Abstract
The majority of Alzheimer’s disease (AD) risk genes are highly and selectively expressed by microglia in the brain. Several of these genes are related to lipid and cholesterol metabolism, lipid synthesis, lipid transport, endocytosis, exocytosis and phagocytosis. Therefore, studying the roles of cellular membrane biophysics in microglial function should improve our understanding of the AD pathology. In this chapter, we discuss how lipid rafts and membrane-cytoskeleton adhesion impact microglial-mediated oxidative stress and clearance of amyloid-β peptide (Aβ). We also discuss potential roles of lipid membrane-bound extracellular vesicles as carriers of pathological factors to promote inflammation and cytotoxicity.
1. Introduction
Microglia are the major neuroimmune cells. They provide three essential functions including constant sensing of their environment changes, promoting neuronal health and operation, providing defense function and neuroprotection (Hickman, Izzy, Sen, Morsett, & El Khoury, 2018).
Microglial functions play pivotal roles in driving the pathology of Alzheimer’s disease (AD). For example, microglia mediate early synapse loss in AD (Hong et al., 2016; Rajendran & Paolicelli, 2018). The presence of triggering receptor expressed on myeloid cells 2 (TREM2) in microglia is essential for microglia to surround and alter amyloid-β peptide (Aβ) plaque structure, thereby limiting neuritic damage to protect from AD (Wang et al., 2016). Microglia mediate synaptic remodeling, inflammation, and oxidative stress (Bisht, Sharma, & Tremblay, 2018), spreading of tau (Asai et al., 2015), and clearance of Aβ (Paolicelli et al., 2017).
In microglia-specific transcriptomic studies, genome wide association study (GWAS) shows that variants of highly expressed microglial transcripts are associated with an increased risk of AD, suggesting microglia play an important role in both the development and onset of the disease (Gosselin et al., 2017; Heneka, Kummer, & Latz, 2014; Karch & Goate, 2015). Importantly, more than 50% of genes associated with noncoding GWAS risk alleles for AD are preferentially expressed in microglia (Gosselin et al., 2017). In addition, numerous AD risk genes expressed by microglia are related to lipid and cholesterol metabolism, lipid synthesis, lipid transport, endocytosis, exocytosis and phagocytosis (McQuade & Blurton-Jones, 2019). Therefore, investigating the roles of cellular membrane biophysics in microglial function should further our understanding of the AD pathology. In this chapter, we discuss how physical properties of cellular membranes impact microglial-mediated oxidative stress and clearance of Aβ. We will also discuss potential roles of extracellular vesicles in promoting pathological spreading from microglia.
2. The roles of lipid rafts in microglial-mediated oxidative stress
Specialized regions of plasma membranes, such as lipid rafts and caveolae, govern the states of reactive oxygen species (ROS) producing systems (Nordzieke & Medrano-Fernandez, 2018). Many redox enzymatic systems exert their activity at the membrane. An integral membrane protein, NADPH oxidases (NOX), produce ROS (DeYulia Jr., Carcamo, Borquez-Ojeda, Shelton, & Golde, 2005). While xanthine oxidases (XOR) generate ROS at the outer part of the membrane (Radi, Rubbo, Bush, & Freeman, 1997), they generate nitric oxide synthases (NOS) at the inner part (Sessa, Barber, & Lynch, 1993). Since increased activation of NOX have been observed in AD brains, and microglial-mediated ROS are the major source of oxidative stress (Ansari & Scheff, 2011; Bruce-Keller et al., 2010; Choi, Aid, Kim, Jackson, & Bosetti, 2012; Shimohama et al., 2000), here we focus on the effects of lipid rafts on NOX activity in microglia.
Microglia produce ROS mainly through activation of NOX (Wilkinson & Landreth, 2006), which is an enzyme complex comprised of the membrane bound cytochrome b558 (p22phox and the enzymatic subunit, gp91phox), cytosolic subunits (p47phox, p67phox, and p40phox), and the Rac G-protein (Babior, 2000, 2004). Phosphorylation of cytosolic subunits and activation of Rac result in their translocation to the membrane forming the active NOX complex with cytochrome b558, and subsequently producing superoxide. NOX activation to produce ROS can be stimulated by amyloid-β peptide (Aβ) (Bianca, Dusi, Bianchini, Dal Pra, & Rossi, 1999; Qin et al., 2002). Consistently, a significant translocation of p47phox and p67phox from the cytosol to the membrane was found in AD brains (Shimohama et al., 2000). Immunohistochemical evaluations of NOX expression indicate that gp91phox in microglia was upregulated specifically in a vulnerable brain region of mild cognitive impairment (MCI) patients (Bruce-Keller et al., 2010). Increased activation and upregulation of NOX in frontal and temporal cortex were found in the MCI and AD groups (Ansari & Scheff, 2011). Another study also showed a twofold increase in p47phox, but not gp91phox in the brain of AD patients (Choi et al., 2012). Increased expression of NOX was also found in the 5xFAD early onset mouse model of AD (Landel et al., 2014). These findings suggest that NOX participate in the early pathogenesis of AD.
Lipid rafts are dynamic structures. They are assemblies of mainly saturated lipids and cholesterol. Modifications of lipid raft structures and composition with nonpathological aging (Díaz, Fabelo, Ferrer, & Marín, 2018) and at different diseased stages including AD and Parkinson’s diseases (PD) (Mesa-Herrera, Taoro-González, Valdés-Baizabal, Diaz, & Marín, 2019) have been observed. In normal aging, major changes in the levels of plasmalogens, polyunsaturated fatty acids (especially docosahexaenoic acid (DHA) and arachidonic acid (ARA)), total polar lipids (mainly phosphatidylinositol, sphingomyelin, sulfatides, and cerebrosides), and total neutral lipids (particularly cholesterol and sterol esters) were detected in brain cortex (Díaz et al., 2018). These membrane and lipid raft modifications may impact activities of membrane molecules including NOX and caveolae (Fig. 1).
Fig. 1.
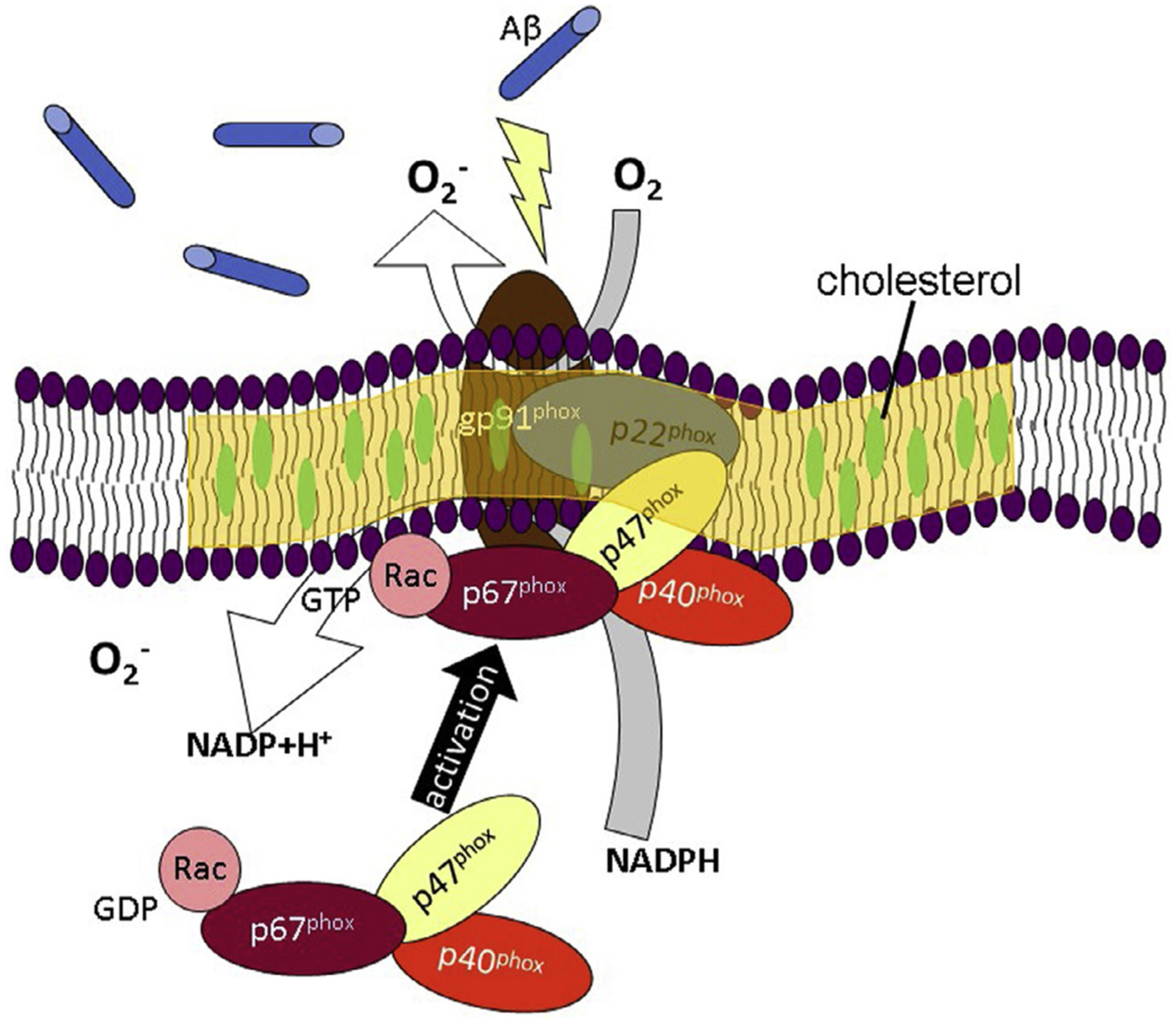
A schematic depicts Aβ induces activation of NADPH oxidase (NOX), in which the cytosolic subunits of NOX translocate to the membrane subunits located at the lipid raft. Therefore, it is reasonable to hypothesize that alterations in nano-structure and composition of lipid rafts affect NOX activation for producing superoxides.
Numerous studies have reported the roles of caveolin-containing lipid rafts in NOX activities. For example, caveolae can serve as the membrane target for p47phox, a cytosolic subunit of NOX in macrophages (Wang et al., 2018), and act as mediators of strain-induced ROS generation through NOX in glomerular mesangial cells (Zhang, Peng, Gao, Ingram, & Krepinsky, 2010). In turn, caveolae function is also dependent of NOX activity. NOX deficiency alters caveolin phosphorylation in vascular smooth muscle cells (Basset et al., 2009). High glucose downregulates caveolae in monocytes through oxidative stress from NOX (Hayashi, Juliet, Miyazaki, Ignarro, & Iguchi, 2007). However, the roles of caveolae in NOX activities in microglia, and their relations to the AD pathology have yet to be fully elucidated.
Besides caveolin, lipid raft modifications due to changes in the membrane cholesterol content have been reported to modulate NOX activity in microglia. Disruption of lipid rafts with methyl-β-cyclodextrin, fumonisin B1 or nystatin inhibit NOX-mediated ROS production and its downstream caspase-1 pathway in microglia stimulated with the pro-inflammatory lysophosphatidylcholine (LPC) (Schilling & Eder, 2010). Consistently, prolonged application of oxysterol significantly enhances lysophosphatidylserine (LPS)-induced association of cytosolic NOX subunit, p47phox, with detergent-resistant microdomains (DRMs) in BV-2 cells, thereby increasing ROS production (Rackova, 2013). (−)-Epigallocatechin-3-gallate (EGCG) inhibits function of voltage-gated proton channels probably through its ability to disturb lipid rafts by sequestering cholesterol and subsequently inhibits NOX-dependent ROS generation (Jin, Park, & Song, 2013).
In addition to cholesterol, polyunsaturated fatty acids (PUFAs), especially docosahexaenoic acid (DHA), have recently been studied for their ability to incorporate into cellular membranes, alter physical properties of membranes, and subsequently alter cellular pathways. In fact, DHA supplement has been found to be beneficial to AD patients and animal models. DHA-enriched diet reduces amyloid burden in an aged AD mouse model (Lim et al., 2005). It has been shown to slow the progression of AD in E4FAD mouse (Ma et al., 2018). Therefore, the mechanisms underlying the beneficial effects of DHA are of research interests.
In fact, DHA can incorporate into and fluidize plasma membranes in neuronal cells, resulting in an upregulation of a neurotrophic α-secretase-dependent amyloid precursor protein processing (Eckert et al., 2011; Yang, Sheng, Sun, & Lee, 2011). Although there is no direct evidence showing the effects of DHA on lipid rafts in microglia resulting in subsequent inhibition of NADPH oxidase activity, numerous studies have demonstrated the ability of DHA to inhibit microglial-mediated oxidative stress. For example, omega-3 fish oils, including DHA, inhibit ROS production induced by lipopolysaccharide (LPS) (Hadad & Levy, 2017; Yang et al., 2018, 2020), interferon-γ (IFNγ) (Hadad & Levy, 2017) and oligomeric amyloid-β peptide (oAβ) in microglial cells (Geng et al., 2020). However, these studies lack examinations of how DHA alters lipid rafts in microglia, and the roles of lipid rafts in the inhibiting effects of DHA on microglial-mediated oxidative stress have yet to be fully elucidated.
3. The roles of membrane-cytoskeleton adhesion in microglial-mediated clearance of Aβ
As the resident macrophage cells, microglia are key cells responsible for scavenging cell debris in the brain, plaques, damaged neurons and synapses, and infectious agents (Gehrmann, Matsumoto, & Kreutzberg, 1995). The clearance of Aβ by microglial cells has become an important AD research direction. Aβ phagocytosis has been found to be mediated by a range of receptors in microglia (Zuroff, Daley, Black, & Koronyo-Hamaoui, 2017), including CD14-Toll-like receptors (TLRs) to decrease phagocytosis of fibrillar Aβ42 (Reed-Geaghan, Savage, Hise, & Landreth, 2009; Udan, Ajit, Crouse, & Nichols, 2008), macrophage scavenger receptor 1 (SCARA1) to facilitate both soluble and fibrillar Aβ42 uptake (Chung, Brazil, Irizarry, Hyman, & Maxfield, 2001; El Khoury et al., 1996; Frenkel et al., 2013; Yang, Shiao, et al., 2011), CD36 to mediate phagocytosis of fibrillar Aβ42 through interactions with two distinct receptor complexes acting as a functional unit (Koenigsknecht & Landreth, 2004; Wilkinson & El Khoury, 2012; Yu & Ye, 2015), and a functional triggering receptor expressed on myeloid cells 2 protein (TREM2) required for microglia to appropriately phagocytose Aβ (Guerreiro et al., 2013; Jonsson et al., 2013). TREM2-mediated uptake of lipoprotein-Aβ complexes and modeling the ApoE-Aβ interactions have been observed in vivo (Yeh, Wang, Tom, Gonzalez, & Sheng, 2016). Increased expression of CD33, a member of the sialic acid-binding immunoglobulin-like lectins (SIGLECS) family was shown to significantly reduce the ability to phagocytose Aβ (Hollingworth et al., 2011; Naj et al., 2011).
In addition to receptor-mediated Aβ phagocytosis, endocytosis as well as exocytosis are not only biochemical but also mechanical processes governed by the physical properties of plasma membranes and the membrane-cytoskeleton adhesion (Fig. 2) (Sheetz, 2001). Yet cellular and mechanical pathways dictating Aβ internalization in microglia are still not fully understood. From the viewpoint of energetics, the formation of an endocytic vesicle is similar to membrane tether formation using modern cell mechanics techniques, such as atomic force microscopy (AFM) or laser tweezers (Sheetz, 2001). There are three mechanical factors governing the formation of an endocytic vesicle. They are in-plane bilayer membrane tension, the membrane bending stiffness, and the membrane-cytoskeleton connectivity (Sheetz, 2001). In turn, the major mechanical energy required to overcome and then produce an endocytic vesicle is the membrane-cytoskeleton adhesion (Fig. 2). Under normal conditions, the main changes in membrane tether force are the consequence of changes in membrane-cytoskeleton adhesion (Dai & Sheetz, 1999; Raucher et al., 2000). A marked rise in the membrane tether force has been found to parallel the drop in endocytosis rate (Raucher & Sheetz, 1999). Decrease in tether force paralleled the rise in endocytosis rate (Dai, Sheetz, Wan, & Morris, 1998; Raucher & Sheetz, 1999). Therefore, measuring membrane tether force is a powerful method to study the mechanical pathway of endocytosis, especially macropinocytosis.
Fig. 2.
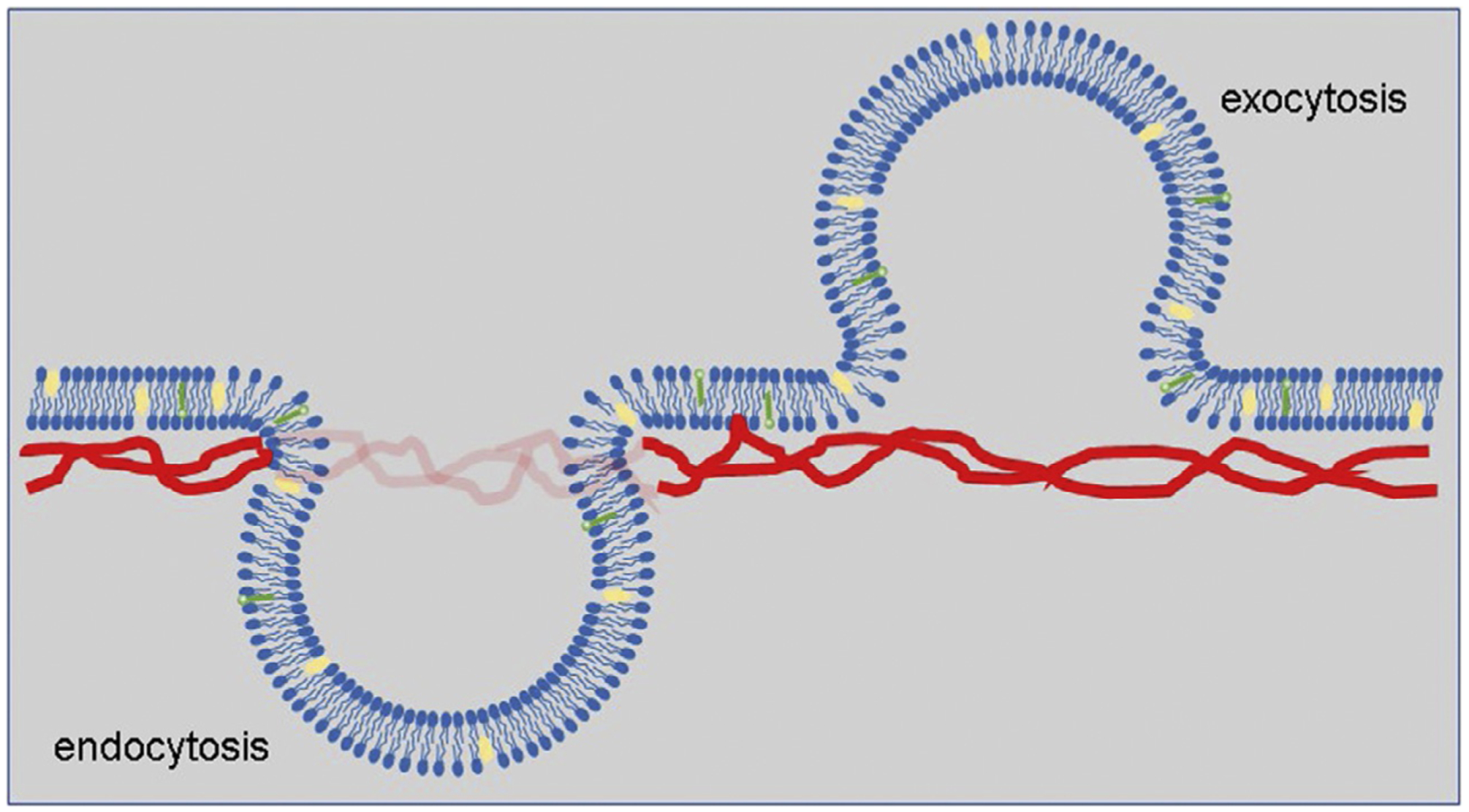
A schematic describes the role of membrane-cytoskeleton adhesion in endocytosis and exocytosis. Mechanical energy is required to overcome the membrane-cytoskeleton adhesion for detaching the membrane from cytoskeleton during the formation of invagination to produce intracellular vesicles for endocytosis and outward membrane budding for exocytosis.
In fact, microglia have been reported to mediate the clearance of soluble Aβ through fluid phase macropinocytosis (Mandrekar et al., 2009). Therefore, it is reasonable to hypothesize that cell signaling molecules involving in lipid metabolism, such as cytosolic phospholipase A2 (cPLA2), play a role in microglial-mediated clearance of Aβ. Indeed aberrant cPLA2 activity has been observed in AD brains (Stephenson et al., 1999). As activated cPLA2 is known for its ability to hydrolyze membrane phospholipids, cPLA2 has been found to be involved in Aβ-induced alterations in the physical properties of cellular membranes, such as membrane molecular order, in astrocytes (Hicks et al., 2008). In addition, cPLA2 has also been found to mediate actin rearrangements (Liu, Zhao, Fang, & Chen, 2012; Moes, Boonstra, & Regan-Klapisz, 2010). Both dynamic rearrangements of cytoskeleton and alterations of cellular membranes due to cPLA2 activity may subsequent alter the mechanical energetics of endocytosis. In fact, cPLA2 has been reported to drive recycling through the clathrin-independent endocytic route (Capestrano et al., 2014). Subsequently, we recently reported that cPLA2 facilitates Aβ oligomer uptake in microglia through its ability to lower the membrane-cytoskeleton adhesion (Teng et al., 2019).
DHA can directly alter physical properties of cell membranes through its ability to integrate into cellular membranes (Yang, Sheng, et al., 2011), but also indirectly through its ability to modulate cPLA2 (Geng et al., 2020; Hicks et al., 2008; Yang et al., 2019, 2020). Therefore, DHA can affect Aβ uptake in microglia through its direct and indirect effects on the mechanical energetics of endocytosis. In fact, omega-3 fatty acids, including DHA and eicosapentaenoic acid (EPA), have been reported to enhance phagocytosis of Aβ by human microglia (Hjorth et al., 2013), yet the underlying mechanism needs further investigations.
4. Roles of microglial-derived extracellular vesicles in AD
Extracellular vesicles (EVs) are lipid membrane-bound, cell-secreted nanoparticles that play important roles in intercellular communication (van Niel, D’Angelo, & Raposo, 2018). EVs are generally classified into exosomes (50–150nm in size), which are formed by fusion of multivesicular bodies, and larger microvesicles (150–500nm in size), which are formed by outward budding of the plasma membrane (Meldolesi, 2018). Interestingly, plasma membrane-derived vesicle budding is known to be driven by phosphatidylserine and phosphatidylethanolamine asymmetry (Hugel, Martínez, Kunzelmann, & Freyssinet, 2005) suggesting potential roles of lipids in EV release.
In neurodegenerative diseases, activation of microglia could result in EV release to promote inflammation and neurotoxicity. For example, microglial EVs were found to be neurotoxic and myelinotoxic in the presence of Aβ (Agosta et al., 2014). Accumulation of pro-inflammatory EVs from microglia was also observed in rat brains of post focal cerebral ischemia, which was inhibited by GW4869 that suppresses inward budding of multivesicular bodies (Gao et al., 2020). Glutaminase C regulates microglial activation and pro-inflammatory exosome release (Gao et al., 2019). Interestingly, the miR-124-3p level in microglial exosomes from injured brain was significantly altered after repetitive mild traumatic brain injury (TBI)—this miRNA was then transferred into hippocampal neurons and able to alleviate post-traumatic neurodegeneration by targeting the Rela/ApoE signaling pathway (Ge et al., 2020). While the roles of microglial-derived EVs have been recognized in brain injury and AD, how cell and membrane mechanics affect EV production in microglia has yet to be fully elucidated.
A recent study shows that EVs have the natural ability to undergo transport in confined tissue environments in part by tuning EV membrane deformability via water flux (Lenzini, Bargi, Chung, & Shin, 2020). Thus, microglial-derived EVs may also facilitate the transport of larger proteins within the brain, such as neurotoxic AD protein aggregates. Aβ Protofibrils are trafficked through microglial-derived EVs (Gouwens et al., 2018). BIN1 favors the spreading of tau via EVs (Crotti et al., 2019). Depletion of microglia and inhibition of their EV synthesis halt Tau propagation (Asai et al., 2015). In turn, microglial-EVs are also involved in clearance of these neurotoxic AD protein aggregates. Statin has been reported to trigger clearance of extracellular Aβ by microglia through the secretion of insulin-degrading enzyme in a nonconventional pathway in association with EVs (Glebov & Walter, 2012; Tamboli et al., 2010).
5. Conclusion
Microglia are reservoirs of lipids and their regulators, which impact biophysical properties of cell membrane. Understanding how lipid rafts and membrane-cytoskeleton adhesion of microglia regulate oxidative stress and clearance of Aβ will help deepen a fundamental insight into AD pathology from the perspective of cellular biophysics. Since EVs are lipid membrane-bound particles, understanding how EVs navigate in brain tissues and transport secreted factors will provide a novel angle to understand AD pathology, which can be helpful to devise effective treatment for the disease.
Acknowledgment
This work was supported, in part, by NIH R01AG044404 (J.C.L.), and NIH R01HL141255 (J.W.S.).
References
- Agosta F, Dalla Libera D, Spinelli EG, Finardi A, Canu E, Bergami A, et al. (2014). Myeloid microvesicles in cerebrospinal fluid are associated with myelin damage and neuronal loss in mild cognitive impairment and Alzheimer disease. Annals of Neurology, 76, 813–825. [DOI] [PubMed] [Google Scholar]
- Ansari MA, & Scheff SW (2011). NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radical Biology & Medicine, 51, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, et al. (2015). Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nature Neuroscience, 18, 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM (2000). Phagocytes and oxidative stress. The American Journal of Medicine, 109, 33–44. [DOI] [PubMed] [Google Scholar]
- Babior BM (2004). NADPH oxidase. Current Opinion in Immunology, 16, 42–47. [DOI] [PubMed] [Google Scholar]
- Basset O, Deffert C, Foti M, Bedard K, Jaquet V, Ogier-Denis E, et al. (2009). NADPH oxidase 1 deficiency alters caveolin phosphorylation and angiotensin II-receptor localization in vascular smooth muscle. Antioxidants & Redox Signaling, 11, 2371–2384. [DOI] [PubMed] [Google Scholar]
- Bianca VD, Dusi S, Bianchini E, Dal Pra I, & Rossi F (1999). Beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. The Journal of Biological Chemistry, 274, 15493–15499. [DOI] [PubMed] [Google Scholar]
- Bisht K, Sharma K, & Tremblay M (2018). Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiology of Stress, 9, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, et al. (2010). NOX activity is increased in mild cognitive impairment. Antioxidants & Redox Signaling, 12, 1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capestrano M, Mariggio S, Perinetti G, Egorova AV, Iacobacci S, Santoro M, et al. (2014). Cytosolic phospholipase A(2)epsilon drives recycling through the clathrin-independent endocytic route. Journal of Cell Science, 127, 977–993. [DOI] [PubMed] [Google Scholar]
- Choi SH, Aid S, Kim HW, Jackson SH, & Bosetti F (2012). Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. Journal of Neurochemistry, 120, 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Brazil MI, Irizarry MC, Hyman BT, & Maxfield FR (2001). Uptake of fibrillar beta-amyloid by microglia isolated from MSR-A (type I and type II) knockout mice. Neuroreport, 12, 1151–1154. [DOI] [PubMed] [Google Scholar]
- Crotti A, Sait HR, McAvoy KM, Estrada K, Ergun A, Szak S, et al. (2019). BIN1 favors the spreading of Tau via extracellular vesicles. Scientific Reports, 9, 9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, & Sheetz MP (1999). Membrane tether formation from blebbing cells. Biophysical Journal, 77, 3363–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sheetz MP, Wan X, & Morris CE (1998). Membrane tension in swelling and shrinking molluscan neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience, 18, 6681–6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYulia GJ Jr., Carcamo JM, Borquez-Ojeda O, Shelton CC, & Golde DW (2005). Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proceedings of the National Academy of Sciences of the United States of America, 102, 5044–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M, Fabelo N, Ferrer I, & Marín R (2018). “Lipid raft aging” in the human frontal cortex during nonpathological aging: Gender influences and potential implications in Alzheimer’s disease. Neurobiology of Aging, 67, 42–52. [DOI] [PubMed] [Google Scholar]
- Eckert GP, Chang S, Eckmann J, Copanaki E, Hagl S, Hener U, et al. (2011). Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochimica et Biophysica Acta, 1808, 236–243. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, & Loike JD (1996). Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature, 382, 716–719. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, et al. (2013). Scara1 deficiency impairs clearance of soluble amyloid-beta by mono-nuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nature Communications, 4, 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Li C, Zhu J, Wang Y, Huang Y, Zhao S, et al. (2020). Glutaminase 1 regulates neuroinflammation after cerebral ischemia through enhancing microglial activation and pro-inflammatory exosome release. Frontiers in Immunology, 11, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Zhao S, Xia X, Li C, Li C, Ji C, et al. (2019). Glutaminase C regulates microglial activation and pro-inflammatory exosome release: Relevance to the pathogenesis of Alzheimer’s disease. Frontiers in Cellular Neuroscience, 13, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Guo M, Hu T, Li W, Huang S, Yin Z, et al. (2020). Increased microglial exosomal miR-124–3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Molecular Therapy: The Journal of the American Society of Gene Therapy, 28, 503–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, & Kreutzberg GW (1995). Microglia: Intrinsic immuneffector cell of the brain. Brain Research. Brain Research Reviews, 20, 269–287. [DOI] [PubMed] [Google Scholar]
- Geng X, Yang B, Li R, Teng T, Ladu MJ, Sun GY, et al. (2020). Effects of docosahexaenoic acid and its peroxidation product on amyloid-beta peptide-stimulated microglia. Molecular Neurobiology, 57, 1085–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov K, & Walter J (2012). Statins in unconventional secretion of insulin-degrading enzyme and degradation of the amyloid-β peptide. Neuro-Degenerative Diseases, 10, 309–312. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, et al. (2017). An environment-dependent transcriptional network specifies human microglia identity. Science, 356(6344), eaal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouwens LK, Ismail MS, Rogers VA, Zeller NT, Garrad EC, Amtashar FS, et al. (2018). Aβ42 protofibrils interact with and are trafficked through microglial-derived microvesicles. ACS Chemical Neuroscience, 9, 1416–1425. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. (2013). TREM2 variants in Alzheimer’s disease. The New England Journal of Medicine, 368, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad N, & Levy R (2017). Combination of EPA with carotenoids and polyphenol synergistically attenuated the transformation of microglia to M1 phenotype via inhibition of NF-κB. Neuromolecular Medicine, 19, 436–451. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Juliet PA, Miyazaki A, Ignarro LJ, & Iguchi A (2007). High glucose downregulates the number of caveolae in monocytes through oxidative stress from NADPH oxidase: Implications for atherosclerosis. Biochimica et Biophysica Acta, 1772, 364–372. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, & Latz E (2014). Innate immune activation in neurodegenerative disease. Nature Reviews. Immunology, 14, 463–477. [DOI] [PubMed] [Google Scholar]
- Hickman S, Izzy S, Sen P, Morsett L, & El Khoury J (2018). Microglia in neurodegeneration. Nature Neuroscience, 21, 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JB, Lai Y, Sheng W, Yang X, Zhu D, Sun GY, et al. (2008). Amyloid-beta peptide induces temporal membrane biphasic changes in astrocytes through cytosolic phospholipase A2. Biochimica et Biophysica Acta, 1778, 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, et al. (2013). Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. Journal of Alzheimer’s Disease: JAD, 35, 697–713. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nature Genetics, 43, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugel B, Martínez MC, Kunzelmann C, & Freyssinet JM (2005). Membrane microparticles: Two sides of the coin. Physiology (Bethesda, Md.), 20, 22–27. [DOI] [PubMed] [Google Scholar]
- Jin S, Park M, & Song JH (2013). (−)-Epigallocatechin-3-gallate inhibits voltage-gated proton currents in BV2 microglial cells. European Journal of Pharmacology, 698, 154–160. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. (2013). Variant of TREM2 associated with the risk of Alzheimer’s disease. The New England Journal of Medicine, 368, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, & Goate AM (2015). Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biological Psychiatry, 77, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht J, & Landreth G (2004). Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 9838–9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landel V, Baranger K, Virard I, Loriod B, Khrestchatisky M, Rivera S, et al. (2014). Temporal gene profiling of the 5XFAD transgenic mouse model highlights the importance of microglial activation in Alzheimer’s disease. Molecular Neurodegeneration, 9, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzini S, Bargi R, Chung G, & Shin J-W (2020). Matrix mechanics and water permeation regulate extracellular vesicle transport. Nature Nanotechnology, 15, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, et al. (2005). A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. The Journal of Neuroscience, 25, 3032–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DX, Zhao WD, Fang WG, & Chen YH (2012). cPLA2alpha-mediated actin rearrangements downstream of the Akt signaling is required for Cronobacter sakazakii invasion into brain endothelial cells. Biochemical and Biophysical Research Communications, 417, 925–930. [DOI] [PubMed] [Google Scholar]
- Ma QL, Teng E, Zuo X, Jones M, Teter B, Zhao EY, et al. (2018). Neuronal pentraxin 1: A synaptic-derived plasma biomarker in Alzheimer’s disease. Neurobiology of Disease, 114, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, & Landreth GE (2009). Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 4252–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade A, & Blurton-Jones M (2019). Microglia in Alzheimer’s disease: Exploring how genetics and phenotype influence risk. Journal of Molecular Biology, 431, 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J (2018). Exosomes and ectosomes in intercellular communication. Current Biology, 28, R435–R444. [DOI] [PubMed] [Google Scholar]
- Mesa-Herrera F, Taoro-González L, Valdés-Baizabal C, Diaz M, & Marín R (2019). Lipid and lipid raft alteration in aging and neurodegenerative diseases: A window for the development of new biomarkers. International Journal of Molecular Sciences, 20(15), 3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moes M, Boonstra J, & Regan-Klapisz E (2010). Novel role of cPLA(2)alpha in membrane and actin dynamics. Cellular and Molecular Life Sciences: CMLS, 67, 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nature Genetics, 43, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordzieke DE, & Medrano-Fernandez I (2018). The plasma membrane: A platform for intra- and intercellular redox signaling. Antioxidants (Basel, Switzerland), 7(11), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Jawaid A, Henstridge CM, Valeri A, Merlini M, Robinson JL, et al. (2017). TDP-43 depletion in microglia promotes amyloid clearance but also induces synapse loss. Neuron, 95, 297–308.e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Cooper C, Liu B, Wilson B, & Hong JS (2002). Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. Journal of Neurochemistry, 83, 973–983. [DOI] [PubMed] [Google Scholar]
- Rackova L (2013). Cholesterol load of microglia: Contribution of membrane architecture changes to neurotoxic power? Archives of Biochemistry and Biophysics, 537, 91–103. [DOI] [PubMed] [Google Scholar]
- Radi R, Rubbo H, Bush K, & Freeman BA (1997). Xanthine oxidase binding to glycosaminoglycans: Kinetics and superoxide dismutase interactions of immobilized xanthine oxidase-heparin complexes. Archives of Biochemistry and Biophysics, 339, 125–135. [DOI] [PubMed] [Google Scholar]
- Rajendran L, & Paolicelli RC (2018). Microglia-mediated synapse loss in Alzheimer’s disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38, 2911–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, & Sheetz MP (1999). Membrane expansion increases endocytosis rate during mitosis. The Journal of Cell Biology, 144, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, et al. (2000). Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell, 100, 221–228. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, & Landreth GE (2009). CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 11982–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T, & Eder C (2010). Importance of lipid rafts for lysophosphatidylcholine-induced caspase-1 activation and reactive oxygen species generation. Cellular Immunology, 265, 87–90. [DOI] [PubMed] [Google Scholar]
- Sessa WC, Barber CM, & Lynch KR (1993). Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circulation Research, 72, 921–924. [DOI] [PubMed] [Google Scholar]
- Sheetz MP (2001). Cell control by membrane-cytoskeleton adhesion. Nature Reviews. Molecular Cell Biology, 2, 392–396. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, et al. (2000). Activation of NADPH oxidase in Alzheimer’s disease brains. Biochemical and Biophysical Research Communications, 273, 5–9. [DOI] [PubMed] [Google Scholar]
- Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, et al. (1999). Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. GLIA, 27, 110–128. [DOI] [PubMed] [Google Scholar]
- Tamboli IY, Barth E, Christian L, Siepmann M, Kumar S, Singh S, et al. (2010). Statins promote the degradation of extracellular amyloid {beta}-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. The Journal of Biological Chemistry, 285, 37405–37414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng T, Dong L, Ridgley DM, Ghura S, Tobin MK, Sun GY, et al. (2019). Cytosolic phospholipase A2 facilitates oligomeric amyloid-beta peptide association with microglia via regulation of membrane-cytoskeleton connectivity. Molecular Neurobiology, 56, 3222–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan ML, Ajit D, Crouse NR, & Nichols MR (2008). Toll-like receptors 2 and 4 mediate Abeta(1–42) activation of the innate immune response in a human monocytic cell line. Journal of Neurochemistry, 104, 524–533. [DOI] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, & Raposo G (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19, 213–228. [DOI] [PubMed] [Google Scholar]
- Wang J, Bai Y, Zhao X, Ru J, Kang N, Tian T, et al. (2018). oxLDL-mediated cellular senescence is associated with increased NADPH oxidase p47phox recruitment to caveolae. Bioscience Reports, 38(3). BSR20180283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, et al. (2016). TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. The Journal of Experimental Medicine, 213, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K, & El Khoury J (2012). Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. International Journal of Alzheimer’s Disease, 2012, 489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson BL, & Landreth GE (2006). The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. Journal of Neuroinflammation, 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Fritsche KL, Beversdorf DQ, Gu Z, Lee JC, Folk WR, et al. (2019). Yin-Yang mechanisms regulating lipid peroxidation of docosahexaenoic acid and arachidonic acid in the central nervous system. Frontiers in Neurology, 10, 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Li R, Liu PN, Geng X, Mooney BP, Chen C, et al. (2020). Quantitative proteomics reveals docosahexaenoic acid-mediated neuroprotective effects in lipopolysaccharide-stimulated microglial cells. Journal of Proteome Research, 19(6), 2236–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Li R, Michael Greenlief C, Fritsche KL, Gu Z, Cui J, et al. (2018). Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. Journal of Neuroinflammation, 15, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Sheng W, Sun GY, & Lee JC (2011). Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing. Neurochemistry International, 58, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CN, Shiao YJ, Shie FS, Guo BS, Chen PH, Cho CY, et al. (2011). Mechanism mediating oligomeric Abeta clearance by naive primary microglia. Neurobiology of Disease, 42, 221–230. [DOI] [PubMed] [Google Scholar]
- Yeh FL, Wang Y, Tom I, Gonzalez LC, & Sheng M (2016). TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron, 91, 328–340. [DOI] [PubMed] [Google Scholar]
- Yu Y, & Ye RD (2015). Microglial Abeta receptors in Alzheimer’s disease. Cellular and Molecular Neurobiology, 35, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Peng F, Gao B, Ingram AJ, & Krepinsky JC (2010). Mechanical strain-induced RhoA activation requires NADPH oxidase-mediated ROS generation in caveolae. Antioxidants & Redox Signaling, 13, 959–973. [DOI] [PubMed] [Google Scholar]
- Zuroff L, Daley D, Black KL, & Koronyo-Hamaoui M (2017). Clearance of cerebral Abeta in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cellular and Molecular Life Sciences, 74(12), 2167–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]


