Severe cases of pneumonia are frequently associated with acute respiratory distress syndrome (ARDS), which carries a mortality rate of about 40% [1]. Uncontrolled host inflammatory response in the lung is a key factor in the transition from pneumonia to ARDS, with alveolocapillary membrane disruption leading to interstitial and alveolar oedema [2]. Neutrophils are part of the innate immune system and are the first responders to local tissue damage and infection. Recruited neutrophils are considered important actors in lung tissue injury [3]. Indeed, their broad arsenal of antimicrobial weaponry can cause direct and indirect collateral damage. Neutrophil serine proteinases (NSPs), including elastase (NE), proteinase 3 (PR3) and cathepsin G (CatG), are released from activated cells and play a part in ARDS pathophysiology, as illustrated in both preclinical and clinical studies [4]. Thus, NSPs emerge as an untapped point for therapeutic interventions in pneumonia-induced ARDS [4]. These NSPs are readily synthesised in neutrophil precursors within the bone marrow and are converted into their active form by cathepsin C (CatC) [5]. They are stored together in cytoplasmic granules and secreted into the extracellular compartment upon stimulation [6].
Short abstract
COVID-19 ARDS is associated with release of biologically active neutrophil elastase-related proteinases to the airways and blood at a comparable level to non-COVID ARDS https://bit.ly/3nihveh
To the Editor:
Severe cases of pneumonia are frequently associated with acute respiratory distress syndrome (ARDS), which carries a mortality rate of about 40% [1]. Uncontrolled host inflammatory response in the lung is a key factor in the transition from pneumonia to ARDS, with alveolocapillary membrane disruption leading to interstitial and alveolar oedema [2]. Neutrophils are part of the innate immune system and are the first responders to local tissue damage and infection. Recruited neutrophils are considered important actors in lung tissue injury [3]. Indeed, their broad arsenal of antimicrobial weaponry can cause direct and indirect collateral damage. Neutrophil serine proteinases (NSPs), including elastase (NE), proteinase 3 (PR3) and cathepsin G (CatG), are released from activated cells and play a part in ARDS pathophysiology, as illustrated in both preclinical and clinical studies [4]. Thus, NSPs emerge as an untapped point for therapeutic interventions in pneumonia-induced ARDS [4]. These NSPs are readily synthesised in neutrophil precursors within the bone marrow and are converted into their active form by cathepsin C (CatC) [5]. They are stored together in cytoplasmic granules and secreted into the extracellular compartment upon stimulation [6].
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can lead to pneumonia with lung hyper-inflammation [7] and, in the most severe cases, to ARDS [8]. However, there are some controversies regarding the clinical presentation and pathophysiology of COVID-19-induced ARDS, as compared to ARDS associated with other microbial infections. In this regard, investigation of the potential role of neutrophils in COVID-19 ARDS is only in its infancy [9–11]. In an attempt to fill this knowledge gap, we investigated neutrophilic inflammation by studying NSPs in endotracheal aspirates (ETAs) and blood collected from mechanically ventilated patients with pneumonia-driven ARDS of non-COVID-19 and COVID-19 aetiology. CatC, identified as a biomarker of neutrophil degranulation [6] and pro-inflammatory NSPs were analysed using enzymatic and immunological methods in concentrated ETA supernatants and sera. To our knowledge, this study is the first to compare the concentrations of proteolytically active CatC and NSPs in samples from patients with distinct pneumonia-related ARDS, including with COVID-19 aetiology.
Patients from non-COVID-19 ARDS (n=15) and COVID-19 ARDS (n=17) presented similar clinical characteristics at admission. Median (interquartile range) age was 62 (57–66.5) years for the non-COVID-19 ARDS group and 64 (59–70) for the COVID-19 ARDS group, with a percentage of males of 60% and 65%, respectively. Median neutrophil count in the blood of the non-COVID-19 group was 8.7 (6.6–13.7) ×109 L−1 and 6.4 (6.0–7.6) ×109 L−1 for the COVID-19 group. All patients met the criteria for ARDS, and median arterial oxygen tension/inspired oxygen fraction ratio at the time of analysis was <200 in both groups (and >100), denoting moderate severity: 197 (158–250) for the non-COVID-19 ARDS group and 162 (117–215) for the COVID-19 ARDS group. Microbial identification, using multiplex PCR and/or conventional cultures on ETA samples, was obtained in 13/15 of the non-COVID-19 patients: influenza A or B (n=8), Streptococcus pneumoniae (n=1), rhinovirus (n=2), respiratory syncytial virus (n=1), Staphylococcus haemolyticus (n=1) and three patients with influenza were co-infected with Streptococcus pneumoniae, Mycoplasma pneumoniae or Streptococcus pneumoniae and Aspergillus fumigatus.
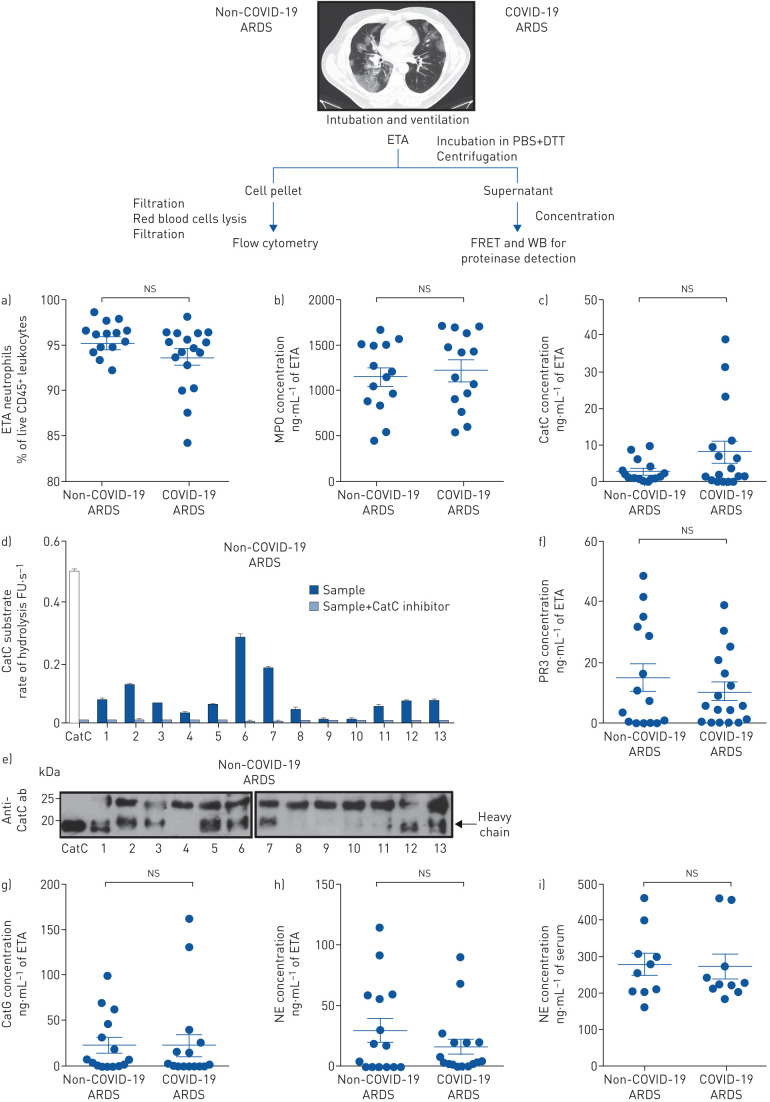
Flow cytometry analysis of the cellular content of ETAs showed that neutrophils (live CD45+/CD14−/CD16+ cells) accounted for the vast majority of leukocytes in the airways of pneumonia-related ARDS and, of note, no significant difference between the two groups was found (non-COVID-19 ARDS: 95±0.7%; COVID-19 ARDS: 94±0.9%, p=0.16) (figure 1a). ETA neutrophils from the two groups expressed similar amount of surface activation marker CD16 (mean fluorescence intensity (MFI) for non-COVID-19 ARDS: 19930±2617; MFI for COVID-19 ARDS: 20057±2112, p=0.7340; Mann–Whitney test). Myeloperoxidase (MPO), a marker of neutrophil activation, was detected in all ETA samples of non-COVID-19 and COVID-19 ARDS patients, with mean concentrations of 1148±102 ng·mL−1 and 1218±111 ng·mL−1 respectively, and no significant difference in concentration between the two groups (p=0.63) (figure 1b). CatC activity was detected in ETA samples of 14 non-COVID-19 ARDS patients (93%; 2.6±0.8 ng·mL−1) and 14 COVID-19 ARDS patients (82%; 8±2.8 ng·mL−1) with no significant difference in concentration between the two groups (p=0.39) (figure 1c). CatC activity was assayed using a CatC-selective FRET substrate [12] in the presence or absence of a selective CatC inhibitor (figure 1d). The 20-kDa heavy chain of CatC, representing the fully processed proteolytically active subunit of the proteinase, was detected by Western blotting using an antibody against CatC in a subset of the non-COVID-19 ARDS group ETA samples. When measured activity levels were low (figure 1d), this was reflected by an absence of the heavy chain band in the corresponding blot (figure 1e). Detection of proteolytically active CatC indicates the presence of activated neutrophils with granule release and consequently NSPs at inflammatory sites.
FIGURE 1.
Neutrophils and proteolytically active proteinases in endotracheal aspirates (ETAs). Schematic of the workflow is shown above. ETAs collected from mechanically ventilated non-COVID-19 acute respiratory distress syndrome (ARDS) and coronavirus disease 2019 (COVID-19) ARDS patients from intensive care unit were weighed and incubated in PBS (5 mL·g−1) with 1 mM dithiothreitol (DTT) for 30 min at 4°C under gentle agitation. After centrifugation, supernatants were collected and then concentrated five times before total protein quantification by the BCA or Bradford assay. Cell pellets were filtered through a 100 µm cell strainer. Red blood cells were removed using a red blood cell lysis buffer and then cell suspensions from ETAs were passed through a 40 µm cell strainer prior to staining for flow cytometry. a) Cells were stained with antibodies against surface markers (CD45, CD14 and CD16) and viability dye. Neutrophils were defined as live CD45+ CD14− CD16+ cells. Neutrophil relative proportion in ETAs of all non-COVID-19 ARDS and COVID-19 ARDS patients is represented as percentage of live leukocytes (CD45+ cells). Individuals are depicted. The data represent mean±sem. Mann–Whitney test. b) Quantitative detection of myeloperoxidase (MPO) in ETA supernatants (dilution: 1/500) from 14 non-COVID-19 ARDS and 14 COVID-19 ARDS patients using a human MPO Elisa kit (DMYE00B, R&D Systems). Individuals are depicted. The data represent mean±sem. Mann–Whitney test. c) The concentration of active cathepsin C (CatC) was estimated by comparison of the rate of hydrolysis of FRET (fluorescence resonance energy transfer) substrate by ETA supernatants of all non-COVID-19 ARDS and COVID-19 ARDS patients and recombinant CatC. Each point represents data of two independent experiments, both in duplicate, from an individual subject. Individuals are depicted. The data represent mean±sem. Mann–Whitney test. d) The CatC activity in five times concentrated ETA supernatants (4 µg protein) was measured spectrofluorometrically at 460 nm with or without the nitrile inhibitor (L)-Thi-(L)-Phe-CN (0.5 µM final, 20 min incubation at 37°C) using Thi-Ala(Mca)-Ser-Gly-Tyr(3-NO2)-NH2 (20 µM final) as selective FRET substrate. The residual activities were inhibited by the CatC inhibitor (L)-Thi-(L)-Phe-CN which demonstrate the presence of CatC activity in samples. Inhibition experiments were performed with all ETA samples used in the study. Results with ETA samples from 13 non-COVID-19 ARDS are shown. Data represent the mean±sd of two independent experiments, both performed with duplicates of the same sample. Similar results were obtained with ETA supernatants of all COVID-19 ARDS patients. FU: fluorescence unit. e) Western blotting (WB) of concentrated ETA supernatants (30 µg of protein) from 13 non-COVID-19 ARDS (no 1–13) using an anti-CatC antibody (sc-74590, 1:1000, Santa Cruz Biotechnology) shows the presence of the CatC heavy chain. Similar results were obtained with ETA supernatants of all non-COVID-19 ARDS and COVID-19 ARDS patients in three independent experiments. f) The proteinase 3 (PR3) activity in ETA supernatants was measured at 420 nm with or without the PR3 inhibitor Ac-PYDAP(O-C6H6-4-Cl)2 (0.5 µM final, 20 min incubation at 37°C) using ABZ-VAD(nor)VADYQ-EDDnp (20 µM final) as a substrate. g) CatG activity in ETA supernatants was measured in the presence or absence of alpha-1-antichymotrypsin using ABZ-TPFSGQ-EDDnp (20 µM final) as a substrate. h) The neutrophil elastase (NE) activity in ETA supernatants was measured with or without NE inhibitor EPI-hNE-4 (0.5 µM final, 20 min incubation at 37°C) using ABZ-APEEIMRRQ-EDDnp as a substrate. The residual activities in ETA samples were inhibited by selective NSP inhibitors demonstrating the specificity of the FRET assays utilised. Inhibition experiments were performed with all ETA samples used in the study. Each point represents data of two independent experiments, both in duplicate, from an individual subject. Individuals are depicted. The data represent mean±sem. Mann–Whitney test. i) Quantitative detection of circulating NE. A human NE ELISA kit (HK319, HycultBiotech) was used for the quantitative detection of NE in sera (dilution: 1/100) from 10 non-COVID-19 ARDS and 10 COVID-19 ARDS patients. Individuals are depicted. The data represent mean±sem. Kruskal–Wallis test. ns: nonsignificant.
The selective and specific FRET substrates designed for each NSP [13] allowed their identification as active proteinases in ETA from patients (figure 1f–h). A reduction of NSP activities was achieved in our samples with purified alpha-1-antitrypsin, a natural serpin inhibitor of NSPs in human plasma. In this way, we were able to exclude the presence of active NSPs as a component of alpha-2-macroglobulin complexes, which encage, but still contain, the active proteinases. NSPs were detected in most samples also containing mature CatC (72%). NSP activity was detected in ETA of 12 non-COVID-19 ARDS patients (80%) and 14 COVID-19 ARDS patients (82%). We estimated the concentration of active NSPs from the rate of substrate hydrolysis using purified NSPs (non-COVID-19 ARDS group: PR3 14.8±4.4 ng·mL−1, NE 29.9±9.6 ng·mL−1, CatG 23.6±8.2 ng·mL−1; COVID-19 ARDS group: PR3 10.2±2.9 ng·mL−1, NE 16±6.2 ng·mL−1, CatG 23.7±11.7 ng·mL−1). We found no significant difference in the total concentration of the three NSPs between the non-COVID-19 ARDS group and COVID-19 ARDS (68.4±19.7 ng·mL−1 of ETA and 49.9±20.2 ng·mL−1 of ETA, respectively, p=0.52). Individual analysis of the three NSPs did not reveal any difference between non-COVID-19 and COVID-19 groups (figure 1f–h). The variation observed in NSP concentrations could be explained by neutrophil counts in the airways, their degranulation status and the level of active extracellular inhibitors. Last, we explored circulating NSP concentration in the serum of non-COVID-19 and COVID-19 patients and compared them to healthy donors (n=10). Because of huge amounts of serpins in sera, it was not possible to detect any proteolytic activity using NSP selective FRET substrates, despite their high sensitivity. We used an ELISA (enzyme-linked immunosorbent assay) that was particularly sensitive for detection of free NE and complexed NE with alpha-1-antitrypsin. There was no significant difference in NE serum concentration between non-COVID-19 and COVID-19 groups (280±29 ng·mL−1 of serum and 273±32 ng·mL−1 of serum, respectively, p=1) as assayed by ELISA (n=10) (figure 1i). However, NE concentration in both groups was significantly higher compared to healthy donors (median (interquartile range) age was 59 (54–63.5) years; male/female ratio was 2/1, n=10) (107±3.5 ng·mL−1 of serum; p<0.001, Kruskal–Wallis test).
Specific identification and detection of proteolytically active NSPs in acute inflammatory lung diseases is an important starting point to infer the simultaneous inhibition of multiple NSPs as a useful and effective therapeutic approach. By analysing ETA samples, we present strong circumstantial evidence for the involvement of NSPs in pneumonia-driven ARDS, in both non-COVID-19 and COVID-19 patients. It should, however, be kept in mind that pneumonia-driven ARDS is an alveolar process and that ETA sampling does not precisely reflect the unfolding inflammatory process in the alveolar compartment. Despite these technical limitations, our study strongly suggests that COVID-19 ARDS is associated with the release of biologically active NSPs into the small airways at a comparable level as observed in non-COVID-19 ARDS. It is thought that elevated levels of NSPs overwhelm the endogenous shield of natural inhibitors. In addition, we identified active CatC, the critical enzyme stored together with mature NSPs in primary granules of neutrophils, in ETA samples of non-COVID-19 and COVID-19 ARDS patients. Although no major difference between the two groups was found, we cannot exclude a difference below the limited statistical power of the study due to small numbers of patients. Since our observations have been made on a limited number of patients and because neutrophils may be involved in different detrimental processes in COVID-19 pathophysiology (including diffuse alveolar damage, thrombosis and microangiopathy [10]), we have not been able so far to establish a clear relationship between the outcome of pneumonia-driven ARDS patients and NSPs activities.
Taken together, our data strongly encourage further mechanistic studies with regard to the precise contribution of NSPs in pneumonia-driven ARDS and strongly support initiatives to evaluate the therapeutic potential and efficacy of CatC inhibitors in COVID-19 to eliminate NSPs already at an early phase of the disease [4]. In this regard, the outcome of an ongoing clinical trial with brensocatib, a reversible nitrile CatC inhibitor approved in a phase 2 clinical study [14], is eagerly awaited by health professionals, scientists and currently affected patients (STOP-COVID19, Superiority Trial of Protease Inhibition in COVID-19, EudraCT number 2020-001643-13 [4]). Brensocatib treatment may help prevent irreversible pulmonary failure of COVID-19 patients with high level of active NSPs. Clinical trial results from the STOP-COVID-19 study will help us to better understand NSP-dependent pathophysiology of neutrophils in COVID-19.
Shareable PDF
Acknowledgements
The authors thank Celia Moss (Birmingham Children's Hospital and University of Birmingham) for English editing.
Footnotes
Author contributions: B. Korkmaz and S. Seren designed the study. S. Seren, L. Derian, I. Keleş, L. Gonzalez, T. Baranek and C. Paget performed the experiments. All authors analysed and discussed the results. B. Korkmaz and Y. Jouan wrote the manuscript and all authors contributed to the writing and revision process of the manuscript. B. Korkmaz supervised the work.
Conflict of interest: S. Seren has nothing to disclose.
Conflict of interest: L. Derian has nothing to disclose.
Conflict of interest: I. Keleş has nothing to disclose.
Conflict of interest: A. Guillon has nothing to disclose.
Conflict of interest: A. Lesner has nothing to disclose.
Conflict of interest: L. Gonzalez has nothing to disclose.
Conflict of interest: T. Baranek has nothing to disclose.
Conflict of interest: M. Si-Tahar has nothing to disclose.
Conflict of interest: S. Marchand-Adam has nothing to disclose.
Conflict of interest: D.E. Jenne has nothing to disclose.
Conflict of interest: C. Paget has nothing to disclose.
Conflict of interest: Y. Jouan has nothing to disclose.
Conflict of interest: B. Korkmaz has been paid for the time spent as a committee member for advisory boards (INSMED), other forms of consulting (Neuprozyme Therapeutics Aps (Denmark), Santhera Pharmaceuticals (Switzerland)), symposium organisation (INSMED) and travel support, lectures or presentations, outside the submitted work.
Support Statement: This work was supported by the “Ministère de l'Enseignement Supérieur et de la Recherche”, the “Région Centre Val de Loire” (Project Pirana) and “National Science Center Poland” (UMO 2014/15/B/ST5/05311) granted to A. Lesner. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377: 562–572. doi: 10.1056/NEJMra1608077 [DOI] [PubMed] [Google Scholar]
- 2.Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet 2016; 388: 2416–2430. doi: 10.1016/S0140-6736(16)00578-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potey PM, Rossi AG, Lucas CD, et al. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J Pathol 2019; 247: 672–685. doi: 10.1002/path.5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korkmaz B, Lesner A, Marchand-Adam S, et al. Lung protection by cathepsin C inhibition: a new hope for COVID-19 and ARDS? J Med Chem 2020; 63: 13258–13265. doi: 10.1021/acs.jmedchem.0c00776 [DOI] [PubMed] [Google Scholar]
- 5.Korkmaz B, Caughey GH, Chapple I, et al. Therapeutic targeting of cathepsin C: from pathophysiology to treatment. Pharmacol Ther 2018; 190: 202–236. doi: 10.1016/j.pharmthera.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 6.Hamon Y, Legowska M, Herve V, et al. Neutrophilic cathepsin C is maturated by a multi-step proteolytic process and secreted by activated cells during inflammatory lung diseases. J Biol Chem 2016; 291: 8486–8499. doi: 10.1074/jbc.M115.707109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillon A, Hiemstra PS, Si-Tahar M. Pulmonary immune responses against SARS-CoV-2 infection: harmful or not? Intensive Care Med 2020; 46: 1897–1900. doi: 10.1007/s00134-020-06170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan C. Neutrophils and COVID-19: nots, NETs, and knots. J Exp Med 2020; 217: e20201439. doi: 10.1084/jem.20201439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med 2020; 217: e20200652. doi: 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020; 5: e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legowska M, Hamon Y, Wojtysiak A, et al. Development of the first internally-quenched fluorescent substrates of human cathepsin C: The application in the enzyme detection in biological samples. Arch Biochem Biophys 2016; 612: 91–102. doi: 10.1016/j.abb.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 13.Korkmaz B, Attucci S, Juliano MA, et al. Measuring elastase, proteinase 3 and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrates. Nat Protoc 2008; 3: 991–1000. doi: 10.1038/nprot.2008.63 [DOI] [PubMed] [Google Scholar]
- 14.Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med 2020; 383: 2127–2137. doi: 10.1056/NEJMoa2021713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03755-2020.Shareable (425.8KB, pdf)