Abstract
BACKGROUND.
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has been found to be epidemiologically and microbiologically distinct from healthcare-associated MRSA. Most CA-MRSA infections are not invasive; however, fatal outcomes have been reported among healthy people with CA-MRSA invasive infections. Epidemiological studies have attributed a major burden of CA-MRSA infections in the United States to the predominant clone USA300. We investigated the association between USA300 invasive infections and mortality by conducting a systematic review and meta-analysis of studies that reported mortality rates associated with USA300 strains.
METHODS.
We searched PubMed, bibliographies of other publications, and gray literature between January 2001 and December 2013. Observational studies of patients with an invasive MRSA infection were included. The exposure of interest was presence of USA300 invasive infection. Studies were included only if they provided MRSA PFGE types and if corresponding mortality data were the measured outcome. We pooled crude odds ratios (cORs) using a random-effects model. Woolf test of homogeneity and Q and I2 statistics were assessed.
RESULTS.
Of 574 articles identified by the search strategy, 8 met the inclusion criteria. Risk of mortality was significantly lower among patients with USA300 MRSA infections (pooled cOR, 0.63 [95% confidence interval (CI)], 0.49–0.81). There was a moderate degree of heterogeneity among study results (P = .29; I2 = 18%). Results were observed to be heterogeneous due to study design, quality of studies, and definition of mortality.
CONCLUSIONS.
MRSA invasive infection with USA300 does not appear to be associated with higher mortality compared with infections due to non-USA300 strains. Nevertheless, larger well-designed studies are warranted to further evaluate this association.
Staphylococcus aureus is an opportunistic pathogen traditionally known to cause healthcare-associated (HCA) infections. Methicillin-resistant S. aureus (MRSA) was first identified among hospitalized patients and considered to be predominantly acquired nosocomially for almost 3 decades.1 However, MRSA infections originating in the community have been reported to be a common occurrence after their first appearance in the United States in 1980.2–5
Community-associated (CA) MRSA strains are phylogenetically distinct from HCA-MRSA strains.6 CA-MRSA strains are known to exhibit increased virulence, a characteristic attributed to their ability to secrete a wide array of toxins and efficient host-to-host transmission.6 In the United States, the USA300 and USA400 strains are the most common cause of CA-MRSA infections in individuals with no known risk factors for S. aureus infection. The strain USA300 has become the most prevalent S. aureus clone to cause infections in community and healthcare settings in the United States, blurring the line defining the origin of infections.7,8 USA300 is 1 of the first 8 MRSA USA strain types reported in 2003.9 MRSA isolates with the spa motif MBQBLO and expressing the Panton-Valentine leukocidin (PVL) and the arginine catabolic mobile element (ACME) genes are classified as USA300.10 Pulsed-field gel electrophoresis (PFGE) is the most common method used to confirm S. aureus pulsed-field types, such as the USA300.9,11 Although USA300 is frequently associated with being a MRSA clone, USA300 methicillin-susceptible S. aureus (MSSA) has also been observed to cause invansive infections.12,13 There is evidence that patients with MRSA infection are at a higher risk for mortality compared with patients with MSSA infections.14,15 In addition, the USA300 MRSA acquires mobile genetic elements (MGEs) and has a volatile core genome, both of which encode and express genes for virulence factors, including PVL and ACME.16–19 These factors motivated us to better understand the effect of USA300 MRSA on the risk of mortality.
Epidemiological studies conducted to assess the impact of the USA300 strain on patient health outcomes have yielded inconsistent results. Studies have been limited by their sample size, inconsistent measures for mortality (eg, in-hospital, 90-day, or attributable), and variable use of molecular methods, such as the PFGE, to distinguish MRSA strains.20 These limitations have resulted in an inability to establish a true association, if one exists, between USA300 MRSA and mortality.
It is crucial to evaluate this association given the wide reach of USA300 and its potential to cause noninvasive infections, such as skin and soft-tissue infections, as well as invasive infections, such as bacteremia, pneumonia, osteomyelitis, and endocarditis.5 In addition, the USA300 MRSA clone with its armamentarium of virulence factors, in conjunction with its ability to develop resistance to commonly used antibiotics, poses a potential challenge in the practice of infection control. For these reasons, we evaluated the association between USA300 MRSA invasive infections and mortality by conducting a systematic review and meta-analysis of studies that reported mortality rates associated with USA300 MRSA. Infections and mortality due to all other strain types were included in the non-USA300 comparison group.
METHODS
Literature Search Strategy
We searched PubMed/MEDLINE, EBSCOhost, and Cochrane Reviews to identify all studies of USA300 invasive infections published during the last 12 years (January 2001-December 2013). We chose to include the last 12 years because the prevalence of all MRSA infections and CA-MRSA infections (including USA300) increased dramatically during this time period.21 Before 2001, there were few reports of CA-MRSA infections.
Our search revealed 574 references using the terms “USA300” OR “community associated MRSA” AND “mortality;” “USA300” OR “community associated MRSA” AND “patient outcomes;” “USA300” OR “community associated MRSA” AND “outcomes;” and “USA300” AND “mortality” (Figure 1). References were also identified from bibliographies of reviewed literature. We extended our literature search to available abstracts presented at conferences such as the Society for Healthcare Epidemiology of America and the American Society for Microbiology between 2010 and 2011. A ProQuest search was also conducted to identify relevant articles from unpublished dissertation studies.
FIGURE 1.
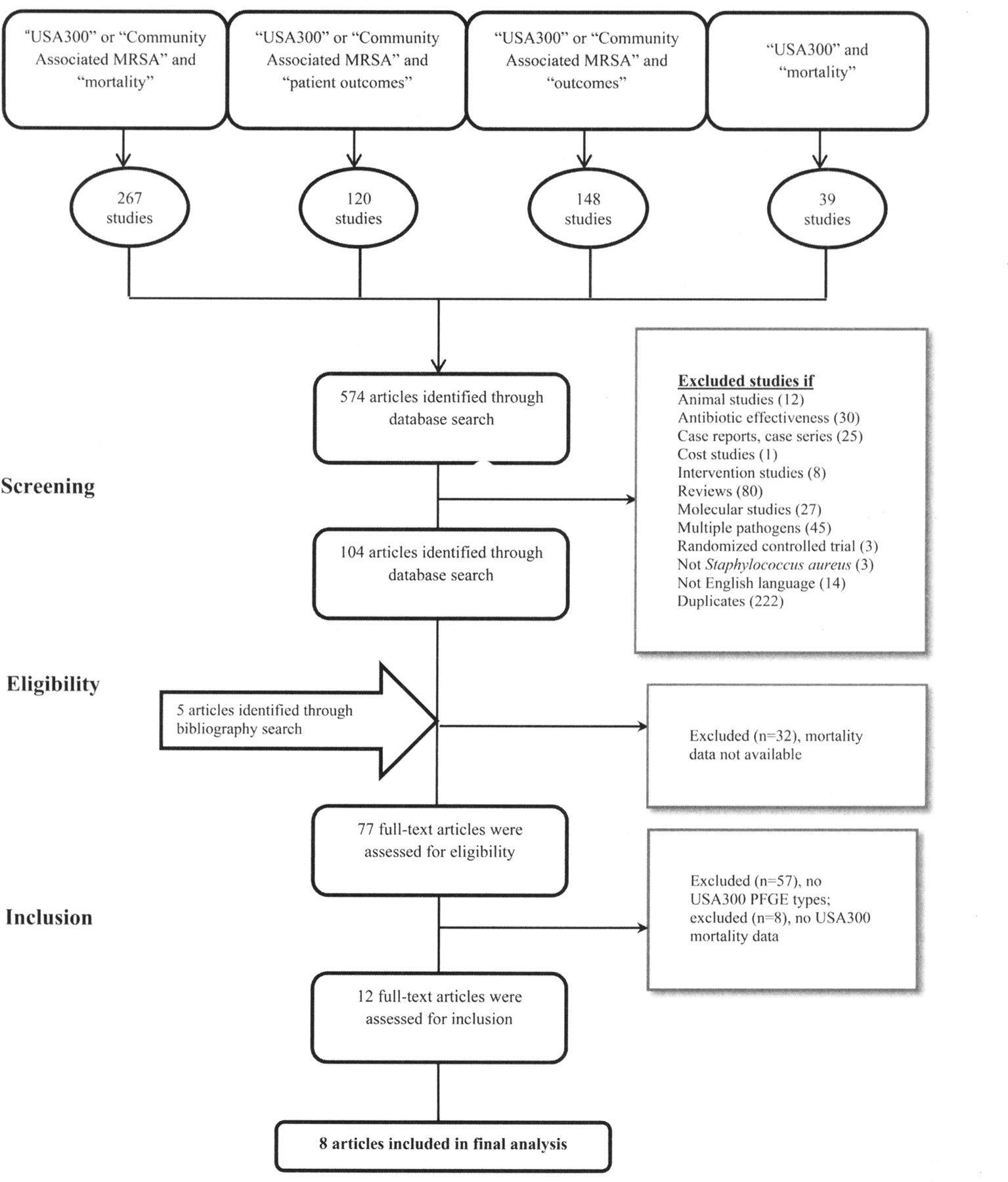
Flow chart for systematic literature review. MRSA, methicillin-resistant Staphylococcus aureus; PFGE, pulsed-field gel electrophoresis.
INCLUSION AND EXCLUSION CRITERIA
Criteria were established by the investigators before reviewing abstracts and articles. We included articles if they met the case definition of an invasive MRSA infection, as defined previously.5 Studies were included if (1) at least 1 of the clinical outcomes was mortality; (2) S. aureus infection isolates were typed by PFGE,9 or polymerase chain reaction (PCR) assays for PVL, ACME, or staphylococcal protein A (spa) were used to distinguish between USA300 and non-USA300; (3) they were written in English; (4) they involved human subjects; and (5) they provided separate mortality counts or rates for patients with USA300 and non-USA300 invasive infections. In cases in which studies provided data on both MRSA and MSSA infections, we included only the outcome of mortality due to MRSA infections.22 We excluded 8 potentially important USA300 studies from the final analysis because of their inability to distinguish between mortality attributed to USA300 invasive infection and non-USA300 invasive infection (Table A1).
Reviews, editorials, commentaries, purely molecular studies, animal studies, multiple pathogen studies, case reports and/or case series, and cost-effectiveness studies were excluded from our analysis. No clinical trials were found to include data on PFGE typing of isolates. Bibliographies of excluded reviews were hand-searched to identify relevant articles. Studies were excluded if more than 1 manuscript was published using the same study population. In the latter case, the study with the larger sample size was included in the analysis. The study population included all age groups.
DATA EXTRACTION AND ANALYSES
The main objective of this study was to summarize the odds of mortality associated with USA300 MRSA invasive infection compared with non-USA300 MRSA invasive infection. We developed a data abstraction form and subjected it to a pilot test. The form was revised as deemed necessary by investigators. Article abstracts were reviewed (R.N.) in accordance with the predefined inclusion criteria. Two of 3 independent researchers (R.N., M.L.S., and E.A.) reviewed each included article in full to extract data. Included articles were discussed and compared for agreement with a third reviewer (M.R.). Inconsistencies were reviewed and disagreements were resolved by consensus. Extracted data were entered in a Microsoft Excel (2007) database and the Cochrane Review Manager, version 5.2.5 for Windows (Nordic Cochrane Center, Cochrane Collaboration) to conduct statistical analysis. This meta-analysis was conducted according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist.23
Data extracted from each study included study design, study period, diversity of population (single- vs multicenter), origin of study population (hospital, community, other), number of patients meeting exposure criteria, definition of mortality (30-day, 90-day, attributable, crude), cOR and adjusted OR (aOR; if provided), and 95% confidence intervals (CIs). Information was extracted on covariates adjusted for in each study. We assigned a quality score to included studies using the Newcastle-Ottawa quality assessment scale (NOS), as described in a previous meta-analysis.24 Subgroup analyses were conducted on the basis of data abstracted for a priori categories.
STATISTICAL ANALYSIS
To evaluate the association between USA300 invasive infections and mortality, cORs and 95% CIs were abstracted from study data. If an included study reported a risk ratio other than the OR, we used the extracted raw data to calculate the natural log of the risk estimate and variance for each study, using the Woolf method. Calculations were performed using Microsoft Excel. We used the aOR for the association between USA300 and mortality if published in the study. We used a random-effects model to obtain pooled estimates of the ORs and 95% CIs.25,26 Heterogeneity was assessed using the Q statistic (large Q suggest heterogeneity and provide a P value to assess the presence of heterogeneity), the I2 statistic (degree of heterogeneity), and results from stratified analyses based on a priori categories. We considered heterogeneity to be significant if the P value was less than .20. Possible publication bias was evaluated by visual inspection of a funnel plot.
RESULTS
Literature search results are illustrated in Figure 1. A total of 104 studies were reviewed in detail for this meta-analysis. Characteristics of the 8 included studies are reported in Table 1.
TABLE 1.
Description of Studies included in Meta-Analysis
| Variable | Seybold et al32 | Carrillo-Marquez et al22 | Kempker et al27 | Kreisel et al10 | Haque et al33 | Lessa et al20 | Tattevin et al34 | Sherwood et al35 |
|---|---|---|---|---|---|---|---|---|
| Study year | 2006 | 2010 | 2010 | 2011 | 2012 | 2012 | 2012 | 2013 |
| Location | Grady Memorial Hospital, Atlanta, GA | Texas Children’s Hospital, Houston, TX | Emerging Infections Program, Atlanta, GA | Baltimore, MD; Buffalo, NY; Washington DC; Richmond, VA | University of Louisville, KY; Ohio State University Medical Center, OH; Henry Ford Health System, MI; University of Miami/Jackson Memorial Hospital, FL; Summa Health System, OH | Active Bacterial Core Surveillance (ABCs) system, CA, CO, GA, MI, NY, TN | San Francisco General Hospital (public, tertiary care) and 13 city-wide outpatient clinics | Walter Reed Army Medical Center and National Naval Medical Center |
| No. of centers | Single | Single | 8 | 4 | 5 | 6 | 14 | 2 |
| Study population | All age groups; MRSA bacteremia | Neonates, infants, children; SA-CRB | All age groups; MRSA bacteremia | Adults, older (≥65 years); MRSA bacteremia | Adults, older (≥65 years); MRSA pneumonia (HAP, VAP, or HCAP) | All age groups; invasive MRSA infection (CLABSI and PNEUMO) | All age groups ≥5 years; MRSA BSI | Adults, older (≥65 years); MRSA BSI |
| Study design | Prospective cohort | Prospective cohort | Prospective cohort | Retrospective cohort | Retrospective cohort | Prospective cohort | Retrospective cohort | Retrospective cohort |
| Comparison group/non-USA300 | USA100, USA500, USA800 | USA100, USA200, USA400, USA700, USA800, unique | USA100, USA500, other (<1%) | One half USA100 | USA100, USA600, other | USA100 | USA100, USA1000, USA500, USA1100, Other (3%) | Predominantly USA100 |
| Staphylococcus aureus typing method | PFGE, SCC mec, PCR for PVL, phenotyping | PFGE, SCCmec, PCR for agr typing and PVL, phenotyping | PFGE | PCR for PVL, ACME, and spa typing, PFGE (used for validation), phenotyping | PFGE, SCCmec, PCR for agr typing and PVL, phenotyping | PFGE | PCR for PVL, ACME, and spa typing, PFGE (used for validation), MLST | PFGE phenotyping |
| No. identified with S. aureus invasive infection | 132a | 112 | 4,344b | 271 | 251 | 336 | 549 | 245c |
| No. (%) with USA300 | 39 (33.6) | 12 (41.4) | 414 (37.5) | 67 (25) | 60 (23.9) | 90 (26.8) | 304 (55.37) | 30 (19.87) |
| No. (%) with non-USA300 | 77 (66.4) | 17 (58.6) | 690 (62.5) | 204 (75) | 191 (76.1) | 246 (73.2) | 245 (44.63) | 121 (80.13) |
| Mortality measured | Crude in-hospital mortality | Crude in-hospital mortality | In-hospital mortality | 90-day mortality | 28-day all-cause mortality | Attributable mortality within 30 days in hospital | Attributable mortality | Crude in-hospital mortality |
| Mortality no./total mortality (%) in invasive infections | ||||||||
| USA300 | 3/25 (12) | 2/5 (40) | 68/204 (33.33) | 20/121 (16.53) | 16 (26.7) | 19/102 (18.63) | 24/47 (51.06) | 5/30 (16.67) |
| Non-USA300 | 22/25 (88) | 3/5 (60) | 136/204 (66.67) | 101/121 (83.47) | 77 (40.3) | 83/102 (81.37) | 23/47 (48.94) | 21/121 (17.35) |
NOTE. ACME, arginine catabolic mobile element; agr, accessory gene regulator; BSI, bloodstream infection; CLABSI, central line-associated bloodstream infection; HAP, hospital-associated pneumonia; HCAP, healthcare-associated pneumonia; MLST, multilocus sequence typing; PCR, polymerase chain reaction; PFGE, pulsed-field gel electrophoresis; phenotyping, antibiotic susceptibility testing; PNEUMO, community-onset pneumonia; PVL, Panton-Valentine leukocidin; SA-CRB, S. aureus catheter-related bacteremia; SCCmec, staphylococcal cassette chromosome mec element; VAP, ventilator-associated pneumonia.
Final analysis included only 116 isolates.
Final analysis included 1,104 isolates typed by PFGE for USA300 vs non-USA300.
Final analysis included only 151 isolates.
We used the NOS developed specifically for cohort studies, because all eligible studies were cohort by design. All included studies possessed equal quality of outcome assessment; all used record linkages for outcome ascertainment and had a sufficient follow-up period for occurrence of the outcome. The quality of included studies varied depending on selection of the study population and comparability of cohorts by design or analysis (Table 2). Overall, 4 studies were of high quality, defined as NOS greater than or equal to 8, and 4 studies were of low quality, defined as NOS less than 8.
TABLE 2.
Risk of Bias Assessment
| Study, year | Selection | Comparabilitya | Outcome | Overall (maximum of 13) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort (USA300)a | Selection of the non-exposed cohort (non-USA300)b | Ascertainment of exposurea | Demonstration that outcome was not present at start of studyb | Outcome ascertainmenta | Follow-up long enough for outcome to occurb | Adequacy of follow-upa | |||
| Seybold et al,32 2006 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Carrillo-Marquez et al,22 2010 | … | … | ★ | ★ | … | ★ | ★ | ★ | 5 |
| Kempker et al,27 2010 | ★ | … | ★ | ★ | ★★ | ★ | ★ | ★ | 8 |
| Kreisel et al10 2011 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Haque et al33 2012 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Lessa et al20 2012 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Tattevin et al34 2012 | ★ | ★ | ★ | ★ | … | ★ | ★ | ★ | 7 |
| Sherwood et al35 2013 | ★ | ★ | ★ | … | … | ★ | ★ | ★ | 6 |
NOTE. Each study received 0–6 stars for “Selection,” 0–2 stars for “Comparability,” and 0–5 stars for “Outcome,” for an overall maximum of 13 stars.
Range, 0–2 stars.
Range, 0–1 star.
The included studies described a total of 2,806 isolates from patients with MRSA invasive infections; of these, 1,016 (36.21%) had USA300 infection and 1,790 (63.79%) had non-USA300 infection as identified by PFGE and other typing methods. The primary analysis observed a lower risk of mortality associated with USA300 invasive infections compared with non-USA300 invasive infections with a pooled cOR (95% CI) of 0.63 (0.49–0.81) and marginal heterogeneity (P = .29) using the random effects model (Figure 2). We attempted to obtain adjusted risk estimates for all studies; however, this information was available only for the study by Kempker et al.27 Inclusion of the adjusted hazard ratio from this study yielded a similar pooled OR as the pooled OR for crude risk estimates, although the association became non-significant (using adjusted rates, OR [95% CI], 0.64 [0.37–1.12]).
FIGURE 2.
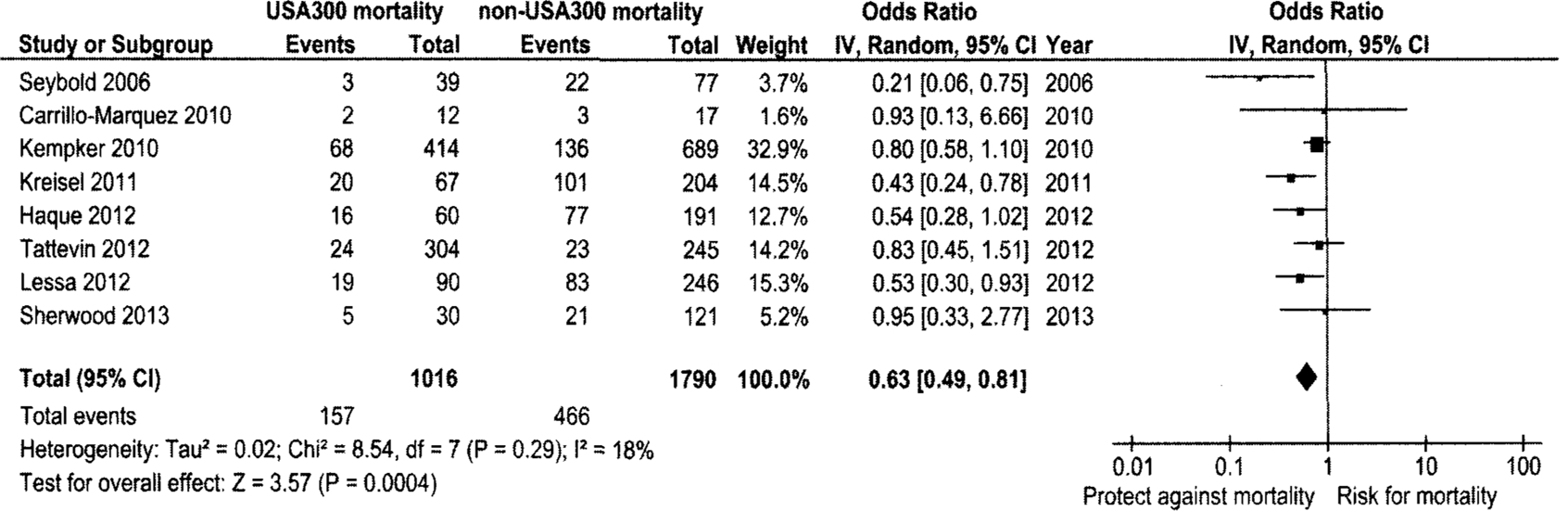
Forest plot for random effects meta-analysis comparing the odds of mortality in USA300 methicillin-resistant Staphylococcus aureus (MRSA) invasive infections with non-USA300 MRSA invasive infections. Vertical line indicates “no difference” point between the effects of USA300 MRSA on mortality; horizontal lines indicate the 95% confidence interval (CI). Black square, odds ratio; black diamond, pooled odds ratio for all studies; df, degrees of freedom.
The cause of heterogeneity was explored within a priori categories by stratified analyses using cORs (Table 3). The summary OR was closer to the null and statistically significant for multicenter studies compared with single-center studies. The summary OR (95% CI) was 0.58 (0.39–0.87) for high-quality studies and 0.65 (0.44–0.95) for low-quality studies. We compared the ORs for studies using infection-related mortality (2 studies) as outcome with studies using non-infection-related mortality (6 studies). Studies included in the category of non-infection-related mortality measured the outcome as crude in-hospital mortality, 90-day mortality, and 28-day all-cause mortality. Although there were not enough studies using infection-related mortality to calculate a pooled OR, both studies found a lower risk of mortality in USA300 infections. The studies of non-infection-related mortality demonstrated a statistically significant lower risk of mortality (Table 3). Stratifying by study design yielded a significant pooled OR (95% CI) of 0.61 (0.43–0.85) for the 4 retrospective studies compared with OR (95% CI) of 0.60 (0.37–0.97) for the 4 prospective studies. All subgroup analyses except study center demonstrated an existing heterogeneity pattern within study groups even after stratification (Table 3).
TABLE 3.
Stratified Analyses for Mortality in USA300 Methicillin-Resistant Staphylococcus aureus Invasive Infections
| Stratification (n = no. of studies) | OR (95% CI) | Heterogeneity | ||
|---|---|---|---|---|
| Fixed-effects model | Random-effects model | Q | P | |
| Main analysis (n = 8) | 0.65 (0.53–0.81) | 0.63 (0.49–0.81) | 8.54 | .29 |
| Stratified analyses | ||||
| Study location | ||||
| Multicentera (n = 6) | 0.67 (0.54–0.83) | 0.67 (0.53–0.84) | 5.27 | .38 |
| Study design | ||||
| Prospective (n = 4) | 0.69 (0.52–0.90) | 0.60 (0.37–0.97) | 5.15 | .16 |
| Retrospective (n = 4) | 0.61 (0.43–0.85) | 0.61 (0.43–0.85) | 3.08 | .38 |
| Overall quality of studies | ||||
| High (8–9 points) (n = 4) | 0.66 (0.51–0.85) | 0.58 (0.39–0.87) | 5.50 | .14 |
| Low (0–7 points) (n = 4) | 0.64 (0.44–0.95) | 0.65 (0.44–0.95) | 3.04 | .39 |
| Definition of mortality | ||||
| Non-infection-related mortalityb (n = 6) | 0.65 (0.51–0.84) | 0.60 (0.42–0.86) | 7.39 | .19 |
NOTE. CI, confidence interval; OR, odds ratio.
There were not enough single-center studies to pool the results.
There were not enough studies assessing attributable mortality to pool the results.
Because of the small number of published studies available for this meta-analysis, we attempted to identify the study that most influenced the pooled cOR. We assessed this by excluding 1 study at a time to calculate the pooled OR and 95% CI. This evaluation identified the study by Kempker et al27 as having maximum influence on the overall estimate. Exclusion of this study further strengthened the observed statistically significant association (Table A2).
We assessed publication bias by visual inspection of a funnel plot (Figure 3). The funnel plot appears symmetrical among studies with low standard error. However, there is an asymmetrical distribution of studies with higher standard error, which suggests the possibility of a moderate publication bias.
FIGURE 3.
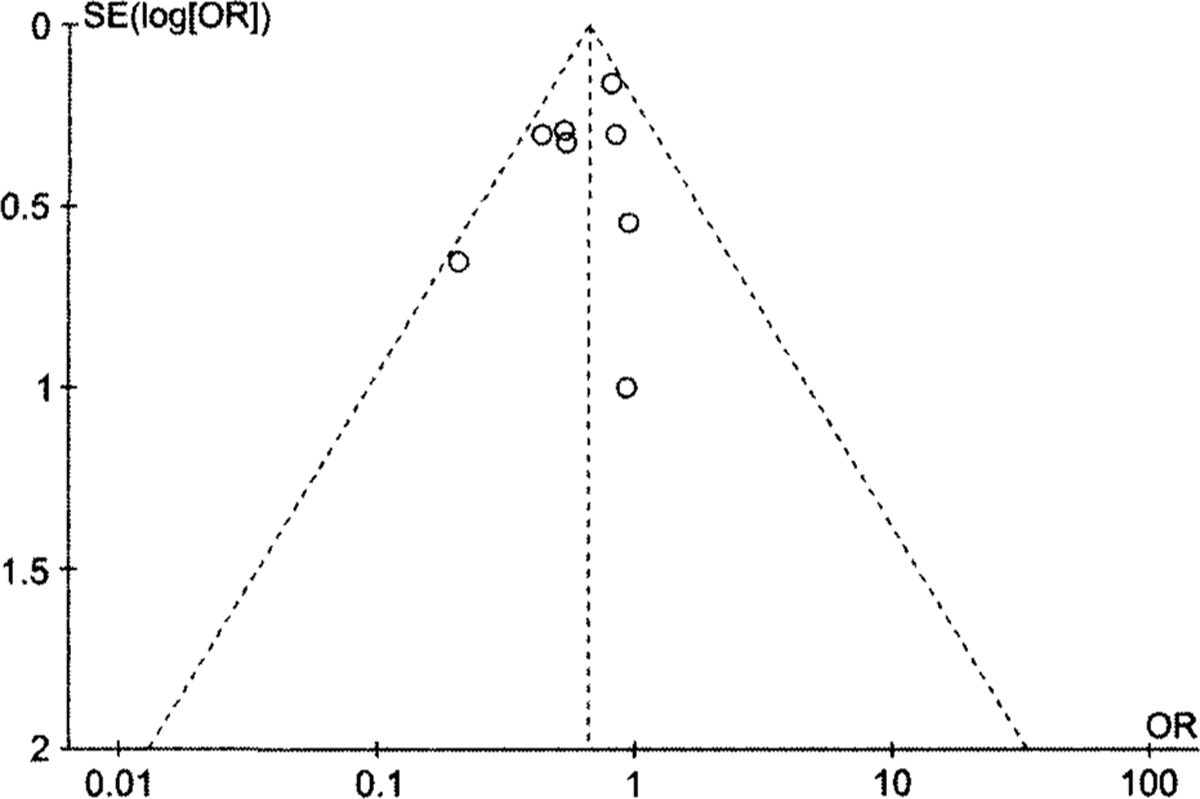
Funnel plot with pseudo 95% confidence limits to assess publication bias in review of mortality in USA300 methicillin-resistant Staphylococcus aureus (MRSA) invasive infections compared with non-USA300 MRSA invasive infections. Funnel plot created in Cochrane Review Manager, version 5.2.5 for Windows, using the fixed-effects model for illustrative purposes. Each circle represents a separate study for the indicated association. Vertical line represents the fixed-effect summary estimate (estimated using inverse variance); sloping lines represent the expected 95% confidence limit for a given SE, assuming no heterogeneity between the studies.
DISCUSSION
Existing studies have observed inconsistent results for an association between USA300 invasive infections and risk of mortality. Our synthesis of published studies indicates that patients with USA300 MRSA invasive infections have a decreased risk of mortality compared with patients with non-USA300 MRSA invasive infections. The lower risk of mortality in USA300 invasive infections was apparent even in subgroup analyses.
Our findings that USA300 MRSA invasive infections are associated with decreased mortality are clinically plausible, because patients infected with USA300 MRSA strains tend to be healthier, with fewer comorbidities and better immune function, compared with patients infected with HCA-MRSA-associated PFGE types, such as USA100. Thus, patients infected with USA300 MRSA may be more likely to survive their infection. A study conducted by Eells et al3 found similar results and observed that patients with CA-MRSA infection were less likely to have treatment failure or die than were patients with infection due to typical HCA-MRSA strains.3 Our results are also consistent with a recent meta-analysis that found reduced crude in-hospital mortality among patients infected with PVL-positive strains (typically USA300) compared with PVL-negative strains.28
Interestingly, a study by Moore et al29 observed that patients with HCA-MRSA USA300 infections had poorer outcomes than patients with CA-MRSA USA300 infections, which suggests that the location of origin of strain type USA300 may also contribute to clinical outcomes of hospitalized patients with MRSA invasive infections. Based on these results, studies conducted to investigate the association of USA300 infections with mortality should be adjusted for confounding factors, such as origin of infection (CA vs HCA) as well as patient comorbidities ascertained using validated severity of illness scales. All studies included in this meta-analysis categorized MRSA isolates as CA and HCA using previously published definitions.5 However, these studies did not provide data on the number of USA300 isolates that could be considered as CA- or HCA-MRSA.
There are several limitations to this meta-analysis. Studies provided different measures of risk, such as OR, relative risk, and hazard ratio, prompting us to calculate the OR for each study using the raw data to obtain the pooled OR. A major limitation was the unavailability of risk estimates adjusted for potential confounders. Only a single included study, published by Kempker et al,27 provided an adjusted association between USA300 and mortality. We calculated a pooled OR with the unadjusted and the adjusted risk estimate reported in the Kempker et al27 study. We observed that the pooled OR was not statistically significant when the adjusted risk ratio for this study was included. It is also important to note that the Kempker et al27 study was observed to be the most influential in deciding the direction of the risk association, reinforcing the importance of reporting adjusted risk estimates for the association between USA300 infection and mortality (Table A2). Additionally, we could not account for information obtained in studies that did not include PFGE typing results on USA300 and non-USA300 infections. Included studies considered various invasive infections, such as bacteremia, pneumonia, and catheter-related bloodstream infections. However, we lacked information on disease severity and host response to the infection, both of which could potentially affect the study outcome, and were unable to conduct stratified analyses on these factors. The funnel plot of the main analysis reflects possible publication bias that could be due to a lack of published studies. Selection of study subjects, comparability between study cohorts, and nonadjustment for potential confounding variables may have contributed to the low quality of some included studies and could also affect our results.
Our finding of a decreased risk of mortality associated with USA300 MRSA infections is consistent with other epidemiological studies.30,31 However, these study findings could be explained by the inability to directly attribute the risk of mortality to invasive infections and inadequate adjustment for confounding factors, such as patient age and comorbidities. Additional research is warranted with sufficiently powered studies and should use molecular methodologies, such as PFGE, to identify MRSA strain types, control for important confounders, and incorporate a clinically relevant, standardized definition of mortality.
ACKNOWLEDGMENTS
We thank Dr. Kristen Kreisel for her contribution to this work. We also thank the Iowa City VA Health Care System for its support.
Financial support.
M.L.S. is supported by a Veterans Affairs Health Services Research and Development Career Development grant (CDA 11–215). M.R. is supported by a National Institutes of Health Medical Scientist Training Program grant (GM007337).
APPENDIX
TABLE A1.
Potentially Relevant USA300 Studies Excluded from Final Analysis
| Authors, journal (year) | Study population | No. identified with Staphylococcus aureus infections | No. identified with USA300 infections | Mortality attributed to USA300, % | Reason for excluding study |
|---|---|---|---|---|---|
| King et al, Annals of Internal Medicine (2006) | S. aureus SSTIs | 389 | 157 | Not reported | No information on mortality attributed to USA300 infections |
| Huang et al, Journal of Clinical Microbiology (2006) | MRSA infections | 283 | 156 | Not reported | No information on mortality attributed to USA300 infections |
| Liu et al, Clinical Infectious Diseases (2008) | All available MRSA infections | 3,985a | 570 | Not reported | No information on mortality attributed to USA300 infections |
| Lalani et al, Journal of Clinical Microbiology (2008) | S. aureus bacteremia and endocarditis | 88 MRSA, 141 MSSA | 23 MRSA, 5 MSSA | Not reported | No information on mortality attributed to USA300 infections |
| Moore et al, International Journal of Antimicrobial Agents (2009) | USA300 infections | NA | 160 | 12.5 (P = .044) | No non-USA300/comparison group |
| Carrillo-Marquez et al, Pediatric Infectious Disease Journal (2009) | S. aureus septic arthritis | 16 MRSA, 28 MSSA | 13 MRSA, 28 MSSA | Not reported | No information on mortality attributed to USA300 infections |
| Jenkins et al, Infection Control and Hospital Epidemiology (2009) | USA300 MRSA BSI | NA | 330 | Not reported | No information on mortality attributed to USA300 infections |
| Hota et al, Clinical Infectious Diseases (2011) | CO-MRSA infections | 360 | 232 | Not reported | No information on mortality attributed to USA300 infections |
NOTE. BSI, bloodstream infection; CO, community onset; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; NA, not applicable; SSTI, skin and soft-tissue infection.
Only 801 of these isolates were used for final molecular analysis.
TABLE A2.
Exclusion Test to Assess Influence of Studies
| Study excluded | Odds ratio (95% CI) | Heterogeneity P | Degree of heterogeneity (I2) |
|---|---|---|---|
| Overall analyses | 0.63 (0.49–0.81) | .29 | 18 |
| Seybold et al32 | 0.68 (0.55–0.84) | .50 | 0 |
| Kreisel et al10 | 0.68 (0.54–0.87) | .38 | 7 |
| Kempker et al27 | 0.56 (0.43–0.74) | .43 | 0 |
| Carrillo-Marquez et al22 | 0.62 (0.47–0.81) | .21 | 29 |
| Haque et al33 | 0.64 (0.48–0.86) | .23 | 26 |
| Tattevin et al34 | 0.60 (0.45–0.80) | .25 | 24 |
| Lessa et al20 | 0.65 (0.48–0.87) | .25 | 24 |
| Sherwood et al35 | 0.61 (0.46–0.80) | .23 | 25 |
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
REFERENCES
- 1.Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PloS ONE 2013;8(1):e52722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naber CK. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 2009;48(suppl 4):S231–S237. [DOI] [PubMed] [Google Scholar]
- 3.Eells SJ, McKinnell JA, Wang AA, et al. A comparison of clinical outcomes between healthcare-associated infections due to community-associated methicillin-resistant Staphylococcus aureus strains and healthcare-associated methicillin-resistant S. aureus strains. Epidemiol Infect 2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Hidron, Low CE Honig EG, Blumberg HM. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis 2009;9(6):384–392. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007;298(15):1763–1771. [DOI] [PubMed] [Google Scholar]
- 6.Thurlow LR, Joshi GS, Richardson AR. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol Med Microbiol 2012;65(1):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deleo FR, Otto M, Kreiswirth BN, Chambers HE Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010;375(9725):1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popovich KJ, Weinstein RA. Commentary: the graying of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2009;30(1):9–12. [DOI] [PubMed] [Google Scholar]
- 9.McDougal LK, Steward CD, Killgore GE, Chaitram JM, Mc-Allister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol 2003;41(11):5113–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreisel KM, Stine OC, Johnson JK, et al. USA300 methicillin-resistant Staphylococcus aureus bacteremia and the risk of severe sepsis: is USA300 methicillin-resistant Staphylococcus aureus associated with more severe infections? Diagn Microbiol Infect Dis 2011;70(3):285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995;33(9):2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCaskill ML, Mason EO Jr, Kaplan SL, Hammerman W, Lamberth LB, Hulten KG. Increase of the USA300 clone among community-acquired methicillin-susceptible Staphylococcus aureus causing invasive infections. Pediatr Infect Dis J 2007;26(12): 1122–1127. [DOI] [PubMed] [Google Scholar]
- 13.Hsiang MS, Shiau R, Nadle J, et al. Epidemiologic similarities in pediatric community-associated methicillin-resistant and methicillin-sensitive in the San Francisco bay area. J Pediatr Infect Dis Soc 2012;1(3):200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 2007;28(3):273–279. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36(1): 53–59. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci USA 2009;106(14):5883–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talan DA, Krishnadasan A, Gorwitz RJ, et al. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis 2011;53(2):144–149. [DOI] [PubMed] [Google Scholar]
- 18.Velazquez-Meza ME, Ayala-Gaytan J, Carnalla-Barajas MN, Soto-Nogueron A, Guajardo-Lara CE, Echaniz-Aviles G. First report of community-associated methicillin-resistant Staphylococcus aureus (USA300) in Mexico. J Clin Microbiol 2011;49(8): 3099–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otter JA, French GL. Community-associated meticillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated infection. J Hosp Infect 2011;79(3):189–193. [DOI] [PubMed] [Google Scholar]
- 20.Lessa FC, Mu Y, Ray SM, et al. Impact of USA300 methicillin-resistant Staphylococcus aureus on clinical outcomes of patients with pneumonia or central line-associated bloodstream infections. Clin Infect Dis 2012;55(2):232–241. [DOI] [PubMed] [Google Scholar]
- 21.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010;23(3): 616–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrillo-Marquez MA, Hulten KG, Mason EO, Kaplan SL. Clinical and molecular epidemiology of Staphylococcus aureus catheter-related bacteremia in children. Pediatr Infect Dis J 2010; 29(5):410–414. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 24.Alavaikko S, Jaakkola MS, Tjaderhane L, Jaakkola JJ. Asthma and caries: a systematic review and meta-analysis. Am J Epidemiol 2011;174(6):631–641. [DOI] [PubMed] [Google Scholar]
- 25.Woolf B On estimating the relation between blood group and disease. Ann Hum Genet 1955;19(4):251–253. [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 27.Kempker RR, Farley MM, Ladson JL, Satola S, Ray SM. Association of methicillin-resistant Staphylococcus aureus (MRSA) USA300 genotype with mortality in MRSA bacteremia. J Infect 2010;61(5):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis 2013;13(1):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore CL, Hingwe A, Donabedian SM, et al. Comparative evaluation of epidemiology and outcomes of methicillin-resistant Staphylococcus aureus (MRSA) USA300 infections causing community- and healthcare-associated infections. Int J Antimicrob Agents 2009;34(2):148–155. [DOI] [PubMed] [Google Scholar]
- 30.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis 2008;198(3):336–343. [DOI] [PubMed] [Google Scholar]
- 31.Furuno JP, Johnson JK, Schweizer ML, et al. Community-associated methicillin-resistant Staphylococcus aureus bacteremia and endocarditis among HIV patients: a cohort study. BMC Infect Dis 2011;11:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis 2006;42(5):647–656. [DOI] [PubMed] [Google Scholar]
- 33.Haque NZ, Arshad S, Peyrani P, et al. Analysis of pathogen and host factors related to clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2012;50(5):1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tattevin P, Schwartz BS, Graber CJ, et al. Concurrent epidemics of skin and soft tissue infection and bloodstream infection due to community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2012;55(6):781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherwood J, Park M, Robben P, Whitman T, Ellis MW. USA300 methicillin-resistant Staphylococcus aureus emerging as a cause of bloodstream infections at military medical centers. Infect Control Hosp Epidemiol 2013;34(4):393–399. [DOI] [PubMed] [Google Scholar]


