Abstract
Background
To date, there has been no practical guidelines for the prescription of antiepileptic drugs (AEDs) in brain tumor patients in Korea. Thus, the Korean Society for Neuro-Oncology (KSNO), a multidisciplinary academic society, had begun preparing guidelines for AED usage in brain tumors since 2019.
Methods
The Working Group was composed of 27 multidisciplinary medical experts in Korea. References were identified through searches of PubMed, MEDLINE, EMBASE, and Cochrane CENTRAL using specific and sensitive keywords as well as combinations of the keywords.
Results
The core contents are as follows. Prophylactic AED administration is not recommended in newly diagnosed brain tumor patients without previous seizure history. When AEDs are administered during peri/postoperative period, it may be tapered off according to the following recommendations. In seizure-naïve patients with no postoperative seizure, it is recommended to stop or reduce AED 1 week after surgery. In seizure-naïve patients with one early postoperative seizure (<1 week after surgery), it is advisable to maintain AED for at least 3 months before tapering. In seizure-naïve patients with ≥2 postoperative seizures or in patients with preoperative seizure history, it is recommended to maintain AEDs for more than 1 year. The possibility of drug interactions should be considered when selecting AEDs in brain tumor patients. Driving can be allowed in brain tumor patients when proven to be seizure-free for more than 1 year.
Conclusion
The KSNO suggests prescribing AEDs in patients with brain tumor based on the current guideline. This guideline will contribute to spreading evidence-based prescription of AEDs in brain tumor patients in Korea.
Keywords: Korean Society for Neuro-Oncology, Guideline, Brain tumors, Antiepileptic drug, Practice
INTRODUCTION
Seizure is one of the most common medical complications in brain tumor patients [1,2]. Up to two third of patients diagnosed with a brain tumor will experience at least 1 seizure throughout their disease course [3]. Antiepileptic drugs (AEDs) are frequently prescribed in brain tumor patients for therapeutic or prophylactic purposes [4]. Seizures can be provoked during the perioperative period of brain tumor resection and it is widely accepted that perioperative seizures must be controlled with AEDs [5], however the adequate duration of AED maintenance is not established [6]. Moreover, clinicians are still controversial about the use of prophylactic AEDs for brain tumor surgery in patients with no prior seizure history [7].
Recently, a web-based survey was conducted on the administration of AEDs in patients with brain tumors among the Korean Society for Neuro-Oncology (KSNO) members [8]. The majority of respondents (95.2%) routinely prescribed AEDs during peri/postoperative period for patients with previous seizure history. Also, 72.8% of respondents routinely prescribed AEDs for seizure-naïve patients. The duration of prophylactic AED administration was widely variable according to the history of epilepsy and the location of tumor. Levetiracetam (82.9%) was the most preferred AED for seizure prophylaxis in brain tumor patients in Korea.
To date, a practical guideline for AED prescription in brain tumor patients is not available in Korea. Therefore, the KSNO, a multidisciplinary academic society for central nervous system (CNS) tumors in Korea, established a new Guideline Working Group chapter to discuss guidelines on the prescription of AEDs in brain tumor patients. The objective of this guideline is to provide physicians with evidence-based recommendations and consensus expert opinion for prescribing AEDs in patients with brain tumor. It will also provide a source of knowledge for institutions and insurance companies involved in the management of brain tumor in Korea.
KSNO GUIDELINE WORKING GROUP
A new chapter of Working Group was appointed by the KSNO in 2019 to develop a clinical guideline for management of patients with brain tumor. These guidelines should be optimized considering the unique medical circumstance in Korea. The KSNO Guideline Working Group was composed of 27 medical experts in Korea, including 15 neurosurgeons, 6 radiation oncologists, 1 medical oncologist, 2 neuroradiologists, 2 pathologists, and 1 neurologist.
References were searched from PubMed, MEDLINE, EMBASE, and Cochrane CENTRAL using specific and sensitive keywords as well as combinations of keywords. The scope of the brain tumors included all histological grades of primary and metastatic brain tumors. The purpose of AED prescriptions covered prophylactic use, symptomatic control and withdrawal strategy. The driving issues regarding seizure control in brain tumor patients were evaluated. The final reference list was generated based on the originality and relevance to the scope of this guideline.
Scientific evidence was evaluated and graded according to the following categories: high level evidence (obtained from multiple populations and derived from randomized clinical trials or meta-analysis or systemic review), and low level evidence (obtained from limited population and derived from non-randomized studies, including observational studies, cohort studies, and case-control studies).
To establish the recommendation levels, the following criteria were used. Level I (strong recommendation) required a high level evidence and uniform agreement among panels. Level II (weak recommendation) required a high level evidence but not uniform agreement among panels or low level evidence but uniform agreement among panels. Level III (no consensus; individual decision) required a low level evidence but not uniform agreement among panels. Level IV (not recommended) required contents being not beneficial or harmful.
PROPHYLACTIC ANTIEPILEPTIC DRUG PRESCRIPTION
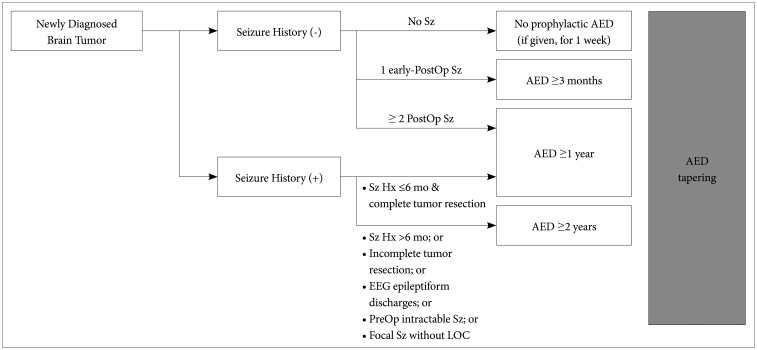
Preventive anticonvulsant administration is not recommended in patients with newly diagnosed brain tumors who do not have a history of seizure (Level I) (Table 1, Fig. 1)
Table 1. Summary of the Korean Society for Neuro-Oncology (KSNO) Guideline for Antiepileptic Drug Usage in Brain Tumor.
| Recommendations on AED prescription | Recommendation level |
|---|---|
| • Preventive anticonvulsant administration is not recommended in patients with newly diagnosed brain tumors who do not have a history of seizure | Level I |
| • In seizure-naïve brain tumor patients when seizure did not occur after surgery, it is advisable to stop or reduce prophylactic AEDs 1 week after surgery | Level II |
| • In seizure-naïve brain tumor patients when seizure occurred once during acute postoperative period (<1 week after surgery), it is advisable to maintain AED and if no additional seizures occur afterward then to stop or reduce AEDs 3 months after surgery | Level III |
| • In seizure-naïve brain tumor patients when seizure occurred more than twice after surgery, AEDs should be maintained and if the patient remains seizure-free for over 1 year, stopping or reducing AEDs can be considered | Level III |
| • In patients with brain tumors who had preoperative epilepsy (or seizures), AED should be maintained for at least a year after surgery | Level II |
| • Drug interactions should be considered when selecting AEDs in patients with brain tumors | Level II |
| • Even in brain tumor patients who previously experienced seizures, when seizure-free period with or without AEDs is longer than 1 year, driving can be allowed. However, it is not allowed to drive during the tapering period of AEDs | Level III |
AED, antiepileptic drug
Fig. 1. Guideline for AED maintenance and withdrawal in brain tumor patients. Sz, seizure; Hx, history; PostOp, postoperative; EEG, electroencephalogram; PreOp, preoperative; LOC, loss of consciousness; AED, antiepileptic drug.
The high risk for development of seizures and epilepsies in brain tumor raised the question of prophylactic AED treatment in patients with newly diagnosed brain tumor. While initiation of AEDs is justified after a first seizure in patients with brain tumors, prophylactic AED should not be used in seizure naïve brain tumor patients [9]. In 2000, the American Academy of Neurology (AAN) recommended against prophylactic AED prescription for patients with brain tumor [10]. Afterward, several randomized controlled studies [11,12,13] and meta-analyses [14,15] have confirmed that prophylactic AED is not beneficial for preventing first seizure in brain tumor patients.
However, prophylactic AEDs may be considered in the presence of the following high-risk factors: 1) low-grade gliomas, 2) tumors invading cortical area of the brain, 3) multifocal tumors, and 4) tumors located in the temporal lobe or in insular region [5]. These factors should be considered when deciding whether to prescribe prophylactic AEDs to each patient.
Low-grade gliomas that are frequently associated with seizure are recently categorized as low-grade developmental and epilepsy associated brain tumors (LEATs). LEAT contains ganglioglioma, dysembryoplastic neuroepithelial tumor, angiocentric glioma, isomorphic diffuse glioma and papillary glioneuronal tumor which account for more than 70% of epilepsy-related brain tumors [16]. The incidence of seizure in patients with LEAT is estimated to be between 70–90% [17], which is substantially higher than in high-grade gliomas (25–60%), meningiomas (25%), or brain metastases (15%) [18,19,20,21]. High-grade tumors have relatively lower rate of seizures [1,22].
The location of the tumor is also important for assessing the risk of seizure occurrence. Brain tumors located in the superficial cortical area is much likely to cause seizure than those in the noncortical deeper area [1,23,24]. Multiple lesions are associated with a higher risk compared to solitary lesions. Brain tumors located in temporal lobe and insular regions are associated with intractable epilepsy [23,24]. Additionally, frontal and parietal tumors are known for higher risk of causing epilepsy than occipital or infratentorial tumors [23].
PERI/POSTOPERATIVE ANTIEPILEPTIC DRUG PRESCRIPTION
Seizure naïve patients
In seizure-naïve brain tumor patients when seizure did not occur after surgery, it is advisable to stop or reduce prophylactic AEDs 1 week after surgery (Level II) (Table 1, Fig. 1)
AED is often prescribed during the peri/postoperative period of brain tumor surgery. According to a National Consensus Survey in Korea, more than 70% of respondents (32 of 44, 72.8%) prescribed prophylactic AEDs for seizure naïve patient in the peri/postoperative period. Only two (4.4%) respondents never prescribed prophylactic AEDs in this situation [8]. This is in line with the survey studies performed in thousands of neurosurgeons about their real clinical practice, that 63–70% of neurosurgeons routinely prescribed AEDs postoperatively to patients without seizure history [11,12,13].
Even when prophylactic AEDs were prescribed to brain tumor patients who have not had seizure, AAN recommends tapering and discontinuing AEDs after the first postoperative week [10]. No subsequent studies have provided evidence against this recommendation.
In seizure-naïve brain tumor patients when seizure occurred once during acute postoperative period (<1 week after surgery), it is advisable to maintain AED and if no additional seizures occur afterward then to stop or reduce AEDs 3 months after surgery (Level III) (Table 1, Fig. 1)
Early postoperative seizures are rare after supratentorial neurosurgery [25]. It has been demonstrated that only rare portion of patients (about 1%) experience seizure within 24 hours after brain tumor surgery [25], and it is unlikely that early postoperative seizure to be caused by postoperative hematoma or metabolic abnormality [26]. Moreover, a large proportion of patients who experienced early postoperative seizure after supratentorial neurosurgery also had preoperative seizures. Therefore, seizure will rarely occur during the early postoperative period in seizure naïve patients. However, when it occurs it is advisable to maintain AED for longer period than 1 week.
There is limited consensus on the timing of AED withdrawal after brain tumor surgery [9]. No studies had systemically examined AED withdrawal in tumor-related seizures, therefore, the decision has to be made based on what is known in general [27]. In patients with single seizure during the acute postoperative period (<1 week after surgery), AED can be gradually withdrawn from 3 months after surgery.
In seizure-naïve brain tumor patients when seizure occurred more than twice after surgery, AEDs should be maintained and if the patient remains seizure-free for over 1 year, stopping or reducing AEDs can be considered (Level III) (Table 1, Fig. 1)
If seizure occurred more than twice after surgery, it is more likely that the patient had subtle seizures from preoperative stage [28]. Therefore, in these conditions, AEDs should be maintained for longer period.
When the patient remains seizure-free for at least 1 year, AED reduction or discontinuation can be considered. A patient can be considered ‘seizure-free’ when the period without seizures has elapsed equal to three times the longest inter-seizure interval over the previous year [29].
In patients with brain tumors who had preoperative epilepsy (or seizures), AED should be maintained for at least a year after surgery (Level II) (Table 1, Fig. 1)
In patients with history of preoperative seizure or epilepsy, AED reduction or discontinuation should be determined in the same way as in other epilepsy. In these conditions, AED withdrawal may be carefully attempted after a sufficient amount of seizure-free period, according to the clinician's judgment considering various clinical factors.
AED can be withdrawn after a minimum of 1 year of seizure freedom when the seizure history of the patient is shorter than 6 months and tumors are completely removed after surgery (Level III) (Fig. 1). The percentage of achieving seizure freedom after brain tumor surgery is highly variable, affected by the type of tumors, residual tumors, duration of uncontrolled epilepsy, and others [9]. More than 50% of brain tumor can reach seizure-freedom after brain surgery so withdrawal of AED should be attempted whenever possible [9]. When tumors are completely resected and the preoperative seizure duration is short it is more likely to achieve seizure-freedom after surgery [30], thus AED withdrawal should be actively considered.
AEDs withdrawal can be attempted after a minimum of 2 years of seizure freedom in the presence of any of the following conditions: 1) patients with a longer seizure history than 6 months, 2) incomplete tumor resection, 3) epileptiform EEG discharges after surgery, 4) preoperative drug-resistant seizures, 5) focal seizure without a loss of consciousness (Level III) (Fig. 1). Gross total resection of tumor is the most important predictor for seizure freedom in brain tumor patients [1]. Therefore, AED withdrawal should be considered carefully after longer seizure-free period when residual tumor is present. Since no studies systematically examined AED withdrawal in brain tumor-related epilepsy, decision has to be made, transferring what is known in general [5,9]. In the general epilepsy population, long duration of active seizure, epileptiform discharges on EEG, multiple AEDs required for seizure control, and focal seizure without a loss of consciousness are considered poor prognostic factor for successful withdrawal of AEDs [31,32,33].
In any cases, when seizure occurs during AED withdrawal, AED dose should be increased again and maintained longer. It is controversial whether to consider AED withdrawal in high-grade glioma (HGG) patients. Some researchers insist that AED withdrawal is not recommended in HGG patient, because of the progressive nature of the tumor [34,35]. On the other hand, some researchers support AED withdrawal even in HGG whenever possible, considering the adverse effect of AEDs and the chance of masking tumor progression that can be detected by worsening seizures [33]. Therefore, the adjustment of AEDs after resection of HGG should be determined case by case according to the clinician's decision.
ANTIEPILEPTIC DRUG SELECTION
Drug interactions should be considered when selecting AEDs in patients with brain tumors (Level II) (Table 1)
Cytochrome P450 (CYP) enzyme inducers and inhibitors affect the blood concentration of anticancer drugs, so care must be taken during prescription (Level II). Several AEDs demonstrate CYP drug interaction, which may affect the level of chemotherapeutic agents. Chemotherapeutic agents often have a narrow therapeutic window close to the maximum tolerated dose, so these interactions can easily result in insufficient anti-tumor effect or in drug toxicity [36]. Carbamazepine, phenytoin, phenobarbital are CYP enzyme inducers, mainly of 2C9, SC19, and 3A4 [37], which may lower the serum level of chemotherapeutic agents and reduce the anti-tumor effects. On the other hand valproate contains CYP enzyme inhibiting properties so that it may increase the serum level of concomitant chemotherapeutic agents which will lead to increased toxicity [36].
Levetiracetam, zonisamide, lacosamide, perampanel, and pregabalin have minimal drug interactions therefore can be administered relatively safely to patients receiving chemotherapy (Level III). Most of second and third generation AEDs are not enzyme-inducing, so their use is preferred in brain tumor patients [36]. Several studies investigated the efficacy and tolerability of levetiracetam in brain tumor patients. All studies reported fairly good efficacy and tolerability [9]. Lacosamide [38,39] and perampanel [40,41] are proven to be well tolerated and effective on seizure control in brain tumor patients, both as monotherapy and add-on therapy.
Adverse effects of AEDs should also be taken into consideration when prescribing them in brain tumor patients. Adverse effects occur more frequently in patients with brain tumor compared with the overall population of people with epilepsy [36,42]. Many AEDs may cause cytopenia or rash, topiramate may induce cognitive dysfunction, and topiramate or zonisamide may cause weight loss, which are critical issues in the management of brain tumor patients [43]. Levetiracetam or perampanel may cause psychiatric adverse events, such as aggressive behavior, agitation, psychosis [44] that can interfere with management of brain tumor patients. Otherwise, lacosamide are less likely to cause cognitive and psychiatric side effects [45].
DRIVING
Even in brain tumor patients who previously experienced seizures, when seizure-free period with or without AEDs is longer than 1 year, driving can be allowed. However, it is not allowed to drive during the tapering period of AEDs (Level III) (Table 1)
Some countries provide detailed recommendations on the driving of brain tumor patients [46]. However, in general, it is similar to recommendations in patients with epilepsy due to other causes. In United Kingdom, government recommends patients not to drive for 6–12 months and 1–2 years after craniotomy of benign and malignant brain tumors, respectively. More strict restrictions are applied to professional drivers [47]. In United States, the prerequisites for allowing epilepsy patients to drive vary from state to state, and specific recommendations for brain tumor patients are not available. According to a survey study, US clinicians tended to restrict driving for longer postoperative period in patients with high-grade brain tumors or in professional drivers [48].
To date, there are no official recommendations on the driving of brain tumor patients in Korea. Thus, it is reasonable to apply the same recommendation on driving suggested in other epilepsy patients. The Korean Epilepsy Society limits epilepsy patients from driving until they are proven seizure-free for more than 1 year [49]. In particular, newly-diagnosed epilepsy patients or patients who underwent reduction or withdrawal of AEDs should not be permitted to drive until remaining seizure-free for more than 1 year. However, driving is allowed for patients who have proven to have only focal seizure that does not cause alteration of consciousness (focal aware seizure) [49,50].
CONCLUSION
To date, no practical guideline for AED prescription in brain tumor patients were available. Thus, KSNO developed the current guideline that could be used by physicians under medical circumstances in Korea. The KSNO Guideline Working Group have previously developed several guidelines regarding the management of gliomas. Now, the working group composed of 27 multidisciplinary medical experts in Korea prepared “The Korean Society for Neuro-Oncology (KSNO) Guideline for Antiepileptic Drug Usage of Brain Tumor: Version 2021.1.”
In summary, prophylactic AED administration is not recommended in newly diagnosed brain tumor patients without previous seizure history. In seizure-naïve patients with no postoperative seizure, it is advisable to stop or reduce AED 1 week after surgery. In seizure-naïve patients with one early postoperative seizure (<1 week after surgery), AED should be maintained for at least 3 months before tapering. In seizure-naïve patients with ≥2 postoperative seizures or in patients with preoperative seizure history, AEDs should be maintained for more than 1 year. The possibility of drug interactions should be considered when selecting AEDs. Driving can be allowed to those proven to be seizure-free for at least 1 year.
Acknowledgments
None
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest.
References
- 1.Ertürk Çetin Ö, İşler C, Uzan M, Özkara Ç. Epilepsy-related brain tumors. Seizure. 2017;44:93–97. doi: 10.1016/j.seizure.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Holthausen H, Blümcke I. Epilepsy-associated tumours: what epileptologists should know about neuropathology, terminology, and classification systems. Epileptic Disord. 2016;18:240–251. doi: 10.1684/epd.2016.0851. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira JA, Santana IA, Caires IQ, et al. Antiepileptic drug prophylaxis in primary brain tumor patients: is current practice in agreement to the consensus? J Neurooncol. 2014;120:399–403. doi: 10.1007/s11060-014-1564-5. [DOI] [PubMed] [Google Scholar]
- 4.Sayegh ET, Fakurnejad S, Oh T, Bloch O, Parsa AT. Anticonvulsant prophylaxis for brain tumor surgery: determining the current best available evidence. J Neurosurg. 2014;121:1139–1147. doi: 10.3171/2014.7.JNS132829. [DOI] [PubMed] [Google Scholar]
- 5.Liang S, Fan X, Zhao M, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma-related epilepsy. Cancer Med. 2019;8:4527–4535. doi: 10.1002/cam4.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimek M, Dammers R. Antiepileptic drug therapy in the perioperative course of neurosurgical patients. Curr Opin Anaesthesiol. 2010;23:564–567. doi: 10.1097/ACO.0b013e32833e14f2. [DOI] [PubMed] [Google Scholar]
- 7.Sughrue ME, Rutkowski MJ, Chang EF, et al. Postoperative seizures following the resection of convexity meningiomas: are prophylactic anticonvulsants indicated? J Neurosurg. 2011;114:705–709. doi: 10.3171/2010.5.JNS091972. [DOI] [PubMed] [Google Scholar]
- 8.Kim SK, Moon J, Cho JM, et al. A national consensus survey for current practice in brain tumor management I: antiepileptic drug and steroid usage. Brain Tumor Res Treat. 2020;8:1–10. doi: 10.14791/btrt.2020.8.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer R, Ortler M, Seiz-Rosenhagen M, Maier R, Anton JV, Unterberger I. Treatment of epileptic seizures in brain tumors: a critical review. Neurosurg Rev. 2014;37:381–388. doi: 10.1007/s10143-014-0538-6. discussion 388. [DOI] [PubMed] [Google Scholar]
- 10.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 11.Siomin V, Angelov L, Li L, Vogelbaum MA. Results of a survey of neurosurgical practice patterns regarding the prophylactic use of anti-epilepsy drugs in patients with brain tumors. J Neurooncol. 2005;74:211–215. doi: 10.1007/s11060-004-6912-4. [DOI] [PubMed] [Google Scholar]
- 12.Dewan MC, Thompson RC, Kalkanis SN, Barker FG, 2nd, Hadjipanayis CG. Prophylactic antiepileptic drug administration following brain tumor resection: results of a recent AANS/CNS Section on Tumors survey. J Neurosurg. 2017;126:1772–1778. doi: 10.3171/2016.4.JNS16245. [DOI] [PubMed] [Google Scholar]
- 13.Parhi A, Gupta T. Antiepileptic drug usage in neuro-oncology: a practice survey. Int J Neurooncol. 2019;2:17–23. [Google Scholar]
- 14.Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004;79:1489–1494. doi: 10.4065/79.12.1489. [DOI] [PubMed] [Google Scholar]
- 15.Tremont-Lukats IW, Ratilal BO, Armstrong T, Gilbert MR. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev. 2008;(2):CD004424. doi: 10.1002/14651858.CD004424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slegers RJ, Blumcke I. Low-grade developmental and epilepsy associated brain tumors: a critical update 2020. Acta Neuropathol Commun. 2020;8:27. doi: 10.1186/s40478-020-00904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aronica E, Leenstra S, van Veelen CW, et al. Glioneuronal tumors and medically intractable epilepsy: a clinical study with long-term followup of seizure outcome after surgery. Epilepsy Res. 2001;43:179–191. doi: 10.1016/s0920-1211(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 18.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 19.Oberndorfer S, Schmal T, Lahrmann H, Urbanits S, Lindner K, Grisold W. [The frequency of seizures in patients with primary brain tumors or cerebral metastases. An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Kaiser Franz Josef Hospital, Vienna] Wien Klin Wochenschr. 2002;114:911–916. [PubMed] [Google Scholar]
- 20.Baumgarten P, Sarlak M, Baumgarten G, et al. Focused review on seizures caused by meningiomas. Epilepsy Behav. 2018;88:146–151. doi: 10.1016/j.yebeh.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Lynam LM, Lyons MK, Drazkowski JF, et al. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. 2007;109:634–638. doi: 10.1016/j.clineuro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. 2014;19:751–759. doi: 10.1634/theoncologist.2014-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudà R, Trevisan E, Soffietti R. Epilepsy and brain tumors. Curr Opin Oncol. 2010;22:611–620. doi: 10.1097/CCO.0b013e32833de99d. [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, Wen PY, Hurwitz S, et al. Morphological characteristics of brain tumors causing seizures. Arch Neurol. 2010;67:336–342. doi: 10.1001/archneurol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonjaret L, Guyonnet M, Berard E, et al. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017;36:213–218. doi: 10.1016/j.accpm.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Kvam DA, Loftus CM, Copeland B, Quest DO. Seizures during the immediate postoperative period. Neurosurgery. 1983;12:14–17. doi: 10.1227/00006123-198301000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Boshuisen K, Arzimanoglou A, Cross JH, et al. Timing of antiepileptic drug withdrawal and long-term seizure outcome after paediatric epilepsy surgery (TimeToStop): a retrospective observational study. Lancet Neurol. 2012;11:784–791. doi: 10.1016/S1474-4422(12)70165-5. [DOI] [PubMed] [Google Scholar]
- 28.Milligan TA, Hurwitz S, Bromfield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology. 2008;71:665–669. doi: 10.1212/01.wnl.0000324624.52935.46. [DOI] [PubMed] [Google Scholar]
- 29.Westover MB, Cormier J, Bianchi MT, et al. Revising the “Rule of Three” for inferring seizure freedom. Epilepsia. 2012;53:368–376. doi: 10.1111/j.1528-1167.2011.03355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol. 2017;16:523–531. doi: 10.1016/S1474-4422(17)30114-X. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick CJ. Withdrawal of antiepileptic drugs in seizure-free adults. Aust Prescr. 2004;27:114–117. [Google Scholar]
- 32.Koekkoek JA, Kerkhof M, Dirven L, et al. Withdrawal of antiepileptic drugs in glioma patients after long-term seizure freedom: design of a prospective observational study. BMC Neurol. 2014;14:157. doi: 10.1186/s12883-014-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerkhof M, Koekkoek JAF, Vos MJ, et al. Withdrawal of antiepileptic drugs in patients with low grade and anaplastic glioma after long-term seizure freedom: a prospective observational study. J Neurooncol. 2019;142:463–470. doi: 10.1007/s11060-019-03117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koekkoek JA, Dirven L, Taphoorn MJ. The withdrawal of antiepileptic drugs in patients with low-grade and anaplastic glioma. Expert Rev Neurother. 2017;17:193–202. doi: 10.1080/14737175.2016.1219250. [DOI] [PubMed] [Google Scholar]
- 35.Koekkoek JA, Dirven L, Sizoo EM, et al. Symptoms and medication management in the end of life phase of high-grade glioma patients. J Neurooncol. 2014;120:589–595. doi: 10.1007/s11060-014-1591-2. [DOI] [PubMed] [Google Scholar]
- 36.Bénit CP, Vecht CJ. Seizures and cancer: drug interactions of anticonvulsants with chemotherapeutic agents, tyrosine kinase inhibitors and glucocorticoids. Neurooncol Pract. 2016;3:245–260. doi: 10.1093/nop/npv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;54:11–27. doi: 10.1111/j.1528-1167.2012.03671.x. [DOI] [PubMed] [Google Scholar]
- 38.Sepúlveda-Sánchez JM, Conde-Moreno A, Barón M, Pardo J, Reynés G, Belenguer A. Efficacy and tolerability of lacosamide for secondary epileptic seizures in patients with brain tumor: a multicenter, observational retrospective study. Oncol Lett. 2017;13:4093–4100. doi: 10.3892/ol.2017.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudà R, Pellerino A, Franchino F, et al. Lacosamide in patients with gliomas and uncontrolled seizures: results from an observational study. J Neurooncol. 2018;136:105–114. doi: 10.1007/s11060-017-2628-0. [DOI] [PubMed] [Google Scholar]
- 40.Maschio M, Zarabla A, Maialetti A, et al. Perampanel in brain tumor-related epilepsy: observational pilot study. Brain Behav. 2020;10:e01612. doi: 10.1002/brb3.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppola A, Zarabla A, Maialetti A, et al. Perampanel confirms to be effective and well-tolerated as an add-on treatment in patients with brain tumor-related epilepsy (PERADET Study) Front Neurol. 2020;11:592. doi: 10.3389/fneur.2020.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gefroh-Grimes HA, Gidal BE. Antiepileptic drugs in patients with malignant brain tumor: beyond seizures and pharmacokinetics. Acta Neurol Scand. 2016;133:4–16. doi: 10.1111/ane.12437. [DOI] [PubMed] [Google Scholar]
- 43.Kerrigan S, Grant R. Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database Syst Rev. 2011;(8):CD008586. doi: 10.1002/14651858.CD008586.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liguori C, Izzi F, Manfredi N, et al. Efficacy and tolerability of perampanel and levetiracetam as first add-on therapy in patients with epilepsy: a retrospective single center study. Epilepsy Behav. 2018;80:173–176. doi: 10.1016/j.yebeh.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Li KY, Huang LC, Chang YP, Yang YH. The effects of lacosamide on cognitive function and psychiatric profiles in patients with epilepsy. Epilepsy Behav. 2020;113:107580. doi: 10.1016/j.yebeh.2020.107580. [DOI] [PubMed] [Google Scholar]
- 46.Kang JY, Mintzer S. Driving and epilepsy: a review of important issues. Curr Neurol Neurosci Rep. 2016;16:80. doi: 10.1007/s11910-016-0677-y. [DOI] [PubMed] [Google Scholar]
- 47.Neurological disorders: assessing fitness to drive. Swansea: Driver and Vehicle Licensing Agency; 2016. [updated Mar 2, 2021]. [Accessed April 9, 2021]. at https://www.gov.uk/guidance/neurological-disorders-assessing-fitness-to-drive. [Google Scholar]
- 48.Thomas S, Mehta MP, Kuo JS, Ian Robins H, Khuntia D. Current practices of driving restriction implementation for patients with brain tumors. J Neurooncol. 2011;103:641–647. doi: 10.1007/s11060-010-0439-7. [DOI] [PubMed] [Google Scholar]
- 49.Kang HG, Lee SD, Lee SA, et al. Epilepsy and driving regulation in Korea. J Korean Neurol Assoc. 2018;36:65–73. [Google Scholar]
- 50.Cho YW, Kim KT. Driving regulations for epilepsy in South Korea. Epilia. 2020;2:36–39. [Google Scholar]