Abstract
Chemoresistance is the main cause of poor prognosis in colorectal cancer (CRC). Nicotinamide N-methyltransferase (NNMT) is a metabolic enzyme that is upregulated in various tumor types. It has been reported that NNMT inhibits apoptosis and enhances resistance to 5-fluorouracil (5-Fu) via inhibition of the apoptosis signal regulating kinase 1 (ASK1)-p38 MAPK pathway in CRC cells. A natural product library was screened, and it was found that vanillin, also known as 4-hydroxy-3-methoxybenzaldehyde, a plant secondary metabolite found in several essential plant oils, mainly Vanilla planifolia, Vanilla tahitensis, and Vanilla pompon, may be a promising anticancer compound targeted to NNMT. The aim of the present study was to explore the effect of vanillin on promoting apoptosis and attenuating NNMT-induced resistance to 5-Fu in CRC. Lentiviral vectors of short hairpin RNA and small interfering RNA were transfected into HT-29 cells to construct NNMT-knockdown HT-29 cell lines. Vectors containing an open reading frame of NNMT were stably transfected into SW480 cells to induce NNMT overexpression in SW480 cell lines. Vanillin was found to inhibit the mRNA and protein expression levels of NNMT following the inhibition of NNMT activity in HT-29 cell lines. Vanillin was able to reverse NNMT-induced increased cell proliferation, decreased cell apoptosis and resistance to 5-Fu by inhibiting NNMT expression. Furthermore, it increased cell apoptosis by activating the ASK1-p38 MAPK pathway, which could be inhibited by NNMT. In addition, vanillin increased cell apoptosis by promoting mitochondrial damage and reactive oxygen species. In vivo, the combination of vanillin with 5-Fu yielded a notable synergy in inhibiting tumor growth and inducing apoptosis. Considering that vanillin is an important flavor and aromatic component used in foods worldwide, vanillin is deemed to be a promising anticancer candidate by inhibiting NNMT and may attenuate NNMT-induced resistance to 5-Fu in human CRC therapy with few side effects.
Keywords: vanillin, nicotinamide N-methyltransferase, cell apoptosis, chemoresistance, colorectal cancer
Introduction
Colorectal cancer (CRC) is a common malignancy and the leading cause of cancer-related mortality worldwide (1). Chemotherapy remains one of the major adjuvant treatment strategies for CRC. 5-Fluorouracil (5-Fu) is a chemotherapeutic drug widely used for the treatment of CRC, but its therapeutic effects are limited by severe side effects and/or drug resistance, which leads to poor CRC prognosis. 5-Fu-based chemotherapy insensitiveness or resistance is a major obstacle in achieving effective treatment for CRC (2). Therefore, the identification of drugs with anti-colorectal cancer activities and a potential synergistic effect with 5-Fu is urgently required.
Nicotinamide N-methyltransferase (NNMT) is a S-adenosylmethionine-dependent enzyme that catalyzes nicotinamide to 1-methylnicotinamide (1-MNA) via N-methylation. It also catalyzes pyridines and other structural analogs. NNMT is upregulated in various tumor types, including CRC (3,4), and is known to contribute to drug resistance, leading to cancer treatment failure. Our previous study showed that NNMT accelerated cell proliferation through a reduction in reactive oxygen species (ROS), promotion of cell cycle and inhibition of apoptosis in human CRC cells (5). NNMT has been previously found to enhance resistance to 5-Fu in CRC cells via inhibition of the apoptosis signal regulating kinase 1 (ASK1)-p38 MAPK pathway (6). Collectively, the aforementioned findings suggest that NNMT plays multiple roles in CRC, making it an attractive therapeutic target.
Drugs targeting NNMT may help reduce NNMT-related chemotherapy resistance. Natural bioactive products play critical roles in the discovery of anticancer drugs; a series of drugs have been developed based on natural products including paclitaxel, vinblastine, camptothecin, irinotecan and topotecan, among others (7). A natural product library was screened and it was found that vanillin could inhibit cell proliferation and NNMT expression in HT-29 cell lines in vitro. We hypothesized that vanillin is an NNMT-targeting compound with great potential for reducing chemoresistance to 5-Fu.
Vanillin, also known as 4-hydroxy-3-methoxybenzaldehyde, is a plant secondary metabolite found in several essential plant oils, mainly Vanilla planifolia, Vanilla tahitensis, and Vanilla pompon (8). It is used as an important flavor and aromatic component in foods worldwide. Studies have shown that vanillin has antitumor potential due to its multiple functions. It has been reported to exert anticancer effects by inducing apoptosis and cell cycle arrest in colon cancer (9,10). Vanillin binds to calcium/calmodulin-dependent protein kinase IV and microtubule affinity regulated kinase 4 with high affinity, resulting in mitochondrial damage and ROS production, eventually leading to apoptosis (11,12). Vanillin exhibits anti-invasive and anti-metastatic activities by suppressing the expression of MMP-9, via signaling pathways such as PI3K, nuclear factor (NF)-κB and signal transducer and activator of transcription 3 (STAT3)/hypoxia-inducible factor 1α (HIF-1α) (13–16). In addition, vanillin has been found to exert synergistically potentiated anticancer effects together with doxorubicin and cisplatin (17,18). Although studies have recognized that vanillin has anticancer effects, the precise molecular mechanism of vanillin-related tumor suppression has not yet been elucidated. Moreover, no research has investigated the effect of vanillin on NNMT and its potential synergistic effect with 5-Fu to data.
Taking all of these facts into consideration, the present study aimed to determine the effect of vanillin on NNMT expression and NNMT-related chemoresistance. In the present study, it was identified that vanillin inhibited cell proliferation and enhanced the effects of 5-Fu in both CRC cell lines SW480 and HT-29. Vanillin was also found to decrease NNMT expression and activity by reducing the 1-MNA level. Flow cytometry and western blot analysis confirmed that vanillin induced cell apoptosis. Further experiments showed that vanillin induced cell apoptosis by activating the ASK1/p38 MAPK pathway, promoting mitochondrial damage and increasing intracellular ROS. SB203580 (a specific inhibitor of p38 phosphorylation) and N-acetyl-L-cysteine (NAC; the scavenger of ROS) were used to affirm the results. In addition, the in vivo results demonstrated that vanillin combined with 5-Fu inhibited tumor growth and induced apoptosis. The present study revealed that vanillin inhibits the expression of NNMT and enhances sensitivity to 5-Fu through the induction of ROS and cell apoptosis in CRC. In addition, considering that vanillin has long been used as a food additive worldwide, it was proposed that vanillin is a promising anticancer drug candidate with low side effects for human CRC therapy that may attenuate NNMT-related resistance to 5-Fu.
Materials and methods
Cells and cell culture
The human CRC cell lines, SW480 and HT-29, which have low and high NNMT expression, respectively, were purchased from the Cell Bank at the Chinese Academy of Sciences (Shanghai, China). The modified cell lines SW480/NC, SW480/NNMT (NNMT overexpression) as well as HT-29/NC and HT-29/shNNMT (NNMT knockdown) were constructed as described in our previous study (5). STR authentication of all the cell lines was completed. All cells were cultured in RMPI-1640 medium (cat. no. 11875-093. Gibco; Thermo Fisher Scientific, Inc.) supplemented with Penicillin-Streptomycin Liquid (cat. no. 15140-122; Merck KGaA) at a final concentration of 100 U/ml penicillin and 100 mg/ml streptomycin, and 10% serum (cat. no. 26010066. Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
Chemical reagents and antibodies
Vanillin (cat. no. V1104, 99% purity, Merck KGaA), 5-Fu (cat. no. V900394, reagent grade, ≥99%, Merck KGaA) and SB203580 (cat. no. S1076, Selleck Chemical) were dissolved in DMSO (cat. no. D8418, Merck KGaA). N-acetyl-L-cysteine (NAC; cat. no. ST1546, Beyotime Institute of Biotechnology) and 1-MNA (cat. no. S3346, Selleck Chemical) were dissolved in deionized water. All reagents used were of molecular biology grade, stored at −80°C, and diluted in culture medium for each experiment. The final concentration of DMSO did not exceed 0.3%. Barbiturate injection was unified supplied by the Laboratory Animal Center of Sir Run Run Shaw Hospital of Zhejiang University. The mouse anti-human NNMT monoclonal antibody (clone: 1E7) was prepared through the hybridoma technique as previously described (19). The anti-β-actin (cat. no. 4970), anti-PARP (cat. no. 9542), anti-p53 (cat. no. 2527), anti-cleaved caspase-3 (Asp175) (cat. no. 9661), anti-phospho-Stat3 (Tyr705) (cat. no. 9145), anti-ASK1 (cat. no. 8662), anti-phospho-ASK1 (Thr845) (cat. no. 3765), anti-p38 MAPK (cat. no. 9212), anti-phospho-p38 MAPK (Thr180/Tyr182) (cat. no. 4511), goat anti-rabbit IgG (cat. no. 7074) and goat anti-mouse IgG (cat. no. 7076) antibodies were all obtained from CST (Cell Signaling Technology, Inc.). Anti-cleaved caspase-9 (cat. no. ab2324) was obtained from Abcam, Inc.
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
Cells were treated and harvested, and total RNA was isolated using TRIzol® reagent (cat. no. 15596026. Thermo Fisher Scientific, Inc.). RNA (2 µg) was then reverse-transcribed into cDNA using a cDNA Synthesis kit (cat. no. 6130. Takara Bio, Inc.), according to the manufacturer's instructions. The reaction mixture for reverse transcription was heated at 65°C for 5 min, then 42°C for 1 h and then iced for 2 min. RT-qPCR was performed using NovoStart SYBR qPCR SuperMix Plus Kit (cat. no. E096-01A; Novoprotein), following the manufacturer's instructions. The reaction mixture for RT-qPCR was performed as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 68°C for 30 sec, using an ABI PRISM 7500 Fast real-time PCR System (Thermo Fisher Scientific, Inc.). The primer sequences were as follows: h-GAPDH-F, GAAGGTGAAGGTCGGAGT and h-GAPDH-R, GAAGATGGTGATGGGATTTC; h-NNMT-F, GAGATCGTCGTCACTGACTACT and h-NNMT-R, CACACACATAGGTCACCACTG. Relative expression levels were calculated using the 2−ΔΔCq method (20). NNMT levels were normalized to those of GAPDH and then compared with the control group, which was set as 1. All of the experiments were independent and conducted at least three times.
Detection of 1-MNA
1-MNA was detected by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Briefly, cells were seeded in 10-cm plates, incubated for 24 h, and treated with different concentration of vanillin for 48 h. The cells were then harvested and washed with PBS twice. Next, 50% of the cells were fixed with methanol for LC-MS/MS, and the other 50% was used to extract total protein for quantification. A total of 5 µl of the samples was injected into an Eclipse XDB-C18 column (4.6×150 mm, 5 µm, Agilent Technologies, Inc.), which was connected to Jasper™ LC system (AB Sciex). The isocratic mobile phase, a mixture of 0.1% formic acid and methanol mixture (v/v), was delivered at a flow rate of 1 ml/min into the mass spectrometer electrospray ionization chamber. Quantitation was achieved by MS/MS Detection in positive ion mode for 1-MNA and internal standard (N-MNA-d4, cat. no. M321172, Tan Mo Quality Inspection Technology Co., Ltd.). Detection of the ions was performed in the multiple reaction monitoring mode. The retention times of 1-MNA and N-MNA-d4 were 1.31 and 1.65 min, respectively. The experiments were independent and conducted a minimum of three times.
Western blot analysis
Total protein was extracted from cells using RIPA lysis buffer (cat. no. P0013B, Beyotime Institute of Biotechnology) with protease inhibitors. Protein concentrations were measured using the BCA Protein Assay Kit (cat. no. P0012, Beyotime Institute of Biotechnology). A total of 40 µg each protein sample was separated by SDS-PAGE and transferred to a PVDF membrane (cat. no. IPVH00010, EMD Millipore). Following blocking and washing, the membranes were incubated with primary antibodies at a dilution of 1:1,000 overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies at 1:2,000 for 80 min at room temperature. Using high sensitivity chemiluminescence detection reagents (FDbio-Femto ECL, cat. no. FD8030, HangZhou FuDe Biological Technology. Co., Ltd.), signals were visualized and captured using Image Lab (Bio-Rad, Laboratories, Inc.). The protein bands were semi-quantified and normalized to β-actin. They were then compared with the control group, which was set as 1. All experiments were conducted a minimum of three times independently.
Cell viability and colony formation assays
Cell viability assay was assessed using a Cell-Counting Kit-8 (CCK-8; cat. no. CK04; Dojindo Molecular Technologies, Inc.), following the manufacturer's instructions. Previous reports have tried different treating times including 24, 48 and 72 h, and found that vanillin treated for 48 h already has an evident effect on CRC cell lines (9,10), thus Ramadoss and Sivalingam selected 48 h for the functional analysis of vanillin (10). Here we used 48 h as the treatment time for vanillin in HT-29 and SW480 cells. Briefly, exponentially growing cells were seeded in 96-well plates at a concentration of 5×103 cells/100 µl per well and incubated for 24 h. Then cells were incubated with different concentrations of vanillin (1, 2, 2.5, 3, 3.5, 4, 5, 6, 7 mM) and/or 5-Fu (1, 2, 4, 6, 8, 12, 16, 24, 36, 48, 72, 100, 150, 200, 250 mg/l) for a further 48 h. Before detection, 10 µl/well CCK-8 solution was added to cells and incubated at 37°C for 2 h. Absorbance was measured at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.). Cells treated with media alone were used as a control. The half maximal inhibitory concentration (IC50) was calculated using GraphPad Prism software version 7.0 (GraphPad Software, Inc.).
For the colony formation assay, 103 exponentially growing cells/well were seeded in 6-well plates and incubated for 24 h. The cells were incubated with different concentrations of vanillin. Cultures with or without vanillin were changed every 48 h. After 2 weeks, the viable colonies were fixed with methanol, stained with methylrosanilinium chloride (cat. no. C0775, Merck KGaA) and counted using ImageJ (Rawak Software Inc.). The experiment was repeated at least three times.
Cell apoptosis analysis
Apoptosis was detected by flow cytometric analysis using Annexin V-PE/7-AAD Apoptosis Detection kit (cat. no. 559763; BD Biosciences) and Annexin V-FITC/PI Apoptosis Detection kit (cat. no. 556547; BD Biosciences), according to the manufacturer's instructions. Briefly, 3×105 cells/well were seeded in a 6-well plate. Following treatment with vanillin (2.5 and 3.5 mM for HT-29 cells, 3 and 4 mM for SW480 cells) and/or 5-Fu (40 mg/l for HT-29 cells, 20 mg/l for SW480 cells) or SB203580 (10 µM) or 1-MNA (1 mM), cells were harvested, incubated with Annexin V-PE and 7-AAD or Annexin V-FITC and PI for 15 min in the dark at room temperature and analyzed immediately by flow cytometry (FACSCalibur flow cytometer; BD Biosciences). Each experiment was conducted a minimum of three times.
ROS determination
The ROS Assay kit (cat. no. S0033S, Beyotime Institute of Biotechnology) was used to measure ROS generation, according to the manufacturer's instructions. In brief, cells were cultured in 12-well plates following treatment with vanillin and/or NAC, and then harvested. They were then stained with 5-(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) at 37°C for 30 min, washed with serum-free medium three times and analyzed by flow cytometry (FACSCalibur flow cytometer; BD Biosciences). DCFH-DA can be oxidized to 2′,7′-dichlorofluorescein (DCF) which emits green fluorescence. Relative ROS levels=DCF fluorescence intensity (treatment group)/DCF fluorescence intensity (control group). Experiments were conducted a minimum of three times independently.
Measurement of mitochondrial transmembrane potential (ΔΨm)
The dissipation of the mitochondrial electrochemical potential gradient is a symbol of mitochondrial damage. A mitochondrial membrane potential assay kit (cat. no. C2006, Beyotime Institute of Biotechnology) with 5,5′,6,′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolyl carbocyanine iodide (JC-1) was used to measure the change in ΔΨm, following the manufacturer's instructions. Briefly, cells were seeded in a 6-well plate following treatment with vanillin and/or NAC, and then harvested. Cells were stained with JC-1 at 37°C for 20 min and then washed and incubated in the assay buffer. ΔΨm was measured using flow cytometry. JC-1 forms aggregates in healthy mitochondrial matrix and emits red fluorescence, while in damaged mitochondria it exists in the cytoplasm as green fluorescence monomers. The changes of ΔΨm are showed as the ratio of the fluorescent intensity of JC-1 aggregates and monomers.
NNMT knockdown by short hairpin (sh)RNA and small interfering (si)RNA
The lentivirus expressing NNMT shRNA was designed and synthesized by Shanghai Genechem Co., Ltd.. The sequences of the shRNA targeting NNMT were shNNMT, 5′-ACCCTCGGGATTACCTAGAAA-3′ and the control shRNA shNC, 5′-TTCTCCGAACGTGTCACGT-3′. The lentivirus was transfected to HT-29 cells as described in our previous study (5). Briefly, HT-29 cells were seeded in 6-well plates and incubated for 24 h. Lentivirus expressing the shRNAs (shNNMT or shNC; MOI=10 for HT-29 cells) was added for 10 h, and the supernatant was replaced with fresh medium. Forty-eight hours after transfection, the cells were sorted using a BD FACSAria II System (BD Biosciences) to obtain the GFP-positive cells, which were then subjected to functional assays.
NNMT siRNA and control siRNA were designed and synthesized by Guangzhou RiboBio Co., Ltd. The control siRNA contained a scrambled sequence that was not specific to any known cellular mRNA. The sequences of the siRNA were as follows: siNNMT (sense, 5′-GCUCAAGAGCAGCUACUACAUdTdT-3′ and antisense, 5′-AUGUAGUAGCUGCUCUUGAGCdTdT-3′) and siNC (sense, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAAdTdT-3′). siRNA was transfected with transfection reagent (Guangzhou RiboBio Co., Ltd.), according to the manufacturer's instructions. Briefly, HT-29 cells were seeded in 6-well plates and incubated for 24 h. siRNA, with a final concentration of 50 nM, was mixed with transfection reagent and then mixed with RMPI-1640 medium supplemented with penicillin, streptomycin and 10% serum. Following culture of the HT-29 cells with the siRNA-RMPI-1640 mixture for 48 h, cells were subjected to functional assays.
Xenograft experiments
Male BALB/c nude mice aged 6 weeks were purchased from the Model Animal Research Center of Nanjing University and housed under pathogen-free conditions on a 12/12 h light/dark cycle with free access to food and water. A total of 3×106 cells from each of the SW480/NC and SW480/NNMT cell lines were subcutaneously injected into nude mice (n=5 per cell line). Then, 2 weeks after injection, the mice were randomly assigned to different treatment groups (n=5 for each group) and treated with 5-Fu (30 mg/kg body weight dissolved in saline every 2 days for 3 weeks) and/or vanillin (100 mg/kg body weight dissolved in 10% PEG, every other day for 3 weeks) by intraperitoneal injection. At the end of the experiment, all mice were intraperitoneally injected with barbiturate (100 mg/kg) and promptly sacrificed by cervical dislocation which resulted in rapid and irreversible loss of animal consciousness with minimal distress leading to eventual death. Mice were also euthanized because of spontaneous pain, sickness, injury or deformity. The maximum size of the xenograft tumors was ~1400 mm3. Volume (V) calculation formula was V=(length × width2)/2. All tumors were harvested and weighed.
TUNEL assay
Tumor tissues were fixed in 4% formaldehyde, and embedded in paraffin. Tissue sections were sliced into 4-µm sections and then dewaxed and rehydrated according to a standard protocol, and an In Situ Apoptosis Detection kit (cat. no. 11684795910, Roche Diagnostics) was used for TUNEL assay following the manufacturer's instructions to detect apoptotic cells in tumor tissue sections, as previously described (6). Signals were visualized and captured using a fluorescence microscopy system (Carl Zeiss AG). In total, 10 fields were randomly selected from each tumor slide, and the rate of positively stained cells was calculated.
Statistical analysis
All data are presented as the mean ± standard deviation from a minimum of three independent experiments. GraphPad Prism version 7.0 software was used for graphic drawing and statistical analysis. The two-sample t-test was used for two-group comparisons. ANOVA was used for multiple comparisons among more than two groups and the Bonferroni method was used to correct the P-value of multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Vanillin inhibits NNMT expression
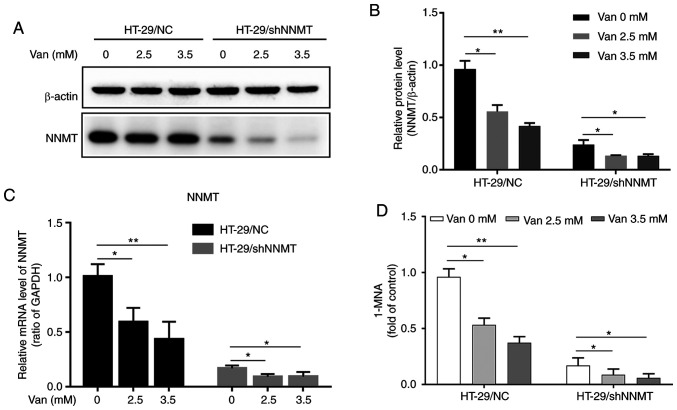
In HT-29/NC and HT-29/shNNMT cells, following treatment with 2.5 or 3.5 mM vanillin (Van) for 48 h, NNMT was downregulated at both the mRNA and protein levels (Fig. 1A-C). Given that the NNMT catalytic activity depends on the expression of NNMT, the 1-MNA level in HT-29 cells was detected following treatment with vanillin to verify the effect of vanillin on NNMT activity. The present results showed that vanillin reduced the 1-MNA level in a dose-dependent manner in HT-29/NC and HT-29/shNNMT cells (Fig. 1D). It was suggested that vanillin could downregulate NNMT expression and in turn depress NNMT activity in HT-29 cells.
Figure 1.
Vanillin downregulates NNMT expression and activity. (A and B) NNMT protein levels were determined by western blot analysis and are displayed as a histogram after treatment with vanillin (0, 2.5 and 3.5 mM) for 48 h in HT-29/NC and HT-29/shNNMT cells. (C) NNMT mRNA was determined by RT-qPCR and is displayed as a histogram after treatment with vanillin for 48 h in the HT-29 cells. (D) Relative 1-MNA level after treatment with vanillin for 48 h in the HT-29 cell lines. The untreated group of HT-29/NC cell was used as the control group which was set as 1 in all the histograms. Data are represented as means ± SD; n=3, *P<0.05, **P<0.01, Van, vanillin; NNMT, nicotinamide N-methyltransferase; 1-MNA, 1-methylnicotinamide.
It was also detected whether vanillin could inhibit NNMT expression in SW480 cells. However, in SW480/NC cells the expression of NNMT was too low to be detected at the protein level, and NNMT was slightly decreased at the mRNA level as detected by RT-qPCR, but no statistically significant difference was detected. This may be because the mRNA level of NNMT was very low as the fold of GAPDH/NNMT was about 25. In SW480/NNMT cells, NNMT was dramatically elevated by the efficient exogenous expression plasmids both at the RNA and protein level, and no decrease by vanillin was detected (Fig. S1A-C). But the 1-MNA level in SW480/NNMT cells was significantly decreased by vanillin (Fig. S1E).
Next, the present study attempted to verify whether vanillin inhibited NNMT expression by affecting the phosphorylation of STAT3 which was reported to be associated with the expression of NNMT in CRC (21). In HT-29 cell lines, the phosphorylation of STAT3 was too low to be detected, but in the SW480/NC and SW480/NNMT cell lines vanillin significantly decreased the phosphorylation of STAT3 (Fig. S1B and D). However, in SW480/NC cells, NNMT was expression at a low level, and in SW480/NNMT cells, the expression of NNMT was not controlled by STAT3, but was influenced by the exogenous plasmids. Collectively, we cannot draw a conclusion that the inhibitory effect of vanillin on NNMT was due to the inhibition of phospho-STAT3.
Vanillin attenuates NNMT-induced cell proliferation and resistance to 5-Fu
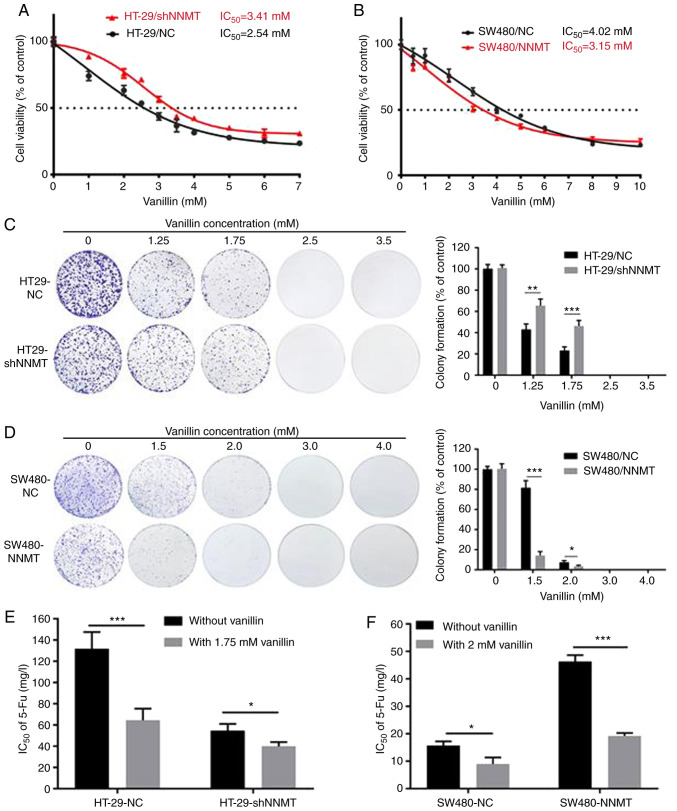
SW480/NNMT, SW480/NC and HT-29/shNNMT, HT-29/NC cells were treated with vanillin for 48 h, and cell viability was detected using the CCK-8 assay. The viability of CRC cells was inhibited by vanillin. The IC50 of vanillin in the four cell lines was IC50=3.15 mM for SW480/NNMT, IC50=4.02 mM for SW480/NC, IC50=3.41 mM for HT-29/shNNMT and IC50=2.54 mM for HT-29/NC (Fig. 2A and B). Moreover, the colony formation assay results showed that vanillin reduced the number of colonies in the CRC cell lines in a dose-dependent manner especially in NNMT high expression cell lines vs. HT-29/shNNMT or SW480/NC cell line where the difference was significant (Fig. 2C and D). It was suggested that vanillin inhibits NNMT-related CRC cell proliferation.
Figure 2.
Vanillin inhibits CRC cell growth and attenuates NNMT-induced resistance to 5-Fu. CRC cell lines, HT-29/NC, HT-29/shNNMT, SW480/NC and SW480/NNMT, were treated with vanillin at different concentrations for 48 h (for the CCK-8 assay) or two weeks (for the colony forming assay). (A and B) CCK-8 analysis for HT-29/NC and HT-29/shNNMT, SW480/NC and SW480/NNMT cell viability. (C and D) Colony forming efficiency in HT-29 and SW480 cell lines. The untreated groups of HT-29/NC and SW480/NC were used as the control group respectively which was set as 100%. (E and F) Cells were treated with vanillin combined with different concentration of 5-Fu (1, 2, 4, 6, 8, 12, 16, 24, 36, 48, 72, 100, 150, 200 and 250 mg/l) for 48 h, and cell viability was determined by CCK-8 assay to evaluate IC50 of 5-Fu. IC50 values for 5-Fu in HT-29/NC, HT-29/shNNMT, SW480/NC, SW480/NNMT are displayed as a histogram. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01, ***P<0.001. CRC, colorectal cancer; NNMT, nicotinamide N-methyltransferase; 5-Fu, 5-fluorouracil.
As our study previously reported, NNMT can reduce the sensitivity to 5-Fu in human CRC cells (6). Herein, following the treatment of cells with 5-Fu combined with the IC50 concentration of vanillin, cells death was extreme; therefore, cells were then treated with 5-Fu combined with a lower concentration of vanillin (1.75 mM vanillin in SW480 cell lines and 2 mM vanillin in HT-29 cell lines, respectively), and the cell viability was detected using a CCK-8 assay to calculate the IC50 of 5-Fu when combined with vanillin. The results showed that the IC50 of 5-Fu was significantly reduced when combined with vanillin; from 131.81 to 64.58 mg/l in HT-29/NC, and from 54.79 to 39.95 mg/l in HT-29/shNNMT; and from 15.52 to 8.5 mg/l in SW480/NC, and from 46.32 to 19.21 mg/l in SW480/NNMT, (Fig. 2E and F). In combination, these data suggest that vanillin attenuates NNMT-related resistance to 5-Fu.
Vanillin reverses the NNMT-induced reduction in apoptosis in CRC cells
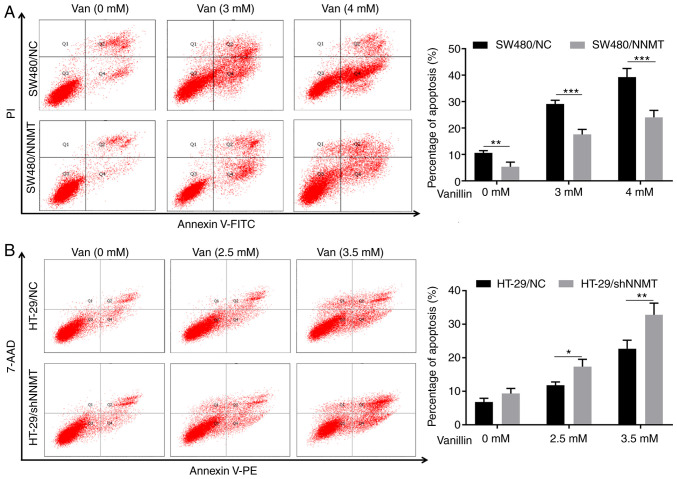
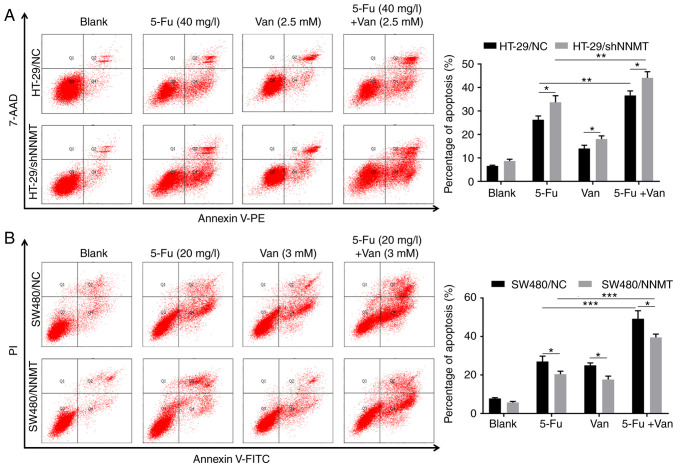
In our previous studies, it was found that NNMT reduced cell apoptosis in CRC cell lines, which was a key reason for NNMT-induced resistance to 5-Fu (5,6). In the following experiments, 3 and 4 mM vanillin was used in the SW480 cell lines (similar concentrations to the IC50 of SW480/NNMT and SW480/NC cells, respectively), and 2.5 and 3.5 mM vanillin in HT-29 cell lines (similar concentrations to the IC50 of HT-29/NC and HT-29/shNNMT cells, respectively). Flow cytometry showed that the proportion of apoptotic cells was increased significantly in the SW480 and HT-29 cell lines, from 10.6 to 29.1 (3 mM vanillin) and 39.25% (4 mM vanillin) in SW480/NC cells, from 5.35 to 17.55 (3 mM vanillin) and 23.96% (4 mM vanillin) in SW480/NNMT cells (Fig. 3A), from 6.7 to 12.3 (2.5 mM vanillin) and 22.7% (3.5 mM vanillin) in HT-29/NC cells, and from 9.3 to 17.35 (2.5 mM vanillin) and 32.95% (3.5 mM vanillin) in HT-29/shNNMT cells (Fig. 3B).
Figure 3.
Vanillin induces cell apoptosis in a dose-dependent manner in CRC cells. CRC cell lines, HT-29/NC, HT-29/shNNMT, SW480/NC and SW480/NNMT, were treated with vanillin of different concentrations for 48 h. (A and B) Cell apoptosis was analyzed by flow cytometry and is displayed as a histogram in SW480/NC and SW480/NNMT (A) and HT-29/NC and HT-29/shNNMT (B) cell lines. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01, ***P<0.001. Van, vanillin; CRC, colorectal cancer; NNMT, nicotinamide N-methyltransferase.
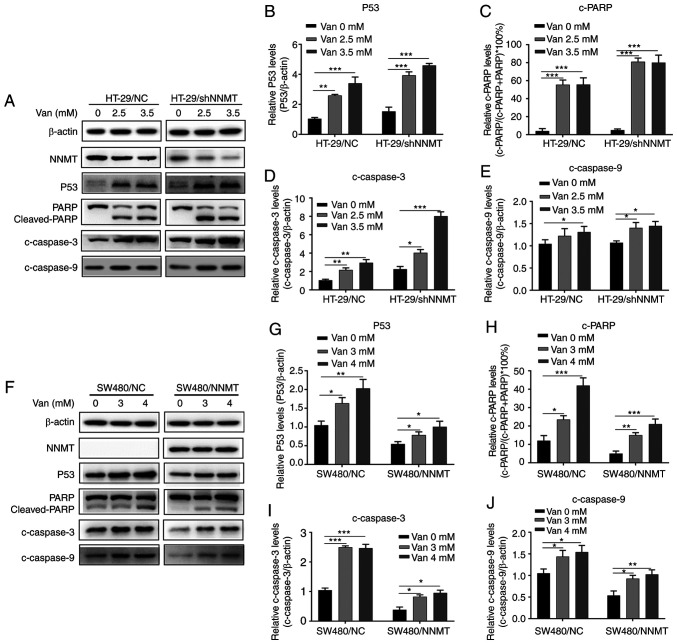
The expression of cell apoptosis-related proteins was further detected following treatment with vanillin in HT-29 and SW480 cell lines. Western blot analysis showed that, following vanillin treatment, p53 was significantly upregulated (Fig. 4A, B, F and G), and cleaved (c)-PARP (Fig. 4A, C, F and H), c-caspase-3 (Fig. 4A, D, F and I), and c-caspase-9 (Fig. 4A, E, F and J) were induced in the HT-29/NC, HT-29/shNNMT, SW480/NC and SW-480/NNMT cell lines.
Figure 4.
Vanillin induces expression of proteins related to cell apoptosis in CRC cells. CRC cell lines, HT-29/NC, HT-29/shNNMT, SW480/NC and SW480/NNMT, were treated with vanillin at different concentrations for 48 h. (A-E) Apoptosis-related proteins were analyzed by western blot analysis in the HT-29/NC, HT-29/shNNMT cells and are displayed as histograms. (F-J) Apoptosis-related proteins were analyzed by western blot analysis in the SW480/NC and SW480/NNMT cells and are displayed as histograms. The untreated groups of HT-29/NC and SW480/NC cells were used as the control group, respectively, which was set as 1 in B, D, E, G, I and J. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01, ***P<0.001. Van, vanillin; CRC, colorectal cancer; NNMT, nicotinamide N-methyltransferase; PARP, poly(ADP-ribose) polymerase; c-, cleaved.
We previously reported that NNMT inhibits apoptosis via its metabolic product 1-MNA (6). Thus, it was also detected whether 1-MNA could reverse the vanillin-induced apoptosis. HT-29/NC and HT-29/shNNMT cells were treated with 1 mM 1-MNA with or without vanillin (2.5 or 3.5 mM) for 48 h. The flow cytometry results showed that following addition of 1 mM of 1-MNA, the proportion of cell apoptosis was decreased from 17.35 to 14.55% (with 2.5 mM vanillin) and from 32.95 to 23.4% (with 3.5 mM vanillin) in HT-29/shNNMT, but in HT-29/NC there was no significant difference in apoptosis with and without 1-MNA (Fig. S2A and B). SW480/NC and SW480/NNMT were also treated with or without 1 mM 1-MNA with vanillin (3 or 4 mM) for 48 h. The flow cytometry results showed that the proportion of SW480/NC apoptotic cells decreased from 29.1 to 23.2% (with 3 mM vanillin) and from 39.25 to 33.4% (with 4 mM vanillin) following addition of 1 mM 1-MNA, but in SW480/NNMT cells 1-MNA did not decrease the apoptosis induced by vanillin. (Fig. S2C and D).
In combination, these results showed that vanillin increased cell apoptosis by attenuating the NNMT-induced reduction in CRC cell apoptosis, and 1-MNA could partly reverse the vanillin-induced apoptosis.
Vanillin has a synergistic effect with 5-Fu by attenuating the NNMT-induced reduction in apoptosis
It was explored whether vanillin has a synergistic effect with 5-Fu through increasing cell apoptosis in CRC cells. Flow cytometry results showed that in the HT-29/NC and HT-29/shNNMT cell lines, cell apoptosis was significantly increased in the groups treated with 2.5 mM vanillin combined with 40 mg/l 5-Fu; from 26.25 to 36.65% in HT-29/NC and from 33.7 to 44.15% in HT-29/shNNMT cells, compared with the groups treated with 40 mg/l 5-Fu alone (Fig. 5A). In SW480 cell lines, cell apoptosis was also significantly increased in the groups treated with 3 mM vanillin combined with 20 mg/l 5-Fu, compared with the groups treated with 20 mg/l 5-Fu alone; from 27.05 to 49.2% in SW480/NC cells, and from 20.35 to 39.6% in SW480/NNMT cells (Fig. 5B). In conclusion, it was determined that vanillin has a synergistic effect with 5-Fu by attenuating the NNMT-induced decreased apoptosis in CRC cell lines.
Figure 5.
Vanillin combined with 5-Fu induces cell apoptosis in CRC cells. CRC cell lines, HT-29/NC, HT-29/shNNMT, SW480/NC and SW480/NNMT, were treated with vanillin and/or 5-Fu (40 mg/l for HT-29 cells, 20 mg/l for SW480 cells) for 48 h. The untreated groups were displayed as blank groups. (A and B) Cell apoptosis was analyzed by flow cytometry and is displayed as histograms in HT-29/NC and HT-29/shNNMT (A) and SW480/NC and SW480/NNMT (B) cell lines. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01, ***P<0.001. Van, vanillin; CRC, colorectal cancer; NNMT, nicotinamide N-methyltransferase; 5-Fu, 5-fluorouracil.
Vanillin induces cell apoptosis by activating the ASK1-p38 MAPK pathway
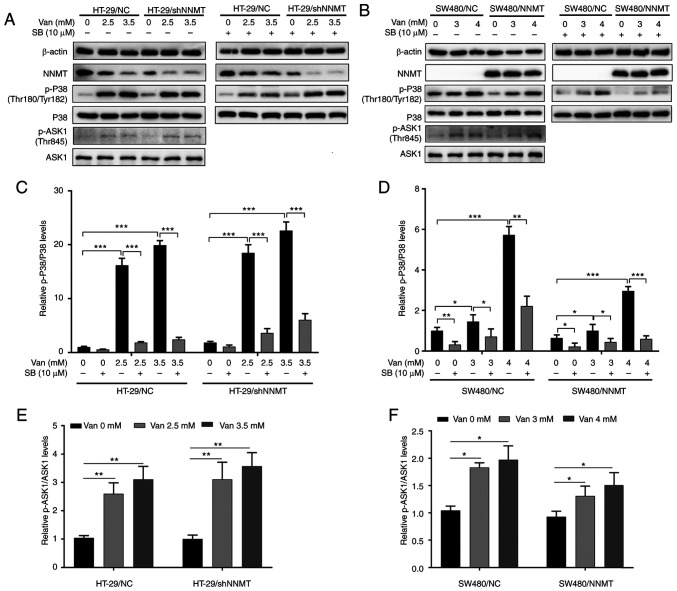
It was aforementioned that NNMT enhances resistance to 5-Fu in CRC cells via inhibition of the ASK1-p38 MAPK pathway. Next, it was examined whether vanillin has an effect on the ASK1-p38 MAPK pathway. The phosphorylation of p38 (p-p38) and ASK1 (p-ASK1) were significantly activated following treatment with vanillin in both HT-29 (Fig. 6A, C and E) and SW480 (Fig. 6B, D and F) cell lines. SB203580, the specific inhibitor of p38 phosphorylation, was used to verify the effect of vanillin on the p38 MAPK pathway. The phosphorylation of p38 was inactivated by SB203580 (10 µM), following incubation with vanillin for 48 h in both HT-29 and SW480 cell lines (Fig. 6A-D).
Figure 6.
Vanillin activates the ASK1-p38 MAPK pathway. CRC cell lines, HT-29/NC, HT-29/shNNMT, SW480/NC and SW480/NNMT, were treated with vanillin and/or SB203580 (SB) for 48 h. (A and B) ASK1-p38 MAPK pathway-related proteins were analyzed by western blot analysis in HT-29/NC and HT-29/shNNMT (A) and SW480/NC and SW480/NNMT (B) cell lines. (C and D) Ratios of phospho-P38 to total P38 (p-P38/P38) were displayed as histograms in HT-29/NC and HT-29/shNNMT (C) and SW480/NC and SW480/NNMT (D) cell lines. (E and F) Ratios of phospho-ASK1 to total ASK1 (p-ASK1/ASK1) are displayed as histograms in HT-29/NC and HT-29/shNNMT (E) and SW480/NC and SW480/NNMT (F) cell lines. The untreated groups of HT-29/NC and SW480/NC were used as the control group, respectively, which was set as 1 in all the histograms. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01, ***P<0.001. Van, vanillin; SB, SB203580; CRC, colorectal cancer; NNMT, nicotinamide N-methyltransferase; ASK1, apoptosis signal regulating kinase 1.
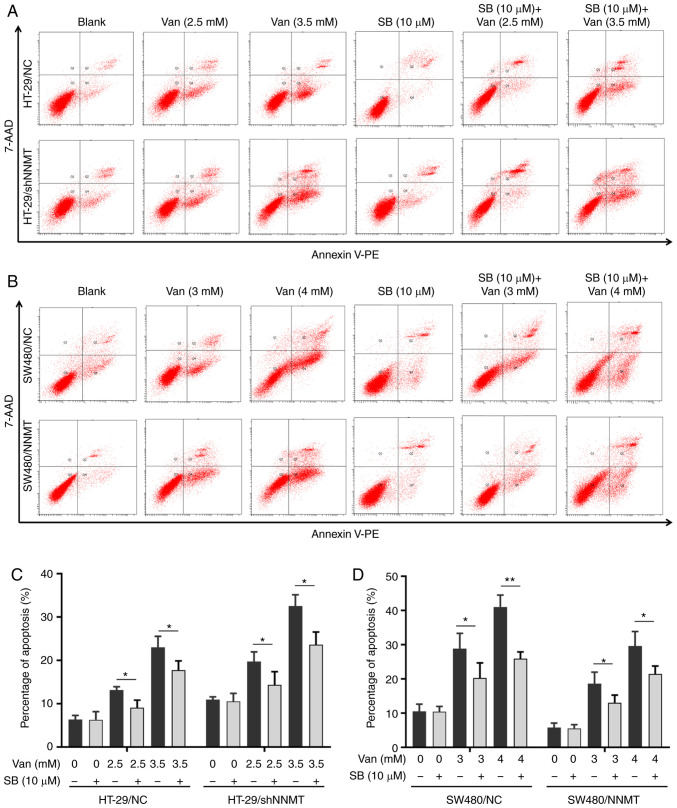
Flow cytometry showed that, following treatment with SB203580 (10 µM) and vanillin for 48 h, the proportion of apoptotic cells was significantly decreased in SW480 and HT-29 cell lines; from 12.3 to 9.06% (with 2.5 mM vanillin), from 22.7 to 17.73% (with 3.5 mM vanillin) in HT-29/NC cells, from 17.35 to 14.36% (with 2.5 mM vanillin), from 32.95 to 23.6% (with 3.5 mM vanillin) in HT-29/shNNMT cells (Fig. 7A and C); from 29.1 to 20.35% (with 3 mM vanillin), from 39.25 to 25.9% (with 4 mM vanillin) in SW480/NC cells, and from 17.55 to 13.04% (with 3 mM vanillin), from 23.96 to 21.46% (with 4 mM vanillin) in SW480/NNMT (Fig 7B and D).
Figure 7.
Inhibition of the ASK1-p38 MAPK pathway reverses the increased apoptosis induced by vanillin. CRC cell lines, HT-29/NC, HT-29/shNNMT, SW480/NC and SW480/NNMT, were treated with vanillin and/or SB203580 for 48 h. The untreated groups were displayed as blank groups. (A and B) Cell apoptosis was analyzed by flow cytometry in HT-29/NC and HT-29/shNNMT (A) and SW480/NC and SW480/NNMT (B) cell lines. (C and D) Cell apoptosis was displayed as a histogram in HT-29/NC and HT-29/shNNMT (C) and SW480/NC and SW480/NNMT (D) cell lines. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01. Van, vanillin; SB, SB203580; CRC, colorectal cancer; NNMT, nicotinamide N-methyltransferase; ASK1, apoptosis signal regulating kinase 1.
These results indicated that p38 MAPK is an important mediator of vanillin-induced apoptosis, and might be a key reason for the vanillin-induced weakening of NNMT-induced 5-Fu resistance in CRC cells.
Vanillin induces cell apoptosis by enhancing mitochondrial damage and ROS production
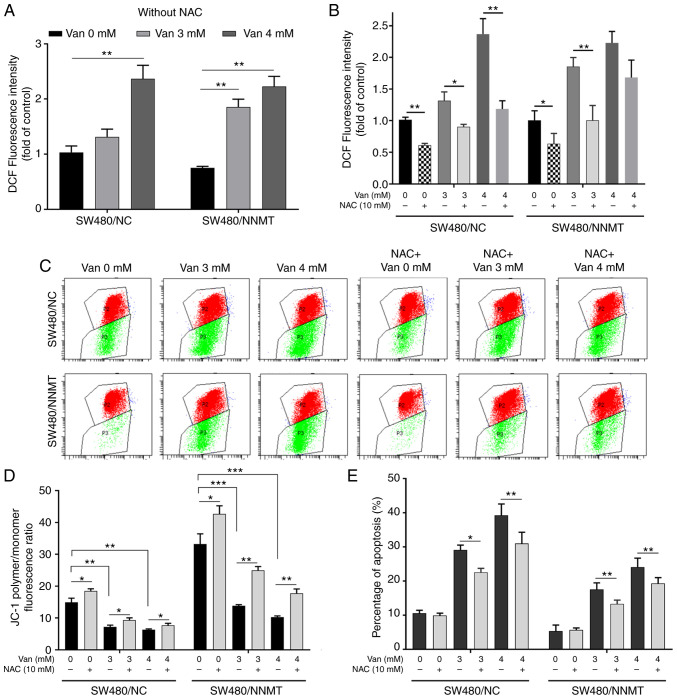
ROS is one of the causes for cell apoptosis. Intracellular ROS can activate ASK1 by dissociating it from glutathione-S-transferase (22). Our previous study showed that NNMT reduced the ROS production in CRC cells after treatment with 5-Fu (6). Herein, it was found that vanillin promoted ROS production in a dose-dependent manner in both SW480/NC and SW480/NNMT cell lines (Fig. 8A). NAC, a known scavenger of ROS, was used to reduce the ROS level that had been increased by vanillin. Flow cytometry showed that ROS was significantly reduced by NAC (Fig. 8B).
Figure 8.
Vanillin increases cell apoptosis by inducing mitochondrial damage and ROS production. SW480/NC and SW480/NNMT cell lines were treated with vanillin for 48 h, or pretreated with 10 mM NAC for 2 h followed by treatment with vanillin for 48 h. (A and B) ROS were detected by flow cytometry using the fluorescent probe DCFH-DA and displayed as a histogram. (C and D) Measurement of mitochondrial transmembrane potential by JC-1 fluorescence and displayed as histogram. (E) Cell apoptosis was displayed as a histogram. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01, ***P<0.001. Van, vanillin; NAC, N-acetyl-L-cysteine; ROS, reactive oxygen species.
Mitochondrial damage, leading to electron leakage from mitochondrial electron transport, is the main source of intracellular ROS, and mitochondria are the primary targets of ROS-induced cellular damage (23). The ΔΨm was detected using JC-1 fluorescent probe to explore whether the promotion of ROS came from mitochondrial damage. Flow cytometry results showed that green fluorescence increased following treatment with vanillin, which meant that vanillin induced mitochondrial damage in the SW480 cell lines (Fig. 8C). The vanillin-induced mitochondrial damage could be partly rescued following NAC-induced ROS inhibition (Fig. 8D)
Next, it was examined whether ROS promotion is a key reason for the induction of vanillin-induced cell apoptosis. SW480 cell apoptosis was detected after the inhibition of ROS following pre-treatment with NAC and treatment with vanillin. Flow cytometry results showed that cell apoptosis was reduced following pre-treatment with NAC in vanillin-treated SW480 cell lines; from 29.1 to 24.56% (with 3 mM vanillin), from 39.25 to 30.87% (with 4 mM vanillin) in SW480/NC, and from 17.55 to 13.43% (with 3 mM vanillin), from 23.96 to 19.3% (with 4 mM vanillin) in SW480/NNMT cells (Fig. 8E). These results showed that the vanillin-induced cell apoptosis was partly reversed following the NAC-induced reduction in ROS.
Since the lentiviral vector used to construct the HT-29/NC and HT-29/shNNMT cell lines contained a green fluorescence protein tag, which conflicts with the green fluorescent signal of ROS and JC-1 monomers, NNMT siRNA was used to knock down NNMT to detect changes in ROS and mitochondrial damage. However, western blot analysis showed that NNMT expression was knocked down by NNMT siRNA although it was not so efficient as the NNMT shRNA by lentiviral vector (Fig. S3A and B). Consistent with the results in SW480 cell lines, we also found that ROS were increased after treatment with 2.5 and 3.5 mM vanillin for 48 h, and that NAC could reduce ROS in HT-29 cells (Fig. S3C and D). But the ROS promotion by vanillin was much more mild than in SW480 cell lines. And JC-1 assays showed that vanillin could induce mitochondrial damage and that NAC could partly reduce the damage in HT-29 cells (Fig. S3E and F). On account of the higher knockdown efficiency, HT-29/NC and HT-29/shNNMT cell lines were used to detect the effect of ROS reduction by NAC on apoptosis. When cells were pre-treated with NAC, the proportion of apoptotic cells was slightly reduced but exhibited no significant difference (Fig. S3G).
Overall, vanillin was considered to attenuate the inhibitory effect of NNMT on ROS production and increase cell apoptosis in CRC cell lines.
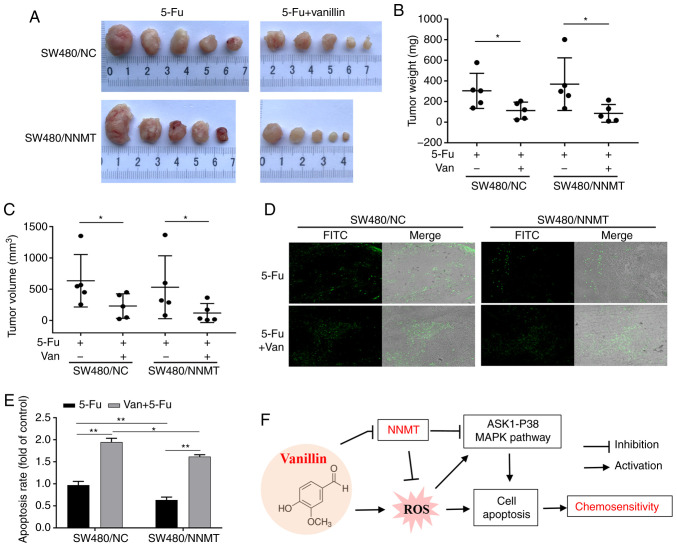
Vanillin combined with 5-Fu decreases tumor growth and induces apoptosis in vivo
The mouse xenograft models established by SW480/NC and SW480/NNMT cells were used to explore the synergistic effect of vanillin with 5-Fu in vivo. A previous study reported no side effects following treatment with 100 mg/kg/day vanillin (15). In the present study, 100 mg/kg vanillin was used every other day, and 30 mg/kg 5-Fu every 2 days, as described in our previous study (6). After 3 weeks of intraperitoneal injections in tumor-bearing nude mice, the tumor volume and weight in the 5-Fu combined with vanillin-treated groups was significantly smaller than that in the 5-Fu-treated groups, both in the SW480/NC and SW480/NNMT cell derived tumors (Fig. 9A-C). Moreover, the TUNEL analysis of tumor sections showed that cell apoptosis was induced more significantly in groups treated with 5-Fu combined with vanillin than in groups treated with 5-Fu alone. Consistent with our previous study (6), it was found that tumors overexpressing NNMT exhibited less 5-Fu-induced cell apoptosis than tumors in the SW480/NC groups, both in the 5-Fu and 5-Fu combined with vanillin-treated groups (Fig. 9D and E).
Figure 9.
Vanillin inhibits CRC tumor growth in a mouse xenograft model. (A) Representative image showing tumors from subcutaneous tumor-bearing nude mice of the SW480/NC or SW480/NNMT groups which were treated with 5-Fu alone or 5-Fu combined with vanillin. (B) Tumor weight of the different groups displayed as a scatter diagram. (C) Tumor volume of the different groups displayed as a scatter diagram. (D) Cell apoptosis detected by TUNEL staining (magnification, ×100). (E) Cell apoptosis rate from TUNEL staining displayed as a histogram showing the 5-Fu alone treated group of SW480/NC cells as a control which was set as 1. (F) Schematic illustration of the regulation of NNMT and chemosensitivity by vanillin. Vanillin downregulates NNMT and enhances sensitivity to 5-Fu via ROS-induced cell apoptosis in CRC cells. Data are represented as means ± SD, n=3; *P<0.05, **P<0.01. Van, vanillin; CRC, colorectal cancer; NNMT, nicotinamide N-methyltransferase; ASK1, apoptosis signal regulating kinase 1; ROS, reactive oxygen species; 5-Fu, 5-fluorouracil.
In conclusion, vanillin was found to downregulate NNMT and enhance sensitivity to 5-Fu via ROS-induced cell apoptosis. A schematic illustration of the regulation of NNMT and chemosensitivity by vanillin in CRC cells is shown in Fig. 9F.
Discussion
Chemoresistance is the main cause of poor prognosis in colorectal cancer (CRC) (24). Nicotinamide N-methyltransferase (NNMT) is an enzyme for nicotinamide metabolism, and has been found to be upregulated in a variety of diseases, including CRC (2,3). Our previous study found that NNMT enhanced resistance to 5-Fu in CRC cells (6). Therefore, it is important to identify compounds targeting NNMT.
Few NNMT inhibitors have been reported to date. 1-MNA is known to inhibit NNMT through enzymatic reaction product inhibition (10), but 1-MNA itself has been shown to play a unique role in tumor proliferation and drug resistance (5,6). A growing number of inhibitors have recently been reported including chloroacetamide-based covalent NNMT inhibitors, nicotinamide analogues, quinolone (inhibitors based on NNMT's alternative substrate), sinefungin (general methyltransferase inhibitor), and bisubstrate-like inhibitors (25–31). All reported inhibitors are chemosynthetic, and more research is required to certify their potential side effects. Inhibitors derived from natural products are a safer choice. Curcumin is the only reported natural product that inhibits NNMT mRNA expression (21). A natural product library was screened, and it was found that vanillin may target NNMT and help reduce NNMT-induced chemotherapy resistance.
Vanillin, a natural dietary flavor component widely used in food, has been reported to exert multiple anticancer effects (9–16). In the present study, it was also found that vanillin could inhibit cell proliferation in CRC cells and attenuate NNMT-induced resistance to 5-Fu. However, following a review of the literature, no study was found on the association between vanillin and NNMT. In the present study, vanillin was found to effectively decrease NNMT mRNA and protein levels in HT-29 cell lines, and then to inhibit NNMT activity by reducing the 1-MNA level in a dose-dependent manner. It was also found that vanillin could decrease the 1-MNA level in SW480/NNMT cells, but the expression of NNMT was not changed significantly. This was reconcilable because in SW480/NNMT cells, the expression of NNMT was dramatically elevated by the efficient exogenous expression plasmids.
Given that STAT3 is one of the transcription factors of NNMT in CRC (21), and vanillin can inhibit NNMT at the mRNA level, it was explored whether vanillin could inhibit the phosphorylation of STAT3. In HT-29 cell lines, no STAT3 phosphorylation was detected by western blot analysis, with or without vanillin treatment. The low phosphorylation of STAT3 in HT-29 cells was also reported previously (32,33). Several other researchers obtained the slight bands of phospho-STAT3 in HT-29 cells (34,35), but we attempted many times and failed to obtain a clear band of phospho-STAT3 in our experiments. In the SW480 cell lines, vanillin markedly decreased the phosphorylation of STAT3, but NNMT had a low expression in SW480 cells and the exogenous expression of NNMT in SW480/NNMT cells was not associated with STAT3. It could not, therefore, be confirmed whether the inhibition of NNMT was due to the inhibition of STAT3 phosphorylation. Thus, the precise molecular mechanism through which vanillin inhibits NNMT expression was not provided and should be further explored.
It was found in the present study that vanillin inhibited the viability and colony formation of CRC cells in a dose-dependent manner, especially in NNMT-overexpressing cell lines. It was suggested that vanillin could inhibit NNMT-promoted CRC cell proliferation. However, in the colony formation assay, it was found that all cells died when the concentration of vanillin peaked at only 2.5 mM. We suggested two reasons for this result: i) In the colony formation assays, if the clone is less than 0.2 mm in diameter, it is difficult to be stained effectively and will be missed; ii) For the colony formation test, we seeded 103 cells per well in 6-well plates and treated cells with vanillin for two weeks, and found that cells were more sensitive to vanillin than in the CCK-8 assays, in which we seeded 5×103 cells per well in 96-well plates and treated only for 48 h. Thus, we supposed that this may be because the lower the cell density, the more sensitive are the cells to vanillin. There are also some other studies that reported a similar phenomenon (36,37).
Cell apoptosis was further investigated following treatment with vanillin. Flow cytometry results showed that vanillin significantly increased cell apoptosis, and western blot analysis results showed that key apoptotic proteins were markedly promoted by vanillin in CRC cells. It was also found that 1-MNA, the metabolic product of NNMT, could reverse the apoptosis induced by vanillin in HT-29/shNNMT and SW480/NC cell lines, which were NNMT low expression cell lines. But in HT-29/NC and SW480/NNMT cells, which are NNMT high expression cell lines, 1-MNA could not reduce the apoptosis increased by vanillin. We supposed that there are naturally high concentrations of 1-MNA in these cell lines and excessive 1-MNA might be cytotoxic. The rescue experiment by 1-MNA, as well as the functional analysis by NNMT knockdown in HT-29 cells and NNMT overexpression in SW480 cells, provided evidence to support the conclusion that vanillin promotes apoptosis by inhibiting NNMT expression.
We previously reported that NNNT could inhibit the ASK1-p38 MAPK pathway (6). Herein, it was also found that vanillin activated the ASK1-p38 MAPK pathway to induce apoptosis. There are two possible underlying mechanisms: i) Vanillin inhibits NNMT expression, therefore decreasing the effect of NNMT on the ASK1-p38 MAPK pathway; ii) Vanillin itself activates the ASK1-p38 MAPK pathway. No conclusion was reached on the exact mechanism in the present study.
In addition, consistent with our previous study, the apoptotic rate was lower in cells with a high, compared with cells with low NNMT expression, which was dependent on the protective effect of NNMT on cell apoptosis reported in our precious study (19). In addition, the present results showed that cell apoptosis was more significantly increased in groups treated with vanillin combined with 5-Fu, than in groups treated with 5-Fu alone. In conclusion, vanillin was deemed to have a synergistic effect with 5-Fu by reversing the NNMT-induced decreased CRC cell apoptosis.
It was shown in our previous study that NNMT could reduce ROS production in CRC cells (6). Herein, it was found that vanillin could increase intracellular ROS in both SW480 and HT-29 cell lines. NAC, the scavenger of ROS, could partly reverse vanillin-mediated ROS promotion and, in turn, partly decrease cell apoptosis, suggesting that vanillin induces cell apoptosis using a mechanism dependent on ROS promotion. However, in HT-29 cell lines, ROS promotion was much lower than that noted in the SW480 cell lines. We assume that ROS promotion was not the main cause of vanillin-induced apoptosis in HT-29 cells. We did not provide more reasons by which vanillin induces apoptosis in HT-29 cells, which should be further investigated. It was also found that the ROS promotion by vanillin arose from mitochondrial damage, and NAC could partly rescue this damage. Nevertheless, the mechanism of mitochondrial damage and precise signaling pathway of ROS production remain unclear. Collectively, it was suggested that mitochondrial damage and its subsequent promotion of ROS are partly to account for the vanillin-induced increase in cell apoptosis.
It was found that the inhibitory effect of vanillin on cell proliferation was more significant in HT-29/NC and SW480/NNMT cells which have a high NNMT expression, and 3 mM vanillin could promote more ROS production in SW480/NNMT cells than in SW480/NC. In HT-29 cells, no significant difference in ROS was found when NNMT was knocked down by siRNA. This meant that vanillin could induce more ROS in HT-29/siNC cells and eliminate the inhibitory effect of NNMT on ROS. This created some confusion in the present study. As was aforementioned that NNMT could increase cell proliferation and inhibit ROS, why vanillin could induce more ROS and inhibition in cell proliferation in NNMT-overexpressing cells must be further investigated. We hypothesized that vanillin could also inhibit the downstream proteins, whose expression depends on NNMT and which are necessary for cell survival under high NNMT expression. Further research should be undertaken to investigate that mechanism.
A murine xenograft model was constructed using SW480/NC and SW480/NNMT cells to assess the synergistic effect of vanillin and 5-Fu in vivo. Vanillin inhibited tumor growth and enhanced the 5-Fu sensitivity of CRC by promoting apoptosis in vivo. Considering that vanillin has long been used as a food additive worldwide, and a previous study showed that no side effects were observed following treatment with vanillin at 100 mg/kg/day (15), it was proposed herein that vanillin is a promising anticancer compound with low side effects for CRC therapy, particularly for CRC with high NNMT expression.
In conclusion, in the present study, it was demonstrated that vanillin targets NNMT and attenuates NNMT-related resistance to 5-Fu. Insight was provided into part of the mechanism that involves the NNMT/mitochondrial damage/ROS axis involved in vanillin-induced cell apoptosis. This study identified vanillin as a useful adjuvant chemotherapy candidate for CRC therapy.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This work was supported by grants from the Key Traditional Chinese Medicine Program of Zhejiang Province (no. 2018ZZ016), the National Natural Science Foundation of China (no. 81972012), and the Health Bureau Foundation of Zhejiang Province (nos. 2018KY482 and 2021PY012).
Funding
This work was supported by grants from the Key Traditional Chinese Medicine Program of Zhejiang Province (no. 2018ZZ016), the National Natural Science Foundation of China (no. 81972012), and the Health Bureau Foundation of Zhejiang Province (nos. 2018KY482 and 2021PY012).
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
GL wrote the original manuscript and analyzed the data. GL, BK, QT, YL, LC, HY and JZ performed the experiments and partial data analysis. XX and JZ confirmed the authenticity of all the raw data. JZ designed the research. All authors read and approved the manuscript and agreed to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The animal experiments were previously approved by the Animal Care and Use Committee of Sir Run Run Shaw Hospital of Zhejiang University (permit no. 20120222-31) and followed internationally recognized guidelines on animal welfare. All animal studies complied with the ARRIVE guidelines and the AVMA euthanasia guidelines 2013.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Testa U, Pelosi E, Castelli G. Colorectal cancer: Genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel) 2018;6:31. doi: 10.3390/medsci6020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong CS, Wong VW, Chan CM, Ma BB, Hui EP, Wong MC, Lam MY, Au TC, Chan WH, Cheuk W, Chan AT. Identification of 5-fluorouracil response proteins in colorectal carcinoma cell line SW480 by two-dimensional electrophoresis and MALDI-TOF mass spectrometry. Oncol Rep. 2008;20:89–98. [PubMed] [Google Scholar]
- 3.Roessler M, Rollinger W, Palme S, Hagmann ML, Berndt P, Engel AM, Schneidinger B, Pfeffer M, Andres H, Karl J, et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin Cancer Res. 2005;11:6550–6557. doi: 10.1158/1078-0432.CCR-05-0983. [DOI] [PubMed] [Google Scholar]
- 4.Jung J, Kim LJ, Wang X, Wu Q, Sanvoranart T, Hubert CG, Prager BC, Wallace LC, Jin X, Mack SC, Rich JN. Nicotinamide metabolism regulates glioblastoma stem cell maintenance. JCI Insight. 2017;2:e90019. doi: 10.1172/jci.insight.90019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X, Yu H, Wang Y, Zhou Y, Li G, Ruan Z, Li F, Wang X, Liu H, Zhang J. Nicotinamide N-methyltransferase enhances the capacity of tumorigenesis associated with the promotion of cell cycle progression in human colorectal cancer cells. Arch Biochem Biophys. 2014;564:52–66. doi: 10.1016/j.abb.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Xie X, Liu H, Wang Y, Zhou Y, Yu H, Li G, Ruan Z, Li F, Wang X, Zhang J. Nicotinamide N-methyltransferase enhances resistance to 5-fluorouracil in colorectal cancer cells through inhibition of the ASK1-p38 MAPK pathway. Oncotarget. 2016;7:45837–45848. doi: 10.18632/oncotarget.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akaberi M, Mehri S, Iranshahi M. Multiple pro-apoptotic targets of abietane diterpenoids from Salvia species. Fitoterapia. 2015;100:118–132. doi: 10.1016/j.fitote.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Priefert H, Rabenhorst J, Steinbuchel A. Biotechnological production of vanillin. Appl Microbiol Biotechnol. 2001;56:296–314. doi: 10.1007/s002530100687. [DOI] [PubMed] [Google Scholar]
- 9.Ho K, Yazan LS, Ismail N, Ismail M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009;33:155–160. doi: 10.1016/j.canep.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Ramadoss DP, Sivalingam N. Vanillin extracted from Proso and Barnyard millets induce apoptotic cell death in HT-29 human colon cancer cell line. Nutr Cancer. 2020;72:1422–1437. doi: 10.1080/01635581.2019.1672763. [DOI] [PubMed] [Google Scholar]
- 11.Naz H, Tarique M, Khan P, Luqman S, Ahamad S, Islam A, Ahmad F, Hassan MI. Evidence of vanillin binding to CAMKIV explains the anti-cancer mechanism in human hepatic carcinoma and neuroblastoma cells. Mol Cell Biochem. 2018;438:35–45. doi: 10.1007/s11010-017-3111-0. [DOI] [PubMed] [Google Scholar]
- 12.Khan P, Rahman S, Queen A, Manzoor S, Naz F, Hasan GM, Luqman S, Kim J, Islam A, Ahmad F, Hassan MI. Elucidation of dietary polyphenolics as potential inhibitor of microtubule affinity regulating Kinase 4: In silico and in vitro studies. Sci Rep. 2017;7:9470. doi: 10.1038/s41598-017-09941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang JA, Wu SL, Lo HY, Hsiang CY, Ho TY. Vanillin inhibits matrix metalloproteinase-9 expression through down-regulation of nuclear factor-kappaB signaling pathway in human hepatocellular carcinoma cells. Mol Pharmacol. 2009;75:151–157. doi: 10.1124/mol.108.049502. [DOI] [PubMed] [Google Scholar]
- 14.Lirdprapamongkol K, Kramb JP, Suthiphongchai T, Surarit R, Srisomsap C, Dannhardt G, Svasti J. Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J Agric Food Chem. 2009;57:3055–3063. doi: 10.1021/jf803366f. [DOI] [PubMed] [Google Scholar]
- 15.Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25:57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Park EJ, Lee YM, Oh TI, Kim BM, Lim BO, Lim JH. Vanillin suppresses cell motility by inhibiting STAT3-Mediated HIF-1α mRNA expression in malignant melanoma cells. Int J Mol Sci. 2017;18:532. doi: 10.3390/ijms18030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durant S, Karran P. Vanillins-a novel family of DNA-PK inhibitors. Nucleic Acids Res. 2003;31:5501–5512. doi: 10.1093/nar/gkg753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsherbiny NM, Younis NN, Shaheen MA, Elseweidy MM. The synergistic effect between vanillin and doxorubicin in ehrlich ascites carcinoma solid tumor and MCF-7 human breast cancer cell line. Pathol Res Pract. 2016;212:767–777. doi: 10.1016/j.prp.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Wang Y, Li G, Yu H, Xie X. Down-regulation of nicotinamide N-methyltransferase induces apoptosis in human breast cancer cells via the mitochondria-mediated pathway. PLoS One. 2014;9:e89202. doi: 10.1371/journal.pone.0089202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Tomida M, Ohtake H, Yokota T, Kobayashi Y, Kurosumi M. Stat3 up-regulates expression of nicotinamide N-methyltransferase in human cancer cells. J Cancer Res Clin Oncol. 2008;134:551–559. doi: 10.1007/s00432-007-0318-6. [DOI] [PubMed] [Google Scholar]
- 22.Ji Y, Dai F, Yan S, Shi JY, Zhou B. Identification of catechol-type diphenylbutadiene as a tyrosinase-activated pro-oxidative chemosensitizer against melanoma A375 cells via glutathione S-transferase inhibition. J Agric Food Chem. 2019;67:9060–9069. doi: 10.1021/acs.jafc.9b02875. [DOI] [PubMed] [Google Scholar]
- 23.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann NY Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horning BD, Suciu RM, Ghadiri DA, Ulanovskaya OA, Matthews ML, Lum KM, Backus KM, Brown SJ, Rosen H, Cravatt BF. Chemical proteomic profiling of human methyltransferases. J Am Chem Soc. 2016;138:13335–13343. doi: 10.1021/jacs.6b07830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruf S, Hallur MS, Anchan NK, Swamy IN, Murugesan KR, Sarkar S, Narasimhulu LK, Putta VP, Shaik S, Chandrasekar DV, et al. Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg Med Chem Lett. 2018;28:922–925. doi: 10.1016/j.bmcl.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 27.Kannt A, Rajagopal S, Kadnur SV, Suresh J, Bhamidipati RK, Swaminathan S, Hallur MS, Kristam R, Elvert R, Czech J, et al. A small molecule inhibitor of nicotinamide N-methyltransferase for the treatment of metabolic disorders. Sci Rep. 2018;8:3660. doi: 10.1038/s41598-018-22081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neelakantan H, Wang HY, Vance V, Hommel JD, McHardy SF, Watowich SJ. Structure-activity relationship for small molecule inhibitors of nicotinamide N-methyltransferase. J Med Chem. 2017;60:5015–5028. doi: 10.1021/acs.jmedchem.7b00389. [DOI] [PubMed] [Google Scholar]
- 29.van Haren MJ, Taig R, Kuppens J, Sastre Toraño J, Moret EE, Parsons RB, Sartini D, Emanuelli M, Martin NI. Inhibitors of nicotinamide N-methyltransferase designed to mimic the methylation reaction transition state. Org Biomol Chem. 2017;15:6656–6667. doi: 10.1039/C7OB01357D. [DOI] [PubMed] [Google Scholar]
- 30.Babault N, Allali-Hassani A, Li F, Fan J, Yue A, Ju K, Liu F, Vedadi M, Liu J, Jin J. Discovery of bisubstrate inhibitors of nicotinamide N-methyltransferase (NNMT) J Med Chem. 2018;61:1541–1551. doi: 10.1021/acs.jmedchem.8b00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y, van Haren MJ, Moret EE, Rood JJ, Sartini D, Salvucci A, Emanuelli M, Craveur P, Babault N, Jin J, Martin NI. Bisubstrate inhibitors of nicotinamide N-methyltransferase (NNMT) with enhanced activity. J Med Chem. 2019;62:6597–6614. doi: 10.1021/acs.jmedchem.9b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sulaiman S, Arafat K, Iratni R, Attoub S. PTC-209 anti-cancer effects involved the inhibition of STAT3 Phosphorylation. Front Pharmacol. 2019;10:1199. doi: 10.3389/fphar.2019.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mojzeš A, Tomljanović M, Milković L, Kujundžić RN, Gašparović AČ, Trošelj KG. Cell-type specific metabolic response of cancer cells to curcumin. Int J Mol Sci. 2020;21:1661. doi: 10.3390/ijms21051661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue H, Li T, Wang P, Mo X, Zhang H, Ding S, Ma D, Lv W, Zhang J, Han W. CMTM4 inhibits cell proliferation and migration via AKT, ERK1/2, and STAT3 pathway in colorectal cancer. Acta Biochim Biophys Sin (Shanghai) 2019;51:915–924. doi: 10.1093/abbs/gmz084. [DOI] [PubMed] [Google Scholar]
- 35.Laudisi F, Cherubini F, Di Grazia A, Dinallo V, Di Fusco D, Franzè E, Ortenzi A, Salvatori I, Scaricamazza S, Monteleone I, et al. Progranulin sustains STAT3 hyper-activation and oncogenic function in colorectal cancer cells. Mol Oncol. 2019;13:2142–2159. doi: 10.1002/1878-0261.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao S, Guan X, Hou R, Zhang X, Guo F, Zhang Z, Hua C. Vitexin attenuates epithelial ovarian cancer cell viability and motility in vitro and carcinogenesis in vivo via p38 and ERK1/2 pathways related VEGFA. Ann Transl Med. 2020;8:1139. doi: 10.21037/atm-20-5586. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Lin S, Pan Y, Xu C. Effects of aspirin on pancreatic cancer cells PANC-1 and its potential molecular mechanism. J BUON. 2020;25:2449–2455. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.