Abstract
Background:
Preclinical drug self-administration procedures are commonly used to investigate expression, mechanisms, and treatment of substance use disorders.
New Method:
The aims were to back-translate an intravenous drug-vs-food choice procedure primarily utilized in monkeys to male and female rats and to develop a surgical method for sustained intravenous catheter patency suitable for long-term drug-choice studies.
Results:
The surgical protocol resulted in a median intravenous jugular catheter patency in male and female rats of 126 days (range: 25–365 days). Drug-vs-food choice was established with opioids (fentanyl and heroin), psychostimulants (cocaine, methamphetamine, and amphetamine), and an opioid/psychostimulant mixture (fentanyl+methamphetamine). The average time from catheter implantation to stable choice behavior across all drugs was 27 sessions (range: 16–44 sessions). Choice behavior stabilized more quickly for cocaine and fentanyl than for other drugs. Manipulations of both environmental variables (e.g., response requirement or food reinforcer magnitude) and pharmacological variables (e.g., extended access drug self-administration or continuous buprenorphine treatment via osmotic pump) significantly shifted opioid-vs-food choice consistent with previous monkey studies.
Comparison with existing methods:
Duration of intravenous catheter patency in rats was suitable for long-term, within-subject drug choice studies. Effects of environmental and pharmacological manipulations in rats confirmed and extended previous results from monkeys.
Conclusions:
The concordance of behavioral results between rats and monkeys using the present drug-vs-food choice procedure supports its utility to improve our basic understanding of the expression and mechanisms of substance use disorders towards to development of more effective therapeutics.
Keywords: drug self-administration, choice, fentanyl, heroin, cocaine, methamphetamine
1. Introduction
Substance Use Disorder (SUD) occurs in a commodity-rich and complex environment that includes access to both drugs of abuse (e.g., methamphetamine, heroin, alcohol) and nondrug reinforcers (e.g., social interaction and food). Because SUD is a pathology of behavior for which there are no accepted biomarkers, this psychiatric disorder is currently diagnosed based on behavioral criteria outlined in Diagnostic and Statistical Manual of Mental Health Disorders (Association, 2013). Thus, both preclinical and clinical research related to the expression, mechanisms, and treatment of SUD requires the inclusion of behavioral endpoints. Although there are numerous behaviors that can be examined in preclinical and clinical SUD research, drug self-administration procedures, in particular, allow for the direct measurement of volitional drug-taking behavior in both nonhumans and humans. Drug self-administration procedures that assess choice between a drug and a non-drug reinforcer offer additional interpretative power for at least two reasons. First, choice procedures model the concurrent availability of multiple reinforcers available to human drug users and permit assessment of variables that promote or retard drug choice at the expense of more adaptive behaviors maintained by nondrug reinforcers. Second, choice procedures provide dependent variables for both behavioral allocation (often expressed as % drug choice) and behavioral rate (often expressed as rate of responding or reinforcement). These two variables facilitate interpretation of effects produced by experimental manipulations, such that selective changes in drug reinforcement are indicated by shifts in drug choice, whereas nonselective changes in general motor competence or motivation are indicated by changes in behavioral rate.
For both rodents (Weeks, 1962) and nonhuman primates (Thompson and Schuster, 1964), the earliest intravenous (IV) drug self-administration experiments used simple “single-operant” schedules of reinforcement in which drug injection was the only reinforcer available and rate of responding or reinforcement was the only dependent measure. The technical demands of IV catheter maintenance limit catheter patency longevity and thus constrain the types of behaviors that can be trained and tested during the period of reliable catheter life. This technical constraint was overcome first in nonhuman primates, and the use of choice procedures emerged early and continue today as a sustained if muted theme (Aigner and Balster, 1978; Findley et al., 1972; Foltin et al., 2015; Gasior et al., 2004; Griffiths et al., 1975; Iglauer and Woods, 1974; Maguire et al., 2013; Nader and Woolverton, 1991; Negus, 2003). Despite the well-documented value of nonhuman primates in drug abuse research (Banks et al., 2017; Weerts et al., 2007), the translation of drug self-administration procedures from humans or monkeys to rodents may facilitate improved understanding of SUD towards the development of safer and more effective treatment strategies. Furthermore, the rapid expansion of molecular neuroscience tools affords unique research opportunities to investigators to combine sophisticated behavioral and neuroscience techniques in rodents that are currently either unavailable or not easily accessible to nonhuman primates.
Therefore, the aims of the present study were to 1) translate and 2) validate an IV drug-vs-food choice procedure originally developed for nonhuman primates to rats (Banks and Blough, 2015; Negus, 2006; Negus, 2003). The drug-vs-food choice procedure described in this manuscript varies from a published drug-vs-food choice procedure in rats that is also based on and originally developed for nonhuman primates (Thomsen et al., 2013; Thomsen et al., 2008). The Thomsen (2008) procedure used a chained schedule and an observing lever response link to initiate the concurrent “choice” schedule link; whereas the present study eliminated the chained schedule and observing response in an effort to shorten training time and align more with the nonhuman primate studies reference above. Towards that goal, a surgical procedure for IV jugular catheter implantation was developed and refined in an attempt to improve longevity in catheter patency modified from previously published techniques (Lenoir et al., 2013a; Thomsen and Caine, 2005) and our experience with venous catheter implantations in nonhuman primates. A total of five drugs of abuse: fentanyl, heroin, cocaine, methamphetamine, and amphetamine were trained in the drug-vs-food choice procedure to determine the broad applicability of the procedure across two pharmacological classes. Once drug-vs-food choice was established, environmental variables (e.g., food reinforcer magnitude, response requirement) and pharmacological variables (continuous buprenorphine treatment) were manipulated to validate translation to rats of published results in nonhuman primates (Nader and Woolverton, 1992; Nader and Woolverton, 1991; Negus, 2006; Negus, 2003). Furthermore, a final experiment determined behavioral interactions between fentanyl and methamphetamine reinforcement because of the rising clinical incidence of polysubstance opioid and psychostimulant abuse (Al-Tayyib et al., 2017; Karilsa et al., 2019; LaRue et al., 2019). For this experiment, a multi-modal drug self-administration procedure was used that included a 2h drug-vs-food choice session component (to assess behavioral allocation) and a 12h extended access drug self-administration component (to allow for increased drug intake) to model aspects of a similar multi-modal drug self-administration procedure in rhesus monkeys (Banks and Negus, 2010; Negus, 2006) and in rats studies with fentanyl alone (Townsend et al., 2019a).
2. Methods
2.1. Subjects
Sprague-Dawley female and male rats were acquired at weights of 240–260g (~12 weeks) and 290–310g (~11 weeks), respectively from a commercial supplier (Envigo, Frederick, MD, USA). Final sample sizes are reported for each experiment and ranged from 6 to 12. Rats were singly housed in a temperature and humidity-controlled vivarium to protect the vascular access port. Lights in the vivarium were programmed to a 12-h light/dark cycle (lights off at 6pm). Water and food (Tekland Rat Diet, Envigo) were provided ad-libitum in the home cage. Animal maintenance and research were conducted in accordance with the 2011 Guidelines of the National Institutes of Health Committee on Laboratory Animal Resources. Both research and enrichment protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
2.2. Intravenous catheter implantation
Rats were aseptically implanted with custom-made jugular catheters and commercially purchased vascular access ports (VABR1B/22, Instech Laboratories, Plymouth Meeting, PA). A list of the surgical instruments and catheter materials is shown in Supplemental Table 1, and Supplemental Figures 1 and 2 show the surgical instruments and method for the custom-made catheters. Prior to surgery, the rat was weighed and the cuffed end of the catheter was trimmed with a scalpel blade so that the distance from catheter tip to behind the first cuff equaled 1 cm for each 100 g of rat weight, with a maximal length of 4 cm. Rats were anesthetized with 2–3% isoflurane in oxygen, and the right neck and back areas were shaved and prepped for surgery with povidone and alcohol three times. An initial incision of ~0.75 cm in length was made directly above the visibly pulsating jugular vein, with the depth minimally sufficient to expose the muscle layer. Forceps were used to separate muscle fibers until the right jugular vein was visible. Subsequently, smaller forceps removed the fascia layer surrounding the vein, allowing for two 10 cm lengths of synthetic nonabsorbable suture (e.g., 21275126, Patterson Vet, Greeley, CO, USA) to be placed underneath a one cm length of vein. The ends of suture #1 were secured with hemostats and placed onto the ribcage (sterile drape placed between rat and hemostats), with the “v” of this suture loosely underlying the end of the vein segment furthest from the rat’s head. The ends of suture #2 were similarly secured with hemostats, which were placed just beyond the nose of the rat. The “v” of suture #2 was made taught underneath the end of the vein segment closest to the rat’s head, allowing for leverage to puncture the top exposed surface of the vein (20 g needle) and insert the custom-made polyurethane catheter (BTPU-040, Instech Laboratories). The distal end of the catheter away from the cuffs was connected to a 5 ml, saline-filled syringe with a luer stub. This saline-filled catheter had two 1-mm cuffs (BTSIL-047, Instech Laboratories) based on our nonhuman primate catheter design and similar to a previously published rat catheter design (Lenoir et al., 2013a). The weight-adjusted portion of the catheter and the cuff closest to the catheter tip was inserted into the vein toward the heart (i.e., toward the rat’s tail) with forceps and Dumont tweezers, such that only the cuff furthest from the catheter tip remained outside of the vein. Venous access was verified by filling the catheter with blood by gently pulling the plunger on the 5 ml syringe, followed by a 0.5 ml saline flush. Next, hemostats were removed from Suture #1 and a knot was placed snuggly around the vein and between the two cuffs without occluding the catheter. A knot was also tied around the uncatheterized portion of the vein with suture #2 to prevent unnecessary bleeding. An additional length of suture #3 was used to place a knot behind the cuff most distal from the catheter tip (i.e., the cuff not inserted into the vein), anchoring the catheter to nearby muscle tissue. Next, an incision (2.5 cm in length, minimally sufficient to expose the muscle layer) was made in the shaved skin of the back and 1 cm posterior to the scapulae. A subcutaneous pocket beneath the incision was opened using curved hemostats to accommodate the eventual implantation of the vascular access port (e.g., Model VABR1B/22, Instech Laboratories). Next, the unsecured end of the catheter was subcutaneously routed to the incision site using curved hemostats, clamped with non-serrated hemostats, trimmed such that approximately 5 cm of catheter material was exposed from the mid-scapular incision, and connected to the vascular access port with suture. As the vascular access port was subcutaneously inserted, excess catheter material (~ 5 cm) was looped under the skin to function as a “shock absorber” and protect against undesirable tension on the catheter, with the loop placed near the scapulae. Skin incisions were closed with suture material (e.g. SN-5698G, Covidien, Dublin, Ireland), and the catheter was flushed with 0.1 ml of gentamicin (4 mg/ml) followed by 0.1 ml of heparinized saline (30 U/ml). Subjects were administered ketoprofen (5 mg/kg, SC) immediately following surgery and 24h post-operatively. Rats were allowed to recover for five days prior to initiating self-administration training.
2.3. Apparatus and catheter maintenance
Modular operant chambers (ENV-007CT or ENV-008CT, Med Associates, St. Albans, VT) located in sound-attenuating cubicles (ENV-017M, ENV-018MD-W, or EMV-018MD, Med Associates) were equipped with two retractable levers (ENV-112CM, Med Associates) on the same side of the operant chamber, a set of three LED lights (ENV-222M, Med Associates) (red, yellow, green) mounted above each lever, and a retractable “dipper” (ENV-202M-S, Med Associates) cup (0.1 ml) located between the levers for presenting liquid food (0–100% v/v vanilla-flavored Ensure® in tap water, Abbott Laboratories, Chicago, IL). Corncob bedding was present in waste pans located under the stainless-steel rod floor of the operant chamber. IV drug infusions were delivered by activation of a syringe pump (PHM-100, Med Associates) located inside the sound-attenuating cubicle. Infusions were delivered through co-extruded 22-gauge PE/PVC tubing (BTCOEX-22, Instech Laboratories) attached via a 22-gauge luer stubs (LS22, Instech Laboratories). This tubing then connects to the top of a fluid swivel (375/22PS, Instech Laboratories) mounted on drug delivery arm (PHM-110-SAI, Med Associates) above the operant chamber. The bottom of the fluid swivel was connected to a 1-channel magnetic tether (VABR1TH/22, Instech Laboratories) that passed through a hole in the operant chamber roof and terminated in a magnetic injector that was inserted into the vascular access port. Behavioral sessions were operated by custom programs written for Med-PC IV (Med Associates), and the drug-vs-food choice Med-PC IV program used in our laboratory can be found in Supplemental Materials. After each behavioral session, catheters were flushed with 0.1 ml of gentamicin (4 mg/ml) followed by 0.1 ml of heparinized saline (30 U/ml). Catheter patency was verified at the end of each experiment by instantaneous muscle tone loss following IV methohexital (1.6 mg) administration.
2.4. Drug-vs-food choice training
At least five days were allowed following internal jugular catheterization surgery to ensure the vascular access port surgical site had healed enough to support the tension of the tether. Rats were trained on the terminal 2-h drug-vs-food choice procedure using a series of steps described below. First, rats are trained to respond for IV drug infusions under an initial fixed-ratio (FR)1 / 20-s time out schedule of reinforcement during daily 2-h behavioral sessions. Each session began with a non-contingent drug infusion followed by a 60-s time out. The response period was signaled by extension of only the right lever and illumination of the right green stimulus light. Following each response requirement completion, the lever retracted, the green light was extinguished, and an IV drug infusion was administered. Initial drug self-administration training doses were: fentanyl (3.2 μg/kg/infusion), heroin (0.032 mg/kg/infusion), cocaine (0.32 mg/kg/infusion), methamphetamine (0.1 mg/kg/infusion), and amphetamine (0.1 mg/kg/infusion). Initial drug doses were based on our published (Townsend et al., 2019b) and unpublished rat self-administration data from dose-effect functions under an FR5 schedule of reinforcement. Selected doses were the first half-log dose on the descending limb of the dose-effect function following the peak dose. This schedule of reinforcement remained until the number of injections earned was >10 injections per 2-h session for at least three consecutive days. Once rates of drug self-administration were stable, the FR requirement was steadily increased over consecutive days from FR1 to FR2 and FR3 until the terminal FR5 schedule was reached. Rats remained on the FR5 schedule for at least five days. Second, rats were trained to respond on the opposite lever for 0.1 mls of diluted liquid food (either 18% or 32% vanilla-flavored Ensure, Abbot Laboratories, Chicago, IL) presentations using a switchable liquid dipper for rats (Model ENV-202M-S, Med Associates) fitted with a 0.1 ml cup (ENV-202C-10). Two different concentrations were used because Abbott changed the Ensure formulation/taste of Ensure in 2019 which resulted in 18% no longer maintaining reliable operant responding. As in Step 1, the response requirement was initially FR1 and those conditions were implemented for at least one day. If high rates of behavior were observed on the food-associated lever (>100 responses), then the FR was increased to the terminal FR5 schedule for at least one day.
Third, once both drug and food-maintained responding had been established separately, rats were then trained on the drug-vs-food choice procedure with exposure to the terminal drug-choice conditions and no intermediary steps. This was similar to our training of a within-session drug-vs-food choice in rhesus monkeys (Banks and Blough, 2015; Negus, 2006; Negus, 2003). The terminal drug-choice session consisted of five 20-min response components each preceded by a 4-min “sample” component. Each sample component started with a non-contingent infusion of the unit drug dose available during the subsequent response component followed by a 2-min time out. Next, a 5-s presentation of liquid food was programmed followed by a 2-min time out. Following this second time out, the response component would begin. During each response component, both levers were extended, a red stimulus light above the left lever was illuminated to signal liquid food availability and a green stimulus light above the right lever was illuminated to signal IV drug availability. Response-requirement (FR5) completion on the left lever resulted in a 5-s presentation of liquid food, whereas response-requirement (FR5) completion on the right lever resulted in infusion of the IV drug dose available for that component. Responding on one lever reset the ratio requirement for the other lever. The liquid-food concentration was held constant across components. A different drug dose was available during each of the five successive response components (e.g., 0, 0.32, 1.0, 3.2, and 10 μg/kg/infusion fentanyl during components 1–5, respectively). Drug dose was varied by changing the infusion duration (300g rat; 0, 0.5, 1.56, 5, and 15.6-s of pump activation during components 1–5, respectively) and visually signaled by the frequency of the flashing of the right green light above the drug-associated lever in 3-s cycles (component 1: off; component 2: on for 0.1 s and off for 2.9 s; component 3: on for 0.3 s and off for 2.7 s; component 4: on for 1 s and off for 2 s; component 5: on) similar to our rhesus-monkey drug-vs-food choice studies. During each response component, rats could complete up to 10 total ratio requirements between the food- and drug-associated levers. Each ratio requirement completion initiated a 20-s time out, the retraction of both levers, and extinction of the red and green stimulus lights. If all 10 ratio requirements were completed before 20 min had elapsed, then both levers retracted, and stimulus lights were extinguished for the remainder of that response component. Drug-vs-food choice behavior was considered stable when the smallest drug dose maintaining ≥80% drug choice did not change ≥0.5 log units over three consecutive days. In instances where rats did not allocate ≥80% of behavior towards drug injections during any response component, interventions were imposed for 1–2 experimental sessions to promote responding on the drug-associated lever. These interventions included increasing the response requirement for the food reinforcer (e.g., FR30) or substituting water for liquid food as the alternative reinforcer. Using the rats included in Table 1 as an example, these training interventions were successfully implemented in 6/12 fentanyl rats, 5/12 methamphetamine rats, 5/12 heroin rats, and 3/9 amphetamine rats. Training interventions were not required for the cocaine or the 1:54 fentanyl/methamphetamine rats included in Table 1.
Table 1:
Group mean (± SD) number of sessions under each of the training conditions until drug-vs-food choice in male and female rats was stable for each of the self-administered drugs listed.
| Self-administered Drug | Drug Only Sessions | Food Only Sessions | Choice Sessions | Total Sessions |
|---|---|---|---|---|
| Cocaine (n=10) | 12 (± 4) | 4 (± 1) | 5 (± 0) | 21 (± 5) |
| Fentanyl (n=12) | 12 (± 4) | 4 (± 1) | 9 (± 5) | 25 (± 5) |
| Methamphetamine (n=12) | 12 (± 4) | 3 (± 0) | 11 (± 5) * | 26 (± 8) |
| Amphetamine (n=9) | 13 (± 2) | 3 (± 1) | 15 (± 6) *,# | 30 (± 6) * |
| Heroin (n=12) | 12 (± 4) | 4 (± 3) | 16 (± 6) *,# | 32 (± 6) *,# |
| 1:54 Fentanyl/Methamphetamine (n=10) | 8 (± 2) | 4 (± 2) | 6 (± 2) | 17 (± 6) |
The sample size for each of the different self-administered drugs is shown.
denote statistical significance (p < 0.05) compared to cocaine or 1:54 fentanyl/methamphetamine and
denotes statistical significance compared to fentanyl.
2.5. Environmental manipulations
Three types of environmental manipulations were performed under conditions of fentanyl- or heroin-vs.-food choice. In experiment #1, the role of discriminative and consequent stimuli in the drug-vs-food choice procedure were examined in six rats (4 females and 2 males). Three different manipulations were conducted across consecutive weeks: 1) removal of non-contingent fentanyl infusions before each response component, 2) removal of response-contingent fentanyl infusions during the response component, and 3) removal of the visual discriminative stimulus on the fentanyl-associated lever. Each experimental week consisted of a baseline fentanyl-vs-food choice session on Monday and one of the experimental manipulations described above for behavioral sessions conducted Tuesday-Friday. The order of these three experimental manipulations was counterbalanced across different rats and the liquid-food concentration was 18%. Data are presented as the average of each 4-day experimental manipulation and the average of all baseline Monday behavioral sessions before each weekly experimental manipulation.
For experiment #2, the effects of manipulating the liquid-food concentration were examined on fentanyl-vs-food choice in 12 rats (6 males and 6 females) and on heroin-vs-food choice in eight rats (3 males and 5 females). Rats were initially trained on the fentanyl-vs-food and heroin-vs-food choice procedure with 18% liquid food. Subsequently, the effects of decreasing (i.e., 1.8%) or increasing (i.e., 100%) the liquid food concentration on opioid-vs-food choice were examined. Each liquid food concentration manipulation was implemented for two days, with data collected on the second day used for subsequent analyses. Rats were then returned to initial liquid food (i.e., 18%) conditions for at least three days and until choice behavior was stable and returned to pre-test levels. Liquid-food concentration manipulations were counterbalanced across rats.
In experiment #3, the effects of manipulating the response requirement on the heroin or food-associated lever were examined in six rats (3 males and 3 females). The liquid-food concentration was 18% for these experiments. For heroin FR (FRheroin) manipulations, the FR on the food associated-lever was held constant at FR5 while the FR on the heroin- associated lever was varied from FR 1 to FR 100. For food FR (FRfood) manipulations, the FR on the drug associated-lever was held constant at FR5 while the FR on the food- associated lever was manipulated from FR 1 to FR 30. Each manipulation was implemented for three consecutive days and data presented are an average of the 3-day period. After each manipulation, baseline FR5: FR5 choice behavior was reestablished in each rat. Baseline FR values were reinstituted for at least three days and until baseline heroin choice stabilized at pre-test levels.
2.6. Pharmacological manipulations
The effects of two different pharmacological manipulations were determined on drug-vs-food choice. For experiment #4, continuous 0.01 – 0.32 mg/kg/h buprenorphine treatment effects on heroin-vs-food choice were determined in six rats (3 females and 3 males). The liquid-food concentration was 18% for these heroin-choice studies. Continuous buprenorphine treatment was achieved with osmotic minipumps (Model 2001 and 2ML1, Alzet, Cupertino, CA, USA) aseptically implanted into a subcutaneous space on the right or left lower flank. Model 2001 pumps delivered vehicle (15% ethanol, 20% DMSO, and 65% sterile water) and the lower buprenorphine doses (0.01 and 0.032 mg/kg/h). Model 2ML1 pumps delivered the higher buprenorphine doses (0.1 mg/kg/h and 0.32 mg/kg/h). Osmotic pumps were implanted on Friday afternoons, and daily heroin-vs-food choice sessions were conducted the following Monday-Thursday. Pumps were aseptically removed on Friday. Data presented are an average of the last three days of pump implantation. All rats received all buprenorphine doses except for one male rat that received only the three smallest buprenorphine doses. After pump removal, heroin-vs-food choice sessions were conducted for at least one week and continued until choice behavior was stable and returned to pre-test conditions. This series of buprenorphine treatment and non-treatment conditions continued until vehicle and all buprenorphine doses were tested. In general, buprenorphine doses were tested in a counterbalanced order, with the exception that 0.32 mg/kg/h was tested as the final dose in all rats because baseline heroin-vs-food choice behavior took several weeks to recover following 0.1 mg/kg/h buprenorphine treatment.
Experiment #5 determined fentanyl and methamphetamine interactions using a multi-modal drug self-administration procedure that retained daily 2-h drug-vs-food choice sessions and added a daily 12-h extended-access drug self-administration session. This is a procedure we have used previously to evaluate effects of exposure to and withdrawal from extended fentanyl access on fentanyl-vs.-food choice (Townsend et al., 2019a). Eleven rats (6 males and 5 females) were trained to self-administer a 1:54 fixed-proportion fentanyl/methamphetamine mixture based on the relative potency (ED50 value) of each drug alone in the drug-vs-food choice procedure using the same methods described above. Once fentanyl/methamphetamine-vs-food choice dose-effect functions were stable as described above with 18% liquid food, 12-h extended access fentanyl/methamphetamine self-administration sessions were scheduled to begin at 6pm on Sunday-Thursday each week and continue until 6am the next morning. During this extended access component, 1:54 μg/kg/infusion fentanyl/methamphetamine was available under an FR5 / 20-s time out schedule of reinforcement. Fentanyl/methamphetamine choice sessions were conducted from 2–4pm Monday-Friday, and thus began 8 h after each extended-access session. This sequence of 12-h extended access and 2-h choice behavior sessions was continued for 14 days. In addition, rats were observed 5-min before each fentanyl/methamphetamine-vs-food choice session and scored for the presence of six somatic opioid withdrawal behavioral signs over 30 seconds (Cobuzzi and Riley, 2011; Stephens and Riley, 2009). The behaviors scored were tremor, teeth chatter, eye twitch, mastication, yawn, wet dog shake, piloerection, ptosis, and presence of diarrhea on the rat. Using this behavioral scoring system, the maximum value for any behavior was 1 and a maximum score was 9.
2.7. Data analysis
The primary dependent measures for each component of the drug choice session were 1) percent drug choice, defined as {(number of ratio requirements, or ‘choices’, completed on the drug-associated lever ÷ total number of choices completed on both the drug- and food-associated levers) × 100}, and 2) reinforcement rate defined as total number of choices completed. Additional dependent measures were percent session drug choice, defined as {(number of drug choices completed for the entire session ÷ total number of drug and food choices completed for the entire session) × 100} and total, food, and drug choices completed during the entire 2-h session. For the extended access drug self-administration sessions, the primary dependent measure was the number of injections earned. Data were analyzed using one-way or two-way repeated-measures ANOVA with self-administered drug dose and experimental manipulation as the main factors, and the Geisser-Greenhouse correction was utilized when appropriate for within-subject analyses (Prism 8, GraphPad, La Jolla, CA, USA). Somatic opioid withdrawal signs were analyzed using the non-parametric Friedman test and Dunn’s multiple-comparisons post-hoc test because this dependent measure violates parametric statistic assumptions. Significant main effects or interactions were followed by post-hoc tests appropriate for the pre-planned comparisons and corrected for multiple comparisons. Significance was established a priori at the 95% confidence level (p<0.05).
2.8. Dose Addition analysis
The ED50 values for fentanyl and methamphetamine alone were defined as the dose that produced 50% choice and were calculated using non-linear regression (Prism 8, GraphPad). For the 1:54 fentanyl/methamphetamine mixture, the ED50 value was defined as the dose of each drug in the mixture that produced 50% choice. In addition, a related quantity, Zmix, was also calculated as the total drug dose (i.e., dose fentanyl + dose methamphetamine) that produced 50% choice. Drug interactions were assessed using both graphical and statistical approaches to dose-addition analysis (Tallarida, 2000; Wessinger, 1986; Woolverton, 1987) as described previously (Negus, 2005; Stevenson et al., 2003). Graphically, mean ED50 values for fentanyl choice alone or as part of the mixture were plotted as a function of ED50 values for methamphetamine choice alone or as part of the mixture. This data presentation is known as an isobologram, and the line that connects the data points for each drug alone shows predicted data for drug mixtures assuming additivity of drug effects. Points that fall below the line are suggestive of super-additivity or synergism and points that fall above the line are suggestive of sub-additivity or antagonism. Statistical evaluation of fentanyl/methamphetamine interactions was accomplished by comparing the experimentally determined ED50 value for the fentanyl/methamphetamine mixture (Zmix) with the predicted additive ED50 value (Zadd) as described by Tallarida (2000). Zmix was determined empirically as described above. Zadd was calculated from the equation Zadd=fA+(1–f)B, where A was the ED50 for fentanyl alone, B was the ED50 for methamphetamine alone, and f was the fractional multiplier of A in the computation of the additive total dose. Any choice of f is related to the proportion of drug A (ρA) in a mixture according to the equation ρA = fA/Zadd. This study tested a mixture that yielded a value of f=0.5. Zmix and Zadd values were determined to be different if the 95% confidence limits were non-overlapping (i.e., p<0.05).
2.9. Drugs
Fentanyl HCl, (−)-Cocaine HCl, and heroin HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD, USA). (+)-Amphetamine hemisulfate and (+)-methamphetamine HCl were purchased from a commercial supplier (Millipore Sigma, St. Louis MO, USA). Buprenorphine HCl was also purchased from a commercial supplier (Spectrum Chemicals, Gardena, CA, USA) and dissolved in 15% ethanol, 20% DMSO (Fisher Scientific, Waltham, MA, USA), and 65% sterile water for injection. All other drugs were dissolved in bacteriostatic sterile saline. All solutions were passed through a 0.22-micron sterile filter (Model #SLGV033RS, Millipore Sigma) before IV or subcutaneous (SC) administration.
3. Results
3.1. Catheter patency longevity
The records from the first 105 rats implanted with their first jugular catheters in the laboratory by three different lab members were retrospectively surveyed for catheter patency longevity. The median first catheter patency was 126 days, the mode was 144 days, and the range was 25 to 365 days. For the experiments reported in this manuscript, a second catheter implantation surgery was not necessary. However, in some of our other published studies, when the first catheter failed, a second catheter was successfully implanted into the left internal jugular vein and attached to the exist vascular access port to complete the experiments.
3.2. Drug-vs-food choice training
Figure 1 shows drug-vs-food choice dose-effect functions in male and female rats with two mu-opioid receptor agonists (A: fentanyl, C: heroin) and three monoamine transporter ligands (E: cocaine, G: methamphetamine, I: amphetamine). Table 1 shows the average number of sessions on drug only training, food only training, and drug-food choice training until stability for each drug. The liquid-food concentration was 18% liquid food for fentanyl and methamphetamine, 18% or 32% for heroin, and 32% only for cocaine and amphetamine. The median number of sessions until choice behavior was stable was 26 sessions, the mode was 25 sessions, and the range was 16 to 44 sessions. There were significant differences between the different drugs on the number of choice training sessions until stability (F5,79 = 11.2, p<0.0001). For example, training of cocaine choice was significantly faster than training heroin, methamphetamine, and amphetamine choice. Training of fentanyl and 1:54 fentanyl/methamphetamine choice occurred significantly faster than training heroin and amphetamine choice. There were also significant differences in the number of total training sessions (F5,79 = 6.0, p<0.0001), with cocaine, fentanyl, and 1:54 fentanyl/methamphetamine being significantly faster than heroin. Cocaine and 1:54 fentanyl/methamphetamine were also significantly faster than amphetamine. There were no significant differences between the number of drug-only and food-only training sessions between the five different self-administered drugs. Furthermore, there were no sex differences observed during any of the training conditions. Infrequently, rats would experience health issues that precluded further self-administration training before stable choice behavior was established. In the present studies, the number of rats that successfully completed the experiments out of the total number of rats that were successfully trained on drug-vs-food choice were: cocaine (10/10), fentanyl (12/15), methamphetamine (12/14), amphetamine (9/10), heroin (12/15), and fentanyl/methamphetamine (10/10). Moreover, with the exception of heroin, we have been able to establish stable drug choice behavior in all rats that have started the training procedure described in this manuscript. For heroin, there have been 10 out of 47 rats that have failed to acquire stable heroin-vs.-food choice behavior.
Figure 1:
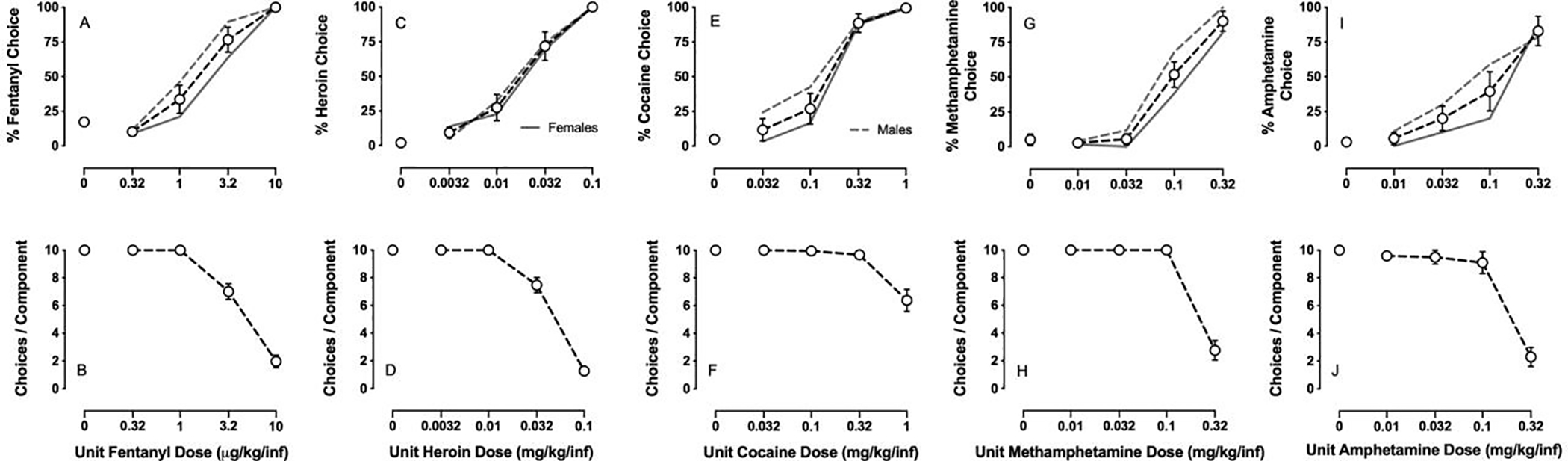
Demonstration of intravenous (IV) drug-vs-food choice with (A, B) fentanyl, (C, D) heroin, (E, F) cocaine, (G, H) methamphetamine, and (I, J) amphetamine in male and female rats. Top panels show percent drug choice as a function of unit drug dose in either micrograms or milligrams per kilogram per infusion. Bottom panels show the number of choices completed per component as a function of the unit dose drug available as the alternative to liquid food (18% for fentanyl and methamphetamine; 18 and 32% for heroin; 32% for cocaine and amphetamine). All points represent the mean ± s.e.m. of the follow group sizes: fentanyl (12 rats); heroin (12 rats); cocaine (10 rats); methamphetamine (12 rats); amphetamine (9 rats). Dashed gray line shows mean data from males whereas solid gray line shows mean data from females.
3.3. Environmental manipulations on drug-vs-food choice
Figure 2 shows effects of removing the visual discriminative stimuli, the non-contingent fentanyl infusion, or the contingent fentanyl infusions on choice between fentanyl and 18% liquid food. Selective removal of either the non-contingent fentanyl infusions before the response component or removal of the contingent fentanyl infusions during the response component significantly decreased behavioral allocation to the fentanyl-associated lever (fentanyl dose/component: F1.1,5.6=59.5, p=0.0003; manipulation: F1.4,7.2=16.5, p=0.003; interaction: F3.2,13=6.5, p=0.006). Selective removal of fentanyl-associated visual stimuli had no effect on percent fentanyl choice. Selective removal of either the non-contingent fentanyl infusions before the response component or removal of the contingent fentanyl infusions during the response component also significantly increased the number of choices completed in the last two choice-session components (fentanyl dose/component: F1.8,8.9=28.2, p=0.0002; manipulation: F1.6,8=13.9, p=0.003; interaction: F2.6,10.9=17.7, p=0.0002).
Figure 2:
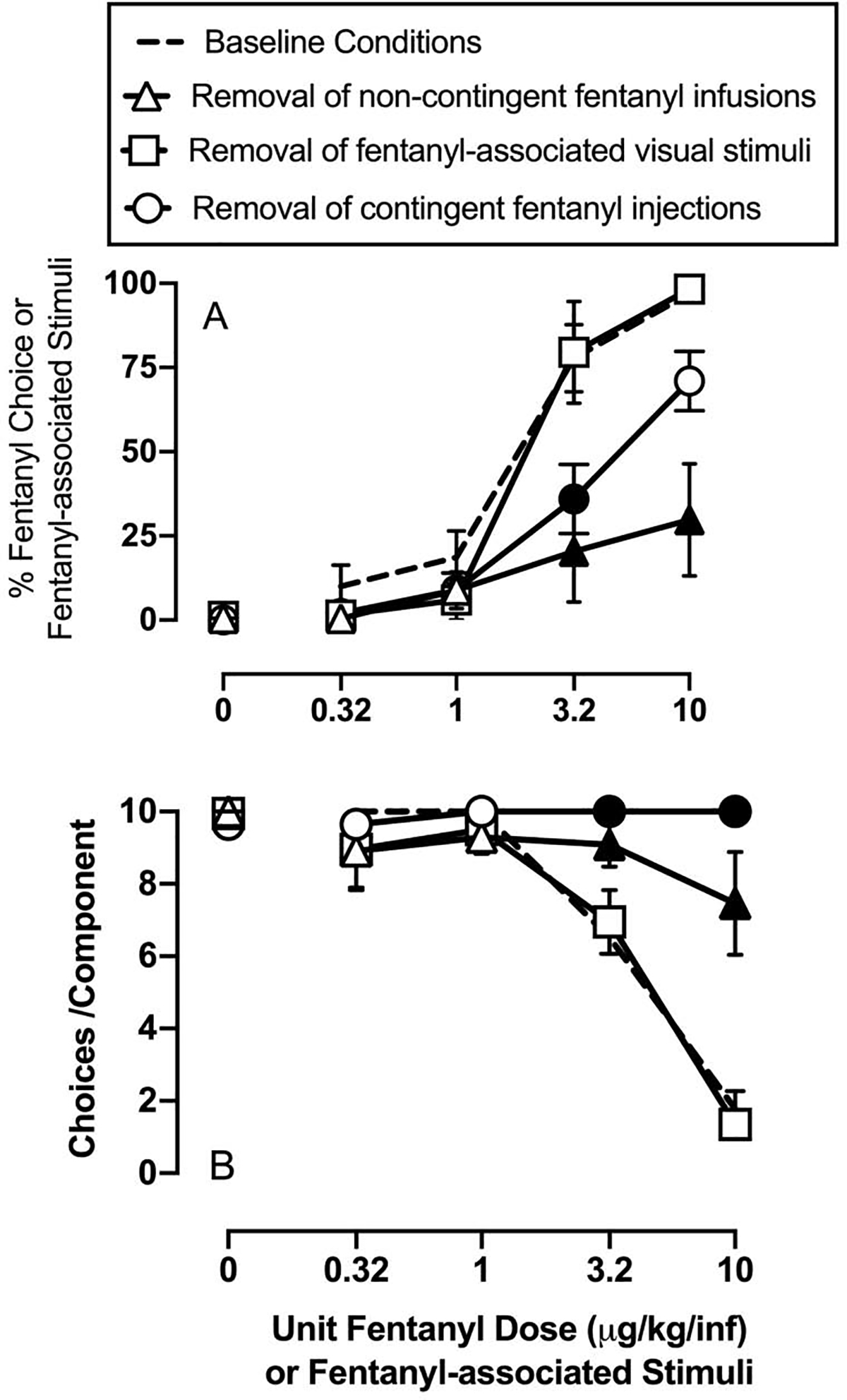
Effects of discriminative- and consequent-stimulus manipulations on fentanyl-vs-food choice in male and female rats. Top panel shows percent choice of fentanyl or fentanyl-associated stimuli as a function of unit fentanyl dose in micrograms per kilogram per infusion. Bottom panels show the number of choices completed per component as a function of the unit fentanyl dose or fentanyl-associated stimuli available as the alternative to 18% liquid food. All points represent the mean ± s.e.m. of 6 rats. Filled symbols denote statistical significance (p<0.05) compared to baseline conditions.
Figure 3 shows effects of manipulating the liquid-food concentration on both fentanyl-vs-food choice (Panels A and C) and heroin-vs-food choice (Panels B and D). Decreasing the liquid-food concentration significantly decreased food choice and produced a reciprocal increase in fentanyl choice, whereas increasing the food concentration significantly increased food choice and decreased fentanyl choice (fentanyl dose: F1.3,14.7 = 104.4, p<0.0001; food concentration: F2.0, 21.4 = 51.1, p<0.0001; interaction: F3.1,34.1 = 5.0, p=0.005). Decreasing the liquid-food concentration also resulted in a decrease in the number of choices completed per component during early components when rats primarily chose food (fentanyl dose: F1.7,18.6 = 219.6, p<0.0001; food concentration: F1.2, 13.3 = 12.6, p=0.003). Because there was no significant fentanyl dose × liquid-food concentration interaction, the results defaulted to a one-way repeated-measures ANOVA for food concentration collapsed across fentanyl doses, and choices completed during availability of 1.8% liquid food was significantly lower compared to 100% liquid food (food concentration: F1.7, 5.1 = 11.6, p=0.014). For the heroin-vs-food choice studies, there was a significant main effect of heroin dose (F1.9,13 = 52.8, p<0.0001) and food concentration (F1.9,13.3 = 10.3, p=0.002) on choice behavior, but no significant heroin dose × food concentration interaction. Thus, the statistical analysis defaulted to a one-way repeated-measures ANOVA for liquid-food concentration manipulations collapsed across heroin doses. There was a significant main effect of food concentration (F1.5,4.5 = 8.0, p=0.037), and heroin choice tended to decrease as liquid-food concentration increased; however, no food concentration was significantly different from any other food concentration after correcting for multiple comparisons upon post-hoc analysis. Manipulating the food concentration did significantly decrease the number of choices completed during early session components (e.g., 0.0032 and 0.01 mg/kg/infusion heroin) when 1.8% liquid food was available and significantly increased the number choices completed during the 0.1 mg/kg/infusion heroin component when 100% liquid food was available (food concentration: F1,7.2 = 15.6, p=0.005; interaction: F2,14.2 = 9, p=0.003).
Figure 3:
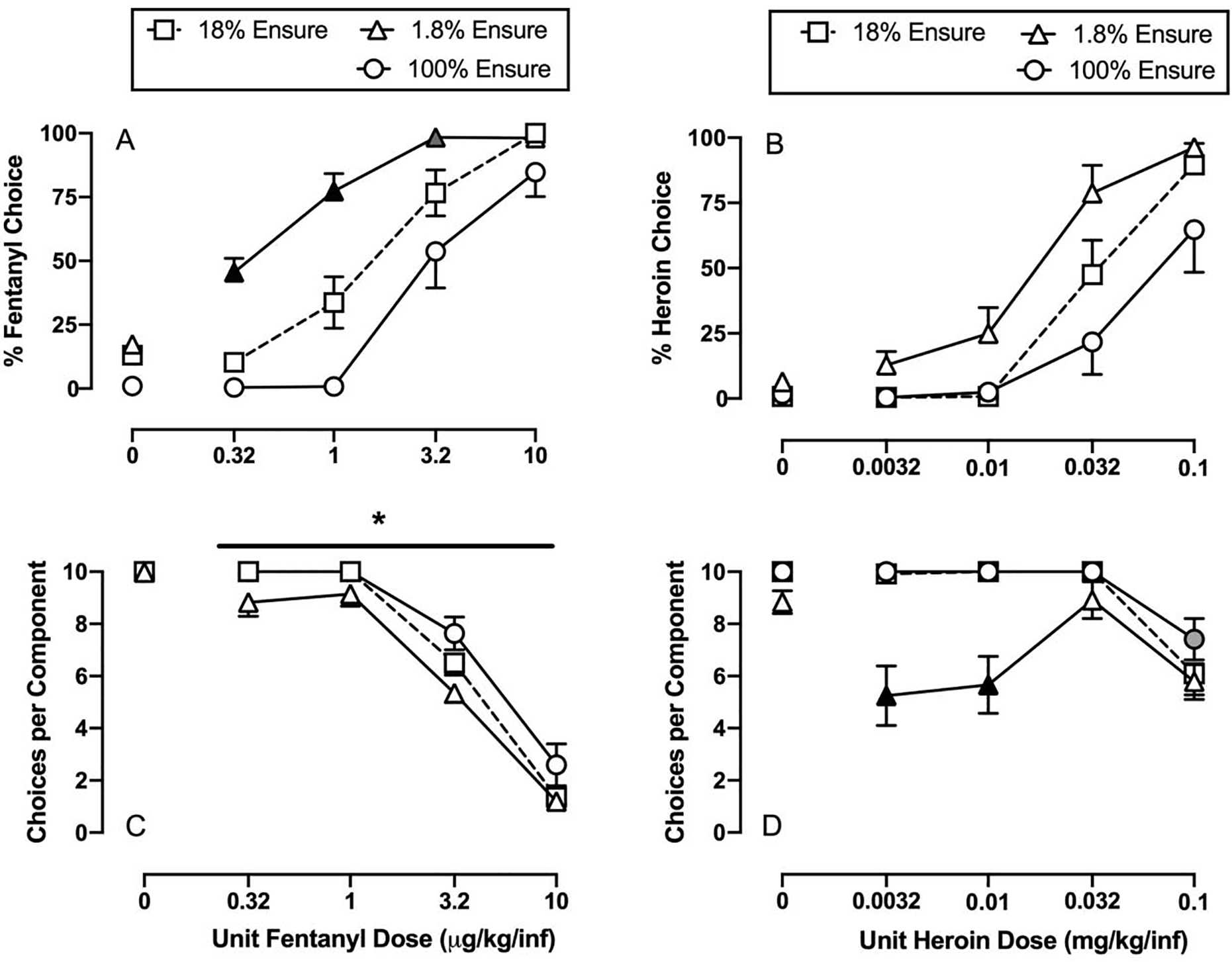
Food reinforcer-magnitude manipulations on fentanyl-vs-food choice (Panels A and C) and heroin-vs-food choice (Panels B and D) in male and female rats. Top panels show percent drug choice as a function of unit drug dose in either micrograms or milligrams per kilogram per infusion. Bottom panels show the number of choices completed per component as a function of the unit dose drug available as the alternative to liquid food (1.8–100%). All points represent the mean ± s.e.m. of 12 rats in the fentanyl experiments and 8 rats in the heroin experiments. Black filled symbols denote statistical significance (p<0.05) compared to all other liquid food concentrations and gray filled symbols denote statistical significance compared to 18% liquid food only. Asterisk denotes statistical significance between 1.8% and 100% liquid food regardless of unit fentanyl dose.
Figure 4 shows effects of manipulating the response requirement on either the heroin- or food-associated lever on behavioral allocation between IV heroin infusions and 18% liquid food. Panels A and B show the effects of manipulating the FR response requirement on the heroin-associated lever (FRheroin) while holding the FR requirement on the food-associated lever constant at FR 5. Increasing FRheroin produced systematic rightward/downward shifts in the heroin choice dose-effect function (FRheroin: F2.9,58.4 = 42.4, p<0.0001; interaction: F12,80 = 9.4, p<0.0001). Increasing the FRheroin to 10 and 30 significantly decreased 0.032 mg/kg/infusion heroin choice, and increasing the FRheroin to 100 significantly decreased both 0.032 and 0.1 mg/kg/infusion heroin choice. The number of choices completed per component increased with increasing FRheroin values as rats reallocated their responding away from heroin and toward food (FRheroin: F2.3,45.4 = 29.6, p<0.0001; interaction: F12,80 = 18.3, p<0.0001). Increasing the FRheroin to 100 resulted in a significant increase in the number of choices completed per component during 0.1 mg/kg/infusion heroin availability. Panels C and D show the effects of manipulating the FR response requirement on the food-associated lever (FRfood) while holding the FR requirement on the heroin- associated lever constant at FR 5. Increasing the FRfood produced a leftward/upward shift in the heroin choice dose-effect function (FRfood: F2.6,51.1 = 37.4, p<0.0001; interaction: F9,60 = 4.9, p<0.0001). Increasing the FRfood to 30 significantly increased 0.032 and 0.01 mg/kg/infusion heroin choice. The number of choices completed per component significantly decreased with increasing FRfood (FRfood: F1.5,29 = 20.9, p<0.0001; interaction: F9,60 = 2.8, p=0.008). Increasing the FRfood to 30 significantly decreased the number of choices completed during components when unit doses of 0 and 0.0032 mg/kg/infusion heroin were available.
Figure 4:
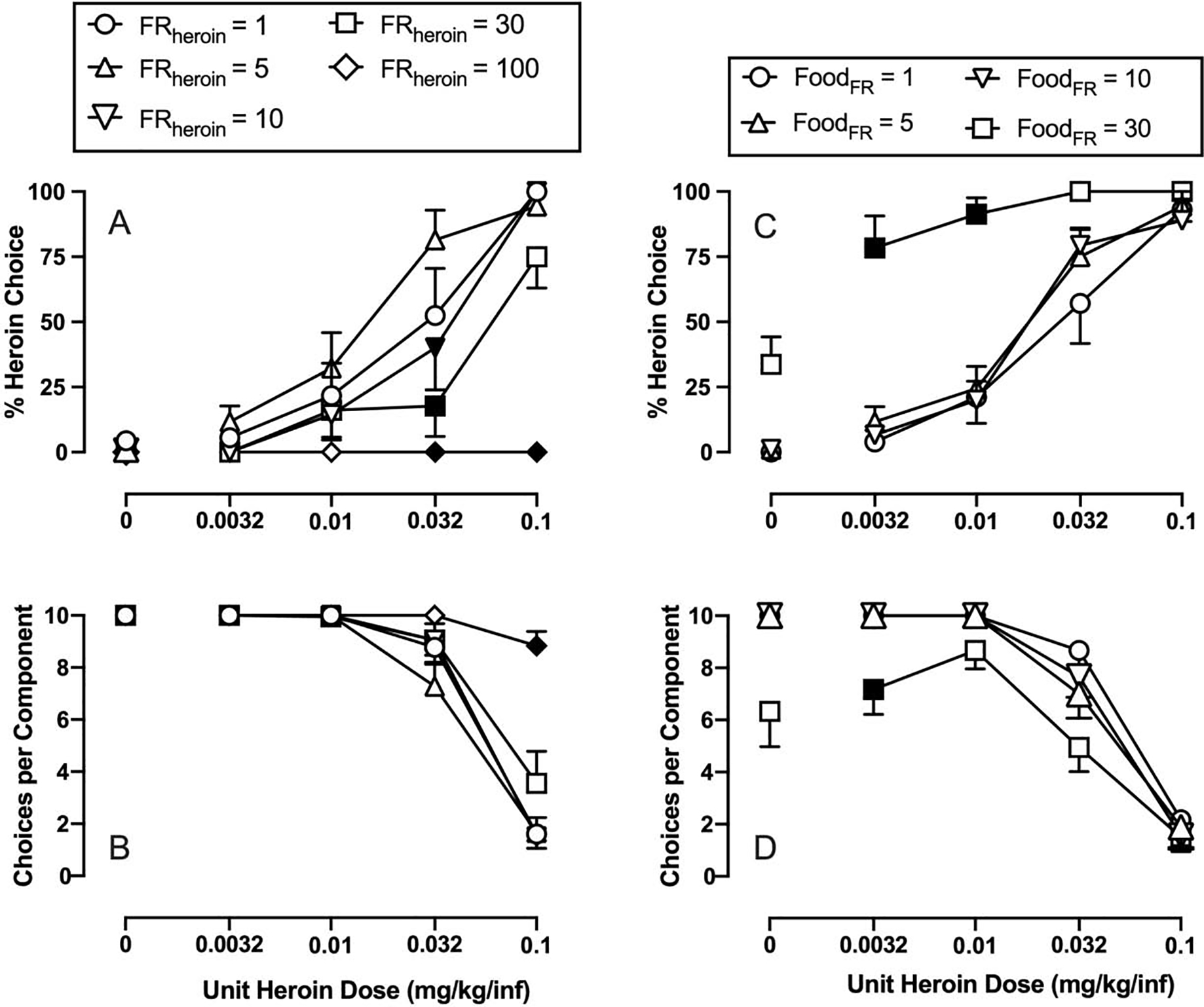
Response-requirement manipulations on either the heroin-associated lever (Panels A and B) or food-associated lever (Panels C and D) on heroin-vs-food choice in male and female rats. Top panels show percent heroin choice as a function of unit heroin dose in milligrams per kilogram per infusion. Bottom panels show the number of choices completed per component as a function of the unit heroin drug dose available as the alternative to 18% liquid food. All points represent the mean ± s.e.m. of 6 rats. Filled symbols denote statistical significance (p<0.05) compared to baseline conditions (i.e. FR5 for both liquid food and heroin).
3.4. Pharmacological manipulations on drug-vs-food choice
Figure 5 shows effects of continuous 7-day vehicle and buprenorphine treatment on heroin-vs-18% food choice. Relative to vehicle treatment, buprenorphine treatment significantly decreased heroin-vs-food choice and produced a rightward/downward shift in the heroin choice-dose effect curve (heroin dose: F1.5,7.4 = 38.7, p=0.0002; buprenorphine dose: F2.7,13.5 = 13.5, p=0.0003). Because there was no significant heroin dose × buprenorphine dose interaction, the statistical analysis defaulted to a one-way repeated-measures ANOVA collapsed across heroin doses, and 0.032 and 0.32 mg/kg/h buprenorphine doses significantly decreased heroin choice compared to vehicle (buprenorphine dose: F1.5,4.6 = 14.6, p=0.012). Panel B shows that 0.1 and 0.32 mg/kg/h buprenorphine treatment significantly increased the number of choices completed per component during 0.1 mg/kg/infusion unit heroin dose availability as subjects reallocated their responding away from heroin and toward food (buprenorphine dose: F2.5,12.5 = 11, p=0.001; heroin dose: F1.3,6.7 = 29.2, p=0.0008; interaction: F2.9,13.5 = 7.1, p=0.005).
Figure 5:
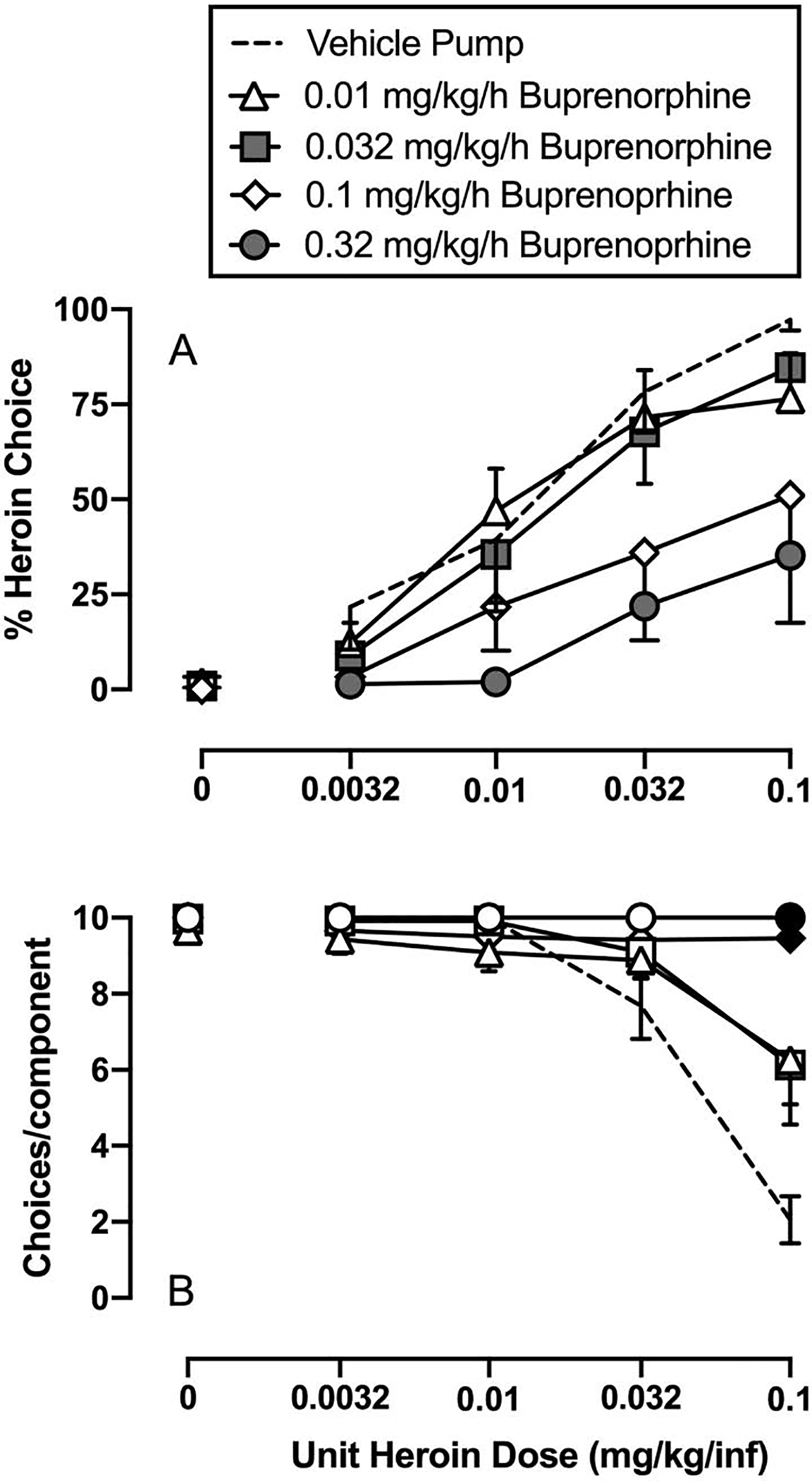
Effects of 7-day continuous 0.01–0.32 mg/kg/h buprenorphine treatment on heroin-vs-food choice in male and female rats. Top panels show percent heroin choice as a function of unit heroin dose in milligrams per kilogram per infusion. Bottom panels show the number of choices completed per component as a function of the unit heroin drug available as the alternative to 18% liquid food. All points represent the mean ± s.e.m. of 6 rats, except the 0.32 mg/kg/h buprenorphine dose, which is 5 rats. Gray filled symbols in Panel A denote statistical significance (p<0.05) compared to vehicle pump conditions regardless of unit heroin dose, whereas black filled symbols in Panel B denote statistical significance compared to vehicle pump conditions within a unit heroin dose.
Figure 6 shows 1:54 fentanyl/methamphetamine-vs-food (18%) choice dose-effect functions for percent drug mixture choice (Panel A), choices per component (Panel B), and the isobologram (Panel C). Table 2 shows ED50 choices values for fentanyl alone (plotted from Figure 1), methamphetamine alone (plotted from Figure 1), and each drug in the fentanyl/methamphetamine mixture. The ED50 choice values for fentanyl and methamphetamine as part of the 1:54 fentanyl/methamphetamine mixture were both significantly smaller compared to each respective drug alone based on non-overlapping confidence limits. Furthermore, Zmix (mean: 34.2; 95% confidence limits: 27.2, 41.2) was significantly lower than Zadd (mean: 57.5; 95% confidence limits: 50.6, 64.5) based on non-overlapping confidence limits indicating a synergistic interaction on drug choice.
Figure 6:
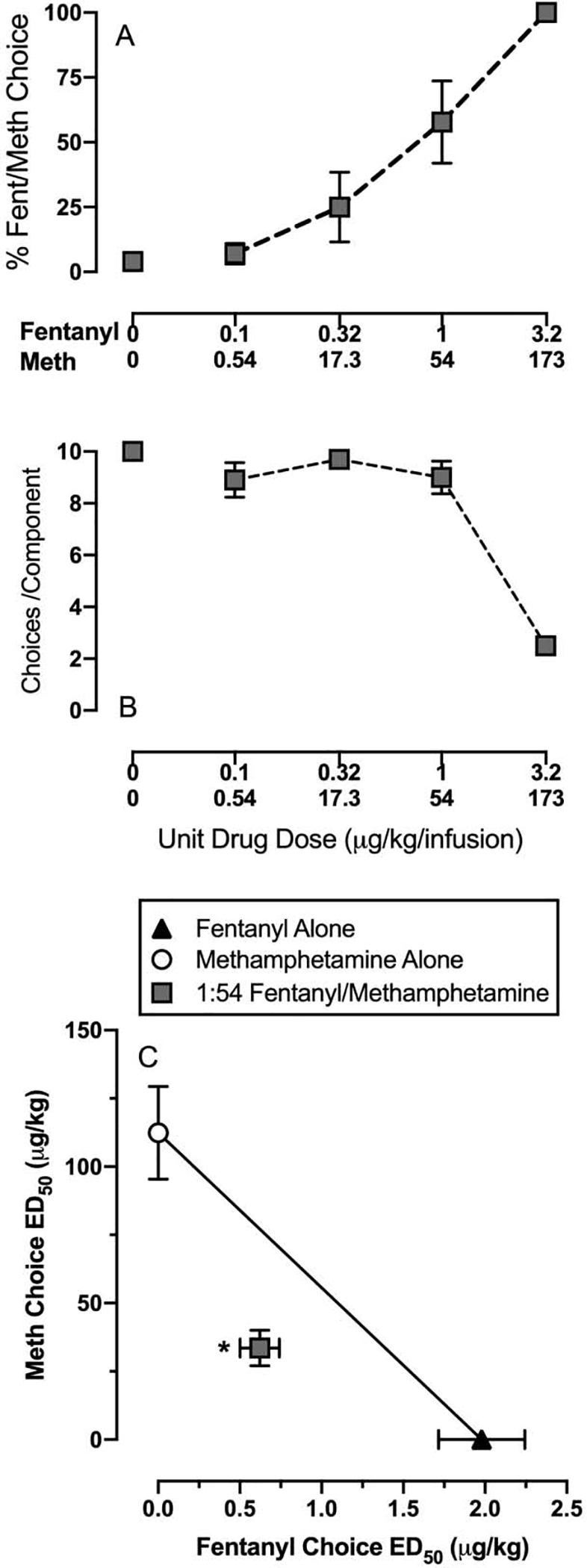
Interactions between fentanyl and methamphetamine in a drug-vs-food choice procedure in nonopioid-dependent male and female rats. Top panel (A) shows percent drug choice for a 1:54 fentanyl/methamphetamine mixture, which corresponds to a 1:1 ED50 choice mixture based on the potency of each drug alone. Middle panel (B) shows the number of choices completed per component as a function of the unit drug-mixture dose available as the alternative to 18% liquid food. Bottom panel (C) shows an isobologram for interactions between fentanyl and methamphetamine on drug choice. All points represent the mean ± s.e.m. of 10 rats. Asterisk denotes statistically significant difference from predicted additivity.
Table 2:
ED50 choice values in μg/kg/infusion (95% confidence limits) for fentanyl alone, methamphetamine alone, and a 1:54 fentanyl/methamphetamine mixture in male and female rats.
| Fentanyl ED50 | Methamphetamine ED50 | |
|---|---|---|
| Fentanyl Alone (n=12) | 2.0 (1.5 – 2.6) | – |
| Methamphetamine Alone (n=12) | – | 112.4 (83 –152.3) |
| 1:54 Fentanyl/Methamphetamine (baseline; n=10) | 0.6 (0.4 – 0.9) | 33.6 (22.7 – 49.7) |
Figure 7 shows effects of extended access 1:54 μg/kg/infusion fentanyl/methamphetamine mixture self-administration on subsequent fentanyl/methamphetamine mixture-vs-food choice. During this 2-week study, weekday 2-h drug-vs-food choice sessions (2–4pm) were preceded by 12-h extended-access drug self-administration sessions (6pm-6am). Thus, under these conditions, extended-access sessions provided opportunities for increased drug intake, and choice sessions began 8 h after conclusion of the preceding extended-access session. Somatic opioid withdrawal signs were assessed immediately before each choice session. Fentanyl/methamphetamine intake during the extended access session was stable over the experimental period and did not significantly change over time (Panel A). Panel B shows somatic opioid withdrawal signs across the experimental period. Relative to Day 1 (i.e., Pre-extended access), opioid withdrawal scores were significantly increased Days 4–6, 9, and 10–14 (Panel B: Friedman statistic = 50.2, p<0.0001). These opioid withdrawal signs were associated with significantly increased fentanyl/methamphetamine choice on Days 2–6, but not Days 9–13, compared to pre-extended access (Panel C: extended access: F1.6,13.8 = 4.3, p=0.041). Extended access fentanyl/methamphetamine self-administration also significantly decreased both total and food choices completed without significantly altering fentanyl/methamphetamine choices (Panel D: dependent measure: F1.1,10.2 = 16.8, p=0.002; extended access week: F1.3,12 = 30.9, p<0.0001; interaction: F1.9,16 = 8.8, p=0.003).
Figure 7:
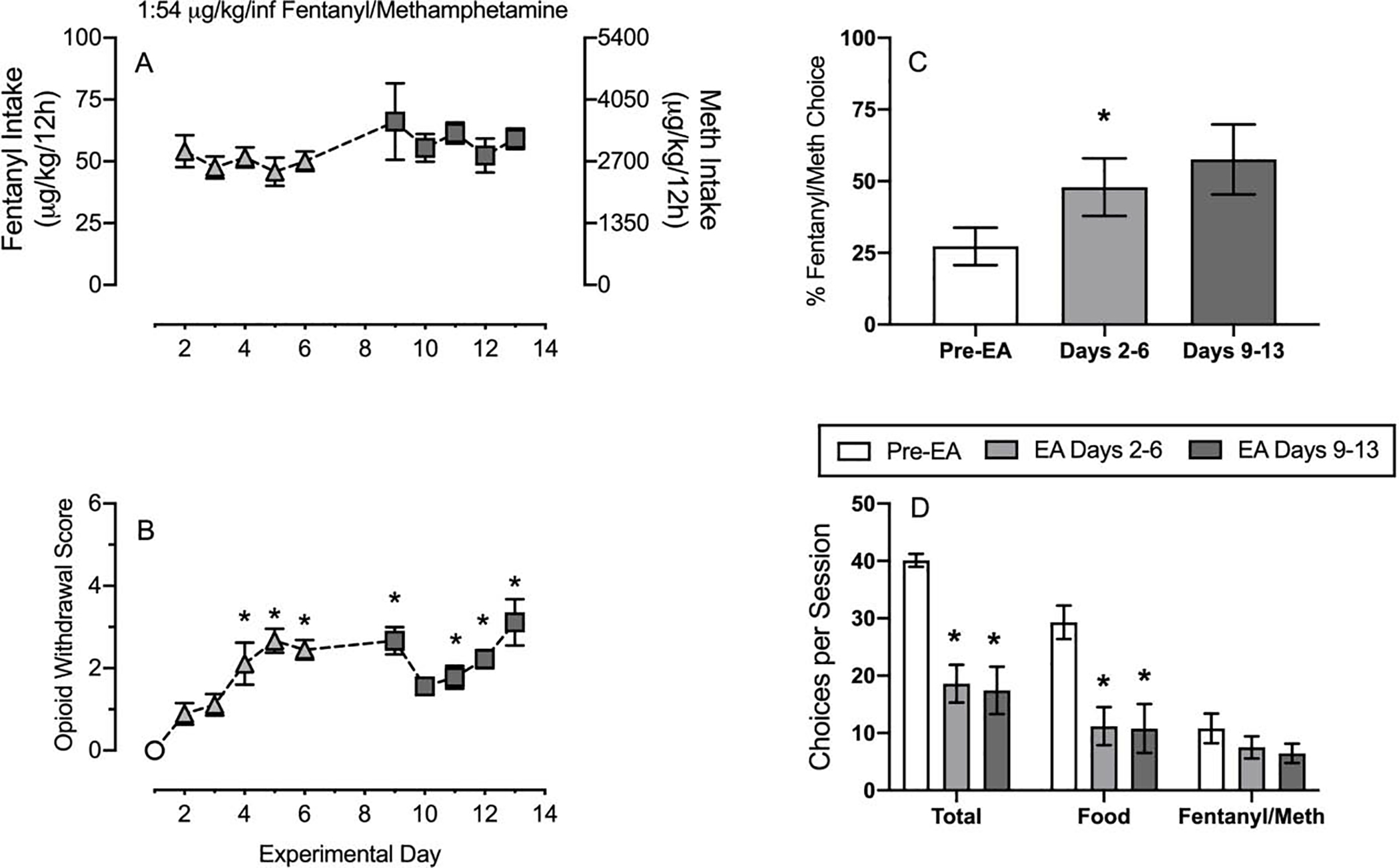
Effects of 12h overnight extended access (EA) 1:54 fentanyl/methamphetamine self-administration on subsequent fentanyl/methamphetamine-vs-food choice in male and female rats. Panel A shows fentanyl (left ordinate) and methamphetamine (right ordinate) intake over the 10 EA sessions. Panel B shows opioid withdrawal scores 5-min before each fentanyl/methamphetamine choice session as a function of experimental period. Panel C shows percent session fentanyl/methamphetamine choice pre-EA and after one week (Days 2–6) and two weeks (Days 9–13) of EA fentanyl/methamphetamine self-administration. Panel D shows total session choices, total food choices, and total fentanyl/methamphetamine choices as a function of experimental period. All points represent the mean ± s.e.m. of 10 rats. Asterisks denote statistical significance (p<0.05) compared to pre-extended access conditions.
4. Discussion
This manuscript described an IV jugular catheter surgical protocol and training method for translation into rats a drug-vs-food choice procedure originally developed for nonhuman primates. Building on previous studies using a similar cocaine-vs.-food choice procedure in rats (Thomsen et al., 2013; Thomsen et al., 2008), there were four main findings for the present study. First, the IV jugular catheter surgical protocol described resulted in a slightly longer first catheter patency duration than previously published methods (Thomsen and Caine, 2005). Second, drug-vs-food choice was established in male and female rats for not only cocaine, but also for the other monoamine transporter ligands methamphetamine and amphetamine and for the opioids fentanyl and heroin. All five drugs of abuse alone and an opioid/psychostimulant mixture maintained dose-dependent increase in choice over an alternative nondrug food reinforcer, consistent with previous results in nonhuman primates (Banks and Blough, 2015; Griffiths et al., 1981; Townsend et al., 2020a; Woolverton and Balster, 1979). Third, opioid-vs-food choice in rats was sensitive to environmental manipulations, such as alternative reinforcer magnitude and response requirement, consistent with and extending previous results from cocaine-vs-food choice studies in nonhuman primates (Foltin et al., 2015; Johnson et al., 2016; Nader and Woolverton, 1992; Negus, 2003) and rats (Cantin et al., 2010; Kerstetter et al., 2012; Thomsen et al., 2013). Lastly, these results illustrate use of opioid-vs.-food choice procedures in rats to examine effects of pharmacological manipulations that have been examined previously in rhesus monkeys and that include effects of chronic treatment with candidate medications (Negus, 2006; Townsend et al., 2020a) and effects of opioid-stimulant interactions (Negus, 2005). Overall, the results presented in this manuscript provide an empirical foundation supporting the feasibility of drug-vs.-food choice procedures in rats and the utility of these procedures to address questions that heretofore have been examined primarily in nonhuman primates. Utilization of these methods could improve our understanding of the expression and mechanisms of different substance use disorders towards the development of safer and more effective therapeutic strategies.
4.1. Establishing drug-vs-food choice in rats
The training method described in this manuscript was effective in establishing drug-vs-food choice behavior in male and female rats with both opioids (i.e., fentanyl and heroin) and monoamine transporter ligands (i.e., cocaine, methamphetamine, and amphetamine). Although there were differences in the number of sessions until stable operant choice behavior was established between the different drugs, all five drugs and an opioid/stimulant mixture could be established as reinforcers under the current drug-vs-food choice procedure. Furthermore, all five drugs maintained dose-dependent increases in choice behavior over an alternative nondrug reinforcer consistent with published results in rats (Beckmann et al., 2019; Chow and Beckmann, 2020; Thomsen et al., 2008), nonhuman primates (Findley et al., 1972; Maguire et al., 2013; Nader and Woolverton, 1992; Negus, 2006; Negus, 2003; Townsend et al., 2020a), and humans (Comer et al., 1998; Foltin et al., 2015; Hart et al., 2000; Heishman et al., 2000; Lile et al., 2020). However, the present results may appear to be inconsistent with other published drug-choice studies in rats using discrete-trial procedures, wherein preference of the drug reinforcer is observed only in a relatively small subset of the experimental subjects (Augier et al., 2018; Canchy et al., 2020; Cantin et al., 2010; Caprioli et al., 2015; Lenoir et al., 2013b; Lenoir et al., 2007; Tunstall et al., 2014; Venniro et al., 2018). We do not interpret our results as inconsistent with these published drug choice studies in rats, but rather a robust demonstration of the effectiveness of alternative nondrug reinforcers in decreasing the potency of abuse drugs to function as reinforcers in a choice context. As has been consistently demonstrated in these discrete trial choice procedures, experimental manipulations that decrease the relative reinforcing effectiveness of these nondrug reinforcers results in orderly behavioral reallocation towards drug and away from the nondrug alternative reinforcer (Canchy et al., 2020; Cantin et al., 2010; Vandaele et al., 2016; Venniro et al., 2018). Thus, we hypothesize that these differences in drug-choice behavior merely reflect differences in individual laboratory research methods and interests as opposed to fundamentally different behavioral phenotypes.
The environmental context has consistently been shown to impact operant drug self-administration behavior since the initial study by Weeks (1962). For example, recent studies have argued that the presence or absence of drug at the time that operant behavior occurs influences subsequent behavioral allocation between drug and nondrug reinforcers (Canchy et al., 2020; Freese et al., 2018; Vandaele et al., 2016). In the present experiments, the non-contingent fentanyl injection functioning as a discriminative stimulus before the response component was an important determinant of subsequent behavioral allocation between fentanyl and food compared to the visual discriminative stimuli. This result was perhaps not surprising given the poor visual acuity of albino rats compared to nonhuman primates. Nonetheless, the present results were consistent with previous results with cocaine as the discriminative or consequent stimulus (Thomsen et al., 2013). Thus, the present results manipulating the non-contingent or contingent fentanyl infusions are somewhat consistent with this interpretation suggesting the presence of drug in the subject (and at a sufficient concentration) can influence subsequent drug-vs-food choice behavior. Furthermore, this non-contingent drug infusion did not appear to significantly alter food-maintained responding as demonstrated by the results in the heroin FR100 experiment where exclusive food choice was reported and no robust decrease in operant responding. Moreover, the present fentanyl results are consistent with similar experiments examining cocaine-vs-food choice behavior in rats (Beckmann et al., 2019; Chow and Beckmann, 2020; Thomsen et al., 2013) and drug-vs-food choice in monkeys (Banks et al., 2011; Gasior et al., 2004; Negus, 2003; Townsend et al., 2019c). Overall, the present results suggest the non-contingent drug infusion in the present drug-vs-food choice procedure was the primary discriminative stimulus, more so than the stimulus lights, that facilitated subsequent drug-choice behavior. To the degree that this non-contingent drug infusion is a limitation of the current procedure, a future direction to refine the drug-vs-food choice procedure would be to remove the non-contingent drug infusion serving as the discriminative stimulus.
4.2. Environmental manipulations of choice behavior
Manipulating either the liquid-food concentration or the response requirement on the food- or opioid-associated lever resulted in systematic changes in behavioral allocation between IV opioid infusions and food. The present results are consistent with the published drug-choice literature in rats (Chow and Beckmann, 2020), monkeys (Cantin et al., 2010; Nader and Woolverton, 1991; Negus, 2003; Thomsen et al., 2013), and humans (Comer et al., 1998; Heishman et al., 2000; Lile et al., 2016). Collectively, the present results and the literature cited above are consistent with the view that drug self-administration is value-based and sensitive to environmental contingencies (Epstein et al., 2018; Pickard, 2020; Venniro et al., 2020). However, implementing these contingencies in our natural environment such as the cost or magnitude of nondrug reinforcers is easier said than done compared to controlled laboratory environments.
4.3. Pharmacological manipulations of choice behavior
One focus of preclinical drug self-administration procedures is the evaluation of candidate medications for substance use disorders. Preclinical drug self-administration procedures have good translational concordance in being sensitive to currently approved substance use disorder medications (Czoty et al., 2016; Haney and Spealman, 2008; Mello and Negus, 1996; Venniro et al., 2020). Moreover, preclinical drug-choice procedures have emerged as being both sensitive to clinically utilized medications and selective against novel therapeutics that have failed in either human laboratory drug self-administration studies or clinical trials (Negus and Banks, 2020; Townsend et al., 2020b; Venniro et al., 2020). In the present study, buprenorphine maintenance-induced decreases in heroin-vs-food choice in rats was consistent with nonhuman primate opioid-vs-food choice studies (Negus, 2006; Townsend et al., 2020a) and human laboratory drug-choice studies and clinical trials (Comer et al., 2005; Greenwald et al., 2002; Mello and Mendelson, 1980; Nasser et al., 2016). The present buprenorphine results were also generally consistent with acute and chronic buprenorphine treatments on opioid self-administration in rats (Bossert et al., 2020; Hammerslag et al., 2020). Overall, the present results and the extant literature cited above support the utility of preclinical drug-vs-food choice procedures in evaluating substance use disorder medications.
4.4. Utility of drug choice procedures for drug interactions and polysubstance abuse
Concurrent abuse of an opioid and a psychostimulant has been and continues to be a common form of polydrug abuse. Drug choice procedures may have utility in investigating drug interactions on reinforcement endpoints because the primary dependent measure (i.e. choice) is less sensitive to disruptions in operant behavior that result from reinforcement-independent drug effects (e.g. motor competence) (Banks and Negus, 2012). Using dose-addition analysis, the present results suggest that a fixed-proportion of fentanyl and methamphetamine interacted synergistically in a drug-vs-food choice procedure in nonopioid-dependent rats. Previous results in nonopioid-dependent rhesus monkeys reported that heroin and cocaine interacted in an additive manner in a drug-vs-food choice context (Negus, 2005). Whether the synergistic interaction between fentanyl and methamphetamine in the present study reflects differences in the mechanism of action of the stimulant (i.e., cocaine as a monoamine transporter inhibitor vs. methamphetamine as a monoamine transporter substrate), species differences, or the relative proportion of fentanyl and methamphetamine in the drug mixture, remains to be empirically determined. Nonetheless, the present results provide empirical evidence for the utility of drug-choice procedures to investigate the expression, mechanisms, and potential candidate medications for polysubstance abuse.
Fentanyl and methamphetamine interactions were also examined in the context of extended access drug self-administration. The length of the drug self-administration session has been an established independent variable in behavioral pharmacology research (Ahmed and Koob, 1998; Ahmed et al., 2000; Aigner and Balster, 1978; Deneau et al., 1969; Johanson, 1975). For example, previous research in both rhesus monkeys (Griffiths et al., 1975; Negus, 2006) and rats (Lenoir et al., 2013b; Townsend et al., 2019a) has demonstrated that extended access opioid self-administration conditions may produce opioid dependence and withdrawal-associated increases in opioid-vs-food choice. In contrast, extended access cocaine self-administration did not significantly impact cocaine-vs-food choice in monkeys (Banks and Negus, 2010) or rats (Lenoir et al., 2007). The fentanyl/methamphetamine mixture in the present study was self-administered to sufficient levels during extended access conditions to produce opioid dependence, expression of spontaneous somatic withdrawal signs, and withdrawal-associated increases in fentanyl/methamphetamine choice despite no time-dependent increase in drug intake or “escalation.” Previous studies examining time-dependent changes in drug intake for either fentanyl or methamphetamine alone demonstrated that a similar fentanyl dose (1.25 μg/kg/infusion) failed to show “escalated” intake (Wade et al., 2015), whereas a similar methamphetamine dose (50 μg/kg/infusion) did show “escalated” methamphetamine intake (Kitamura et al., 2006). The present fentanyl/methamphetamine results also suggest that “escalated” drug intake during extended access self-administration sessions was not necessary for enhancing drug-vs-food choice during periods of drug withdrawal. Moreover, these fentanyl and methamphetamine interaction results highlight one potential method of evaluating the effects of different drug access conditions on measures of drug reinforcement that are less sensitive to reinforcement-independent rate-disrupting effects of the self-administered drug or drug mixture.
5. Conclusions
The training protocols and experiments described in this manuscript demonstrate the broad applicability of preclinical drug-vs-food choice procedures in rats to address translational research questions related to the expression, mechanisms, and treatment of substance use disorders. Drug-vs-food choice in rats was found to be sensitive to environmental manipulations that were consistent with results in both nonhuman primates and human laboratory studies. Opioid-vs-food choice was also sensitive to pharmacological treatments known to be effective in reducing opioid self-administration in both nonhuman primates and humans. Drug-choice procedures may also have utility in improving our mechanistic understanding of drug mixture interactions on reinforcement-related endpoints. In summary, our hope is this manuscript will serve as a useful resource for scientists interested in incorporating drug choice procedures into their research programs.
Supplementary Material
Highlights.
Surgical technique resulted in median first catheter life of 18 weeks
Intravenous drug-vs-food choice could be trained in rats within ~27 sessions
Opioid, stimulant, and opioid+stimulant drug-vs-food choice was established
Consistency between monkey and rat drug choice results supports translatability
Acknowledgements
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers F32DA047026, F31DA043921, T32DA007027, R01DA026946, P30DA033934, and UG3DA050311. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge Steve Negus for providing comments on an earlier version of the manuscript.
Footnotes
Conflict of Interests
The authors report no potential or perceived conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science, 1998; 282: 298–300. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent Increase in the Motivation to Take Heroin in Rats with a History of Drug Escalation. Neuropsychopharmacology, 2000; 22: 413–21. [DOI] [PubMed] [Google Scholar]
- Aigner TG, Balster RL. Choice behavior in rhesus monkeys: cocaine versus food. Science, 1978; 201: 534–5. [DOI] [PubMed] [Google Scholar]
- Al-Tayyib A, Koester S, Langegger S, Raville L. Heroin and Methamphetamine Injection: An Emerging Drug Use Pattern. Substance Use & Misuse, 2017; 52: 1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric. Association. Diagnositc and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association: Arlington, VA, 2013. [Google Scholar]
- Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, Barchiesi R, Farris S, Nätt D, Mayfield RD, Adermark L, Heilig M. A molecular mechanism for choosing alcohol over an alternative reward. Science, 2018; 360: 1321–6. [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE. Effects of environmental maniuplations and bupropion and risperidone treatments on choice between methamphetamine and food in rhesus monkeys. Neuropsychoharmacology, 2015; 40: 2198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol, 2011; 22: 824–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Negus SS. Utility of Nonhuman Primates in Substance Use Disorders Research. ILAR J, 2017: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Effects of extended cocaine access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys. Neuropsychopharmacology, 2010; 35: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv Pharmacol Sci, 2012; 2012: 281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Chow JJ, Hutsell BA. Cocaine-associated decision-making: Toward isolating preference. Neuropharmacology, 2019; 153: 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Kiyatkin EA, Korah H, Hoots JK, Afzal A, Perekopskiy D, Thomas S, Fredriksson I, Blough BE, Negus SS, Epstein DH, Shaham Y. In a Rat Model of Opioid Maintenance, the G Protein–Biased Mu Opioid Receptor Agonist TRV130 Decreases Relapse to Oxycodone Seeking and Taking and Prevents Oxycodone-Induced Brain Hypoxia. Biological Psychiatry, 2020; 88: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canchy L, Girardeau P, Durand A, Vouillac-Mendoza C, Ahmed SH. Pharmacokinetics trumps pharmacodynamics during cocaine choice: a reconciliation with the dopamine hypothesis of addiction. Neuropsychopharmacology, 2021; 46: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine Is Low on the Value Ladder of Rats: Possible Evidence for Resilience to Addiction. PLoS ONE, 2010; 5: e11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Zeric T, Thorndike EB, Venniro M. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addiction Biol, 2015; 20: 913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JJ, Beckmann JS. Remifentanil-food choice follows predictions of relative subjective value. Drug Alcohol Depend, 2020: 108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobuzzi JL, Riley AL. Spontaneous withdrawal in opiate-dependent Fischer 344, Lewis and Sprague–Dawley rats. Pharmacol Biochem Behav, 2011; 98: 28–34. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol, 1998; 345: 13–26. [DOI] [PubMed] [Google Scholar]
- Comer SD, Walker EA, Collins ED. Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers. Psychopharmacology, 2005; 181: 664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol Rev, 2016; 68: 533–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia, 1969; 16: 30–48. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Heilig M, Shaham Y. Science-Based Actions Can Help Address the Opioid Crisis. Trends Pharmacol Sci, 2018; 39: 911–6. [DOI] [PubMed] [Google Scholar]
- Findley JD, Robinson WW, Peregrino L. Addiction to secobarbital and chlordiazepoxide in the rhesus monkey by means of a self-infusion preference procedure. Psychopharmacologia, 1972; 26: 93–114. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, Evans SM. Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacol Biochem Behav, 2015; 134: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese L, Durand A, Guillem K, Ahmed SH. Pre-trial cocaine biases choice toward cocaine through suppression of the nondrug option. Pharmacol Biochem Behav, 2018; 173: 65–73. [DOI] [PubMed] [Google Scholar]
- Gasior M, Bergman J, Kallman MJ, Paronis CA. Evaluation of the Reinforcing Effects of Monoamine Reuptake Inhibitors Under a Concurrent Schedule of Food and I.V. Drug Delivery in Rhesus Monkeys. Neuropsychopharmacology, 2004; 30: 758–64. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Schuh KJ, Hopper JA, Schuster CR, Johanson C-E. Effects of buprenorphine sublingual tablet maintenance on opioid drug-seeking behavior by humans. Psychopharmacology, 2002; 160: 344–52. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Choice between food and heroin: effects of morphine, naloxone, and secobarbital. J Exp Anal Behav, 1981; 35: 335–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Discrete-Trial Choice Procedure: Effects of Naloxone and Methadone on Choice between Food and Heroin. Pharmacol Rev, 1975; 27: 357–65. [PubMed] [Google Scholar]
- Hammerslag LR, Hofford RS, Kang Q, Kryscio RJ, Beckmann JS, Bardo MT. Changes in fentanyl demand following naltrexone, morphine, and buprenorphine in male rats. Drug Alcohol Depend, 2020; 207: 107804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology, 2008; 199: 403–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol, 2000; 11: 87–91. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Schuh KJ, Schuster CR, Henningfield JE, Goldberg SR. Reinforcing and subjective effects of morphine in human opioid abusers: effect of dose and alternative reinforcer. Psychopharmacology, 2000; 148: 272–80. [DOI] [PubMed] [Google Scholar]
- Iglauer C, Woods JH. CONCURRENT PERFORMANCES: REINFORCEMENT BY DIFFERENT DOSES OF INTRAVENOUS COCAINE IN RHESUS MONKEYS1. J Exp Anal Behav, 1974; 22: 179–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE. Pharmacological and Environmental Variables Affecting Drug Preference in Rhesus Monkeys. Pharmacol Rev, 1975; 27: 343–55. [PubMed] [Google Scholar]
- Johnson AR, Banks ML, Blough BE, Lile JA, Nicholson KL, Negus SS. Development of a translational model to screen medications for cocaine use disorder I: Choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karilsa M, Scholl L, Wilson N, Seth P, Hoots B. Drug Overdose Deaths Involving Cocaine and Psychostimulants with Abuse Potential - United States, 2003–2017. MMWR Morb Mortal Wkly Rep, 2019; 68: 388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex Differences in Selecting Between Food and Cocaine Reinforcement are Mediated by Estrogen. Neuropsychopharmacology, 2012; 37: 2605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio S, Koob G, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose–effect function. Psychopharmacology, 2006; 186: 48–53. [DOI] [PubMed] [Google Scholar]
- LaRue L, Twillman RK, Dawson E, Whitley P, Frasco MA, Huskey A, Guevara MG. Rate of Fentanyl Positivity Among Urine Drug Test Results Positive for Cocaine or Methamphetamine. JAMA Network Open, 2019; 2: e192851–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Augier E, Vouillac C, Ahmed SH. A Choice-Based Screening Method for Compulsive Drug Users in Rats. Curr Protoc Neurosci, 2013a; 64: 9.44.1–9..17. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended Heroin Access Increases Heroin Choices Over a Potent Nondrug Alternative. Neuropsychopharmacology, 2013b; 38: 1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense Sweetness Surpasses Cocaine Reward. PLoS ONE, 2007; 2: e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Johnson AR, Banks ML, Hatton KW, Hays LR, Nicholson KL, Poklis JL, Rayapati AO, Rush CR, Stoops WW, Negus SS. Pharmacological validation of a translational model of cocaine use disorder: Effects of d-amphetamine maintenance on choice between intravenous cocaine and a nondrug alternative in humans and rhesus monkeys. Exp Clin Psychopharmacol, 2020; 28: 169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Rush CR, Negus SS, Glaser PEA, Hatton KW, Hays LR. Development of a translational model to screen medications for cocaine use disorder II: Choice between intravenous cocaine and money in humans. Drug Alcohol Depend, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP. Delay discounting of food and remifentanil in rhesus monkeys. Psychopharmacology, 2013; 229: 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine Suppresses Heroin Use by Heroin Addicts. Science, 1980; 207: 657–9. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical Evaluation of Pharmacotherapies for Treatment of Cocaine and Opioid Abuse Using Drug Self-Administration Procedures. Neuropsychopharmacology, 1996; 14: 375–424. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology, 1992; 108: 295–300. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology, 1991; 105: 169–74. [DOI] [PubMed] [Google Scholar]
- Nasser AF, Greenwald MK, Vince B, Fudala PJ, Twumasi-Ankrah P, Liu Y, Jones JP 3rd, Heidbreder C. Sustained-Release Buprenorphine (RBP-6000) Blocks the Effects of Opioid Challenge With Hydromorphone in Subjects With Opioid Use Disorder. J Clin Psychopharmacol, 2016; 36: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Choice between Heroin and Food in Nondependent and Heroin-Dependent Rhesus Monkeys: Effects of Naloxone, Buprenorphine, and Methadone. J Pharmacol Exp Ther, 2006; 317: 711–23. [DOI] [PubMed] [Google Scholar]
- Negus SS. Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharmacology, 2005; 180: 115–24. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid Assessment of Choice between Cocaine and Food in Rhesus Monkeys: Effects of Environmental Manipulations and Treatment with d-Amphetamine and Flupenthixol. Neuropsychopharmacology, 2003; 28: 919–31. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. Learning from lorcaserin: lessons from the negative clinical trial of lorcaserin to treat cocaine use disorder. Neuropsychopharmacology, 2020; 45: 1967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard H What We’re Not Talking about When We Talk about Addiction. Hastings Center Report, 2020; 50: 37–46. [DOI] [PubMed] [Google Scholar]
- Stephens MK, Riley AL. Naloxone-precipitated conditioned taste aversions in morphine-dependent Fischer (F344) and Lewis rat strains. Pharmacol Biochem Behav, 2009; 92: 60–7. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS. Opioid Interactions in Rhesus Monkeys: Effects of δ + μ and δ + κ Agonists on Schedule-Controlled Responding and Thermal Nociception. J Pharmacol Exp Ther, 2003; 307: 1054–64. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Chapman and Hall/CRC: Boca Raton, FL, 2000. [Google Scholar]
- Thompson T, Schuster CR. Morphine Self-Administration, Food-Reinforced, and Avoidance Behaviors in Rhesus Monkeys. Psychopharmacologia, 1964; 5: 87–94. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav, 2013; 99: 211–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic Intravenous Drug Self-Administration in Rats and Mice. Curr Protoc Neurosci. John Wiley & Sons, Inc., 2005. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DPD, Wörtwein G, Sager TN, Holm R, Pepe LM, Barak Caine S. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology, 2008; 201: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Blake S, Faunce KE, Hwang CS, Natori Y, Zhou B, Bremer PT, Janda KD, Banks ML. Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology, 2019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Bremer PT, Jacob NT, Negus SS, Janda KD, Banks ML. A synthetic opioid vaccine attenuates fentanyl-vs-food choice in male and female rhesus monkeys. Drug Alcohol Depend, 2020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Banks ML. Medications Development for Treatment of Opioid Use Disorder. Cold Spring Harbor Persp Med, 2020b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology, 2019b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Poklis JL, Banks ML. Lorcaserin maintenance fails to attenuate heroin vs. food chocie in rhesus monkeys. Drug Alcohol Depend, 2019c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Riley AL, Kearns DN. Drug specificity in drug versus food choice in male rats. Exp Clin Psychopharmacol, 2014; 22: 364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandaele Y, Cantin L, Serre F, Vouillac-Mendoza C, Ahmed SH. Choosing Under the Influence: A Drug-Specific Mechanism by Which the Setting Controls Drug Choices in Rats. Neuropsychopharmacology, 2016; 41: 646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, Shaham Y. Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci, 2020. [DOI] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci, 2018; 21: 1520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-Like Responding for Opioid Analgesics in Rats with Extended Access. Neuropsychopharmacology, 2015; 40: 421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Experimental Morphine Addiction: Method for Automatic Intravenous Injections in Unrestrained Rats. Science, 1962; 138: 143–4. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol, 2007; 15: 309–27. [DOI] [PubMed] [Google Scholar]
- Wessinger WD. Approaches to the study of drug interactions in behavioral pharmacology. Neurosci Biobehav Rev, 1986; 10: 103–13. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav, 1987; 26: 835–9. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. The effects of lithium on choice between cocaine and food in the rhesus monkey. Commun Psychopharmacol, 1979; 3: 309–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


