options to participate online or by phone were available.
2.2. Survey content
The survey (Supplementary material) included questions about the participant’s sociodemographics, presence of other long-term conditions (LTCs), and treatment received. Generic HRQOL was assessed with the five-level EQ-5D (EQ-5D-5L) [23], [24] and European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ)-C30 [25]. BC treatment–specific outcomes were assessed using EORTC QLQ-NMIBC24 [18] and EORTC QLQ-BLM30 [26]. The EQ-5D-5L covers five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), plus a 0–100 rating of self-assessed health (SAH). EQ-5D-5L comparative data are available from other large studies on prostate [27] and colorectal cancer PROMs [28] and population survey [29]. EORTC QLQ-C30 consists of five functional scales, three symptom scales, and six single items assessing symptoms (including financial impact and a global scale of quality of life) [25]. For BC-specific assessment relevant to both NMIBC and MIBC, whilst limiting item duplication and participant burden, we obtained permission to combine the two EORTC questionnaires QLQ-BLM30 and QLQ-NMIBC24 (N. Aaronson, personal communication, 2017). The final merged questionnaire contained 34 questions comprising eight scales and single items (Supplementary material). Table 1 provides an overview of the outcome measures, and their scoring and interpretation.
Table 1.
Overview of patient-reported outcome measures in our questionnaire
| Outcome and instrument | Item summary | Response scale | Scoring | Clinically meaningful differences |
|---|---|---|---|---|
| Generic HRQOL | ||||
| EQ-5D-5L | 5 items assessing mobility, self-care, usual activities, pain/discomfort, and anxiety/depression | 5-point scale: 1 (no problem) to 5 (extreme problem) | Individual and combined responses summarised to percentage of participants reporting at least one problem (any severity) on any domain and those reporting no problems | |
| EQ-5D visual analogue scale | Single item rating overall self-assessed heath | 0 (worse) to 100 (best) scale of health you can imagine | Higher score = better QOL | ≥7 points |
| Cancer-specific QOL | ||||
| EORTC QLQ-C30 | 5-functional scales measuring physical, emotional, cognitive, social, and role | 4-point scale: 1 (not at all) to 4 (very much) | All EORTC responses are linearly transformed to 0–100 scales. Higher score = better functioning | ≥10 points |
| 3-symptom scales measuring fatigue, pain, and nausea/vomiting | 4-point scale: 1 (not at all) to 4 (very much) | Higher score = worse symptoms | ||
| Single items assessing symptoms (dyspnoea, insomnia, appetite, constipation, diarrhoea) and financial impact of cancer | 4-point scale: 1 (not at all) to 4 (very much) | Higher score = worse symptoms | ||
| 2 items assessing global health status | 7-point scale: 1 (very poor) to 7 (excellent) | Higher score = better QOL | ||
| Treatment and cancer-specific QOL | ||||
| Combined EORTC QLQ-BLM30 and QLQ-NMIBC24 (34 items in total)* | Urinary symptoms scale (7 items) | 4-point scale: 1 (not at all) to 4 (very much) | Higher scores = worse symptoms/more problems for all scales and items excluding sexual function and enjoyment | |
| Urostomy problems (6 items) | ||||
| Catheter use problems (1 item) | ||||
| Intravesical treatment issues (1 item) | ||||
| Bloating and flatulence scale (2 items) | ||||
| Malaise scale (2 items) | ||||
| Body image scale (3 items) | ||||
| Sexual function scale (2 items) | Higher scores = better functioning | |||
| Sexual enjoyment (single item) | Higher scores = more enjoyment | |||
| Sexual intimacy (1 item) | ||||
| Male sexual problems (2 items) | ||||
| Female sexual problems (1 item) | ||||
| Risk of contaminating partner (1 item) | ||||
| Future worries scale (4 items) | ||||
EORTC = European Organization for Research and Treatment of Cancer; HRQOL = health-related quality of life; QOL = quality of life.
Permission obtained from EORTC to combine items from QLQ-BLM30 and QLQ-NMIBC24.
2.3. Data linkage and treatment grouping
Responses were linked to patient, tumour, and treatment data collected by NCRAS, including extracts from national cancer registration, Hospital Episodes Statistics, Radiotherapy Data Set, and Systemic Anti-Cancer Therapy datasets. Using a combination of these datasets, respondents were categorised into treatment groups (Supplementary Table 1). RT regimens were classified according to radical and palliative intent [30].
2.4. Statistical analysis
Descriptive statistics were used to report respondent characteristics and questionnaire responses. Age was grouped into <65, 65–74, 75–85, and ≥85 yr. The number of LTCs was counted and categorised into groups with none, one, two or three, or four or more LTCs. EQ-5D-5L responses were split into groups of those by individuals who reported one or more problems (of any severity) on each dimension and by individuals who reported no problems. Mean SAH ratings (0–100) were calculated. The EORTC QLQ-C30 and merged BC modules were linearly transformed to a 0–100 scale, as per the scoring manual, with mean scores calculated for all scales and single items. EQ-5D, QLQ-C30, and the merged EORTC BC module outcomes were analysed by the treatment group, with adjustment for age and sex (using multivariable logistic regression for EQ-5D binary outcomes and multivariable linear regression for SAH, QLQ-C30, and the merged EORTC bladder modules). In addition, outcomes for each treatment group were stratified by age group. Where relevant, differences in scores between groups were assessed using previously defined clinically meaningful differences (a difference of 7 points for EQ-5D SAH [27] and 10 points for QLQ-C30 [31]). BC HRQOL scores were compared with the available PROM datasets for patients with prostate [27] and colorectal cancer [28], and the general population [29]. Analyses were performed using Stata version 16 (Stata Corp., College Station, TX, USA).
2.5. Ethical and regulatory approval
This study received the following approvals: Yorkshire & Humber, South Yorkshire Research Ethics Committee (17/YH/0095), Health Research Authority Confidentiality Advisory Group (17/CAG/0054); Office for Data Release (ODR1718_089), and NHS Digital Data Access Request Service (DARS-NIC-129819-V5P5Z-v2.4).
2.6. Patient and public involvement
Patient and public involvement was embedded in study design and delivery. Initial focus groups helped develop the study concept. Patient feedback contributed to refining patient-facing information and gaining necessary ethical and governance approvals. Throughout the study, patient representatives attended advisory group meetings and helped with the interpretation of the results. Two patient representatives are contributing authors of this manuscript.
3. Results
3.1. Participants, response rates, and treatments
Overall, 3279 eligible participants were identified, of whom 19 died during the survey period and 1796 returned a completed survey (completion rate: 55% [1796/3260], including 29 online and 13 telephone completions). Compared with survey responders, nonresponders were older, lived in more deprived areas, and were more likely to have unknown disease stage (all p < 0.01, Supplementary Table 2). Men and women were equally likely to respond. Question completeness was high (>95%) for all components of EQ-5D-5L and QLQ-C30, but lower for items relating to sexual issues, for example, sexual intimacy (39% completion), sexual enjoyment (36%), and female sexual problems (28%; Supplementary Table 3).
Over three-quarters of respondents were male (77%), and the average age at diagnosis was 69 yr (Table 2). At the time of survey, the average age of respondents was 75 yr (11% aged <65 yr). Coexisting LTCs were common; 76% reporting at least one and 11% reporting four or more. The most common LTCs were hypertension (24%), coronary artery disease (14%), and diabetes (11%). Only 1.0% of respondents were nonwhite in racial origin.
Table 2.
Characteristics of the survey participants
| Sociodemographics |
All treatments (n = 1796) |
TURBT only (n = 306) |
TURBT and BCG/MMC (n = 562) |
Radical cystectomy (n = 405) |
RC and other treatments a (n = 299) |
Radical RT treatments b (n = 155) |
Difference across treatment groups c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | IQR | Age | IQR | Age | IQR | Age | IQR | Age | IQR | Age | IQR | |||
| Median age at diagnosis | 70 | 64–76 | 71 | 64–78 | 69 | 63–75 | 69 | 62–75 | 68 | 63–72 | 74 | 67–78 | <0.001 | |
| Median age at survey | 76 | 70–82 | 78 | 71–84 | 76 | 70–82 | 75 | 69–80 | 74 | 70–78 | 79 | 73–84 | <0.001 | |
| N | % | N | % | N | % | N | % | N | % | N | % | |||
| Sex | Male | 1,376 | 76.6 | 235 | 76.8 | 449 | 79.9 | 296 | 73.1 | 224 | 74.9 | 125 | 80.7 | 0.090 |
| Female | 420 | 23.4 | 71 | 23.2 | 113 | 20.1 | 109 | 26.9 | 75 | 25.1 | 30 | 19.4 | ||
| No. of other long-term conditions | None | 433 | 24.1 | 76 | 24.8 | 122 | 21.7 | 111 | 27.4 | 79 | 26.4 | 29 | 18.7 | 0.168 |
| 1 | 545 | 30.4 | 84 | 27.5 | 176 | 31.3 | 121 | 29.9 | 96 | 32.1 | 47 | 30.3 | ||
| 2 | 384 | 21.4 | 61 | 19.9 | 113 | 20.1 | 92 | 22.7 | 62 | 20.7 | 38 | 24.5 | ||
| 3 | 237 | 13.2 | 48 | 15.7 | 80 | 14.2 | 42 | 10.4 | 42 | 14.0 | 21 | 13.6 | ||
| 4 | 197 | 11.0 | 37 | 12.1 | 71 | 12.6 | 39 | 9.6 | 20 | 6.7 | 20 | 12.9 | ||
| Socioeconomic deprivation | 1—least deprived | 397 | 22.1 | 62 | 20.3 | 135 | 24.0 | 85 | 21.0 | 67 | 22.4 | 28 | 18.1 | 0.838 |
| 2 | 457 | 25.5 | 71 | 23.2 | 136 | 24.2 | 99 | 24.4 | 84 | 28.1 | 46 | 29.7 | ||
| 3 | 378 | 21.1 | 67 | 21.9 | 120 | 21.4 | 85 | 21.0 | 62 | 20.7 | 37 | 23.9 | ||
| 4 | 273 | 15.2 | 49 | 16.0 | 84 | 15.0 | 68 | 16.8 | 38 | 12.7 | 19 | 12.3 | ||
| 5—most deprived | 291 | 16.2 | 57 | 18.6 | 87 | 15.5 | 68 | 16.8 | 48 | 16.1 | 25 | 16.1 | ||
| Stage at diagnosis | I | 773 | 43.0 | 195 | 63.7 | 333 | 59.3 | 149 | 36.8 | 57 | 19.1 | 20 | 12.9 | <0.001 |
| II | 203 | 11.3 | – | 2.3 | – | 1.3 | 53 | 13.1 | 62 | 20.7 | 66 | 42.6 | ||
| III | 69 | 3.8 | – | 1.0 | – | 0.2 | 37 | 3.1 | 17 | 5.7 | – | 6.5 | ||
| IV | 57 | 3.2 | – | 1.0 | – | 0.2 | 17 | 4.2 | 24 | 8.0 | – | 4.5 | ||
| Unknown | 694 | 38.6 | 98 | 32.0 | 220 | 39.2 | 149 | 36.8 | 139 | 46.5 | 52 | 33.6 | ||
BCG = bacillus Calmette-Guerin; IQR = interquartile range; MMC = intravesical mitomycin C; RC = radical cystectomy; RT = radiotherapy; TURBT = transurethral resection of a bladder tumour.
Including radical cystectomy with intravenous chemotherapy (77%), intravenous chemotherapy and radiotherapy (6%) or immunotherapy (5%), and radiotherapy (1%) or immunotherapy (10%).
Four patients received <52 Gy but were classified by consensus as radical (one received 44 Gy in 22 fractions and three received 50 Gy in 20 fractions).
Kruskal-Wallis tests were used to compare age across treatment groups and chi-square tests were used to compare the categorical variables—small numbers suppressed to preserve patient anonymity.
At diagnosis, NCRAS staged 43% of tumours as NMIBC and 18% as MIBC, and 39% did not have a stage recorded. Using information from the linked datasets, 48% (n = 868) of respondents had treatment for NMIBC (transurethral resection of bladder tumour [TURBT] ± intravesical treatment), 50% (n = 893) had radical treatment (RT or RC), and in 1.9% (n = 35) treatment was unknown (Supplementary Table 1). Of those receiving radical treatment, 47% (n = 405/859) underwent RC alone (following TURBT), 35% (n = 299) had radical surgery with other treatments (such as intravenous [IV] neoadjuvant chemotherapy or prior intravesical bacillus Calmette-Guerin [BCG]), and 18% (n = 155) received RT with radical intent. Excluding stage (which is directly linked to treatment), only age varied significantly across the treatment groups; participants receiving RT were older at diagnosis (median: 74 yr) than those undergoing RC or TURBT (median: 69 yr, p < 0.001; Table 2). Finally, 14 participants received palliative RT and 20 received only IV chemotherapy. Given the expected low number in this cohort, we excluded the palliative population from treatment-specific analysis.
3.2. General HRQOL: EQ-5D-5L
Over two-thirds of respondents (69%) reported at least one problem in any EQ-5D-5L dimension. Most problems were reported for mobility, followed by usual activities and pain. The overall mean SAH rating was 74/100. Outcomes were similar across all stages and treatment groups (Fig. 1A and 1B, and Supplementary Table 4). For example, after adjustment for age and sex, SAH ranged from 75 (95% confidence interval [CI]: 73–77) in the RC alone group to 71 (95% CI: 68–74) in the radical RT group. When stratified by treatment modality (Fig. 1C–H), the frequency of reported problems increased in all dimensions with increasing age and number of LTCs, with the exception being anxiety/depression, which was highest in the younger age groups. Multimodal treatment combinations did not impact HRQOL (no differences were seen between patients who received TURBT with/without intravesical treatment and those who received RC with/without other treatments).
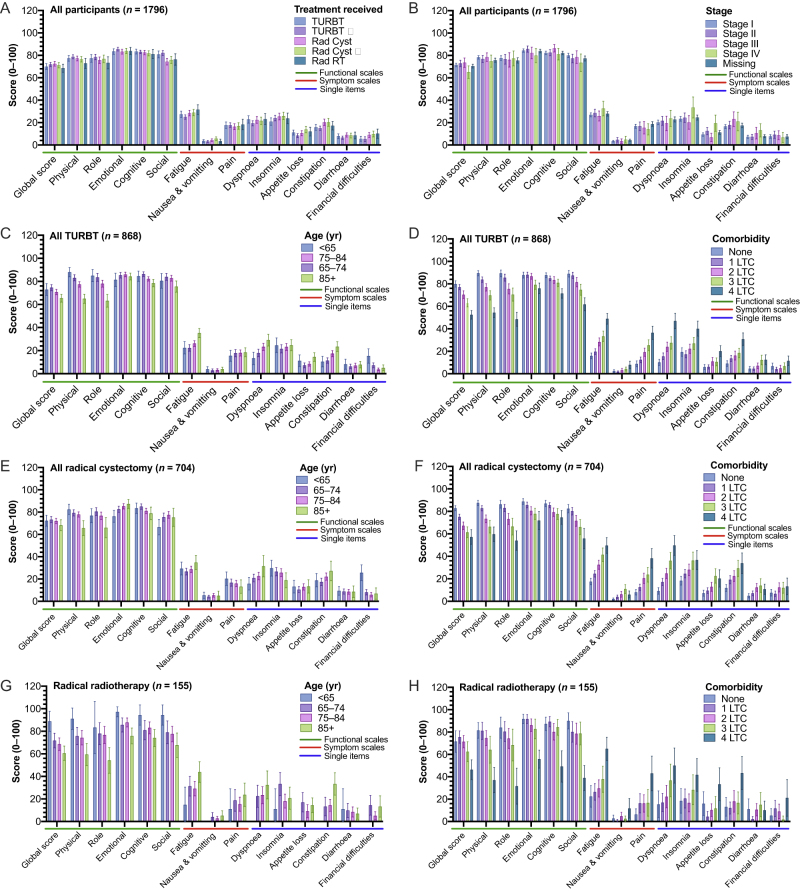
Fig. 1.
HRQOL using the EQ-5D-5L questionnaire. Percentage of patients with a problem in one or more EQ-5D-5L dimensions and scores for self-assessed health (SAH), all adjusted for age and sex, and presented for (A) each treatment and (B) tumour stage (using cancer registration data). Percentage of patients with a problem in one or more EQ-5D-5L dimensions and SAH scores stratified by age and number of long-term conditions (LTCs) in participants who received (C and D) TURBT for NMIBC (including those who also received additional treatments), (E and F) radical cystectomy (including those who also received additional treatments), and (G and H) radical radiotherapy for MIBC (including those who also received additional treatments). Error bars indicate 95% confidence intervals.
Cyst = cystectomy; HRQOL = health-related quality of life; MIBC = muscle-invasive bladder cancer; NMIBC = non–muscle-invasive bladder cancer; Rad = radical; RT = radiotherapy; TURBT = transurethral resection of bladder tumour.
3.3. Cancer generic HRQOL: EORTC QLQ-C30
The mean global health score was 71/100. This ranged from 72 (95% CI: 70–75) after RC to 69 (95% CI: 65–72) after radical RT (age/sex adjusted). No meaningful differences in scales or symptom scores were seen between the treatment groups or by stage (Fig. 2A and 2B, and Supplementary Table 5). Across treatments (Fig. 2C–H), as with EQ-5D-5L, worse function and symptom scores were seen with increasing age and LTC burden. One notable outlier was the higher rate of financial difficulties reported by younger patients: <65 yr old (mean 20 [95% CI: 15–25]) versus ≥65 yr old (6.6 [95% CI: 4.9–8.2]). Of note, these financial difficulties were greater in younger patients undergoing RC (mean 26 [95% CI: 19–33]) than in those undergoing TURBT (15 [95% CI: 9.1–22]).
Fig. 2.
HRQOL using the EORTC QLQ-C30 questionnaire. Mean (±95% CIs) scores (0–100) for each scale, adjusted for age and sex, are shown for the whole population and presented by (A) each treatment and (B) tumour stage (using cancer registration data). Mean scores, stratified by age and number of long-term conditions (LTCs), in participants who received (C and D) TURBT for NMIBC (including those who also received additional treatments, (E and F) radical cystectomy (including those who also received additional treatments), and (G and H) radical radiotherapy for MIBC (including those who also received additional treatments). Error bars indicate 95% confidence intervals.
CI = confidence interval; Cyst = cystectomy; HRQOL = health-related quality of life; MIBC = muscle-invasive bladder cancer; NMIBC = non–muscle-invasive bladder cancer; Rad = radical; RT = radiotherapy; TURBT = transurethral resection of bladder tumour.
3.4. Treatment-specific HRQOL: EORTC BC modules
EORTC bladder symptom scores varied considerably across different treatments, between stages (Fig. 3A and 3B and Supplementary Table 6), and when treatments were stratified by age and LTCs (Fig. 3C–F). In participants who received RT, changes in most symptom scores were less directly associated with age (Fig. 3G).
Fig. 3.
Merged EORTC QLQ-NMIBC24 and EORTC QLQ-BLMC30 scores. Mean (±95% CIs) scores (0–100) for each scale, adjusted for age and sex, are shown for the whole population and presented by (A) each treatment and (B) tumour stage (using cancer registration data). Mean scores, stratified by age and number of long-term conditions (LTCs), in participants who received (C and D) TURBT for NMIBC (including those who also received additional treatments), (E and F) radical cystectomy (including those who also received additional treatments), and (G and H) radical radiotherapy for MIBC (including those who also received additional treatments). Error bars indicate 95% confidence intervals.
CI = confidence interval; Cyst = cystectomy; EORTC QLQ = European Organization for Research and Treatment of Cancer quality of life questionnaire; MIBC = muscle-invasive bladder cancer; NMIBC = non–muscle-invasive bladder cancer; Rad = radical; RT = radiotherapy; TURBT = transurethral resection of bladder tumour.
Worse urinary symptoms were reported following radical RT (mean 32 [95% CI: 28–36]) compared with TURBT (24 [95% CI: 22–26], age/sex adjusted p < 0.001). Urinary symptoms in participants following RC with neobladder (25 [95% CI: 19–31]) were similar to those following TURBT. Addition of intravesical treatments to TURBT did not worsen urinary symptoms or increase concerns about contaminating partners when compared with TURBT (Fig. 3A).
Across treatment groups, respondents who underwent RC (alone or in combination) reported worse problems with body image, sexual intimacy, sexual enjoyment, and male sexual problems (Fig. 3A). In patients who underwent TURBT, sexual function and enjoyment declined with age and increasing LTCs, whilst sexual problems increased (Fig. 3C and 3D). In males who underwent RC, whilst all symptom scores worsened with increasing age, the most dramatic changes were for sexual function, sexual intimacy, and male sexual problem scores (Fig. 3E). Sexual problems in females could not be evaluated accurately due to high rates of missing data (28% completion rate; Supplementary Table 3). Comparisons between reconstruction choices in the RC population were underpowered (neobladder, n = 88 and ileal conduit, n = 616) and potentially mismatched (eg, those receiving neobladder were younger [median age 66 vs 75 yr for ileal conduit] and had fewer LTCs [no LTCs: 41% vs 25% for ileal conduit, and more than two LTCs: 23% vs 45% for ileal conduit]), but suggested few changes beyond improved sexual function in the neobladder cohort (Supplementary Fig. 2).
3.5. Generic and treatment-specific HRQOL over time since diagnosis
We observed little difference in the scores for each questionnaire when analysed by time from diagnosis and treatment (Supplementary Fig. 3).
3.6. Comparison of BC HRQOL with that in other pelvic cancer patients and the general population
In comparison with the general population and groups with prostate and colorectal cancer, our BC respondents (as a whole) reported more problems across all EQ-5D-5L dimensions, except for anxiety/depression, which was comparable with that for colorectal and prostate cancer (in men; Supplementary Table 7 and Supplementary Fig. 4). The differences were greatest for problems with mobility and usual activities. For example, 48% (95% CI: 46–52) of men (male-only figures to facilitate direct comparison with other common pelvic cancers) with BC reported problems (of any level) with mobility compared with 36% (95% CI: 35–37) of men with colorectal cancer, 34% (95% CI: 33–34) of men with prostate cancer, and 25% (95% CI: 23–27) of the male general population (adjusted for age and deprivation, all comparisons p < 0.05). Pain/discomfort was worse in men with BC (than the other populations), but similar for females with BC and the general population.
4. Discussion
We report HRQOL in individuals up to 10 yr after a diagnosis of BC. This is the largest study to date in this hard to reach, under-reported disease group and delivers novel insights: we have documented large variations in overall HRQOL by age and LTCs, with fewer differences according to stage or treatment received. Higher rates of sexual dysfunction were reported, particularly by men, and financial toxicity was reported in younger patients. Compared with the general population and those with other common pelvic cancers, BC patients had lower HRQOL.
Firstly, there were larger variations in long-term overall HRQOL according to patient age and LTCs, rather than treatment type, disease stage, or time since diagnosis. This important observation has direct clinical relevance in treatment decision-making, where robust evidence to understand the trade-offs (in terms of overall survival and quality of survival) between two approaches of different extents is vital. Examples include the choice between intravesical BCG and RC for high-grade NMIBC [32] and between RC and RT for MIBC [33].
Secondly, treatment intensity and multimodality did not appear to be associated with adverse HRQOL outcomes (both generic and treatment-specific symptoms). For example, participants who received TURBT alone or TURBT with intravesical therapies had similar urinary symptoms and sexual function scores. Participants who received RC alone or RC with systemic chemotherapy had similar functional and symptom scores across all domains (including fatigue and gastrointestinal symptoms). Collectively, these data provide reassuring evidence for clinicians and patients considering multimodal treatment options, and justifies treatment choices based on symptoms, patient preferences, and survival.
Thirdly, some of the highest problem scores were seen for sexual function in men. Scores varied according to age, LTCs, and treatment, suggesting a multifactorial origin. Participants undergoing RC or RT have treatments that directly affect erectile ability and ejaculation (and vaginal length in women). The sexual impact of radical treatments is well known [13], [14], and should be managed by pretreatment counselling and post-treatment support. High problem scores and patient experience [7], [34] suggest that this may often be omitted. Surprisingly, we saw participants receiving TURBT (ie, anatomical preserving treatment) had high scores for male sexual problems and low scores for sexual function, intimacy, and enjoyment. These scores were directly related to age and LTC burden. The aetiology of sexual dysfunction in this population probably reflects other health factors (similar to transurethral resection of the prostate [35]) rather than BC or side effects of BC treatment. However, interaction with health care professionals should be seen as an opportunity to help this cohort. One important observation was that most women did not answer questions regarding female sexual issues. This prevents us from drawing any observations in women and warrants further investigation.
Fourthly, financial toxicity was reported by younger patients receiving RC or TURBT [36]. Markedly lower rates were reported by older persons receiving the same treatments, which will be addressed in a subsequent publication in detail, and suggests an impact of the disease and its treatment on employment. Patterns of employment disruption differ between treatments; RC typically requires hospital stay and 3-mo recovery [37], whilst TURBT pathways include multiple outpatient visits (eg, 15 cystoscopies/treatments in year 1 for maintenance BCG [38]). Many BC patients are employed in manual work [39], [40], [41], and so are unable to work whilst recovering from procedures or when suffering from complications. Our survey was conducted in the UK (free public health care) and may grossly under-represent this issue in private health care systems.
Finally, a comparison of the overall HRQOL in BC patients with that in prostate and colorectal cancer patients and a matching general population revealed that all three cancer cohorts had lower HRQOL than the general population, and that BC participants had the lowest HRQOL of all. These findings match with those of prior NHS England surveys [6] reporting that BC cancer patients have poor experiences. This likely reflects a lack of investment in supportive aspects of their care, multiple visits necessary to manage BC, and unchanging cancer outcomes [42]. Further research is needed to understand these differences in more detail and to compare with other pelvic cancers (such as ovarian cancer; see http://www.ncin.org.uk/view?rid=2920]).
Our population-based approach, using cancer registration data, enabled inclusion of all BC phenotypes and treatments, without selection by hospital, speciality, trial participation, or geographic location. Through the collection of generic and specific HRQOL domains, utilising validated instruments, we were able to make comparisons against other major cancer groups and the general population, thereby facilitating important and novel observations. Few data were missing (the exception being questions related to sexual issues, which had particularly low completion rates in females).
Limitations include that the ethnicity of respondents did not fully represent the population of Yorkshire and Humber, where over 10% are nonwhite British [43]. Further work is mandated to explore the HRQOL of other ethnic groups. Response rates were marginally lower than for similar UK cancer surveys (63% for colorectal and 61% for prostate cancer) [27], [28], but similar to that reported by the Department of Health, England, for BC patients (53%) [17] and so may reflect this population (ie, typically more deprived, more manual workers, [39], [40], [41], and lower literacy rates than other cancers [44], [45], [46]). Nonrespondents were more likely to be older and live in more socioeconomically deprived areas, groups that may be expected to experience poorer HRQOL. Within the registry data, we were unable to account for participants whose tumours increased in stage (eg, progressed) and around one-third of BCs had missing tumour stage. This rate is higher than for other cancers and may reflect that UK registries stage tumours as T1-T4, whilst many BCs are pTa and pTis (a level of detail that could not be extracted). When stratifying by treatment, the small radical RT cohort limited some analyses (eg, there were fewer than five respondents aged <65 yr and little information was known about RT/maximal TURBT/chemotherapy use), as did the small number of patients with neobladder formation after RC, and our lack of knowledge regarding clinical outcomes or the time of last treatment. Finally, following discussion with the EORTC quality life group, to avoid duplication of content, we merged the BC outcome measures (EORTC-QLQ BLM30 and EORTC-QLQ NMIBC24) with the potential to disrupt their psychometric properties. This new approach is now being used by others in on-going surveys [47].
5. Conclusions
HRQOL in individuals living with and beyond BC is worse than that reported by the general population and those with other common cancers, and appears to be independent of therapy received and disease stage. The poor outcomes largely reflect age and presence of other LTCs. Further in-depth investigation of financial toxicity in those aged under 65 yr and sexual problems experienced by male and females is necessary to guide risk stratification of aftercare support.
Author contributions: James W.F. Catto had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Catto, Glaser, Wright, Bottomley, Hounsome.
Acquisition of data: Mason, Bottomley, Downing, Hounsome, Raw, Kelly.
Analysis and interpretation of data: Catto, Downing, Glaser, Varughese, Hussain, Mason, Absolom.
Drafting of the manuscript: Catto, Downing, Glaser, Varughese, Hussain, Mason, Absolom.
Critical revision of the manuscript for important intellectual content: Catto, Downing, Glaser, Varughese, Hussain, Mason, Absolom.
Statistical analysis: Mason, Downing.
Obtaining funding: Catto, Glaser.
Administrative, technical, or material support: Bottomley.
Supervision: Catto, Glaser.
Other: None.
Financial disclosures: James W.F. Catto certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James W.F. Catto has received reimbursement for consultancy from Astra Zeneca, Roche, and Janssen; speaker fees from BMS, MSD, Nucleix, and Roche; and honoraria for membership of advisory boards from Astra Zeneca, Ferring, Roche, and Janssen. Syed Hussain has received research funding from CR UK, MRC/NIHR, UHB charities, CCC charities, and North West Cancer Research; reimbursement for consultancy from Bayer, Janssen, Boehringer Ingelheim, Pierre Fabre, and Eli Lilly; and advisory board/consultancy fess from Roche, MSD, AstraZeneca, BMS, Janssen, Pfizer, Astellas, Bayer, Pierre Fabre, Sotio, GSK, Ipsen, and Eisai. Mohini Varughese has received speaker fees from Pfizer, GSK, Genomic Health, and Roche; educational grants from Roche, Janssen, Pfizer, and MSD; and honoraria for membership of advisory boards from Astra Zeneca. The remaining authors declare no potential conflicts of interest.
Funding/Support and role of the sponsor: This study was funded by Yorkshire Cancer Research (study S385: The Yorkshire Cancer Research Bladder Cancer Patient Reported Outcomes Survey). The funder had no role in the design, analysis, or collection of the data; in writing the manuscript; or in the decision to submit the manuscript for publication. James W.F. Catto is funded by an NIHR Research Professorship.
Acknowledgements: We gratefully acknowledge the support of participants and local principal investigators. We acknowledge the support of the User, Clinical and Scientific Advisory Group: Linda Sharpe (Chair), Jo Cresswell, Louise Goodwin, Mohini Varughese, Sally Appleyard, Ananya Choudhury, Rik Bryan, Duncan Nekeman, Andrew Winterbottom (deceased), Caroline Raw, Sophie Jose, Charlotte Eversfield, Hannah Roberts, Ashok Nikapota, and Sunjay Jain. Colleagues at Quality Health supported survey distribution and result collation. This work uses data provided by patients and collected by the NHS as part of their care and support. This work is dedicated to patients who died before its completion, and in particular Andrew Winterbottom from Fight Bladder Cancer UK and Stanley Wilson.
Associate Editor: Giacomo Novara
Statistical Editor: Melissa Assel
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2021.01.032.
Contributor Information
James W.F. Catto, Email: j.catto@sheffield.ac.uk.
Adam W. Glaser, Email: a.glaser@nhs.net.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Cumberbatch M.G.K., Jubber I., Black P.C. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74:784–795. doi: 10.1016/j.eururo.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M., Burger M., Comperat E.M. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)— 2019 update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Witjes J.A., Babjuk M., Bellmunt J. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer—an international collaborative multistakeholder effort: under the auspices of the EAU-ESMO Guidelines Committees. Eur Urol. 2020;77:223–250. doi: 10.1016/j.eururo.2019.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Milowsky M.I., Rumble R.B., Booth C.M. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology Guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34:1945–1952. doi: 10.1200/JCO.2015.65.9797. [DOI] [PubMed] [Google Scholar]
- 5.Noon A.P., Albertsen P.C., Thomas F., Rosario D.J., Catto J.W. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013;108:1534–1540. doi: 10.1038/bjc.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders Cl, Abel Ga, Lyratzopoulos G. Inequalities in reported cancer patient experience by socio-demographic characteristic and cancer site: evidence from respondents to the English Cancer Patient Experience Survey. Eur J Cancer Care (Engl) 2015;24:85–98. doi: 10.1111/ecc.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edmondson A.J., Birtwistle J.C., Catto J.W.F., Twiddy M. The patients’ experience of a bladder cancer diagnosis: a systematic review of the qualitative evidence. J Cancer Surviv. 2017;11:453–461. doi: 10.1007/s11764-017-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaser A.W., Corner J.L. Prostate cancer outcomes: the three questions. Eur Urol. 2015;67:357–358. doi: 10.1016/j.eururo.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Mason S.J., Downing A., Wright P. Life and bladder cancer: protocol for a longitudinal and cross-sectional patient-reported outcomes study of Yorkshire (UK) patients. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali A.S., Hayes M.C., Birch B., Dudderidge T., Somani B.K. Health related quality of life (HRQoL) after cystectomy: comparison between orthotopic neobladder and ileal conduit diversion. Eur J Surg Oncol. 2015;41:295–299. doi: 10.1016/j.ejso.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Mansson A., Davidsson T., Hunt S., Mansson W. The quality of life in men after radical cystectomy with a continent cutaneous diversion or orthotopic bladder substitution: is there a difference? BJU Int. 2002;90:386–390. doi: 10.1046/j.1464-410x.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 12.Caffo O., Fellin G., Graffer U., Luciani L. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self-administered questionnaire.[Erratum appears in Cancer 1996;76:2037] Cancer. 1996;78:1089–1097. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1089::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda T., Aptel I., Exbrayat C., Grosclaude P. Determinants of quality of life of bladder cancer survivors five years after treatment in France. Int J Urol. 2003;10:423–429. doi: 10.1046/j.1442-2042.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- 14.Allareddy V., Kennedy J., West M.M., Konety B.R. Quality of life in long-term survivors of bladder cancer. Cancer. 2006;106:2355–2362. doi: 10.1002/cncr.21896. [DOI] [PubMed] [Google Scholar]
- 15.Imbimbo C., Mirone V., Siracusano S. Quality of life assessment with orthotopic ileal neobladder reconstruction after radical cystectomy: results from a prospective Italian Multicenter Observational Study. Urology. 2015;86:974–980. doi: 10.1016/j.urology.2015.06.058. [DOI] [PubMed] [Google Scholar]
- 16.Hedgepeth R.C., Gilbert S.M., He C., Lee C.T., Wood D.P., Jr Body image and bladder cancer specific quality of life in patients with ileal conduit and neobladder urinary diversions. Urology. 2010;76:671–675. doi: 10.1016/j.urology.2010.01.087. [DOI] [PubMed] [Google Scholar]
- 17.Mason S.J., Downing A., Wright P. Health-related quality of life after treatment for bladder cancer in England. Br J Cancer. 2018;118:1518–1528. doi: 10.1038/s41416-018-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blazeby J.M., Hall E., Aaronson N.K. Validation and reliability testing of the EORTC QLQ-NMIBC24 questionnaire module to assess patient-reported outcomes in non-muscle-invasive bladder cancer. Eur Urol. 2014;66:1148–1156. doi: 10.1016/j.eururo.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed N.E., Gilbert F., Lee C.T. Pursuing quality in the application of bladder cancer quality of life research. Bladder Cancer. 2016;2:139–149. doi: 10.3233/BLC-160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strong L.E. The past, present, and future of patient-reported outcomes in oncology. Am Soc Clin Oncol Educ Book. 2015;35:e616–20. doi: 10.14694/EdBook_AM.2015.35.e616. [DOI] [PubMed] [Google Scholar]
- 21.Partington J., Hounsome L., Byrne E., Dowling M., Blackburn J., Bruce L. Public Health England; 2015. Living with and beyond bladder cancer: a descriptive summary of responses to a pilot of patient reported outcome measures for bladder cancer. [Google Scholar]
- 22.NHS Digital Patient objections (type 2) directions 2016. 2016. https://digital.Nhs.uk/about-nhs-digital/corporate-information-and-documents/directions-and-data-provision-notices/secretary-of-state-directions/patient-objections-type-2-directions-2016
- 23.Herdman M., Gudex C., Lloyd A. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams A. EUROQOL—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 25.Aaronson N.K., Ahmedzai S., Bergman B. The European Organisation for Research and Treatment of Cancer QLQ-C30. A quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 26.European Organisation for the Research and Treatment of Cancer. QLQ-BLM30: muscle invasive bladder cancer. https://qol.eortc.org/questionnaire/qlq-blm30/.
- 27.Downing A., Wright P., Hounsome L. Quality of life in men living with advanced and localised prostate cancer in the UK: a population-based study. Lancet Oncol. 2019;20:436–447. doi: 10.1016/S1470-2045(18)30780-0. [DOI] [PubMed] [Google Scholar]
- 28.Downing A., Morris E.J., Richards M. Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol. 2015;33:616–624. doi: 10.1200/JCO.2014.56.6539. [DOI] [PubMed] [Google Scholar]
- 29.Devlin N.J., Shah K.K., Feng Y., Mulhern B., van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27:7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royal College of Radiologists . ed. 3. The Royal College of Radiologists; London, UK: 2019. Bladder cancer. [Google Scholar]
- 31.King M.T. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 32.Catto J.W.F., Gordon K., Collinson M. Radical cystectomy against intra-vesical BCG immunotherapy for high risk non-muscle invasive bladder cancer: results from the randomised controlled BRAVO-feasibility study. J Clin Oncol. 2021;39:202–214. doi: 10.1200/JCO.20.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni G.S., Hermanns T., Wei Y. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35:2299–2305. doi: 10.1200/JCO.2016.69.2327. [DOI] [PubMed] [Google Scholar]
- 34.Elliott J., Rotterud R., Maddox-Smith A. The bladder cancer patient survey: global perspectives on awareness and treatment of bladder cancer. J Cancer Policy. 2019;22:100207. [Google Scholar]
- 35.Muntener M., Aellig S., Kuettel R., Gehrlach C., Sulser T., Strebel R.T. Sexual function after transurethral resection of the prostate (TURP): results of an independent prospective multicentre assessment of outcome. Eur Urol. 2007;52:510–515. doi: 10.1016/j.eururo.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 36.Desai A., Gyawali B. Financial toxicity of cancer treatment: moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine. 2020;20 doi: 10.1016/j.eclinm.2020.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catto J.W.F., Khetrapal P., Ambler G. Multidomain quantitative recovery following radical cystectomy for patients within the robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy randomised controlled trial: the first 30 patients. Eur Urol. 2018;74:531–534. doi: 10.1016/j.eururo.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Kamat A.M., Bellmunt J., Galsky M.D. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma. J Immunother Cancer. 2017;5:68. doi: 10.1186/s40425-017-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed O., Jubber I., Griffin J. Occupational bladder cancer: a cross section survey of previous employments, tasks and exposures matched to cancer phenotypes. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noon A.P., Martinsen J.I., Catto J.W.F., Pukkala E. Occupation and bladder cancer phenotype: identification of workplace patterns that increase the risk of advanced disease beyond overall incidence. Eur Urol Focus. 2018;4:725–730. doi: 10.1016/j.euf.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Cumberbatch M.G., Cox A., Teare D., Catto J.W. Contemporary occupational carcinogen exposure and bladder cancer: a systematic review and meta-analysis. JAMA Oncol. 2015;1:1282–1290. doi: 10.1001/jamaoncol.2015.3209. [DOI] [PubMed] [Google Scholar]
- 42.Smith A.B., Chisolm S., Deal A. Patient-centered prioritization of bladder cancer research. Cancer. 2018;124:3136–3144. doi: 10.1002/cncr.31530. [DOI] [PubMed] [Google Scholar]
- 43.GOV.UK Regional ethnic diversity. 2019. https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/regional-ethnic-diversity/latest
- 44.Bassett J.C., Gore J.L., Chi A.C. Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol. 2012;30:1871–1878. doi: 10.1200/JCO.2011.36.6518. [DOI] [PubMed] [Google Scholar]
- 45.Scarpato K.R., Kappa S.F., Goggins K.M. The impact of health literacy on surgical outcomes following radical cystectomy. J Health Commun. 2016;21:99–104. doi: 10.1080/10810730.2016.1193916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luckenbaugh A.N., Moses K.A. The impact of health literacy on urologic oncology care. Urol Oncol. 2019 doi: 10.1016/j.urolonc.2019.06.016. Jul 16;S1078-1439(19)30245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ripping T.M., Kiemeney L.A., van Hoogstraten L.M.C., Witjes J.A., Aben K.K.H. Insight into bladder cancer care: study protocol of a large nationwide prospective cohort study (BlaZIB) BMC Cancer. 2020;20:455. doi: 10.1186/s12885-020-06954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.