Key Points
Question
What are the safety and efficacy of atrial fibrillation ablation in patients with paroxysmal atrial fibrillation?
Findings
In this systematic review and meta-analysis of 6 randomized clinical trials including 1212 patients with paroxysmal atrial fibrillation, catheter ablation use was associated with a 38% reduction in atrial arrhythmias and a 68% reduction in hospitalizations compared with use of antiarrhythmic drugs. There was no difference in major adverse events between both groups.
Meaning
Findings of this meta-analysis suggest the potential utility of catheter ablation as a first-line strategy in patients with paroxysmal atrial fibrillation compared with use of antiarrhythmic drugs.
Abstract
Importance
Early rhythm control of atrial fibrillation (AF) with either antiarrhythmic drugs (AADs) or catheter ablation has been reported to improve cardiovascular outcomes compared with usual care; however, the optimal therapeutic modality to achieve early rhythm control is unclear.
Objective
To assess the safety and efficacy of AF ablation as first-line therapy when compared with AADs in patients with paroxysmal AF.
Data Sources
PubMed/MEDLINE, Scopus, Google Scholar, and various major scientific conference sessions from January 1, 2000, through November 23, 2020.
Study Selection
Randomized clinical trials (RCTs) published in English that had at least 12 months of follow-up and compared clinical outcomes of ablation vs AADs as first-line therapy in adults with AF. The quality of individual studies was assessed using the Cochrane risk of bias tool. Six RCTs met inclusion criteria, including 1212 patients.
Data Extraction and Synthesis
Two investigators independently extracted data. Reporting was performed in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines. Analysis was performed using a random-effects model with the Mantel-Haenszel method, and results are presented as 95% CIs.
Main Outcomes and Measures
Main outcomes were safety and efficacy of AF ablation as first-line therapy when compared with AADs. Trials were evaluated as having low risk of selection and attrition biases, high risk of performance bias, and with unclear risk for detection biases due to unblinding and open-label designs.
Results
A total of 6 RCTs involving 1212 patients with AF were included (609 were randomized to AF ablation and 603 to drug therapy; mean [SD] age, 56 [11] years). Compared with AADs, catheter ablation use was associated with reductions in recurrent atrial arrhythmia (32.3% vs 53%; risk ratio [RR], 0.62; 95% CI, 0.51-0.74; P < .001; I2 = 40%), with a number needed to treat with ablation to prevent 1 arrhythmia of 5. Use of ablation was also associated with reduced symptomatic atrial arrhythmia (11.8% vs 26.4%; RR, 0.44; 95% CI, 0.27-0.72; P = .001; I2 = 54%) and hospitalization (5.6% vs 18.7%; RR, 0.32; 95% CI, 0.19-0.53; P < .001) with no significant difference in serious adverse events between the groups (4.2% vs 2.8%; RR, 1.52; 95% CI, 0.81-2.85; P = .19).
Conclusions and Relevance
In this meta-analysis of randomized clinical trials including first-line therapy of patients with paroxysmal AF, catheter ablation compared with antiarrhythmic drugs was associated with reductions in recurrence of atrial arrhythmias and hospitalizations, with no difference in major adverse events.
This systematic review and meta-analysis of 6 randomized clinical trials evaluates the safety and efficacy of atrial fibrillation ablation as first-line therapy compared with antiarrhythmic drugs in patients with paroxysmal atrial fibrillation.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia diagnosed in clinical practice. It is estimated to affect 6 to 12 million people in the US by 2050 and approximately 18 million in Europe by 2060.1,2 Two decades ago, the clinical trials AFFIRM, RACE, and STAF were conducted to assess the relative clinical utility of rate vs rhythm control in patients with AF.3,4,5 While the primary analyses reported no mortality reductions of rhythm control, conclusions were limited by the ineffectiveness and potential toxic effects of the antiarrhythmic drugs (AADs) used (primarily amiodarone) and the allowance for oral anticoagulation cessation for presumed sinus rhythm maintenance, which differentially occurred in the rhythm control arm.3,4,5 More recently, the EAST-AFNET 4 trial addressed the limitation using contemporary rhythm control strategies (with pharmacotherapy focused on less toxic agents, such as class 1c drugs or dronedarone) and the inclusion of catheter ablation; rhythm control provided superior outcomes.6
Future guidelines may expand the current recommendation goal of sinus rhythm maintenance for patients with symptomatic paroxysmal AF to improve quality of life.7,8 However, the efficacy of first-line AAD therapy is limited, and it results in a substantial proportion of patients discontinuing therapy owing to drug toxic effects.9,10 For AAD ineffectiveness or intolerance, catheter ablation has proven to be superior to an additional AAD for preventing AF recurrence.11,12,13 Furthermore, shortening the time between diagnosis and ablation is a modifiable factor independently associated with improved AF ablation outcomes, suggesting the utility of early ablation before AADs for preventing recurrent arrhythmia, repeated ablation, atrial remodeling, and hospitalization.14,15,16 However, ablation was associated with a high rate of repeated procedures and associated with severe adverse events.
Since 2005, randomized clinical trials (RCTs) have compared catheter ablation with AADs as first-line therapy for paroxysmal AF, that is, randomization in patients who had not experienced failure with membrane-active AAD (Class I or III).17,18,19 A meta-analysis of earlier studies20 reported improvements in AF recurrence but not in symptomatic AF recurrence, cardiovascular outcomes, or the need for repeated procedures; furthermore, there were more serious adverse events with AF ablation than with AADs. A number of similar RCTs21,22,23 have recently been reported, prompting us to conduct a systematic review and meta-analysis of all randomized clinical trials to address confounding factors, such as crossover and need for repeated ablation, and evaluate the safety and efficacy of AF ablation as first-line therapy compared with AADs in patients with paroxysmal AF.
Methods
Data Sources and Search Strategy
A protocol developed and followed for this systematic review and meta-analysis is available in the eMethods in the Supplement. A systematic digital search using PubMed/MEDLINE, Scopus, Google Scholar, and various major scientific conference sessions (American Heart Association, American College of Cardiology, European Society of Cardiology Congress, European Heart Rhythm Association Congress and Heart Rhythm Society) for published abstracts and manuscripts from January 1, 2000, through November 23, 2020, was performed. Two of us (M.K.T. and D.M.) independently performed searches that included the following keywords: atrial fibrillation, ablation, antiarrhythmic, and random. Information on unpublished studies was obtained from ClinicalTrials.gov databases and Google. Details of the search strategies and filters are provided in the eMethods in the Supplement. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for systematic reviews and meta-analyses and was registered with PROSPERO (CRD42020223003).
Study Selection
Two of us (M.K.T. and D.M.) independently screened all titles, abstracts, and full-text material to select studies that met the following eligibility criteria: (1) prospective randomized clinical trials with at least 12 months of follow-up, (2) studies that included adults aged at least 18 years comparing AF ablation vs AADs as first-line therapy in patients with AF, and (3) studies reporting at least one clinical outcome. Retrospective studies, case reports, nonrandomized studies, meta-analysis, case reports, editorials, reviews, expert opinions, and studies published in languages other than English were excluded.
Data Extraction and Quality Appraisal
Data from the individual studies were extracted using a standardized protocol and data extraction form (eMethods in the Supplement) by 2 of us (M.K.T. and D.M.). These investigators together assessed the quality of individual studies using the Cochrane risk of bias tool for each clinical outcome.24
Clinical Outcomes
The primary outcome of this analysis was recurrence of any atrial arrhythmia (both symptomatic and asymptomatic) including AF, atrial flutter, or atrial tachycardia. Secondary outcomes of interest included (1) symptomatic atrial arrhythmias, (2) hospitalizations (excluding hospitalization for catheter ablation procedures), (3) additional ablation in patients allocated to either catheter ablation or AADs, (4) trial crossover to alternate therapy, (5) composite of major adverse events, and (6) mortality.
Serious adverse events included a composite of vascular complications (femoral bleeding, hematoma, pseudoaneurysm, and groin infection), pericardial effusion (with and without tamponade), pulmonary vein stenosis, phrenic palsy, systemic thromboembolism, bradycardia, syncope, and atrial flutter with 1:1 conduction. Because antiarrhythmic drugs, especially class 1c drugs, can cause bradycardia, syncope, and atrial flutter with 1:1 ventricular conduction, leading to drug discontinuation, we included them in the composite of major adverse events.
The definition of bradycardia varied among the included trials. The trials RAAFT-2,19 MANTRA-PAF,18 and EARLY- AF21 included patients with bradycardia who required pacemaker implantation. The STOP AF22 trial included patients with bradycardia resulting in a life-threatening injury, hospitalization, or intervention, and the RAAFT17 trial included only patients with documented bradycardia with no other prespecified criteria.
Statistical Analysis
All statistical analyses were performed using Review Manager 5.3 software (The Nordic Cochrane Centre; The Cochrane Collaboration). Because prior studies reported comparable safety and efficacy of radiofrequency ablation and cryoablation, we aggregated both therapies in the ablation group.25 We also pooled outcomes of studies regardless of the follow-up assessment timing for each trial, as the effectiveness of ablation or AADs diminishes with time. However, we also performed a sensitivity analysis based on follow-up duration and sequentially removing each trial from the pooled effect estimates. A prespecified subgroup analysis was also performed according to the use of radiofrequency or cryoablation.
Risk ratios (RRs) were used to pool differences in binary events, and mean differences were used to pool differences in continuous outcomes. The RR using inverse weighting was used for sensitivity and subgroup analysis. Analysis was performed using random-effects model with the Mantel-Haenszel method and results presented as 95% CIs. We pooled within-group recurrence rates for the trials reporting AF-free survival and reported summaries based on Freedman-Tukey double arcsine transformation.26 The Hartung-Knapp small-sample adjustment was used because the number of studies is less than 10.27 The Q Cochran test and Higgins I2 statistics were used to estimate heterogeneity among studies, with I2 less than25% indicating low heterogeneity, 25% to 50% indicating moderate heterogeneity, or greater than 50% indicating high heterogeneity.24 Publication bias assessment and meta-regressions were not performed given that the number of included studies was less than 10. Number needed to treat was calculated for clinical outcomes based on absolute risk difference. A 2-tailed P < .05 was considered statistically significant.
Results
Overview of Trials
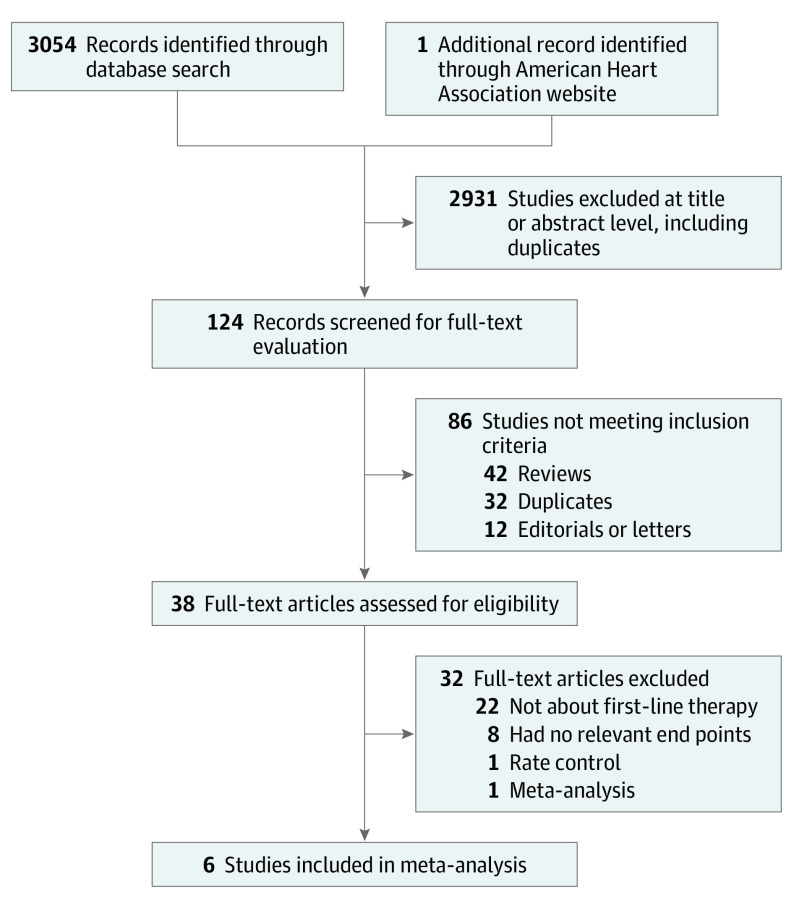
Of 3054 identified records, 6 trials met inclusion criteria (Figure 1).17,18,19,21,22,23 The study characteristics are presented in Table 1 and the inclusion and exclusion criteria for each trial are presented in eTable 1 in the Supplement. We judged trials as having low risk of selection and attrition biases, high risk of performance bias, and with unclear risk for detection biases due to unblinding and open-label designs (eTables 2-8 in the Supplement). We judged risks of reporting biases as unclear for reporting symptomatic atrial arrhythmias, and low for the rest of the outcomes.
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) Flow Diagram.
Table 1. Baseline Characteristics of Clinical Trials.
| Variable | RAAFT-1,17 2005 | MANTRA-PAF,18 2012 | RAAFT-2,19 2014 | STOP AF,22 2020 | EARLY AF,21 2020 | CRYO-FIRST,23a |
|---|---|---|---|---|---|---|
| Funding source | Acuson | Danish Heart Foundation; Biosense Webster | Biosense Webster; Population Health Research Institute | Medtronic | Cardiac Arrhythmia Network of Canada; Medtronic | Medtronic |
| Mean (SD) age ablation vs AADs, y | 53 (8) vs 54 (8) | 56 (9) vs 54 (10) | 56 (9) vs 54 (12) | 60 (11) vs 62 (11) | 58 (12) vs 60 (11) | 51 (13) vs 54 (13) |
| Mean (SD) CHA2DS2-VASc score (ablation vs AADs) | NA | NA | 0.5 (0.7)b vs 0.7 (0.8)b | NA | 1.9 (1.0) vs 1.9 (1.1) | NA |
| Patients randomized, No. | 70 | 294 | 127 | 203 | 303 | 218 |
| Randomization | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 |
| Primary end point | First recurrence of AF >15 s | AF+ burden and cumulative burdenc | First recurrence of AF/AFL/AT >30 s | First recurrence of AF/AFL/AT ≥30 s | First recurrence of AF/AFL/AT ≥30 s | Free from any AF/AFL/AT >30 s |
| Total follow-up | 1 y | 2 y | 2 y | 1 y | 1 y | 1 y |
| Paroxysmal AF, % | ||||||
| Ablation | 97 | 100 | 98.5 | 100 | 95.5 | 100 |
| AAD | 95 | 100 | 96.7 | 100 | 94 | 100 |
| Withdrew/lost to follow-up, No. | ||||||
| Ablation | 1 | 5 | 1 | 1 | 0 | 17 |
| Drugs | 2 | 7 | 1 | 6 | 1 | 13 |
| First-line AADs, % | ||||||
| Class IC | 77 | 99 | 94 | 69 | 81 | 92 |
| Class III | 23 | 1 | 3.3 | 21 | 19 | 8 |
| Oral anticoagulant at baseline (ablation/AAD), % | NR | 47 vs 44 | 53 vs 31 | 69 vs 69 | 67 vs 64 | 35.5 vs 44.0 |
| Time since AF diagnosis (ablation/AAD), mo | 5 (2) vs 5 (2.5)d | NR | NR | 1.3 (2.5) vs 1.3 (2.3) | 1.3 (2.2) vs 1.7 (3.0) | 0.7 (1.5) vs 0.8 (2.1) |
| Left atrial diameter (ablation/AAD), mm | 41 (8) vs 42 (7) | 40 (6) vs 40 (5) | 40 (5) vs 43 (5) | 39 (6) vs 38 (5) | 39 (5) vs 38 (7) | 37 (6) vs 38 (5) |
| LVEF (ablation/AAD), % | 53 (5) vs 54 (6) | LVEF >60% in 80% vs 82% | 61 (5) vs 61 (7) | 61 (6) vs 61 (6) | 60 (7) vs 60 (8) | 63 (5.4) vs 64 (5.4) |
| Frequency of monitoring, mo | 1 mo Event monitor at 1 and 3 mo, 24-h Holter recording before discharge, and at 3, 6, and 12 mo | 7-d Holter monitor at 3, 6, 12, 18, and 24 mo | Biweekly transtelephonic monitoring | 12-Lead ECG at 1,3,6,12 mo Patient-activated telephone monitoring weekly. 24-h ambulatory. ECG monitoring at 6 and 12 mo | Implantable loop recorder (Reveal LINQ, Medtronic) | 7-d Holter monitor at 1, 3, 6, 9, 12 mo follow-up |
| Type of ablation | Radiofrequency ablation | Radiofrequency ablation | Radiofrequency ablation | Cryoablation | Cryoablation | Cryoablation |
| Ablation strategy | Pulmonary vein isolation | Pulmonary vein isolation, roof catheter, CTI catheter, mitral catheter | Pulmonary vein isolation, CFAE ablation, roof catheter, SVC isolation, CTI catheter | Pulmonary vein isolation | Pulmonary vein isolation | Pulmonary vein isolation |
| Acute isolation of pulmonary veins, % | 100 | NR | 87 | 98 | 100 | 100 |
| Blanking period | 2 mo | 3 mo | 3 mo | 3 mo | 3 mo | 3 mo |
| Additional ablation, (ablation/AAD), % | 12.5 vs 51.4e | 47 vs 36 | 15 vs 43 | 0 vs 34f | 12 vs 24f | 5.6 vs 17 |
| Free from atrial arrhythmias in catheter ablation group on follow-up, % | 87 | 64.4 | 45.5 | 79.9 | 57.8 | 82.2 |
| Free from atrial arrhythmias in the drug therapy group on follow-up, % | 37 | 44.6 | 27.9 | 64.7 | 32.2 | 67.6 |
Abbreviations: AADs, antiarrhythmic drugs; AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; CFAE, complex fractionated electrogram; CHA2DS2-VASc score, congestive heart failure, hypertension, age 75 years or older, diabetes, prior stroke/transient ischemic attack–vascular disease, age 65 to 74 years, sex category; CTI, cavotricuspid isthmus; ECG, electrocardiogram; LVEF, left ventricular ejection fraction; NA, not applicable; NR, not reported; SVC, superior vena cava.
Abstract published.
CHADS2 score, with CHA2DS2-VASC, 0 to 1 meaning low risk for stroke and CHA2DS2-VASc 2 or higher meaning high risk for stroke.
Only episodes of AF lasting longer than 1 minute.
Months.
Ablation outside the prespecified time period
Number of repeated ablations after meeting the primary end point.
All trials were open-label, used intention-to-treat analysis, and allowed crossover from ablation to drug therapy or vice versa in patients who experienced failure with initial therapy. There were 1212 patients in the 6 trials; 609 were randomized to AF ablation and 603 to drug therapy. The mean (SD) age of patients was 56 (11) years. Hypertension was the predominant comorbidity, and 98% of patients had paroxysmal AF.17,18,19,21,22,23 The mean (SD) duration of AF was 1.5 (2.3) years . The mean (SD) left atrial diameter was 40 (6) mm, and the left ventricular ejection fraction was normal in 95.5% of patients. Study follow-up ranged from 12 to 24 months (mean [SD] 16 [6] months).
In RAAFT, RAAFT-2, and MANTRA-PAF, ablation was performed using radiofrequency ablation catheters,17,18,19 while cryoballoon ablation was used in the STOP AF, EARLY AF, and CRYO-FIRST clinical trials.21,22,23 Pulmonary vein isolation was the primary ablation strategy in all studies, but additional ablation was performed outside the pulmonary veins in MANTRA-PAF and RAAFT-2. The antiarrhythmic drug therapy arm primarily used class 1c drugs in 82% and class III drugs in 14%. Other ablation details are reported in eTable 9 in the Supplement.
Primary Outcome
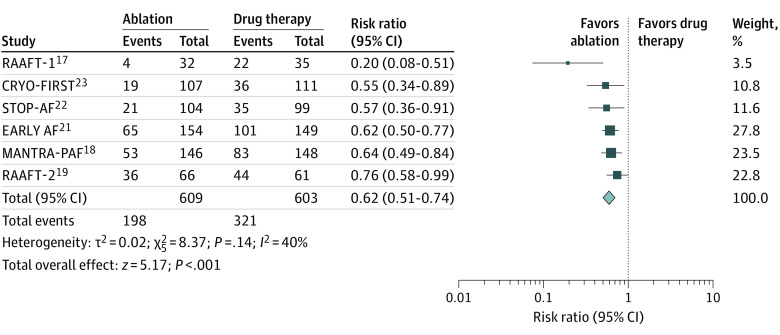
The clinical outcome of atrial arrhythmia recurrence (including atrial fibrillation, atrial flutter, or atrial tachycardia) was reported in all 6 clinical trials. Catheter ablation was associated with a significantly higher rate (38% reduction) of being free from recurrent atrial arrhythmia (32.3% vs 53%; RR, 0.62; 95% CI, 0.51-0.74; P < .001; I2 = 40%) (Figure 2). The number needed to treat with ablation to prevent 1 arrhythmia was 5.
Figure 2. Primary Clinical Outcome.
Squares represent mean values, with the size of the squares indicating weight and horizontal lines representing 95% CIs. The diamond represents the pooled mean with the points of the diamond representing 95% CIs.
Prespecified subgroup analysis based on the type of ablation energy used also showed significantly lower recurrence of atrial arrhythmias with either radiofrequency ablation (RR, 0.58; 95% CI, 0.38-0.89; P = .01) or cryoablation (RR, 0.60; 95% CI, 0.50-0.72; P < .001). Similar findings were also observed when the data were pooled based on study follow-up duration (1 year vs 2 years): there was a 46% reduction in atrial arrhythmia recurrence at 1 year of follow-up and a 30% reduction in atrial arrhythmia recurrence at 2 years of follow-up with ablation, compared with drug therapy (eFigures 1 and 2 in the Supplement).
Secondary Outcomes
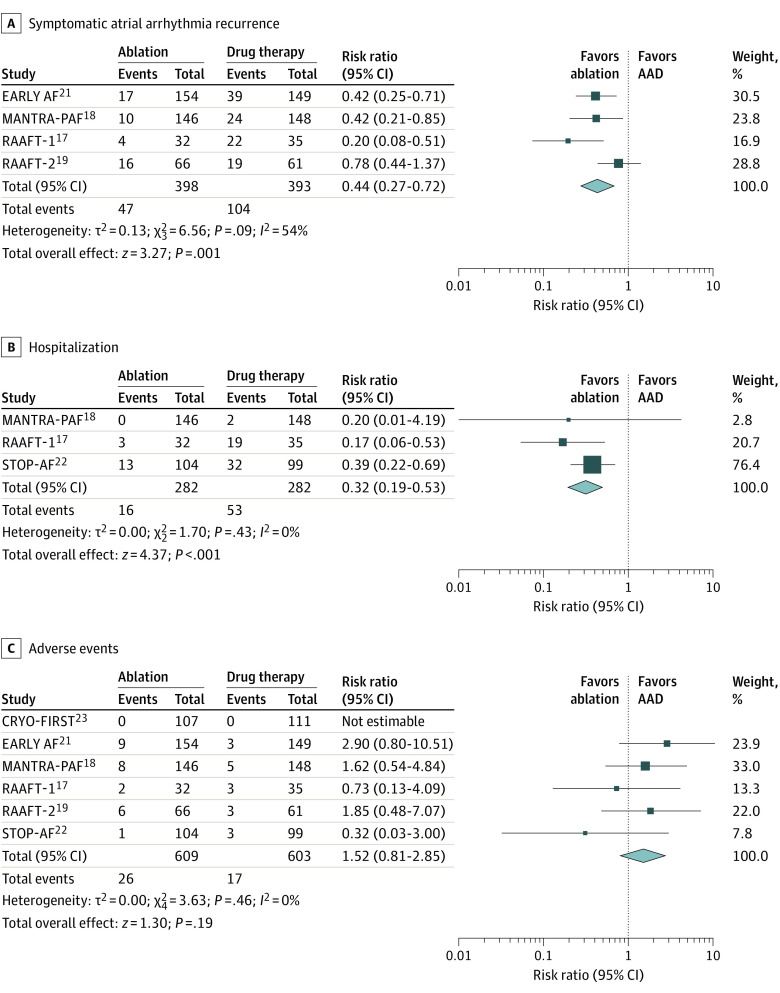
Symptomatic atrial arrhythmia was reported in 4 clinical trials.17,18,19,21 The AF ablation was associated with a reduced recurrence of symptomatic atrial arrhythmia (11.8% vs 26.4%; RR, 0.44; 95% CI, 0.27-0.72; P = .001; I2 = 54%) (Figure 3A).
Figure 3. Secondary Outcomes.
A, Symptomatic atrial arrhythmia. B, Hospitalizations. C, Major adverse events. Squares represent mean values, with the size of the squares indicating weight and horizontal lines representing 95% CIs. Diamonds represent the pooled mean with the points of the diamonds representing 95% CIs.
Hospitalizations were reported in 3 clinical trials.17,18,22 The rate of hospitalizations was significantly lower (68% reduction) with ablation compared with drug therapy (5.6% vs 18.7%; RR, 0.32; 95% CI, 0.19-0.53; P < .001) without statistically significant heterogeneity (I2 = 0%) (Figure 3B).
Study crossover to alternate therapy was reported in all 6 clinical trials.17,18,19,21,22,23 In RAAFT-1, crossover data were reported after the prespecified follow-up period, and in STOP AF and EARLY AF, crossover was permitted only after meeting the primary end point. Assuming no crossover occurred in the given 3 trials, study crossover occurred significantly less frequently with ablation compared with AADs (3.3% vs 16.4%; RR, 0.21; 95% CI, 0.13-0.35; P < .001; I2 = 6%) (eFigure 3 in the Supplement). Furthermore, the rate of additional ablation after randomization was similar in both the catheter ablation and AAD arms (14% vs 16.4%; RR, 0.54; 95% CI, 0.189-1.65; P = .28; I2 = 91%) (eFigure 4 in the Supplement).
Mortality was reported in 5 trials; there was no mortality in 4 of the 5 trials,19,21,22,23 and there was no difference in the treatment groups in the fifth trial.18 Overall, the mortality was low, without significant difference between groups (0.5% vs 0.7%; RR, 0.76; 95% CI, 0.17-3.34; P = .72).
All trials reported percentages of patients with major adverse events (Table 2). There was 1 death (0.1%) related to stroke after an ablation procedure.18 There were no instances of atrio-esophageal fistula or anesthesia-related complications with ablation. The most common major adverse event in the ablation group was pericardial effusion, which occurred in 1.3%, and bradycardia was the most common adverse event in the AAD group and occurred in 1.1%. Pulmonary vein stenosis was rare, occurring in 4 patients (0.6%) in the ablation group, of which the severity was mild in 2 patients. The overall major adverse event percentages were higher with ablation than AADs, though the difference was not statistically significant (ablation, 4.2% vs AADs, 2.8%; RR, 1.52; 95% CI, 0.81-2.85; P = .19) (Table 2; Figure 3C); there was no statistically significant heterogeneity (I2 = 0%). Notably, the type of major adverse events was different between groups. This finding was similar for both radiofrequency ablation and cryoablation. The higher complication rate observed with ablation was predominantly owing to a single trial, EARLY AF,21 in which complications occurred in 9 of 154 ablation patients (5.8%) compared with 3 of 149 patients (2%) with AADs.
Table 2. Aggregate Complications During Ablation vs Drug Therapy.
| Complication | No. (%) | |
|---|---|---|
| Ablation group (n = 609) | Drug group (n = 603) | |
| Vascular puncture site complicationsa | 3 (0.5) | 0 |
| Pericardial effusionb | 8 (1.3) | 1 (0.1) |
| Phrenic nerve palsy | 3 (0.5) | 0 |
| Pulmonary venous stenosis | 4 (0.7) | 0 |
| Thromboembolic eventsc | 3 (0.5) | 1 (0.1) |
| Bradycardia | 3 (0.5) | 7 (1.1) |
| Atrial flutter with 1:1 conduction | 0 | 3 (0.5) |
| Syncope | 2 (0.3) | 5 (0.8) |
| Composite of major adverse events | 26 (4.2) | 17 (2.8) |
Composite of groin hematoma, pseudoaneurysm, and groin infection.
With or without cardiac tamponade physiology.
Composite of all thromboembolic events including stroke and transient ischemic attack.
Sensitivity Analyses
The results of the sensitivity analysis are presented in eTable 10 in the Supplement. Overall, there was no significant change in the results for any of the clinical outcomes on serial exclusion of each trial, except that the rate of additional ablation was significantly lower in the catheter ablation group compared with AADs when MANTRA PAF was excluded (3.2% vs 9.9%; RR, 0.32; 95% CI, 0.19-0.55; P < .001; I2 = 0%).
Discussion
The main findings in this analysis are that as first-line therapy in patients with paroxysmal AF, catheter ablation was associated with a 38% reduction in recurrence of atrial arrhythmias and 68% reduction in hospitalizations compared with antiarrhythmic drugs. A similar finding with ablation was observed irrespective of study follow-up (1-year vs 2 years) or type of ablation energy used (radiofrequency ablation vs cryoablation). There was no significant difference in the composite of major adverse events between the 2 groups. In the drug therapy group, crossover was more frequent, with 16.4% of patients subsequently receiving ablation due to drug toxic effects or failure.
One other systematic review and meta-analysis of first-line AF therapy20 reported a similar finding with AF catheter ablation compared with AADs. Compared with the prior analyses,20 we included additional data from 3 recently published RCTs, which together constitute approximately 60% of the studied patient population.21,22,23 Notably, study crossover and the need for additional ablation was substantially lower when compared with the prior meta-analysis20 because both STOP AF22 and EARLY AF21 prevented any crossover between arms during the prespecified period.
In patients with drug-refractory paroxysmal AF, several clinical trials have reported superiority of catheter ablation compared with additional AAD therapy for the long-term maintenance of sinus rhythm and improvement of quality of life.28,29,30 Consistent with these findings, the 2019 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommend a trial of AADs before catheter ablation.7,8 However, accumulating evidence suggests that in patients with shorter time between diagnosis and treatment, a strategy of early rhythm control using catheter ablation is associated with a higher likelihood of AF-free survival, fewer repeated procedures, fewer recurrent hospitalizations, and a decrease in the progression to persistent AF.15,16,31
The recently published multicenter EAST-AFNET 4 trial6 reported that compared with usual care, early rhythm control with either AADs or ablation was associated with improved cardiovascular outcomes in patients with AF. The EAST-AFNET 4 trial6 included the primary composite end point of cardiovascular death, stroke, or hospitalization for heart failure or acute coronary syndrome (hazard ratio, 0.79; 96% CI, 0.66-0.94; P = .01) and the individual components of cardiovascular death (hazard ratio, 0.72; 96% CI, 0.52-0.98) and stroke (hazard ratio, 0.65; 96% CI, 0.44-0.97) at a median follow-up of 5 years.6 The question now is less about whether to achieve rhythm control than which therapeutic modality one should use. In EAST-AFNET 4, catheter ablation was used in 20% of patients. For those patients randomized to the rhythm control strategy, only 8% of patients received catheter ablation as the initial ablation strategy, and this fraction increased to only 19.4% by the 2-year time point. Thus, the precise role of catheter ablation remains unclear.
Our meta-analysis including all 6 RCTs with relatively uniform study design and strict inclusion and exclusion criteria suggests that early rhythm control using AF ablation as first-line therapy is more effective than AADs in patients with paroxysmal AF. Furthermore, the study inclusion and exclusion criteria were relatively open and accepting of most patients with paroxysmal AF even when the upper range limit on left atrial size (ranging between 5-5.5 cm) was of a magnitude that there were likely to be few patients presenting for first-line treatment of paroxysmal AF that did not fit within this criterion.
An important challenge in interpreting these data are understanding the methodologic differences among the included trials and the heterogeneity in AF monitoring.17,18,19,21,22,23 In MANTRA-PAF, the primary end point was AF burden, and time to first AF recurrence was a secondary end point.18 Atrial fibrillation episodes lasting at least 15 seconds were recorded in RAAFT-1,17 and AF episodes lasting at least 60 seconds were recorded in MANTRA-PAF.18 Additional differences include the type of energy used (radiofrequency ablation vs cryoablation), ablation lesion sets (pulmonary vein isolation vs pulmonary vein isolation with additional lines), and whether irrigated or nonirrigated radiofrequency ablation catheters were used. Nevertheless, these minor variations had no apparent association with the proportional efficacy between the study groups as reported in our sensitivity analyses. Most trials (4 of 6) focused on AF recurrence of at least 30 seconds as a binary end point. The burden of AF measured as percentage of time may prove to be more clinically relevant. Moreover, because methods for AF detection have improved in the last decade with the availability of implantable loop recorders, monitoring AF burden may have increased value.
Clinical Implications
The finding that first-line catheter ablation was superior to drugs does not ipso facto indicate that all patients with AF should immediately undergo catheter ablation. Guidelines currently recommend catheter ablation to improve symptoms and quality of life for patients with paroxysmal AF, and the recommendations range from class I to IIA depending on prior intolerance or whether AAD use was unsuccessful.7,8 There is no guideline indication to perform ablation to reduce hard end points including major adverse cardiovascular events such as stroke and mortality. However, a new AF diagnosis is associated with high risk of mortality (approximately 49%), heart failure (approximately 14%), and stroke (approximately 7%) within 5 years.32 Some trials and observational studies indicate that catheter ablation may be associated with improvement in survival, including patients with heart failure and depressed ejection fraction,13,33,34 in addition to improving quality of life.
Catheter ablation is an invasive procedure with the potential for complications. The major adverse event rate observed in our meta-analysis was 4.2% in the ablation group and 2.8% in the standard therapy group (Table 2). While this finding was not statistically significant, catheter ablation may be disadvantaged by the temporal distribution of these events; the complications of ablation occur immediately, and those related to drugs occur over time (including additional events expected beyond the end of the trial). Moreover, major adverse events and mortality from long-term AADs are mostly related to persistent AF and structural heart disease.35 Thus, even with suboptimal efficacy, antiarrhythmic drugs are a reasonable option as first-line therapy, with a plan to consider catheter ablation if they fail. In contrast, considering the long-term outcomes of catheter ablation in being free from AF, hospitalization, repeated ablation, and overall clinical outcomes, future guidelines might accept this strategy as a first-line therapy for the management of paroxysmal AF. In addition, the importance of patient selection, operator experience, and shared decision-making interactions with patients should not be underestimated.36,37
Limitations
This study has several limitations. First, compared with real-world situations, patients included in this analysis were younger with minimal cardiovascular comorbidities, predominantly structurally normal hearts with normal left ventricular ejection fractions, and with a minimally larger left atrium diameter. Thus, it may be problematic to extrapolate these results to other patient populations. Second, there was heterogeneity regarding arrhythmia detection on follow-up. However, heterogeneity is unlikely to differentially affect one group over the other. Third, because patients and physicians were not blinded to the treatment assignment, it is possible that medical care was different postablation. Fourth, the included RCTs enrolled patients from 2005 to 2020, involving temporal changes in both invasive and drug therapy; however, in our sensitivity analysis, including patients in the last decade after removal of RAAFT-1 (published in 2005) did not alter the results. Fifth, the patients included in our analysis had only 1 to 2 years of follow-up, and our sensitivity analysis raises the possibility that effects of ablation diminished with time (1 vs 2 years) (eFigures 1 and 2 in the Supplement), although ablation was associated with lower recurrence of AF compared with drug therapy alone at both time points. Sixth, the favorable effects of catheter ablation observed in the real-world population may not accurately reflect these trial results. Seventh, supplementary ablation outside pulmonary vein isolation, performed in MANTRA-PAF18 and RAAFT-2,19 perhaps could have affected the clinical outcomes. However, in sensitivity analyses, after sequentially removing the 2 trials, no significant change was observed in outcomes. Eighth, although it is possible that the variation among clinical trials with regard to patients receiving additional ablation is a potential confounder, the 14% rate of additional ablation in the catheter ablation group was primarily associated with one trial, MANTRA-PAF,18 in which a large proportion of patients (47%) underwent subsequent ablation. Furthermore, in a sensitivity analysis removing MANTRA-AF, the rates of additional ablation were significantly lower with catheter ablation compared with drug therapy (eTable 10 in the Supplement). Finally, the absence of patient-level data prevented detailed assessment of baseline characteristics in relationship to clinical outcomes. However, the baseline characteristics of all the studies were well balanced in both groups.
Conclusions
In this meta-analysis of first-line therapy of patients with paroxysmal AF, compared with use of antiarrhythmic drugs, use of catheter ablation was associated with reductions in AF recurrence and hospitalizations, with no difference in major adverse events.
eMethods. Study Protocol and Data Collection Form
eReferences
eTable 1. Inclusion and Exclusion Criteria of The Included Studies
eTable 2. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Atrial Arrhythmia Recurrence Using The Cochran Risk Of Bias Tools
eTable 3. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Symptomatic AF Using The Cochran Risk Of Bias Tools
eTable 4. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Hospitalization Using The Cochran Risk Of Bias Tools
eTable 5. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Mortality Using The Cochran Risk Of Bias Tools
eTable 6. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Composite Of Major Adverse Events Using The Cochran Risk Of Bias Tools
eTable 7. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Outcomes Of Additional Ablation Using The Cochran Risk Of Bias Tools
eTable 8. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Outcomes Of Study Cross Over Using The Cochran Risk Of Bias Tools
eTable 9. Procedure Details Across the Included Trials
eTable 10. Results of The Sensitivity Analysis
eFigure 1. Forest Plot Demonstrating Atrial Arrhythmia Recurrence After Ablation vs AAD at 1-Year Follow Up
eFigure 2. Forest Plot Demonstrating Atrial Arrhythmia Recurrence After Ablation vs AAD at 2-Year Follow Up
eFigure 3. Forest Plot Demonstrating Study Cross Over After Ablation vs AAD
eFigure 4. Forest Plot Demonstrating Additional Ablation After Ablation vs AAD
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837-847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746-2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyse DG, Waldo AL, DiMarco JP, et al. ; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators . A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. doi: 10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
- 4.Van Gelder IC, Hagens VE, Bosker HA, et al. ; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group . A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834-1840. doi: 10.1056/NEJMoa021375 [DOI] [PubMed] [Google Scholar]
- 5.Carlsson J, Miketic S, Windeler J, et al. ; STAF Investigators . Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41(10):1690-1696. doi: 10.1016/S0735-1097(03)00332-2 [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Camm AJ, Goette A, et al. ; EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;2020.32860505 [Google Scholar]
- 9.Doyle JF, Ho KM. Benefits and risks of long-term amiodarone therapy for persistent atrial fibrillation: a meta-analysis. Mayo Clin Proc. 2009;84(3):234-242. doi: 10.4065/84.3.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy D, Talajic M, Dorian P, et al. ; Canadian Trial of Atrial Fibrillation Investigators . Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med. 2000;342(13):913-920. doi: 10.1056/NEJM200003303421302 [DOI] [PubMed] [Google Scholar]
- 11.Khan AR, Khan S, Sheikh MA, Khuder S, Grubb B, Moukarbel GV. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation: systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7(5):853-860. doi: 10.1161/CIRCEP.114.001853 [DOI] [PubMed] [Google Scholar]
- 12.Poole JE, Bahnson TD, Monahan KH, et al. ; CABANA Investigators and ECG Rhythm Core Lab . Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol. 2020;75(25):3105-3118. doi: 10.1016/j.jacc.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packer DL, Mark DB, Robb RA, et al. ; CABANA Investigators . Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. doi: 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisbal F, Alarcón F, Ferrero-De-Loma-Osorio A, et al. Diagnosis-to-ablation time in atrial fibrillation: a modifiable factor relevant to clinical outcome. J Cardiovasc Electrophysiol. 2019;30(9):1483-1490. doi: 10.1111/jce.14000 [DOI] [PubMed] [Google Scholar]
- 15.Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol. 2016;9(1):e003669. doi: 10.1161/CIRCEP.115.003669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew DS, Black-Maier E, Loring Z, et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol. 2020;13(4):e008128. doi: 10.1161/CIRCEP.119.008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634-2640. doi: 10.1001/jama.293.21.2634 [DOI] [PubMed] [Google Scholar]
- 18.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367(17):1587-1595. doi: 10.1056/NEJMoa1113566 [DOI] [PubMed] [Google Scholar]
- 19.Morillo CA, Verma A, Connolly SJ, et al. ; RAAFT-2 Investigators . Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311(7):692-700. doi: 10.1001/jama.2014.467 [DOI] [PubMed] [Google Scholar]
- 20.Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17(3):370-378. doi: 10.1093/europace/euu376 [DOI] [PubMed] [Google Scholar]
- 21.Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 22.Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 23.Velagic V, Pavlovic N, Chierchia GB, et al. . Cryoballoon catheter ablation versus antiarrhythmic drug as a first-line therapy for patients with paroxysmal atrial fibrillation: results of the Cryo-FIRST study. Circulation. 2020;142(suppl 3):A13915. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortuni F, Casula M, Sanzo A, et al. Meta-analysis comparing cryoballoon versus radiofrequency as first ablation procedure for atrial fibrillation. Am J Cardiol. 2020;125(8):1170-1179. doi: 10.1016/j.amjcard.2020.01.016 [DOI] [PubMed] [Google Scholar]
- 26.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607-611. doi: 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 27.Hartung J KG, Sinha BK. Statistical Meta-Analysis with Applications: Wiley & Blackwell; 2008. doi: 10.1002/9780470386347 [DOI] [Google Scholar]
- 28.Wilber DJ, Pappone C, Neuzil P, et al. ; ThermoCool AF Trial Investigators . Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303(4):333-340. doi: 10.1001/jama.2009.2029 [DOI] [PubMed] [Google Scholar]
- 29.Andrade JG, Macle L, Verma A, et al. ; CIRCA-DOSE Study Investigators . Quality of life and health care utilization in the CIRCA-DOSE Study. JACC Clin Electrophysiol. 2020;6(8):935-944. doi: 10.1016/j.jacep.2020.04.017 [DOI] [PubMed] [Google Scholar]
- 30.Packer DL, Kowal RC, Wheelan KR, et al. ; STOP AF Cryoablation Investigators . Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713-1723. doi: 10.1016/j.jacc.2012.11.064 [DOI] [PubMed] [Google Scholar]
- 31.Proietti R, Hadjis A, AlTurki A, et al. A systematic review on the progression of paroxysmal to persistent atrial fibrillation: shedding new light on the effects of catheter ablation. JACC Clin Electrophysiol. 2015;1(3):105-115. doi: 10.1016/j.jacep.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 32.Piccini JP, Hammill BG, Sinner MF, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35(4):250-256. doi: 10.1093/eurheartj/eht483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noseworthy PA, Gersh BJ, Kent DM, et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J. 2019;40(16):1257-1264. doi: 10.1093/eurheartj/ehz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turagam MK, Garg J, Whang W, et al. Catheter ablation of atrial fibrillation in patients with heart failure: a meta-analysis of randomized controlled trials. Ann Intern Med. 2019;170(1):41-50. doi: 10.7326/M18-0992 [DOI] [PubMed] [Google Scholar]
- 35.Kirchhof P, Andresen D, Bosch R, et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet. 2012;380(9838):238-246. doi: 10.1016/S0140-6736(12)60570-4 [DOI] [PubMed] [Google Scholar]
- 36.Deshmukh A, Patel NJ, Pant S, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128(19):2104-2112. doi: 10.1161/CIRCULATIONAHA.113.003862 [DOI] [PubMed] [Google Scholar]
- 37.Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111(9):1100-1105. doi: 10.1161/01.CIR.0000157153.30978.67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Protocol and Data Collection Form
eReferences
eTable 1. Inclusion and Exclusion Criteria of The Included Studies
eTable 2. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Atrial Arrhythmia Recurrence Using The Cochran Risk Of Bias Tools
eTable 3. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Symptomatic AF Using The Cochran Risk Of Bias Tools
eTable 4. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Hospitalization Using The Cochran Risk Of Bias Tools
eTable 5. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Mortality Using The Cochran Risk Of Bias Tools
eTable 6. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Composite Of Major Adverse Events Using The Cochran Risk Of Bias Tools
eTable 7. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Outcomes Of Additional Ablation Using The Cochran Risk Of Bias Tools
eTable 8. Detailed Risk Bias Assessment Of All The Included Trials For Reporting Outcomes Of Study Cross Over Using The Cochran Risk Of Bias Tools
eTable 9. Procedure Details Across the Included Trials
eTable 10. Results of The Sensitivity Analysis
eFigure 1. Forest Plot Demonstrating Atrial Arrhythmia Recurrence After Ablation vs AAD at 1-Year Follow Up
eFigure 2. Forest Plot Demonstrating Atrial Arrhythmia Recurrence After Ablation vs AAD at 2-Year Follow Up
eFigure 3. Forest Plot Demonstrating Study Cross Over After Ablation vs AAD
eFigure 4. Forest Plot Demonstrating Additional Ablation After Ablation vs AAD