Abstract
The demographics of the population with cystic fibrosis (CF) is continuously changing, with nowadays adults outnumbering children and a median predicted survival of over 40 years. This leads to the challenge of treating an aging CF population, while previous research has largely focused on pediatric and adolescent patients. Chronic inflammation is not only a hallmark of CF lung disease, but also of the aging process. However, very little is known about the effects of an accelerated aging pathology in CF lungs. Several chronic lung disease pathologies show signs of chronic inflammation with accelerated aging, also termed “inflammaging”; the most notable being chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). In these disease entities, accelerated aging has been implicated in the pathogenesis via interference with tissue repair mechanisms, alterations of the immune system leading to impaired defense against pulmonary infections and induction of a chronic pro-inflammatory state. In addition, CF lungs have been shown to exhibit increased expression of senescence markers. Sustained airway inflammation also leads to the degradation and increased turnover of cystic fibrosis transmembrane regulator (CFTR). This further reduces CFTR function and may prevent the novel CFTR modulator therapies from developing their full efficacy. Therefore, novel therapies targeting aging processes in CF lungs could be promising. This review summarizes the current research on CF in an aging population focusing on accelerated aging in the context of chronic airway inflammation and therapy implications.
Keywords: cystic fibrosis, aging, inflammaging, oxidative stress, mitochondrial dysfunction, senescence
Introduction
30 years ago, mutations in the cystic fibrosis transmembrane regulator (CFTR) gene were characterized as single gene defect leading to the pathology of cystic fibrosis (CF) (Kerem et al., 1989; Tsui, 1995). Since then, our understanding of the pathological processes has largely improved with CFTR targeted modulator therapies in clinical use (Wainwright et al., 2015; Rowe et al., 2017; Taylor-Cousar et al., 2017; Davies et al., 2018; Keating et al., 2018). Nevertheless, progressive lung disease is still the major cause for morbidity and mortality in individuals born with CF (Elborn, 2016). Functional failure of CFTR results in impaired ciliary function, mucus obstruction, bacterial colonization and chronic inflammation (Matsui et al., 1998; Mall et al., 2004; Boucher, 2007). This sets the stage for smoldering chronic infections interrupted by episodic exacerbations due to newly acquired pathogens leading to progressive loss of lung function (Nichols et al., 2008; Elborn, 2016). Elevated levels of pro-inflammatory mediators, reactive oxygen species (ROS), and proteases, which further propagate airway inflammation, can accelerate cellular senescence (Bezzerri et al., 2019).
Major improvements in supportive therapies and the development of CFTR targeted therapies increased life expectancy in CF patients to a median survival of 40 years or more in industrialized nations (Dodge et al., 2007; MacKenzie et al., 2014). Hence, CF is no longer considered primarily a pediatric disease. This entails new challenges, including treatment of CF in an aging population with CF dependent and independent comorbidities. Diabetes, osteopenia and osteoporosis, renal, and vestibulocholear complications from aminoglycoside toxicity, cardiovascular disease, and worsening lung disease are medical issues often emerging in the aging CF population (Hodson et al., 2008; Simmonds et al., 2009). Chronic local and systemic inflammation is a key pathogenic mechanisms suspected of promoting these comorbidities (Nichols et al., 2008; Bezzerri et al., 2019). Aging impairs lung function through several mechanisms, a central being chronic, low-grade inflammation termed “inflammaging” (Franceschi and Campisi, 2014). Accelerated aging has been implicated in several chronic inflammatory lung disease pathologies. As chronic inflammation is a hallmark of CF airway disease, the question arises whether this chronic inflammation may also cause premature aging in CF lung tissue; and if so whether novel therapies targeting “inflammaging” and aging pathomechanisms in CF lungs might prove to be beneficial.
Hallmarks of Aging in CF Lung Disease
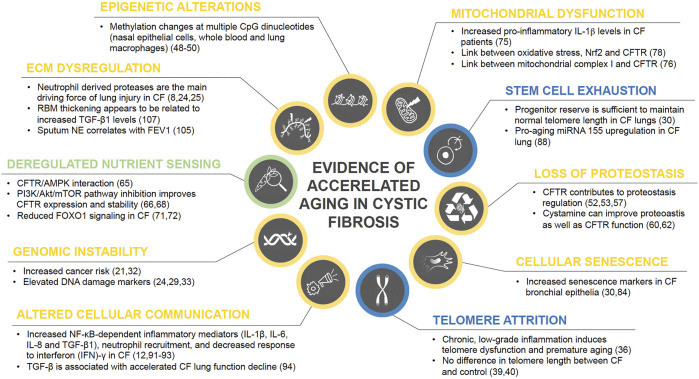
Physiological aging is associated with considerable changes in lung structure and cell function, giving rise to reduced lung function, pulmonary remodeling, diminished regenerative capacity, and enhanced susceptibility to lung injury, and disease (Cho and Stout-Delgado, 2020). Considering the increased life expectancy of CF patients, the question whether chronic inflammation contributes to accelerated aging becomes more and more relevant. Interestingly, healthy aging lungs show a proinflammatory state with increased neutrophils and IL-8 (Meyer et al., 1998), bearing some similarity with chronic CF lung inflammation. In the following paragraphs we aim to provide an overview on the hallmarks of aging and their potential implications for CF lung disease. Central findings on accelerated aging in CF are also summarized in Figure 1.
FIGURE 1.
Current state of evidence on accelerated aging processes in CF. Research findings on the role of each of the ten hallmarks of aging in CF disease are summarized and color coded based on their level of evidence with blue = no evidence for involvement in CF pathology, green = weak evidence, yellow = strong evidence.
Genomic Instability
Non-transplanted CF patients have a higher risk for lymphoid leukemia, testicular cancer and digestive tract cancers than the general population (Maisonneuve et al., 2013). Similarly, cancer risk in CF lung transplant recipients is more increased than in non-CF recipients, particularly for colorectal cancer, esophageal cancer, and non-Hodgkin lymphoma (Fink et al., 2017). Defects in CFTR themselves have been linked to cancers (Scott et al., 2020). The striking overlap of organ systems with elevated cancer risk and those that are primarily affected by CF-associated mucus hypersecretion, i.e., the respiratory, digestive, and reproductive systems, strongly implicates chronic inflammation as a potential causative factor. In CF airway inflammation, large numbers of activated neutrophils release reactive oxygen species (ROS) and proteases, such as neutrophil elastase, thereby inducing additional ROS production in lung cells (Brown and Kelly, 1994; Aoshiba et al., 2001). In addition, reduced antioxidant defense with systemic glutathione deficiency, reduced vitamin E plasma levels, and increased mitochondrial oxidative stress has been reported in CF patients (Roum et al., 1985; Brown et al., 1995; Starosta et al., 2006; Velsor et al., 2006). Not surprisingly, CF lungs are exposed to significantly higher oxidative stress levels (Starosta et al., 2006). As marker of oxidant-induced DNA damage, urinary 8-hydroxydeoxyguanosine (8-OHdG) was found to be significantly elevated in children with CF (Brown and Kelly, 1994). Further, airway epithelia from CF patients show increased expression of two DNA damage markers (phospho-Histone H2A.X (γH2A.X) and phospho-checkpoint 2 kinase (phospho-Chk2)) (Fischer et al., 2013). However, it would be too simplistic to attribute the increased cancer risk solely to oxidative stress. CFTR has been proposed a tumor suppressor protein and CFTR deficiency is implicated in airway and intestinal cancer progression and poor prognosis (Than et al., 2016; Li et al., 2015; Tu et al., 2016). In summary, aging-associated DNA repair deficiency in combination with increased oxidative damage can lead to accumulating genome damage and thus to genomic instability (Moskalev et al., 2013).
Telomere Attrition
The proliferative capacity of cells is limited by the gradual loss of their protective telomeric DNA with each division ultimately leading to cellular senescence (Blackburn et al., 2006). Chronic inflammation can not only increase cell turnover, it has also been shown to lead to telomere dysfunction and accelerated aging (Jurk et al., 2014; Gorenjak et al., 2020). Telomeres are highly sensitive to oxidative damage as it is ineffectively repaired causing accelerated telomere loss (Gorenjak et al., 2020; Hewitt et al., 2012). This raises the question whether oxidative stress associated with chronic airway inflammation can cause accelerated telomere shortening in CF patients.
In CF, decreased telomere length in peripheral blood leukocytes has been reported to be associated with disease severity (Lammertyn et al., 2017). However, telomere length was not reduced in explant lungs from CF patients, and no correlation between telomere length and disease severity at the sampling location was found (Everaerts et al., 2018). Similarly, telomere length in CF airway epithelial cells was not significantly different from control donor lungs; only a small subgroup of CF subjects had shorter telomeres (Fischer et al., 2013). Based on the limited scientific data available, we cannot conclude whether telomere attrition is a common feature in CF lungs. Further research is needed to determine its contribution to CF airway disease pathogenesis.
Epigenetic Alterations
Epigenetic alterations are stable, heritable changes to the chemical DNA structure by alterations in its methylation pattern, post-translational histone modifications, and chromatin remodeling. They regulate gene expression without modifying the DNA sequence (Gonzalo, 1985; Berger et al., 2009). Specific changes in the histone modification pattern such as increased H4K20 and H3K4 trimethylation, and decreased H3K9 methylation as well as H3K27 trimethylation constitute markers of age-associated epigenetic alterations (Gonzalo, 1985; López-Otín et al., 2013). DNA methylation has been shown to play an important role in aging and disease. Global DNA methylation changes over the lifespan and methylation of specific CpG (cytosine guanine) sites correlate with age (Jones et al., 2015; Ashapkin et al., 2017; Levine et al., 2018). Alterations in DNA methylation patterns are associated with cancer, several genetic diseases and inflammatory lung conditions including CF (Robertson, 2005; Scott and De Sario, 2020). In CF, methylation changes were found at multiple CpG dinucleotides in nasal epithelial cells, whole blood and lung macrophages. Interestingly, these epigenetic alterations were preferentially located at genes important for the respective tissue, such as for cell adhesion, immune response or inflammation, and correlated with lung function (Scott and De Sario, 2020; Magalhães et al., 2018; Chen et al., 2018). Though the specific research is yet in its infancy, CFTR has been shown to be epigenetically regulated too which may open up novel therapeutic strategies to restore CFTR dysfunction in CF pathology (Sirinupong and Yang, 2015).
Loss of Proteostasis
Molecular chaperones and the proteolytic machinery assure continuous proteome renewal protecting cells from accumulation of misfolded or damaged proteins (López-Otín et al., 2013). Loss of adequate proteostasis occurs during the aging process and has been shown to be altered in CF epithelia. There is increasing evidence that CFTR function can regulate the proteostasis network (Villella et al., 2013; Esposito et al., 2016). CFTR carrying the ∆F508 mutation as well as other less frequent mutations does not mature into the fully glycosylated protein. Instead, the misfolded protein is mostly retained at the endoplasmic reticulum, where it is sequestered for degradation (Cheng et al., 1990; Van Goor et al., 2011). ∆F508 overexpression causes ER stress and activates the unfolded protein response in vitro leading to decreased wild type CFTR mRNA levels (Bartoszewski et al., 2008). This implicates defective CFTR leads to suppressed CFTR expression. The severity of the clinical disease spectrum varies considerably even within patients carrying the same mutation. Variations in the proteostasis network are suspected to be modifying factors contributing to those differences (Balch et al., 2011). A promising new therapeutic strategy aims at improving protein trafficking by the proteostasis network (Strub and McCray Jr, 2020). The proteostasis regulator cystamine promotes ∆F508-CFTR trafficking to the apical cell membrane and thereby allows it to respond to CFTR potentiators (Luciani et al., 2012). Cystamine combined with epigallocatechin gallate restored CFTR function, reduced lung inflammation and restored autophagy in a phase-2 trial, particularly in patients carrying the ∆F508 mutation (Tosco et al., 2016).
CFTR is not only subjected to degradation by the proteostasis network, it is a key player in its own regulation. Inhibition of CFTR function leads to CFTR protein ubiquitination and drastically reduced stability in the plasma membrane of bronchial epithelial cells (Esposito et al., 2016), with cystamine correcting this deranged proteostasis (Villella et al., 2013). In addition, siRNA depleting CFTR interferes with endosomal trafficking of cell surface proteins demonstrated for transferrin receptor, epidermal growth factor receptor, and CFTR itself (Villella et al., 2013). Hence, insufficient CFTR function derails the proteostasis network in a feed-forward loop fostering its own degradation. Dysfunctional autophagy also appears to contribute to the exaggerated CF lung inflammation. Improving autophagosome clearance attenuates the hyperinflammatory response (Mayer et al., 2013). Oxidative stress from defective CFTR function inhibits ubiquitination and proteasome degradation of the pro-fibrotic tissue trans-glutaminase 2, thereby driving chronic inflammation (Esposito et al., 2016; Luciani et al., 2009). Autophagy restoration by cystamine has been shown to suppress trans-glutaminase 2 and diminish inflammation in CF mice and in patient derived nasal epithelial cells in vitro. In summary, loss of proteostasis as an aging hallmark in CF disease has been established by current evidence and might be a valuable target for future therapies.
Deregulated Nutrient Sensing
Aging is regulated by nutrient-sensing pathways including insulin/insulin-like growth factor (IGF-1), mTOR, AMP kinase, sirtuins, and FOXO transcription factors (Vellai, 2009; Kenyon, 2010). Pro-inflammatory signals are closely integrated in stress and nutrient signaling (Jurk et al., 2014). Hence, both inflammaging and chronic inflammatory conditions such as CF lung disease have the potential to interfere with nutrient sensing signaling pathways. An in vitro study identified the α1 (catalytic) subunit of AMP-activated protein kinase (AMPK) as a dominant and novel protein interacting with CFTR demonstrating a potential link between transepithelial transport and cell metabolic state (Hallows et al., 2000).
Oxidative stress as seen in CF airway inflammation leads to increased pro-growth signaling by the mTOR pathway (Chen et al., 2011). So far, only little is known about deregulated nutrient sensing in CF lung disease. CFTR has been found to interact with mTOR signaling pathway components, which can be affected by CFTR mutations such that ∆F508 CFTR exhibits a specific interactome different from wild type CFTR (Pankow et al., 2015; Reilly et al., 2017). mTOR activity in CF bronchial epithelial cells is upregulated, and PI3K/Akt/mTOR pathway inhibition improves CFTR expression and stability (Reilly et al., 2017). FOXO transcription factors down stream of insulin/IGF-1 signaling (IIS) regulate cellular processes involved in stress resistance, metabolism as well as cell cycle arrest, and are central to IIS attenuation-mediated lifespan expansion (Martins et al., 2016). FOXO1 and 3 have been shown to regulate innate immune mechanisms in respiratory epithelia in response to bacterial infections (Seiler et al., 2013). In a human CF bronchial epithelial cell line, reduced FOXO1 was found related to loss of CFTR function (Smerieri et al., 2014). Interestingly, four miRNAs that are predicted FOXO1 regulators were differently expressed in CF patient sera (Montanini et al., 2016). However, the implications of altered FOXO transcription factor signaling in terms of accelerated aging in CF patients remains unclear and calls for further investigation.
Mitochondrial Dysfunction
Age-associated mitochondrial dysfunction is most commonly caused by increased ROS ultimately leading to cell senescence (Chapman et al., 2019). Excessive oxidant levels from dysfunctional mitochondria can trigger inflammatory cytokine release inducing chronic inflammation and progression of airway diseases such as CF (Prakash et al., 2017). In addition, as cells age, DNA damage occurs and activates DNA damage response pathways. These chronically stimulated pathways cause mitochondrial stress leading to increased release of mitochondrial damage-associated molecular patterns (DAMPs). DAMPs along with ROS production, and/or ATP and K+ efflux contribute to NLRP3 inflammasome activation (Prakash et al., 2017; dos Santos et al., 2012). This leads to caspase-1 activation and release of the proinflammatory cytokines IL-1β and IL-18, initiating a cycle of chronic inflammation, disease progression, and further accumulative damage to mitochondria resulting in accelerated aging (dos Santos et al., 2012). Recent research is showing that mitochondrial dysfunction could be involved in disease progression in CF. Cells with impaired CFTR function exhibit reduced mitochondrial complex I activity (Valdivieso et al., 2012). And increased IL-1β levels are very common among CF patients, which has been linked to inflammation triggered by underlying chronic Pseudomonas aeruginosa infections (Rimessi et al., 2015). As discussed, elevated IL-1β is not only a sign of inflammation, but also a hallmark of mitochondrial dysfunction. These two intrinsically connected hallmarks of aging both contribute to disease progression in CF. Furthermore, it will be of benefit to assess whether the triple CFTR modulator therapy affects these pathogenetic mechanisms favorably. A recent study has shown that CFTR modulation can restore Nrf2 phosphorylation, a major regulator of oxidative balance and inflammatory signaling (Borcherding et al., 2019).
Cellular Senescence
Accumulation of senescent cells is seen in normal aging and chronic pulmonary disease (Jeyapalan et al., 2007; Meiners et al., 2015). Senescence is triggered by a range of insults such as oxidative stress, DNA damage, telomere shortening, and inflammation, and is associated with a characteristic secretory profile termed senescence-associated secretory phenotype (SASP) (Naylor et al., 2013; Jurk et al., 2014). This includes release of proinflammatory mediators, growth factors, and matrix-remodeling proteases that may perpetuate inflammatory processes resulting in further accumulation of senescent cells (Naylor et al., 2013; Parikh et al., 2019). In CF, the liquid lung lining layer contains high amounts of neutrophil elastase that has been shown to trigger cell cycle arrest through elevated p27Kip1 expression resulting in G1 arrest in normal human bronchial epithelial cells in vitro (Nakamura et al., 1992; Fischer et al., 2007). Cell cycle arrest may lead to cellular senescence (Fischer et al., 2013; Naylor et al., 2013). Indeed, a study by Fischer and colleagues confirmed airway epithelia from CF lungs have increased expression of the senescence markers p16INK4a, γH2A.X, and phospho-Chk2 (Fischer et al., 2013). Their study showed that neutrophil elastase increases p16 expression resulting in inhibition of cyclin-dependent kinase 4 activity in vitro (Fischer et al., 2007). This suggests cellular senescence due to excessive neutrophil elastase release in CF lungs may contribute to accelerated aging. A recent review concluded there was consistent data supporting that cellular senescence may also be involved in CF lung disease, but the exact mechanisms leading from loss of CFTR function to cellular senescence and the precise role in CF lung disease remain unknown (Bezzerri et al., 2019).
Stem Cell Exhaustion
Lung tissue has a low steady-state cell turnover with the ability to increase proliferation of stem/progenitor cells in response to injury (Hogan et al., 2014). Aging is associated with a progressive decline of stem/progenitor cells that maintain homeostatic and regenerative capacity (Oh et al., 2014). Chronic inflammation is considered a main factor accelerating the deterioration of stem cell function with transforming growth factor (TGF)-β and ROS accumulation as important pathological factors (Oh et al., 2014). miRNA-155 is upregulated in aging and has been suggested to contribute to inflammation-associated stem cell dysfunction (Teramura and Onodera, 2018). High expression of this specific miRNA has been found in CF lung epithelial cells and circulating neutrophils (Bhattacharyya et al., 2011). Further, chronic airway inflammation in combination with recurrent exacerbations causes recurrent tissue damage thereby increasing the need for stem cell proliferation. This may exceed the supply the stem cell niche is capable of providing resulting in its depletion in CF lungs (Mora and Rojas, 2013). To this point, there are no reports on the exact role of stem cell exhaustion in CF lung disease. One study observed no telomere shortening in CF airway epithelial cells leading to the conclusion that the epithelial stem/progenitor reserve is sufficient to maintain normal telomere length despite enhanced cell turnover (Fischer et al., 2013). But this does not exclude an involvement of stem cell exhaustion in accelerated aging in CF lungs, and further studies are needed to draw definite conclusions.
Altered Cellular Communication
In addition to chronic lung inflammation, CFTR deficiency has been reported to causes abnormalities in diverse signaling pathways. Functional CFTR has been reported to downregulate NF-κB activity and CF associated hyper-inflammation may represent a consequence of insufficient inhibition of NF-κB signaling (Hunter et al., 2010). Chronic, progressive low-grade inflammation promoted by NF-κB can cause premature aging (Jurk et al., 2014). CF airway inflammation is associated with excessive production of NF-κB- dependent inflammatory mediators such as interleukin-1β (IL-1β), IL-6, IL-8, and TGF-β1, neutrophil recruitment, and decreased response to interferon (IFN)-γ as well as further abnormalities in various signaling pathways (Nichols et al., 2008; Harris et al., 2009; Peterson-Carmichael et al., 2009; Lara-Reyna et al., 2020). The profibrotic cytokine TGF-β is considered a pro-aging factor and is associated with accelerated lung function decline in CF patients (Arkwright et al., 2000). Several reports show that TGF-β causes CFTR dysfunction (Manzanares et al., 2015; Snodgrass et al., 2013; Sun et al., 2014). Chronic bacterial infections stimulate persistent IL-8 release from airway epithelia (McCuaig and Martin, 2013). Attracted neutrophils further potentiate airway inflammation by releasing high concentrations of inflammatory mediators such as TNFα and IL-8, oxidants, and proteases (Petit-Bertron et al., 2008). Neutrophil elastase promotes CFTR degradation and dysfunction by calpains (Le Gars et al., 2013). Recent drug developments have focused on restoring CFTR function; these therapies significantly improve lung function and reduce exacerbations in CF patients (Wainwright et al., 2015; Rowe et al., 2017; Davies et al., 2018; Keating et al., 2018). However, sustained airway inflammation leads to degradation and increased turnover of CFTR, hindering the targeted therapies from developing their full potential by inducing a state of accelerated aging at the tissue level (Rowe et al., 2014). Oxidative stress from ROS associated with neutrophilic airway inflammation can lead to accumulation of modified or damaged biomolecules impairing their function. Oxidatively damaged cellular structures can act as DAMPs that are recognized by receptors of the innate immune system, i.e., Toll-like-receptors (TLRs) and the NLRP3 inflammasome (Meiners et al., 2015). Hence, oxidative stress can further enhance cytokine production in the ongoing inflammation and maintain the proinflammatory state in inflammaging (Franceschi and Campisi, 2014; Meiners et al., 2015).
Extracellular Matrix Dysregulation
ECM remodeling in lung diseases is not only a consequence of tissue injury, it also contributes to disease progression by impaired repair mechanisms (Parker et al., 2014). The neutrophilic inflammation in CF is accompanied by protease/antiprotease imbalance with increased neutrophil elastase and matrix metalloprotease-9 and reduced tissue inhibitor of metalloprotease-1 (Gaggar et al., 2007). While proteolytic enzymes are beneficial for tissue repair, excessive release due to chronic inflammation may overwhelm anti-protease activity causing airway remodeling and obstruction (Gaggar et al., 2011). Neutrophil derived proteases are the main driving force of lung injury in CF (Elborn, 2016) and sputum neutrophil elastase correlates with FEV1 in children with CF (Sagel et al., 2002). Furthermore, neutrophil elastase and proteolytic collagen and elastin breakdown products play a pathogenic role in fibrotic lung remodeling (Chua and Laurent, 2006). Ultrastructural evidence for ECM degradation includes lysis of elastic and collagen fibers, loss of arborescent elastic network distribution and alterations in the reticular basement membrane structure (RBM) (Durieu et al., 1998; Hilliard et al., 2007; Regamey et al., 2011). In CF, RBM thickening appears to be related to increased TGF-β1 levels (Hilliard et al., 2007). The elastic network reduction combined with increased collagen deposition in bronchial walls of CF patients resembles fibrotic ECM changes in aging lungs (Hilliard et al., 2007). Together, these data suggest that ECM dysregulation plays an important role in accelerated lung aging in CF.
Therapeutic Implications
Current Effective CF Therapies Targeting Chronic Inflammation
Various drugs targeting inflammation have been studied in CF since 1990, but only few are recommended for clinical use. Due to the length of this review, only significant ones will be discussed here.
Corticosteroids were one of the first anti-inflammatory drugs studied as a chronic therapy attenuating CF airway inflammation (Auerbach et al., 1985; Eigen et al., 1995). Although beneficial effects on lung function were shown, overall adverse effects outweighed the benefits and long-term use of systemic corticosteroids to slow lung function decline is currently not recommended (Flume et al., 2007). Several studies investigated the effects of inhaled corticosteroids (ICS) on lung function decline, but their anti-inflammatory effect was never confirmed (Ren et al., 2008; De Boeck et al., 2011). The CF Foundation therefore is advising against the use of long-term ICS in patients with CF older than 6 years without coexistent asthma or allergic bronchopulmonary aspergillosis (Flume et al., 2007).
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been studied as therapeutic options for CF due to similar properties as corticosteroids, but fewer adverse effects. Ibuprofen has specific activity against neutrophils and twice-daily high-dose ibuprofen use was linked to a slower lung function decline, a decreased exacerbation frequency and less weight loss in the pediatric CF population (Konstan et al., 1995; Konstan et al., 2003; Konstan et al., 2007; Lands et al., 2007). Nevertheless, ibuprofen is not widely used, but the CF Foundation expert panel recommends its use for CF patients with mild lung disease (Flume et al., 2007).
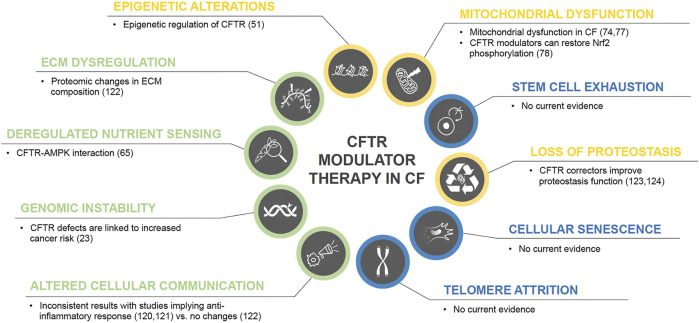
CFTR modulator therapy has demonstrated its efficacy and beneficial effect on restoration of CFTR function for approximately 90% CF genotypes (Wainwright et al., 2015; Rowe et al., 2017; Davies et al., 2018; Keating et al., 2018; Middleton et al., 2019). Interestingly, CFTR dysfunction has been linked to contribute to chronic inflammation as well as other hallmarks of aging, and two recent studies have demonstrated an anti-inflammatory effect of CFTR modulators (Jarosz-Griffiths et al., 2020; Hisert et al., 2017). A recent observational study though did not show any significant alterations in sputum inflammatory markers, but changes in ECM using whole proteome analysis (Kopp et al., 2020). Therefore, the validation of potential anti-inflammatory effects of CFTR correctors needs further investigation. CFTR correctors also have been shown to act as proteostasis regulators (Lopes-Pacheco et al., 2015; Lopes-Pacheco et al., 2016) and there is evidence that CFTR modulators can regulate mitochondrial dysfunction. Figure 2 summarizes current evidence of CFTR modulators and their link to aging hallmarks.
FIGURE 2.
CFTR modulator therapy in CF and potential effects on the hallmarks of aging. Color code for current level of evidence with blue = no evidence for involvement in CF pathology, green = weak evidence, yellow = strong evidence.
Potential Novel CF Therapies
Modulation of ion channels other than CFTR has been evaluated as potential therapies. Most recently, potentiation of TMEM16A using ETX001 significantly increased the Ca2+-activated Cl− channel activity and anion secretion both in vitro and in vivo ovine models leading to improved mucociliary clearance without impacting calcium signaling (Danahay et al., 2020). Whether ETX001 also affects airway inflammation will be of great interest.
Anti-inflammatory cytokines and antibodies targeting cytokines may be valuable novel CF therapies. IL-10 has been demonstrated to terminate the inflammatory response and is deficient in CF patients (Bonfield et al., 1999). And its administration is anti-inflammatory in Pseudomonas infected mice (Chmiel et al., 1999; Soltys et al., 2002). Treatment with the anti-inflammatory cytokine IFN-γ was tested in a multicenter clinical trial but did not show any beneficial effect on pulmonary function or inflammatory sputum markers (Moss et al., 2005). This is in line with the above-mentioned reduced response of CF airways to IFN-γ. As an alternative to the treatment with anti-inflammatory cytokines, various blocking antibodies targeting pro-inflammatory cytokines have been assessed. Anti-IL-17 antibodies have been shown to decrease neutrophil recruitment in murine airways when exposed to lipopolysaccharide (Ferretti et al., 2003). Anti-IL-17 has been tested clinically in rheumatoid arthritis and psoriasis (Ly et al., 2019; Taams, 2020) and might be a potential novel therapeutic strategy for CF-associated airway inflammation. Anti-ICAM-1 and -IL-8 have been assessed preclinically, but never reached the clinical stage. Because of high concentrations of LTB4 in CF airways (Konstan et al., 1993) BIIL 284 BS (amelubant), a specific LTB4 receptor antagonist, has been tested in CF. Due to serious pulmonary adverse events, the clinical trial was terminated early (Konstan MW et al., 2005).
Fatty acid modulation may proof beneficial as alterations in fatty acid concentrations contributing to airway inflammation have been demonstrated in CF such as increases in arachidonic acid and decreases in docosahexaenoic acid (DHA) (Freedman et al., 1999; Horati et al., 2020). Cumulative data from in vitro studies and some small, non-placebo controlled trials using oral DHA supplementation imply this may be beneficial for pulmonary function preservation (Beharry et al., 2007; Van Biervliet et al., 2008; Aldámiz-Echevarría et al., 2009). To validate these results, larger placebo-controlled trials will be needed. Furthermore, Myriocin, an inhibitor of sphingolipid synthesis, induced changes in a transcriptional program of cell metabolism in vitro in CF airway epithelial cells. Therefore, the authors speculated that sphingolipid de novo synthesis could attenuate chronic inflammation, optimize energy supply, and anti-oxidant responses, implying a novel future therapeutic strategy for CF (Mingione et al., 2020)
Inhibiton of NF-κB activation has been demonstrated by both ibuprofen at high doses and IL-10. Furthermore, NF-κB signaling can be attenuated by glitazones through peroxisome proliferator activating receptor (PPAR) elevation (Zingarelli et al., 2003; Ruan et al., 2003) and there seems to be a deficiency of PPAR in CF (Perez et al., 2008). Both tiglitazone and troglitazone can activate PPAR in the CF epithelium and attenuate the inflammatory response to P. aeruginosa (Perez et al., 2008). One clinical trial using glitazones has not shown a beneficial effect towards inflammation, but this trial was very small and did not assess lung function outcomes (Konstan MW et al., 2009). Long term use of Azithromycin has not only been shown to improve functional outcomes, but some of the underlying mechanisms can be attributed to STAT and NF-κB inhibition (Haydar et al., 2019; Nichols et al., 2020). In summary, targeting NF-κB signaling in CF disease seems to be an attractive future therapeutic direction.
Inhibition of the NLRP3 Inflammasome has also been discussed as a potential anti-inflammatory strategy (Scambler et al., 2019). A recent report utilized MCC950 as a specific NLRP3 inhibitor in vivo in CF animal models, which resulted in significantly reduced airway inflammation and improved Pseudomonas clearance (McElvaney et al., 2019).
Statins exhibit anti-inflammatory effects through various mechanisms including inhibition of neutrophil migration, RhoGTPase/increase in nitric oxide (NO)/IL-8 production and other proinflammatory cytokines as well as increase of PPAR transcription (Dunzendorfer et al., 1997; Kraynack et al., 2002; Zelvyte et al., 2002). Agents that can increase NO, such as arginine, have been studied, since it has been shown that there is increased arginase activity in blood and sputum of CF patients. This leads to degradation of L-arginine, which is a substrate for NO production (Grasemann et al., 2005a). A small pilot study assessed oral supplementation with L-arginine in CF patients, which led to increased exhaled NO (Grasemann et al., 2005b). In order to show efficacy, large prospective clinical trials are needed.
The antioxidant N-Acetyl cysteine (NAC) is frequently used as a mucolytic, but has spiked recent interest as antioxidant due to its ability to increase glutathione levels and inhibit H2O2-induced damage. Supplementation of oral NAC was linked to significantly increased blood glutathione levels and decreased neutrophils, IL-8, and elastase activity in sputum of CF patients (Tirouvanziam et al., 2006) A randomized double-blind placebo-controlled trial including 70 CF patients for 24 weeks showed stability or a slight increase in spirometric lung function in NAC treated participants compared to the control group (Conrad et al., 2015). A second open-label randomized controlled trial demonstrated a non-significant improvement in lung function in adults with CF and chronic Pseudomonas infection (Skov et al., 2015). P3001, a mucolytic agent was also assessed and directly compared with NAC in a more recent study using both in vitro and in vivo CF models. Results indicated that P3001 acted faster and was more effective than NAC and DNAse, but investigators did not study its anti-oxidant or anti-inflammatory properties (Ehre et al., 2019). Since glutathione is transported by CFTR (Linsdell and Hanrahan, 1998), CFTR defects could potentially decrease levels of this antioxidant in the airway epithelium increasing susceptibility to oxidative stress. Indeed, decreased glutathione levels have been observed in CF mouse models and patients (Roum et al., 1985; Velsor et al., 2001). A small pilot study using twice daily treatment of aerosolized glutathione in CF patients led to a reduction in superoxide production, but no changes in oxidative stress markers in bronchoalveolar lavage fluid (Bishop et al., 2005; Hartl et al., 2005). A more recent Cochrane meta-analysis summarized antioxidant therapy approaches in CF and concluded that there does not seem to be a beneficial effect of antioxidant micronutrients on clinical outcomes; but oral supplementation with glutathione showed some benefit to lung function, nutritional status, and decrease in oxidative stress (Ciofu et al., 2019). Nevertheless, due to concurrent treatments including intensive antibiotic therapies, there is not sufficient evidence yet justifying their use in the CF population without larger trials and longer follow up.
Therapeutic use of protease inhibitors has been investigated in CF since 1990. Several groups demonstrated that α1-antitrypsin delivered as an aerosol formulary decreases inflammatory markers and neutrophils (McElvaney et al., 1991; Griese et al., 2007). rSLPI and EPI-hNE4 are two neutrophil elastase inhibitors, which have shown beneficial effects in CF, but large cohort trials are still lacking (McElvaney et al., 1993; Grimbert et al., 2003).
Other potential anti-inflammatories are also being investigated. SB-656933, a CXCR2 antagonist, has shown a trend toward attenuating airway inflammation (Moss et al., 2013). Low-dose cyclosporin A was characterized as a potential steroid sparing agent in a small case series (Bhal et al., 2001). Furthermore, methotrexate was studied as a potential anti-inflammatory agent in cystic fibrosis, but studies were inconclusive with one showing beneficial effects on lung function and total serum immunoglobulin levels, whereas another study showed less tolerance and increased exacerbations (Ballmann et al., 2003; Oermann and Wheeler C, 2007).
Micro RNAs as a potential anti-inflammatory approach have recently become a focus of interest. mi-RNAs have been implicated in various diseases including cystic fibrosis (Bardin et al., 2019; Bartoszewska et al., 2017). Vencken et al. suggested the use of nebulized lipid-polymer hybrid nanoparticles for the delivery of a therapeutic anti-inflammatory microRNA, miR-17, to reduce IL-8 secretion, which was successfully tested in vitro (Vencken et al., 2019) In addition, targeting miRNAs to restore CFTR activity has also been suggested as a novel therapeutic strategy (De Santi et al., 2020). Please see Table 1 for a summary of the evidence for accelerated aging in CF shown in in vitro and in vivo studies.
TABLE 1.
Summary of in vitro, animal and human studies pointing towards an involvement of accelerated aging in CF disease.
| In vitro studies | |||
|---|---|---|---|
| Hallmark of aging | Cell culture model | Main findings | Ref |
| Genomic instability | Human fetal lung fibroblast cell line IMR-90, primary human bronchial epithelial cells, and lymphocytes isolated from human blood | ROS and proteases from activated neutrophils increase ROS in mitochondria and cytoplasm, which is associated with oxidative cell injury and death | (Aoshiba et al., 2001) |
| Human CF lung epithelial cell line IB3-1 (compound heterozygot for ∆F508 and 128W2X) and isogenic stably wild-type (wt) CFTR transfected C38 cells | Decreased mitochondrial reduced glutathione (GSH) and increased ROS in CFTR deficient human lung epithelial cells | (Velsor et al., 2006) | |
| CFTR overexpression and knockdown in A549 cell line | Inhibition of CFTR activity promotes epithelial-mesenchymal transition through the uPA/uPAR pathway | (Li et al., 2015) | |
| Loss of proteostasis | COS-7 cell line | Mutated CFTR such as ∆F508 remains in the endoplasmatic reticulum (ER) and is sequester for degradation | (Cheng et al., 1990) |
| Calu-3 (wt and stably ∆F508-CFTR transfected) and CFPAC-1 (endogenous ∆F508-CFTR) cell line | ∆F508-CFTR overexpression causes ER stress and activates the unfolded protein response leading to decreased wt CFTR mRNA and protein maturation | (Bartoszewski et al., 2008) | |
| Normal and CFTR mutated CFBE41° bronchial epithelial cells, primary human bronchial/tracheal epithelial cells and HeLa cells | Functional CFTR controls its own surface expression in a positive feed-forward loop through its effects on the proteostasis network. siRNA depleting CFTR interferes with endosomal trafficking of cell surface proteins. Proteostasis regulator cystamine corrects the deranged proteostasis | (Villella et al., 2013) | |
| FRT cell line, HEK-293 cells, and primary human bronchial epithelial (HBE) cells | CFTR corrector VX-809 improves F508del-CFTR processing in the ER, leading to plasma residence time and susceptibility to proteolysis similar to normal CFTR. | (Van Goor et al., 2011) | |
| Human normal and CF bronchial epithelial cell lines (CFBE41o-, IB3-1, 16HBE14o-), ex-vivo cultures of nasal polyp mucosal biopsies and brushed nasal epithelial cells from ∆F508 homozygous patients and matched controls | Proteostasis regulators cystamine and EUK-134 (superoxide dismutase/catalase-mimetic) improve ∆F508-CFTR trafficking and stability at the epithelial cell surface by overexpressing BECN1 and depleting SQSTM1. This facilitates its response to CFTR potentiators and suppresses inflammation | (Luciani et al., 2012) | |
| IB3-1 and isogenic stably rescued C38 cells and peripheral blood mononuclear cells (PBMCs) from pediatric CF patients and healthy controls | Dysfunctional autophagy appears to contribute to the exaggerated CF lung inflammation. Improving autophagosome clearance attenuates the hyperinflammatory response | (Mayer et al., 2013) | |
| IB3-1 and isogenic stably rescued C38, 16HBE and A549 cell line, ex vivo cultures of nasal polyp mucosal biopsies from CF patients and controls | Defective CFTR function generates oxidative stress that leads to PIASy mediated tissue trans-glutaminase 2 (TG2) SUMOylation inhibiting its ubiquitination and proteasome degradation. TG2 inhibition increases NF-κB inhibitor Ikκα | (Luciani et al., 2009) | |
| Deregulated nutrient sensing | CHO (wt and stably expressing CFTR), T84 and Calu-3 cell line, and Xenopus oocytes | AMPK and CFTR are endogenously expressed in the same tissue types and have been found to interact with each other leading to CFTR phosphorylation and altered CFTR Cl− conductance. This may represent a link between transepithelial transport and cell metabolic state | (Hallows et al., 2000) |
| CF human bronchial epithelial cell line CFBE41o- (∆F508 mutation) and isogenic HBE41o- cells (wt CFTR) | ∆F508-CFTR interactome differs highly from its wt counterpart including differences in the mTOR, JAK/STAT and several other pathways, showing the catastrophic effects from one misfolded protein on protein-protein interactions | (Pankow et al., 2015) | |
| CFBE41o- and HBE41o- cells | Inhibition of the PI3K/Akt/mTOR pathway leads to improved CFTR stability, while select inhibitors of this pathway leads to restored autophagy and reduced ∆F508-CFTR aggregates | Reilly et al. (2017) | |
| CFBE41o- and 16HBE14o- cells | Reduced level of transcription factor FOXO1 and β2 arrestin, along with increased ERK1/2 in CF cells. FOXO1 reduction is linked to loss of CFTR function and increased after insulin-like growth factor 1 (IGF-1) administration. Reduced FOXO1 may explain insulin insensitivity in CF, with IGF-1 constituting a potential treatment of CF-related diabetes | (Smerieri et al., 2014) | |
| CFBE41o-, 16HBE14o-, and IB3-1 cells | Altered transcriptional profile of miRNAs in CF cells, four of which are potential FOXO1 regulators. These four miRNAs are also differentially expressed in CF patients, and dependent on genotype and glucose tolerance state. This may explain some of the variability in metabolism among CF patients | (Montanini et al., 2016) | |
| Mitochondrial dysfunction | IB3-1 cell line | Pseudomonas eruginosa induced inflammation shows importance of mitochondria in the pro-inflammatory condition in CF including their role in Ca2+ signaling along with NLRP3 recruitment and activation | Rimessi et al. (2015) |
| CF and non-CF HBE cells | ∆F508-CFTR correctors recover diminished function of the major redox balance and inflammatory signaling regulator Nrf2, inducing its nuclear translocation and transcription of target genes. Nrf2 rescue is dependent on CFTR function | (Borcherding et al., 2019) | |
| Cellular senescence | Normal HBE cells | Neutrophil elastase (NE) triggers cell cycle arrest through elevated p27Kip1 expression resulting in G1 arrest in normal HBE cells | (Fischer et al., 2007) |
| Normal HBE cells | NE increases p16 expression and decreases CDK4 activity in HBE cells, which may explain how NE treatment triggers cell cycle arrest | (Fischer et al., 2013) | |
| Stem cell exhaustion | HBE cells | No general telomere shortening in CF HBE cells leading to the conclusion that progenitor reserve is sufficient to maintain normal telomere length despite enhanced cell turnover | (Fischer et al., 2013) |
| IB3-1 and control CFTR repaired IB3-S9 cells | CF lung epithelial cells hyperexpress miRNA-155, also upregulated in aging. This activates PI3K/Akt signaling through reduced SHIP1. Resulting activation of downstream MAPKs stabilizes IL-8 mRNA and thus increases IL-8 expression promoting inflammation | (Bhattacharyya et al., 2011) | |
| Altered cellular communication | NCI-H441 and 16HBE14o- cells | Functional CFTR downregulates NF-κB activity. CF associated hyper-inflammation may represent a consequence of insufficient inhibition of NF-κB signaling | Hunter et al. (2010) |
| HBE cells | TGF-β1 decreases expression of the γ-subunit LRRC26 of the apically located large-conductance Ca2+- and voltage-dependent K+ (BK) channels. Thereby, TGF-β1 reduces BK activity, airway surface liquid volume and ciliary beat frequency | Manzanares et al. (2015) | |
| Normal and homozygous △508-CFTR HBE cells | TGF-β1, which is frequently elevated in CF patients, reduces CFTR mRNA and protein level in non-CF HBE cells. TGF-β1 also impairs functional rescue of △508-CFTR suggesting it may interfere with therapies aiming at correcting the processing defect of △508-CFTR. | Snodgrass et al. (2013) | |
| T84 cell line and HBE cells | TGF-β reduces calcium activated chloride conductance (CaCC) and CFTR-dependent chloride currents. It reduces expression and activity of TMEM16A and CFTR, and reverses △508-CFTR correction by VX-809. Inhibition of Smad3 and p38 MAPK partially reverses TMEM16A and CFTR downregulation | Sun et al. (2014) | |
| NCI-H292 cell line infected with wt or △508-CFTR | NE promotes degradation of wt and △508-CFTR through activation of intracellular calpain protease causing loss of channel function | Le Gars et al. (2013) | |
| In vivo animal studies | |||
| Hallmark of aging | Animal model | Main findings | Ref |
| Genomic instability | Congenic CFTR KO strains (S489X and FABP) and C57B6 control mice | Decreased mitochondrial GSH in lungs of CFTR-knockout mice, and increased mitochondrial DNA oxidation and oxidative stress | Velsor et al. (2006) |
| Athymic balb/c mice injected with CFTR knockdown or control A-549 cells | CFTR status affects cell invasion and migration. No difference in primary tumor growth between control and CFTR-knockdown A549-injected mice, but increased lung metastasis and increased tumor burden by CFTR-knockdown cells | Li et al. (2015) | |
| Telomere attrition | Nfkb1-/- mice (C57B1/6 background) and fibroblast cultures from ear clippings, p55△ns/△ns mice, and late-generation (F3-F4) terc−/- mice bred from B6/Cg-TERCtm1Rdp/J | Chronic, low-grade inflammation induces telomere dysfunction, senescence, impaired tissue regeneration and premature aging. Conversely, telomere dysfunction leads to a pro-inflammatory state. Anti-inflammatory or antioxidant treatment blocks accumulation of telomere-dysfunctional senescent cells in nfkb1-/- tissues | Jurk et al. (2014) |
| Loss of proteostasis | CftrF508del/F508del mice (129/FVB outbred background) and wt littermates | Autophagy restoration by cystamine treatment, BECN1 overexpression and SQSTM1 depletion all considerably increase ∆F508-CFTR at the epithelial surface and decrease lung inflammation | Luciani et al. (2012) |
| CftrF508del/F508del, cftr -/- and CftrF508/- mice, CftrF508/+ and CftrF508del/F508del mice in Becn1 haploinsufficient background (Becn1+/−) | Cystamine plus epigallocatechin gallate restore CFTR function and reduce lung inflammation in CftrF508del/F508del and CftrF508/- mice. ∆F508-CFTR rescue is linked to autophagy restoration, i.e., no rescue in Becn1+/− background | Tosco et al. (2016) | |
| CftrF508del/F508del mice (129/FVB outbred background) and wt littermates | TG2 inhibition by cystamine restores PPARγ levels and nuclear localization, and reduces TNFα in lungs of CF mice | Luciani et al. (2009) | |
| Deregulated nutrient sensing | Young adult CF mice homozygous for F508del-CFTR and wt litter mates | Reduced FOXO1 in muscle, but not in liver and adipose tissue of CF mice. Insulin-like growth factor 1 (IGF-1) increases FOXO1 in CF muscle tissue similar to the wt level, and increases it in adipose tissue of both mouse models | Smerieri et al. (2014) |
| Mitochondrial dysfunction | CF mouse models (Cftrtm1kth, Cftrtm2Mrc, and Cftrtm1Unc in C57BL/6J background) | Reduced Nrf2-CFTR colocalization in CF mouse models with concomitantly reduced expression of Nrf2 target geneses HMOX1, NQO1, and GCLC. | Borcherding et al. (2019) |
| Cellular senescence | Nfkb1-/- mice (C57B1/6 background) and fibroblast cultures from ear clipping, p55△ns/△ns mice, and late-generation (F3-F4) terc−/- mice bred from B6/Cg-TERCtm1Rdp/J | Chronic, low-grade inflammation induces telomere dysfunction, senescence, impaired tissue regeneration and premature aging. Anti-inflammatory or antioxidant treatment blocks accumulation of telomere-dysfunctional senescent cells in nfkb1-/- tissues | Jurk et al. (2014) |
| Altered cellular communication | Nfkb1-/- mice (C57B1/6 background) and fibroblast cultures from ear clippings, p55△ns/△ns mice, and late-generation (F3-F4) terc−/- mice bred from B6/Cg-TERCtm1Rdp/J | Chronic, progressive low-grade inflammation promoted by NF-κB can cause premature aging | Jurk et al. (2014) |
| C57/B16 and NE−/- mice | NE promotes CFTR degradation through activation of intracellular calpain protease causing loss of channel function | Le Gars et al. (2013) | |
| Human studies | |||
| Hallmark of aging | Study design | Main findings | Ref |
| Genomic instability | 20 years follow up of CF patients receiving care in United States CF care center programs comparing their cancer incidence with that of the general population | Increased risk for lymphoid leukemia, testicular cancer and digestive tract (esophago-gastric junction, biliary tract, small bowel and colon) cancers in non-transplanted patients compared to the general population. Particularly high risk for digestive tract (mostly bowel) cancers in transplanted patients | Maisonneuve et al. (2013) |
| Cancer incidence in CF and non-CF lung transplant recipients compared to the general population based on the United States transplant and 16 cancer registries | Overall cancer risk in CF lung transplant recipients is more increased than in non-CF recipients, particularly for colorectal cancer, esophageal cancer and non-Hodgkin lymphoma | Fink et al. (2017) | |
| CFTR expression in non-small cell lung cancer (NSCLC) and normal lung samples | Downregulated CFTR expression in NSCLC samples; correlation of low CFTR expression with advanced stage, lymph node metastasis and poor prognosis | Li et al. (2015) | |
| Urinary 8-hydroxydeoxyguano-sine (oh8dG, marker of free radical-induced DNA damage) from CF patients and age matched healthy controls and correlation with clinical status | Significantly higher urinary oh8dG in CF group, but no correlation with markers of lung function (FEV1, FVC) or clinical status assessed by Taussig-Schwachman score in CF patients; highly significant, positive correlation between urinary oh8dG and plasma vitamin E | Brown et al. (1995) | |
| Bronchoalveloar lavage (BAL) of CF and nonsmoking control subjects | Glutathione deficiency with significant reduction in GSH in CF BAL fluid. Simultaneously marked deficiency of plasma GSH. | Roum et al. (1985) | |
| BAL of children with CF and normal control subjects | Highest oxidative stress level assessed by protein carbonyls in patients with FEV1 < 80% of predicted or highly elevated neutrophils | Starosta et al. (2006) | |
| Telomere attrition | Telomere length of DNA extracted from airway epithelial cells of CF patients and controls | No significant difference in telomere length between CF and control airway epithelial cells, apart for a small subgroup of CF subjects showing shorter telomeres | Fischer et al. (2013) |
| Telomere length of DNA extracted from peripheral blood leukocyte of CF patients | Decreased telomere length associated with severe disease characterized by lower FEV1, ∆F508 homozygosity and CF asthma | Lammertyn et al. (2017) | |
| Telomere length of DNA extracted from tissue cores of healthy and diseased human lungs from (re-)transplantation or autopsy | Decreasing telomere length with age in normal, but not in CF lungs. No reduction in telomere length in CF explant lungs compared to normal lungs and no correlation between telomere length and disease severity | Everaerts et al. (2018) | |
| Epigenetic alterations | DNA methylation profiling in nasal epithelial cells and whole blood from CF and control subjects | Substantial DNA methylation differences between CF and control samples, and different methylation levels between mild and severe CF disease. DNA methylation changes in genes responsible for epithelial integrity, inflammatory and immune response, and over-represented in enhancers active in lung tissue | Magalhães et al. (2018) |
| DNA methylation profiling in BAL cells, i.e., primarily lung macrophages, from CF and normal subjects | Multiple differentially methylated CpG sites in CF BAL cells, mostly hypo-methylated and located in non-promoter CpG island as well as putative enhancer regions and DNase hypersensitive regions. Altered DNA methylation significantly associated with CF disease status and may be a driving factor in CF innate immune dysfunction | Chen et al. (2018) | |
| Loss of proteostasis | Single-center, open-label phase-2 clinical trial with CF patients orally treated with cysteamine and epigallocatechin | Beneficial effects of proteostasis regulators in patients with rescuable CFTR with decreased sweat chloride, increased CFTR expression and function, restored autophagy, decreased sputum CXCL8 and TNF-α and tendency for improved respiratory function | Tosco et al. (2016) |
| Cellular senescence | Immunohistochemistry for senescence markers on tissue sections of lungs from CF patients and normal controls | Significantly increased expression of senescence/DNA damage markers (p16INK4a, γH2A.X and phospho-Chk2) in CF airways | Fischer et al. (2013) |
| Stem cell exhaustion | Bronchial epithelial cells from lung brush biopsies and blood neutrophils from CF patients homozygous for ∆F508 mutation and normal controls | miRNA-155, upregulated in aging and suspected to contribute to inflammation-associated stem cell dysfunction (Teramura and Onodera, 2018), is highly expressed in CF lung epithelial cells and blood neutrophils, where it reduces SHIP1 levels promoting PI3K/Akt activation that drives IL-8 expression | Bhattacharyya et al. (2011) |
| Altered cellular communication | TGF-β1 gene polymorphism in CF patients homozygous for ∆F508 | Patients with high levels of the profibrotic, pro-aging cytokine TGF-β1 show accelerated lung function decline | Arkwright et al. (2000) |
| TGF-β1 level in BAL fluid of pediatric CF patients and non-CF controls, and assessment of clinical disease indicators | Elevated TGF-β1 in CF patients is associated with neutrophilic inflammation, diminished lung function and recent hospitalization. No association of TGF-β1 with presence or quantity of bacterial pathogens | Harris et al. (2009) | |
| Estimation of lung volumes and forced expiratory flows in children before clinically indicated BAL | TGF-β1 and matrix metalloprotease (MMP)-2, both previously linked to airway remodeling, are associated with diminished flows as well as hyperinflation and air trapping | Peterson-Carmichael et al. (2009) | |
| ECM dysregulation | Matrix metalloprotease (MMP) profile in lower airway secretions of CF in- and outpatients and normal controls | Increased active MMP-9 and NE, and decreased tissue inhibitor of metalloprotease-1 (TIMP-1) in CF. NE can activate pro-MMP-9 and degrade TIMP-1, hence not surprisingly a strong correlations between NE and MMP-9 activity in CF inpatient samples | Gaggar et al. (2007) |
| BAL and endobronchial biopsies in children with CF and controls without lower airway symptoms | Increased elastin, glycosaminoglycan and collagen in BAL fluid from children with CF indicating matrix breakdown that positively correlates with age, neutrophil count and protease concentration. Increased reticular basement membrane thickness in CF group correlates with TGF-β1 level in BAL fluid | Hilliard et al. (2007) | |
Conclusion and Further Directions
Increasing evidence suggests that accelerated aging processes are involved in CF lung pathology. Based on the current state of research summarized in the paragraphs above, it appears sufficiently established that accelerated aging in CF patients is not only a consequence of chronic lung inflammation, but aging-associated processes are also driving disease progression. Of the ten hallmarks of aging, according to recent literature all but stem cell depletion and telomere attrition appear to be involved to varying degrees in CF lung disease (cp. Figure 1). For some hallmarks there is already considerable data on the specific mechanisms of their pathologic involvement, as in the case of altered proteostasis and intercellular communication. Others, such as mitochondrial dysfunction, deregulated nutrient sensing and cellular senescence, have been indicated to play a role in CF lung pathology and accelerated aging, but further research is needed to elucidate underlying pathomechanisms. And although each of the ten hallmarks was discussed individually, they should be considered as interdependent processes that influence each other.
Growing evidence on the role of accelerated aging processes in CF lung disease and progress in deciphering involved mechanisms also provide new therapeutic targets for future treatment strategy complementing established therapies as summarized in Therapeutic Implications. Some therapies targeting the ten hallmarks of aging are already in clinical use, and further can be expected to follow which may aid further reducing mortality and improving quality of life in CF patients.
Acknowledgments
The authors would like to thank Eva Maria Künzi for her assistance in creating the illustrations.
Author Contributions
LK, ME, MH, and SK contributed to the writing of the manuscript. LK designed the figures and helped to draft the manuscript. LK, ME, and MH contributed to the literature search. SK critically revised the manuscript. All authors read and approved the manuscript for publication.
Funding
This work was supported by the Flight Attendant Medical Research Institute (YFAC152003 to SK), the Cystic Fibrosis Foundation (P30 DK072482 and Rowe19RO to SK), and the National Institutes of Health (R03AG059994 to SK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BAL, bronchoalveolar lavage; BK, large-conductance Ca2+- and voltage-dependent K+; ECM, extracellular matrix; FEV1, forced expiratory volume in 1s; FVC, forced ventilator capacity; GSH, reduced glutathione; HBE, human bronchial epithelial; IGF-1, Insulin-like growth factor 1; MAPK, mitogen-activated protein kinase; NE, neutrophil elastase; oh8dG, urinary 8-hydroxydeoxyguanosine; PBMCs, peripheral blood mononuclear cells; PMN, polymorphnuclear leukocyte; ROS, reactive oxygen species; TG2, tissue trans-glutaminase 2; TIMP-1 tissue inhibitor of metalloprotease-1; wt, wild-type.
References
- Aldámiz-Echevarría L., Prieto J. A., Andrade F., Elorz J., Sojo A., Lage S., et al. (2009). Persistence of essential fatty acid deficiency in cystic fibrosis despite nutritional therapy. Pediatr. Res. 66 (5), 585–589. 10.1203/pdr.0b013e3181b4e8d3 [DOI] [PubMed] [Google Scholar]
- Aoshiba K., Yasuda K., Yasui S., Tamaoki J., Nagai A. (2001). Serine proteases increase oxidative stress in lung cells. Am. J. Physiology-Lung Cell Mol. Physiol. 281 (3), L556–L564. 10.1152/ajplung.2001.281.3.l556 [DOI] [PubMed] [Google Scholar]
- Arkwright P. D., Laurie S., Super M., Pravica V., Schwarz M. J., Webb A. K., et al. (2000). TGF-beta 1 genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax 55 (6), 459–462. 10.1136/thorax.55.6.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashapkin V. V., Kutueva L. I., Vanyushin B. F. (2017). Aging as an epigenetic phenomenon. Curr. Genomics 18 (5), 385–407. 10.2174/1389202918666170412112130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach H., Kirkpatrick J., Williams M., Colten H. (1985). Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. The Lancet 326 (8457), 686–688. 10.1016/s0140-6736(85)92929-0 [DOI] [PubMed] [Google Scholar]
- Balch W. E., Roth D. M., Hutt D. M. (2011). Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harb Perspect. Biol. 3 (2). 10.1101/cshperspect.a004499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmann M., Junge S., von der Hardt H. (2003). Low-dose methotrexate for advanced pulmonary disease in patients with cystic fibrosis. Respir. Med. 97 (5), 498–500. 10.1053/rmed.2002.1471 [DOI] [PubMed] [Google Scholar]
- Bardin P., Foussignière T., Rousselet N., Rebeyrol C., Porter J. C., Corvol H., et al. (2019). miR-636: a newly-identified actor for the regulation of pulmonary inflammation in cystic fibrosis. Front. Immunol. 10, 2643. 10.3389/fimmu.2019.02643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewska S., Kamysz W., Jakiela B., Sanak M., Króliczewski J., Bebok Z., et al. (2017). miR-200b downregulates CFTR during hypoxia in human lung epithelial cells. Cell Mol Biol Lett 22, 23. 10.1186/s11658-017-0054-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewski R., Rab A., Jurkuvenaite A., Mazur M., Wakefield J., Collawn J. F., et al. (2008). Activation of the unfolded protein response by ΔF508 CFTR. Am. J. Respir. Cel Mol Biol 39 (4), 448–457. 10.1165/rcmb.2008-0065oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharry S., Ackerley C., Corey M., Kent G., Heng Y.-M., Christensen H., et al. (2007). Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am. J. Physiology-Gastrointestinal Liver Physiol. 292 (3), G839–G848. 10.1152/ajpgi.00582.2005 [DOI] [PubMed] [Google Scholar]
- Berger S. L., Kouzarides T., Shiekhattar R., Shilatifard A. (2009). An operational definition of epigenetics. Genes Development 23 (7), 781–783. 10.1101/gad.1787609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerri V., Piacenza F., Caporelli N., Malavolta M., Provinciali M., Cipolli M. (2019). Is cellular senescence involved in cystic fibrosis? Respir. Res. 20 (1), 32. 10.1186/s12931-019-0993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhal G. K., Maguire S. A., Bowler I. M. (2001). Use of cyclosporin A as a steroid sparing agent in cystic fibrosis. Arch. Dis. Child. 84 (1), 89. 10.1136/adc.84.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Balakathiresan N. S., Dalgard C., Gutti U., Armistead D., Jozwik C., et al. (2011). Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J. Biol. Chem. 286 (13), 11604–11615. 10.1074/jbc.m110.198390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C., Hudson V. M., Hilton S. C., Wilde C. (2005). A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest 127 (1), 308–317. 10.1378/chest.127.1.308 [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Greider C. W., Szostak J. W. (2006). Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 12 (10), 1133–1138. 10.1038/nm1006-1133 [DOI] [PubMed] [Google Scholar]
- Bonfield T. L., Konstan M. W., Berger M. (1999). Altered respiratory epithelial cell cytokine production in cystic fibrosis☆☆☆★. J. Allergy Clin. Immunol. 104 (1), 72–78. 10.1016/s0091-6749(99)70116-8 [DOI] [PubMed] [Google Scholar]
- Borcherding D. C., Siefert M. E., Lin S., Brewington J., Sadek H., Clancy J. P., et al. (2019). Clinically approved CFTR modulators rescue Nrf2 dysfunction in cystic fibrosis airway epithelia. J. Clin. Invest. 129 (8), 3448–3463. 10.1172/jci96273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C. (2007). Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol. Med. 13 (6), 231–240. 10.1016/j.molmed.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Brown R. K., Kelly F. J. (1994). Evidence for increased oxidative damage in patients with cystic fibrosis. Pediatr. Res. 36 (4), 487–492. 10.1203/00006450-199410000-00013 [DOI] [PubMed] [Google Scholar]
- Brown R. K., McBurney A., Lunec J., Kelly F. J. (1995). Oxidative damage to DNA in patients with cystic fibrosis. Free Radic. Biol. Med. 18 (4), 801–806. 10.1016/0891-5849(94)00172-g [DOI] [PubMed] [Google Scholar]
- Chapman J., Fielder E., Passos J. F. (2019). Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 593 (13), 1566–1579. 10.1002/1873-3468.13498 [DOI] [PubMed] [Google Scholar]
- Chen T., Shen L., Yu J., Wan H., Guo A., Chen J., et al. (2011). Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 10 (5), 908–911. 10.1111/j.1474-9726.2011.00722.x [DOI] [PubMed] [Google Scholar]
- Chen Y., Armstrong D. A., Salas L. A., Hazlett H. F., Nymon A. B., Dessaint J. A., et al. (2018). Genome-wide DNA methylation profiling shows a distinct epigenetic signature associated with lung macrophages in cystic fibrosis. Clin. Epigenetics 10 (1), 152. 10.1186/s13148-018-0580-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., et al. (1990). Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63 (4), 827–834. 10.1016/0092-8674(90)90148-8 [DOI] [PubMed] [Google Scholar]
- Chmiel J. F., Konstan M. W., Knesebeck J. E., Hilliard J. B., Bonfield T. L., Dawson D. V., et al. (1999). IL-10 attenuates excessive inflammation in ChronicPseudomonasInfection in mice. Am. J. Respir. Crit. Care Med. 160 (6), 2040–2047. 10.1164/ajrccm.160.6.9901043 [DOI] [PubMed] [Google Scholar]
- Cho S. J., Stout-Delgado H. W. (2020). Aging and lung disease. Annu. Rev. Physiol. 82, 433–459. 10.1146/annurev-physiol-021119-034610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua F., Laurent G. J. (2006). Neutrophil elastase: mediator of extracellular matrix destruction and accumulation. Proc. Am. Thorac. Soc. 3 (5), 424–427. 10.1513/pats.200603-078aw [DOI] [PubMed] [Google Scholar]
- Ciofu O., Lykkesfeldt J., Lykkesfeldt J. (2019). Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database Syst. Rev. 10, CD007020. 10.1002/14651858.CD007020.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C., Lymp J., Thompson V., Dunn C., Davies Z., Chatfield B., et al. (2015). Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J. Cystic Fibrosis 14 (2), 219–227. 10.1016/j.jcf.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Danahay H. L., Lilley S., Fox R., Charlton H., Sabater J., Button B., et al. (2020). TMEM16A potentiation: a novel therapeutic approach for the treatment of cystic fibrosis. Am. J. Respir. Crit. Care Med. 201 (8), 946–954. 10.1164/rccm.201908-1641oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. C., Moskowitz S. M., Brown C., Horsley A., Mall M. A., McKone E. F., et al. (2018). VX-659-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 379 (17), 1599–1611. 10.1056/nejmoa1807119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boeck K., Vermeulen F., Wanyama S., Thomas M. (2011). Inhaled corticosteroids and lower lung function decline in young children with cystic fibrosis. Eur. Respir. J. 37 (5), 1091–1095. 10.1183/09031936.00077210 [DOI] [PubMed] [Google Scholar]
- De Santi C., Fernández Fernández E., Gaul R., Vencken S., Glasgow A., Oglesby I. K., et al. (2020). Precise targeting of miRNA sites restores CFTR activity in CF bronchial epithelial cells. Mol. Ther. 28 (4), 1190–1199. 10.1016/j.ymthe.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J. A., Lewis P. A., Stanton M., Wilsher J. (2007). Cystic fibrosis mortality and survival in the UK: 1947-2003. Eur. Respir. J. 29 (3), 522–526. 10.1183/09031936.00099506 [DOI] [PubMed] [Google Scholar]
- dos Santos G., Kutuzov M. A., Ridge K. M. (2012). The inflammasome in lung diseases. Am. J. Physiology-Lung Cell Mol. Physiol. 303 (8), L627–L633. 10.1152/ajplung.00225.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunzendorfer S., Rothbucher D., Schratzberger P., Reinisch N., Kähler C. M., Wiedermann C. J. (1997). Mevalonate-dependent inhibition of transendothelial migration and chemotaxis of human peripheral blood neutrophils by pravastatin. Circ. Res. 81 (6), 963–969. 10.1161/01.res.81.6.963 [DOI] [PubMed] [Google Scholar]
- Durieu I., Peyrol S., Gindre D., Bellon G., Durand D. V., Pacheco Y. (1998). Subepithelial fibrosis and degradation of the bronchial extracellular matrix in cystic fibrosis. Am. J. Respir. Crit. Care Med. 158 (2), 580–588. 10.1164/ajrccm.158.2.9707126 [DOI] [PubMed] [Google Scholar]
- Ehre C., Rushton Z. L., Wang B., Hothem L. N., Morrison C. B., Fontana N. C., et al. (2019). An improved inhaled mucolytic to treat airway muco-obstructive diseases. Am. J. Respir. Crit. Care Med. 199 (2), 171–180. 10.1164/rccm.201802-0245oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigen H., Rosenstein B. J., FitzSimmons S., Schidlow D. V. (1995). A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. J. Pediatr. 126 (4), 515–523. 10.1016/s0022-3476(95)70343-8 [DOI] [PubMed] [Google Scholar]
- Elborn J. S. (2016). Cystic fibrosis. The Lancet 388 (10059), 2519–2531. 10.1016/s0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- Esposito S., Tosco A., Villella V. R., Raia V., Kroemer G., Maiuri L. (2016). Manipulating proteostasis to repair the F508del-CFTR defect in cystic fibrosis. Mol. Cel Pediatr 3 (1), 13. 10.1186/s40348-016-0040-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts S., Lammertyn E., Martens D. S., Sadeleer L. D., Maes K., Goldschmeding R., et al. (2018). The aging lung: tissue telomere shortening in health and disease. Respir. Res. 19 (1), 95. 10.1186/s12931-018-0794-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti S., Bonneau O., Dubois G. R., Jones C. E., Trifilieff A. (2003). IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170 (4), 2106–2112. 10.4049/jimmunol.170.4.2106 [DOI] [PubMed] [Google Scholar]
- Fink A. K., Yanik E. L., Marshall B. C., Wilschanski M., Lynch C. F., Austin A. A., et al. (2017). Cancer risk among lung transplant recipients with cystic fibrosis. J. Cystic Fibrosis 16 (1), 91–97. 10.1016/j.jcf.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B. M., Wong J. K., Degan S., Kummarapurugu A. B., Zheng S., Haridass P., et al. (2013). Increased expression of senescence markers in cystic fibrosis airways. Am. J. Physiology-Lung Cell Mol. Physiol. 304 (6), L394–L400. 10.1152/ajplung.00091.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B. M., Zheng S., Fan R., Voynow J. A. (2007). Neutrophil elastase inhibition of cell cycle progression in airway epithelial cells in vitro is mediated by p27kip1. Am. J. Physiology-Lung Cell Mol. Physiol. 293 (3), L762–L768. 10.1152/ajplung.00067.2007 [DOI] [PubMed] [Google Scholar]
- Flume P. A., O'Sullivan B. P., Robinson K. A., Goss C. H., Mogayzel P. J., Willey-Courand D. B., et al. (2007). Cystic fibrosis pulmonary guidelines. Am. J. Respir. Crit. Care Med. 176 (10), 957–969. 10.1164/rccm.200705-664oc [DOI] [PubMed] [Google Scholar]
- Franceschi C., Campisi J. (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Journals Gerontol. Ser. A: Biol. Sci. Med. Sci. 69 (Suppl. 1), S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Freedman S. D., Katz M. H., Parker E. M., Laposata M., Urman M. Y., Alvarez J. G. (1999). A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr-/- mice. Proc. Natl. Acad. Sci. 96 (24), 13995–14000. 10.1073/pnas.96.24.13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A., Hector A., Bratcher P. E., Mall M. A., Griese M., Hartl D. (2011). The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur. Respir. J. 38 (3), 721–727. 10.1183/09031936.00173210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A., Li Y., Weathington N., Winkler M., Kong M., Jackson P., et al. (2007). Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am. J. Physiology-Lung Cell Mol. Physiol. 293 (1), L96–L104. 10.1152/ajplung.00492.2006 [DOI] [PubMed] [Google Scholar]
- Gonzalo S. (1985). Epigenetic alterations in aging. J. Appl. Physiol. 109 (2), 586–597. 10.1152/japplphysiol.00238.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenjak V., Petrelis A. M., Stathopoulou M. G., Visvikis-Siest S. (2020). Telomere length determinants in childhood. Clin. Chem. Lab. Med. 58 (2), 162–177. 10.1515/cclm-2019-0235 [DOI] [PubMed] [Google Scholar]
- Grasemann H., Grasemann C., Kurtz F., Tietze-Schillings G., Vester U., Ratjen F. (2005a). Oral L-arginine supplementation in cystic fibrosis patients: a placebo-controlled study. Eur. Respir. J. 25 (1), 62–68. 10.1183/09031936.04.00086104 [DOI] [PubMed] [Google Scholar]
- Grasemann H., Schwiertz R., Matthiesen S., Racké K., Ratjen F. (2005b). Increased arginase activity in cystic fibrosis airways. Am. J. Respir. Crit. Care Med. 172 (12), 1523–1528. 10.1164/rccm.200502-253oc [DOI] [PubMed] [Google Scholar]
- Griese M., Latzin P., Kappler M., Weckerle K., Heinzlmaier T., Bernhardt T., et al. (2007). alpha1-Antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur. Respir. J. 29 (2), 240–250. 10.1183/09031936.00047306 [DOI] [PubMed] [Google Scholar]
- Grimbert D., Vecellio L., Delépine P., Attucci S., Boissinot E., Poncin A., et al. (2003). Characteristics of EPI-hNE4 aerosol: a new elastase inhibitor for treatment of cystic fibrosis. J. Aerosol Med. 16 (2), 121–129. 10.1089/089426803321919889 [DOI] [PubMed] [Google Scholar]
- Hallows K. R., Raghuram V., Kemp B. E., Witters L. A., Foskett J. K. (2000). Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Invest. 105 (12), 1711–1721. 10.1172/jci9622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. T., Muhlebach M. S., Oster R. A., Knowles M. R., Noah T. L. (2009). Transforming growth factor-β1 in bronchoalveolar lavage fluid from children with cystic fibrosis. Pediatr. Pulmonol. 44 (11), 1057–1064. 10.1002/ppul.21079 [DOI] [PubMed] [Google Scholar]
- Hartl D., Starosta V., Maier K., Beck-Speier I., Rebhan C., Becker B. F., et al. (2005). Inhaled glutathione decreases PGE2 and increases lymphocytes in cystic fibrosis lungs. Free Radic. Biol. Med. 39 (4), 463–472. 10.1016/j.freeradbiomed.2005.03.032 [DOI] [PubMed] [Google Scholar]
- Haydar D., Cory T. J., Birket S. E., Murphy B. S., Pennypacker K. R., Sinai A. P., et al. (2019). Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-κB signaling pathways. J.I. 203 (4), 1021–1030. 10.4049/jimmunol.1801228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt G., Jurk D., Marques F. D. M., Correia-Melo C., Hardy T., Gackowska A., et al. (2012). Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 3, 708. 10.1038/ncomms1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard T. N., Regamey N., Shute J. K., Nicholson A. G., Alton E. W. F. W., Bush A., et al. (2007). Airway remodelling in children with cystic fibrosis. Thorax 62 (12), 1074–1080. 10.1136/thx.2006.074641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisert K. B., Heltshe S. L., Pope C., Jorth P., Wu X., Edwards R. M., et al. (2017). Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am. J. Respir. Crit. Care Med. 195 (12), 1617–1628. 10.1164/rccm.201609-1954oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson M. E., Simmonds N. J., Warwick W. J., Tullis E., Castellani C., Assael B., et al. (2008). An international/multicentre report on patients with cystic fibrosis (CF) over the age of 40 years. J. Cystic Fibrosis 7 (6), 537–542. 10.1016/j.jcf.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Hogan B. L. M., Barkauskas C. E., Chapman H. A., Epstein J. A., Jain R., Hsia C. C. W., et al. (2014). Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15 (2), 123–138. 10.1016/j.stem.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horati H., Janssens H. M., Margaroli C., Veltman M., Stolarczyk M., Kilgore M. B., et al. (2020). Airway profile of bioactive lipids predicts early progression of lung disease in cystic fibrosis. J. Cystic Fibrosis 19 (6), 902–909. 10.1016/j.jcf.2020.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M. J., Treharne K. J., Winter AK, Cassidy D. M., Land S. (2010). Expression of wild-type CFTR suppresses NF-kappaB-driven inflammatory signalling. PLoS One 5 (7), e11598. 10.1371/journal.pone.0011598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz-Griffiths H. H., Scambler T., Wong C. H., Lara-Reyna S., Holbrook J., Martinon F., et al. (2020). Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife 9. 10.7554/elife.54556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan J. C., Ferreira M., Sedivy J. M., Herbig U. (2007). Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Development 128 (1), 36–44. 10.1016/j.mad.2006.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. J., Goodman S. J., Kobor M. S. (2015). DNA methylation and healthy human aging. Aging Cell 14 (6), 924–932. 10.1111/acel.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D., Wilson C., Passos J. F., Oakley F., Correia-Melo C., Greaves L., et al. (2014). Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2, 4172. 10.1038/ncomms5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating D., Marigowda G., Burr L., Daines C., Mall M. A., McKone E. F., et al. (2018). VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 379 (17), 1612–1620. 10.1056/nejmoa1807120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J. (2010). The genetics of ageing. Nature 464 (7288), 504–512. 10.1038/nature08980 [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J., Buchanan J., Markiewicz D., Cox T., Chakravarti A., et al. (1989). Identification of the cystic fibrosis gene: genetic analysis. Science 245 (4922), 1073–1080. 10.1126/science.2570460 [DOI] [PubMed] [Google Scholar]
- Konstan Mw D. G., Lands L. C., Hilliard K. A., Koker P., Bhattacharya S., Staab A., et al. (2005). Results of a phase II clinical trial of BIIL 284 BS (a LTB4 receptor antagonist) for the treatment of CF lung disease. Pediatr. Pulmonol 40, 125–127. [Google Scholar]
- Konstan M. W., Krenicky J. E., Hilliard K. A., Hilliard J. B. (2009). A pilot study evaluating the effect of pioglitazone, simvastatin and ibuprofen on neutrophil migration in vivo in healthy subjects. Pediatr. Pulmonol 44, 289–290. [Google Scholar]
- Konstan M. W., Byard P. J., Hoppel C. L., Davis P. B. (1995). Effect of high-dose ibuprofen in patients with cystic fibrosis. N. Engl. J. Med. 332 (13), 848–854. 10.1056/nejm199503303321303 [DOI] [PubMed] [Google Scholar]
- Konstan M. W., Krenicky J. E., Finney M. R., Kirchner H. L., Hilliard K. A., Hilliard J. B., et al. (2003). Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J. Pharmacol. Exp. Ther. 306 (3), 1086–1091. 10.1124/jpet.103.052449 [DOI] [PubMed] [Google Scholar]
- Konstan M. W., Schluchter M. D., Xue W., Davis P. B. (2007). Clinical use of ibuprofen is associated with slower FEV1Decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 176 (11), 1084–1089. 10.1164/rccm.200702-181oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan M. W., Walenga R. W., Hilliard K. A., Hilliard J. B. (1993). Leukotriene B4Markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am. Rev. Respir. Dis. 148 (4 Pt 1), 896–901. 10.1164/ajrccm/148.4_pt_1.896 [DOI] [PubMed] [Google Scholar]
- Kopp B. T., Fitch J., Jaramillo L., Shrestha C. L., Robledo-Avila F., Zhang S., et al. (2020). Whole-blood transcriptomic responses to lumacaftor/ivacaftor therapy in cystic fibrosis. J. Cystic Fibrosis 19 (2), 245–254. 10.1016/j.jcf.2019.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynack N. C., Corey D. A., Elmer H. L., Kelley T. J. (2002). Mechanisms of NOS2 regulation by Rho GTPase signaling in airway epithelial cells. Am. J. Physiology-Lung Cell Mol. Physiol. 283 (3), L604–L611. 10.1152/ajplung.00459.2001 [DOI] [PubMed] [Google Scholar]
- Lammertyn E. M. D., Colpaert K., Goeminne P., Proesmans M., Vanaudenaerde B., Nawrot T., et al. (2017). The association between leukocyte telomere length, telomere attrition and disease severity in cystic fibrosis patients. Eur. Respir. J., OA4402. [Google Scholar]
- Lands L. C., Milner R., Cantin A. M., Manson D., Corey M. (2007). High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J. Pediatr. 151 (3), 249–254. 10.1016/j.jpeds.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Lara-Reyna S., Holbrook J., Jarosz-Griffiths H. H., Peckham D., McDermott M. F. (2020). Dysregulated signalling pathways in innate immune cells with cystic fibrosis mutations. Cell. Mol. Life Sci. 77 (22), 4485–4503. 10.1007/s00018-020-03540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gars M., Descamps D., Roussel D., Saussereau E., Guillot L., Ruffin M., et al. (2013). Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel FunctionIn VitroandIn vivo. Am. J. Respir. Crit. Care Med. 187 (2), 170–179. 10.1164/rccm.201205-0875oc [DOI] [PubMed] [Google Scholar]
- Levine M. E., Lu A. T., Quach A., Chen B. H., Assimes T. L., Bandinelli S., et al. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging 10 (4), 573–591. 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang J. T., Jiang X., Shi X., Shen J., Feng F., et al. (2015). The cystic fibrosis transmembrane conductance regulator as a biomarker in non-small cell lung cancer. Int. J. Oncol. 46 (5), 2107–2115. 10.3892/ijo.2015.2921 [DOI] [PubMed] [Google Scholar]
- Linsdell P., Hanrahan J. W. (1998). Glutathione permeability of CFTR. Am. J. Physiology-Cell Physiol. 275 (1), C323–C326. 10.1152/ajpcell.1998.275.1.c323 [DOI] [PubMed] [Google Scholar]
- Lopes-Pacheco M., Boinot C., Sabirzhanova I., Morales M. M., Guggino W. B., Cebotaru L. (2015). Combination of correctors rescue df508-CFTR by reducing its association with Hsp40 and Hsp27. J. Biol. Chem. 290 (42), 25636–25645. 10.1074/jbc.m115.671925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Pacheco M., Sabirzhanova I., Rapino D., Morales M. M., Guggino W. B., Cebotaru L. (2016). Correctors rescue CFTR mutations in nucleotide-binding domain 1 (NBD1) by modulating proteostasis. Chembiochem 17 (6), 493–505. 10.1002/cbic.201500620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. (2013). The hallmarks of aging. Cell 153 (6), 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani A., Villella V. R., Esposito S., Gavina M., Russo I., Silano M., et al. (2012). Targeting autophagy as a novel strategy for facilitating the therapeutic action of potentiators on ΔF508 cystic fibrosis transmembrane conductance regulator. Autophagy 8 (11), 1657–1672. 10.4161/auto.21483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani A., Villella V. R., Vasaturo A., Giardino I., Raia V., Pettoello-Mantovani M., et al. (2009). SUMOylation of tissue transglutaminase as link between oxidative stress and inflammation. J. Immunol. 183 (4), 2775–2784. 10.4049/jimmunol.0900993 [DOI] [PubMed] [Google Scholar]
- Ly K., Smith M. P., Thibodeaux Q., Reddy V., Liao W., Bhutani T. (2019). Anti IL-17 in psoriasis. Expert Rev. Clin. Immunol. 15 (11), 1185–1194. 10.1080/1744666x.2020.1679625 [DOI] [PubMed] [Google Scholar]
- MacKenzie T., Gifford A. H., Sabadosa K. A., Quinton H. B., Knapp E. A., Goss C. H., et al. (2014). Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann. Intern. Med. 161 (4), 233–241. 10.7326/m13-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães M., Tost J., Pineau F., Rivals I., Busato F., Alary N., et al. (2018). Dynamic changes of DNA methylation and lung disease in cystic fibrosis: lessons from a monogenic disease. Epigenomics 10 (8), 1131–1145. 10.2217/epi-2018-0005 [DOI] [PubMed] [Google Scholar]
- Maisonneuve P., Marshall B. C., Knapp E. A., Lowenfels A. B. (2013). Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J. Natl. Cancer Inst. 105 (2), 122–129. 10.1093/jnci/djs481 [DOI] [PubMed] [Google Scholar]
- Mall M., Grubb B. R., Harkema J. R., O'Neal W. K., Boucher R. C. (2004). Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 10 (5), 487–493. 10.1038/nm1028 [DOI] [PubMed] [Google Scholar]
- Manzanares D., Krick S., Baumlin N., Dennis J. S., Tyrrell J., Tarran R., et al. (2015). Airway surface dehydration by transforming growth factor β (TGF-β) in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can Be rescued by the drug pirfenidone. J. Biol. Chem. 290 (42), 25710–25716. 10.1074/jbc.m115.670885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R., Lithgow G. J., Link W. (2016). Long live FOXO : unraveling the role of FOXO proteins in aging and longevity. Aging Cell 15 (2), 196–207. 10.1111/acel.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Grubb B. R., Tarran R., Randell S. H., Gatzy J. T., Davis C. W., et al. (1998). Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95 (7), 1005–1015. 10.1016/s0092-8674(00)81724-9 [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Blohmke C. J., Falsafi R., Fjell C. D., Madera L., Turvey S. E., et al. (2013). Rescue of dysfunctional autophagy attenuates hyperinflammatory responses from cystic fibrosis cells. J.I. 190 (3), 1227–1238. 10.4049/jimmunol.1201404 [DOI] [PubMed] [Google Scholar]
- McCuaig S., Martin J. G. (2013). How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: implications for airway hyper-responsiveness and asthma in cystic fibrosis. Lancet Respir. Med. 1 (2), 137–147. 10.1016/s2213-2600(12)70058-9 [DOI] [PubMed] [Google Scholar]
- McElvaney N. G., Doujaiji B., Moan M. J., Burnham M. R., Wu M. C., Crystal R. G. (1993). Pharmacokinetics of recombinant secretory leukoprotease inhibitor aerosolized to normals and individuals with cystic fibrosis. Am. Rev. Respir. Dis. 148 (4 Pt 1), 1056–1060. 10.1164/ajrccm/148.4_pt_1.1056 [DOI] [PubMed] [Google Scholar]
- McElvaney N. G., Hubbard R. C., Birrer P., Crystal R. G., Chernick M. S., Frank M. M., et al. (1991). Aerosol α1 -antitrypsin treatment for cystic fibrosis. The Lancet 337 (8738), 392–394. 10.1016/0140-6736(91)91167-s [DOI] [PubMed] [Google Scholar]
- McElvaney O. J., Zaslona Z., Becker-Flegler K., Palsson-McDermott E. M., Boland F., Gunaratnam C., et al. (2019). Specific inhibition of the NLRP3 inflammasome as an antiinflammatory strategy in cystic fibrosis. Am. J. Respir. Crit. Care Med. 200 (11), 1381–1391. 10.1164/rccm.201905-1013oc [DOI] [PubMed] [Google Scholar]
- Meiners S., Eickelberg O., Königshoff M. (2015). Hallmarks of the ageing lung. Eur. Respir. J. 45 (3), 807–827. 10.1183/09031936.00186914 [DOI] [PubMed] [Google Scholar]
- Meyer K. C., Rosenthal N. S., Soergel P., Peterson K. (1998). Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech. Ageing Development 104 (2), 169–181. 10.1016/s0047-6374(98)00065-7 [DOI] [PubMed] [Google Scholar]
- Middleton P. G., Mall M. A., Dřevínek P., Lands L. C., McKone E. F., Polineni D., et al. (2019). Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 381 (19), 1809–1819. 10.1056/nejmoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingione A., Dei Cas M., Bonezzi F., Caretti A., Piccoli M., Anastasia L., et al. (2020). Inhibition of sphingolipid synthesis as a phenotype-modifying therapy in cystic fibrosis. Cell Physiol Biochem 54 (1), 110–125. 10.33594/000000208 [DOI] [PubMed] [Google Scholar]
- Montanini L., Smerieri A., Gullì M., Cirillo F., Pisi G., Sartori C., et al. (2016). miR-146a, miR-155, miR-370, and miR-708 are CFTR-dependent, predicted FOXO1 regulators and change at onset of CFRDs. J. Clin. Endocrinol. Metab. 101 (12), 4955–4963. 10.1210/jc.2016-2431 [DOI] [PubMed] [Google Scholar]
- Mora A. L., Rojas M. (2013). Adult stem cells for chronic lung diseases. Respirology 18 (7), 1041–1046. 10.1111/resp.12112 [DOI] [PubMed] [Google Scholar]
- Moskalev A. A., Shaposhnikov M. V., Plyusnina E. N., Zhavoronkov A., Budovsky A., Yanai H., et al. (2013). The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 12 (2), 661–684. 10.1016/j.arr.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Moss R. B., Mayer-Hamblett N., Wagener J., Daines C., Hale K., Ahrens R., et al. (2005). Randomized, double-blind, placebo-controlled, dose-escalating study of aerosolized interferon gamma-1b in patients with mild to moderate cystic fibrosis lung disease. Pediatr. Pulmonol. 39 (3), 209–218. 10.1002/ppul.20152 [DOI] [PubMed] [Google Scholar]
- Moss R. B., Mistry S. J., Konstan M. W., Pilewski J. M., Kerem E., Tal-Singer R., et al. (2013). Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J. Cystic Fibrosis 12 (3), 241–248. 10.1016/j.jcf.2012.08.016 [DOI] [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., McElvaney N. G., Crystal R. G. (1992). Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Invest. 89 (5), 1478–1484. 10.1172/jci115738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor R. M., Baker D. J., van Deursen J. M. (2013). Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin. Pharmacol. Ther. 93 (1), 105–116. 10.1038/clpt.2012.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D., Chmiel J., Berger M. (2008). Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clinic Rev. Allerg Immunol. 34 (2), 146–162. 10.1007/s12016-007-8039-9 [DOI] [PubMed] [Google Scholar]
- Nichols D. P., Odem-Davis K., Cogen J. D., Goss C. H., Ren C. L., Skalland M., et al. (2020). Pulmonary outcomes associated with long-term Azithromycin therapy in cystic fibrosis. Am. J. Respir. Crit. Care Med. 201 (4), 430–437. 10.1164/rccm.201906-1206oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oermann C. M. K. M., Wheeler C Cumming. S. (2007). A pilot study evaluating the potential use of low-dose methotrexate as an anti-inflammatory therapy for cystic fibrosis lung disease. Pediatr. Pulmonol 42, 292–293. [Google Scholar]
- Oh J., Lee Y. D., Wagers A. J. (2014). Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20 (8), 870–880. 10.1038/nm.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow S., Bamberger C., Calzolari D., Martínez-Bartolomé S., Lavallée-Adam M., Balch W. E., et al. (2015). ∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature 528 (7583), 510–516. 10.1038/nature15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P., Wicher S., Khandalavala K., Pabelick C. M., Britt R. D., Prakash Y. S. (2019). Cellular senescence in the lung across the age spectrum. Am. J. Physiology-Lung Cell Mol. Physiol. 316 (5), L826–L842. 10.1152/ajplung.00424.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Rossi D., Peterson M., Smith K., Sikström K., White E. S., et al. (2014). Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Invest. 124 (4), 1622–1635. 10.1172/jci71386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A., van Heeckeren A. M., Nichols D., Gupta S., Eastman J. F., Davis P. B. (2008). Peroxisome proliferator-activated receptor-γ in cystic fibrosis lung epithelium. Am. J. Physiology-Lung Cell Mol. Physiol. 295 (2), L303–L313. 10.1152/ajplung.90276.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson-Carmichael S. L., Harris W. T., Goel R., Noah T. L., Johnson R., Leigh M. W., et al. (2009). Association of lower airway inflammation with physiologic findings in young children with cystic fibrosis. Pediatr. Pulmonol. 44 (5), 503–511. 10.1002/ppul.21044 [DOI] [PubMed] [Google Scholar]
- Petit-Bertron A.-F., Tabary O., Corvol H., Jacquot J., Clément A., Cavaillon J.-M., et al. (2008). Circulating and airway neutrophils in cystic fibrosis display different TLR expression and responsiveness to interleukin-10. Cytokine 41 (1), 54–60. 10.1016/j.cyto.2007.10.012 [DOI] [PubMed] [Google Scholar]
- Prakash Y. S., Pabelick C. M., Sieck G. C. (2017). Mitochondrial dysfunction in airway disease. Chest 152 (3), 618–626. 10.1016/j.chest.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regamey N., Jeffery P. K., Alton E. W. F. W., Bush A., Davies J. C. (2011). Airway remodelling and its relationship to inflammation in cystic fibrosis. Thorax 66 (7), 624–629. 10.1136/thx.2009.134106 [DOI] [PubMed] [Google Scholar]
- Reilly R., Mroz M. S., Dempsey E., Wynne K., Keely S. J., McKone E. F., et al. (2017). Targeting the PI3K/Akt/mTOR signalling pathway in Cystic Fibrosis. Sci. Rep. 7 (1), 7642. 10.1038/s41598-017-06588z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C. L., Pasta D. J., Rasouliyan L., Wagener J. S., Konstan M. W., Morgan W. J. (2008). Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. J. Pediatr. 153 (6), 746–751. 10.1016/j.jpeds.2008.07.010 [DOI] [PubMed] [Google Scholar]
- Rimessi A., Bezzerri V., Patergnani S., Marchi S., Cabrini G., Pinton P. (2015). Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 6, 6201. 10.1038/ncomms7201 [DOI] [PubMed] [Google Scholar]
- Robertson K. D. (2005). DNA methylation and human disease. Nat. Rev. Genet. 6 (8), 597–610. 10.1038/nrg1655 [DOI] [PubMed] [Google Scholar]
- Roum J. H., Buhl R., McElvaney N. G., Borok Z., Crystal R. G. (1985). Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 75 (6), 2419–2424. 10.1152/jappl.1993.75.6.24191993 [DOI] [PubMed] [Google Scholar]
- Rowe S. M., Daines C., Ringshausen F. C., Kerem E., Wilson J., Tullis E., et al. (2017). Tezacaftor-Ivacaftor in residual-function heterozygotes with cystic fibrosis. N. Engl. J. Med. 377 (21), 2024–2035. 10.1056/nejmoa1709847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe S. M., Heltshe S. L., Gonska T., Donaldson S. H., Borowitz D., Gelfond D., et al. (2014). Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 190 (2), 175–184. 10.1164/rccm.201404-0703oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X. Z., Moorhead J. F., Fernando R., Wheeler D. C., Powis S. H., Varghese Z. (2003). PPAR agonists protect mesangial cells from interleukin 1 -induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J. Am. Soc. Nephrol. 14 (3), 593–600. 10.1097/01.asn.0000050414.52908.da [DOI] [PubMed] [Google Scholar]
- Sagel S. D., Sontag M. K., Wagener J. S., Kapsner R. K., Osberg I., Accurso F. J. (2002). Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J. Pediatr. 141 (6), 811–817. 10.1067/mpd.2002.129847 [DOI] [PubMed] [Google Scholar]
- Scambler T., Jarosz-Griffiths H. H., Lara-Reyna S., Pathak S., Wong C., Holbrook J., et al. (2019). ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. Elife 8. 10.7554/elife.49248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M., De Sario A. (2020). DNA methylation changes in cystic fibrosis: cause or consequence? Clin. Genet. 98 (1), 3–9. 10.1111/cge.13731 [DOI] [PubMed] [Google Scholar]
- Scott P., Anderson K., Singhania M., Cormier R. (2020). Cystic fibrosis, CFTR, and colorectal cancer. Int. J. Mol. Sci. 21 (8). 10.3390/ijms21082891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler F., Hellberg J., Lepper P. M., Kamyschnikow A., Herr C., Bischoff M., et al. (2013). FOXO transcription factors regulate innate immune mechanisms in respiratory epithelial cells. J.I. 190 (4), 1603–1613. 10.4049/jimmunol.1200596 [DOI] [PubMed] [Google Scholar]
- Simmonds N. J., Cullinan P., Hodson M. E. (2009). Growing old with cystic fibrosis - the characteristics of long-term survivors of cystic fibrosis. Respir. Med. 103 (4), 629–635. 10.1016/j.rmed.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Sirinupong N., Yang Z. (2015). Epigenetics in cystic fibrosis: epigenetic targeting of a genetic disease. Cdt 16 (9), 976–987. 10.2174/1389450116666150416114514 [DOI] [PubMed] [Google Scholar]
- Skov M., Pressler T., Lykkesfeldt J., Poulsen H. E., Jensen P. Ø., Johansen H. K., et al. (2015). The effect of short-term, high-dose oral N-acetylcysteine treatment on oxidative stress markers in cystic fibrosis patients with chronic P. aeruginosa infection - a pilot study. J. Cystic Fibrosis 14 (2), 211–218. 10.1016/j.jcf.2014.09.015 [DOI] [PubMed] [Google Scholar]
- Smerieri A., Montanini L., Maiuri L., Bernasconi S., Street M. (2014). FOXO1 content is reduced in cystic fibrosis and increases with IGF-I treatment. Ijms 15 (10), 18000–18022. 10.3390/ijms151018000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass S. M., Cihil K. M., Cornuet P. K., Myerburg M. M., Swiatecka-Urban A. (2013). Tgf-beta1 inhibits Cftr biogenesis and prevents functional rescue of DeltaF508-Cftr in primary differentiated human bronchial epithelial cells. PLoS One 8 (5), e63167. 10.1371/journal.pone.0063167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys J., Bonfield T., Chmiel J., Berger M. (2002). Functional IL-10 deficiency in the lung of cystic fibrosis (cftr−/−) and IL-10 knockout mice causes increased expression and function of B7 costimulatory molecules on alveolar macrophages. J. Immunol. 168 (4), 1903–1910. 10.4049/jimmunol.168.4.1903 [DOI] [PubMed] [Google Scholar]
- Starosta V., Rietschel E., Paul K., Baumann U., Griese M. (2006). Oxidative changes of bronchoalveolar proteins in cystic fibrosis. Chest 129 (2), 431–437. 10.1378/chest.129.2.431 [DOI] [PubMed] [Google Scholar]
- Strub M. D., McCray P. B., Jr. (2020). Transcriptomic and proteostasis networks of CFTR and the development of small molecule modulators for the treatment of cystic fibrosis lung disease. Genes (Basel) 11 (5). 10.3390/genes11050546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Harris W. T., Kortyka S., Kotha K., Ostmann A. J., Rezayat A., et al. (2014). Tgf-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PLoS One 9 (9), e106842. 10.1371/journal.pone.0106842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taams L. S. (2020). Interleukin-17 in rheumatoid arthritis: trials and tribulations. J. Exp. Med. 217 (3). 10.1084/jem.20192048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Cousar J. L., Munck A., McKone E. F., van der Ent C. K., Moeller A., Simard C., et al. (2017). Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 377 (21), 2013–2023. 10.1056/nejmoa1709846 [DOI] [PubMed] [Google Scholar]
- Teramura T., Onodera Y. (2018). Stem cell depletion by inflammation-associated miR-155. Aging 10 (1), 17–18. 10.18632/aging.101374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than B. L., Linnekamp J. F., Starr T. K., Largaespada D. A., Rod A., Zhang Y., et al. (2016). CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene 35 (32), 4179–4187. 10.1038/onc.2015.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirouvanziam R., Conrad C. K., Bottiglieri T., Herzenberg L. A., Moss R. B., Herzenberg L. A. (2006). High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. 103 (12), 4628–4633. 10.1073/pnas.0511304103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosco A., De Gregorio F., Esposito S., De Stefano D., Sana I., Ferrari E., et al. (2016). A novel treatment of cystic fibrosis acting on-target: cysteamine plus epigallocatechin gallate for the autophagy-dependent rescue of class II-mutated CFTR. Cell Death Differ 23 (8), 1380–1393. 10.1038/cdd.2016.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui L.-C. (1995). The cystic fibrosis transmembrane conductance regulator gene. Am. J. Respir. Crit. Care Med. 151 (3 Pt 2), S47–S53. 10.1164/ajrccm/151.3_pt_2.s47 [DOI] [PubMed] [Google Scholar]
- Tu Z., Chen Q., Zhang J. T., Jiang X., Xia Y., Chan H. C. (2016). CFTR is a potential marker for nasopharyngeal carcinoma prognosis and metastasis. Oncotarget 7 (47), 76955–76965. 10.18632/oncotarget.12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivieso A. G., Clauzure M., Marín M. C., Taminelli G. L., Massip Copiz M. M., Sánchez F., et al. (2012). The mitochondrial complex I activity is reduced in cells with impaired cystic fibrosis transmembrane conductance regulator (CFTR) function. PLoS One 7 (11), e48059. 10.1371/journal.pone.0048059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Biervliet S., Devos M., Delhaye T., Van Biervliet J. P., Robberecht E., Christophe A. (2008). Oral DHA supplementation in ΔF508 homozygous cystic fibrosis patients. Prostaglandins, Leukot. Essent. Fatty Acids 78 (2), 109–115. 10.1016/j.plefa.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Van Goor F., Hadida S., Grootenhuis P. D. J., Burton B., Stack J. H., Straley K. S., et al. (2011). Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. 108 (46), 18843–18848. 10.1073/pnas.1105787108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T. (2009). Autophagy genes and ageing. Cel Death Differ 16 (1), 94–102. 10.1038/cdd.2008.126 [DOI] [PubMed] [Google Scholar]
- Velsor L. W., Kariya C., Kachadourian R., Day B. J. (2006). Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am. J. Respir. Cel Mol Biol 35 (5), 579–586. 10.1165/rcmb.2005-0473oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velsor L. W., van Heeckeren A., Day B. J. (2001). Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am. J. Physiology-Lung Cell Mol. Physiol. 281 (1), L31–L38. 10.1152/ajplung.2001.281.1.l31 [DOI] [PubMed] [Google Scholar]
- Vencken S., Foged C., Ramsey J. M., Sweeney L., Cryan S. A., MacLoughlin R. J., et al. (2019). Nebulised lipid-polymer hybrid nanoparticles for the delivery of a therapeutic anti-inflammatory microRNA to bronchial epithelial cells. ERJ Open Res. 5 (2). 10.1183/23120541.00161-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella V. R., Esposito S., Bruscia E. M., Vicinanza M., Cenci S., Guido S., et al. (2013). Disease-relevant proteostasis regulation of cystic fibrosis transmembrane conductance regulator. Cel Death Differ 20 (8), 1101–1115. 10.1038/cdd.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright C. E., Elborn J. S., Ramsey B. W., Marigowda G., Huang X., Cipolli M., et al. (2015). Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 373 (3), 220–231. 10.1056/nejmoa1409547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelvyte I., Dominaitiene R., Crisby M., Janciauskiene S. (2002). Modulation of inflammatory mediators and pparγand nfκb expression by pravastatin in response to lipoproteins in human monocytes in vitro . Pharmacol. Res. 45 (2), 147–154. 10.1006/phrs.2001.0922 [DOI] [PubMed] [Google Scholar]
- Zingarelli B., Sheehan M., Hake P. W., O’Connor M., Denenberg A., Cook J. A. (2003). Peroxisome proliferator activator receptor-γ ligands, 15-deoxy-d12,14-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J. Immunol. 171 (12), 6827–6837. 10.4049/jimmunol.171.12.6827 [DOI] [PubMed] [Google Scholar]