Abstract
Thyroid cancer accounts for 1% of all malignancies, and is becoming increasingly common worldwide. The literature reports a prevalence of ~50% of thyroid nodules detected during autopsies in subjects with unknown thyroid pathology. An extended retrospective study of 526 autopsy cases was performed to identify the prevalence of thyroid carcinoma, among various types of thyroid nodules identified incidentally. Tissue samples were taken from thyroid nodules, for investigation of the presence of thyroid carcinoma, along with their macroscopic and microscopic features by means of histopathology and immunohistochemistry (IHC) methods. Histopathological diagnosis of malignancy was found in 51 cases of analyzed thyroid samples. Systematic detailed studies demonstrated that a thyroid gland, apparently normal on macroscopic examination, may be the site of pathological manifestations, sometimes presenting carcinomatous findings. Among thyroid carcinomas, the highest frequency was that of papillary microcarcinomas, which have a long evolution, and are incidentally detected during autopsies. Papillary microcarcinoma is an extremely common incidental finding and the vast majority of these tumors pursue a benign course. Furthermore, it is therefore necessary to create national screening programs for the early detection of thyroid carcinoma.
Keywords: thyroid nodule, papillary microcarcinoma, tumor immunophenotype
Introduction
Among the incidental carcinoma of thyroid, the overall incidence of papillary carcinoma is higher than other types. This type of carcinoma has a high incidence, is associated with follicular adenoma, an may present as an isolated thyroid nodule or as multifocal lesions and should be considered with malignant potential (1-3).
Despite advanced understanding of the biological characteristics of papillary thyroid carcinoma and the development of guidelines for its treatment, a number of practical questions remain unsolved; for instance, the extension of surgery, such as lobectomy vs. total thyroidectomy remains controversial (4,5).
The increased incidence of thyroid cancer is the likely result of two coexisting processes: Increased detection and increased number of cases, due to unrecognized thyroid-specific carcinogens. This increase is apparent due to low identification of a large reservoir of subclinical papillary lesions that may never affect patient health (6,7).
The thyroid gland, thoroughly investigated in systematic studies that include complex histopathological examinations, can present a pathology that is often unknown. During autopsies, the thyroid gland is rarely analyzed, being overlooked or incompletely visualized.
When systematic studies are performed to investigate macroscopically found nodules, including complex histopathological examination, an increased incidence of thyroid tumors is detected. Under these circumstances, the literature reports a prevalence of ~50% of thyroid nodules, detected during autopsies in subjects with unknown thyroid pathology (8-10). The prevalence of latent thyroid carcinomas is reported to average between 1.0 and 35.6% in different systemic autopsy series (11,12).
Thyroid cancer accounts for 1% of all malignancies, and is on the increase globally (13,14). Consequently, extensive sessions are dedicated to thyroid pathology in medical endocrinology meetings, where the news regarding the etiological mechanisms, the paraclinical diagnosis and the therapeutic methods of this type of cancer are discussed.
The incidence of thyroid cancer has tripled in the last 30 years, with a constant and latent numerical growth (mainly including small tumors), while mortality remained stable, according to data provided by the American Association of Endocrinopathologists and the American College of Endocrinopathology (15).
In the present study, an extended retrospective study of 526 autopsy cases was performed to identify the prevalence of thyroid carcinoma, among various types of thyroid nodules identified incidentally. The findings indicated that, papillary microcarcinoma is an extremely common incidental finding and the vast majority of these tumors pursue a benign course.
Materials and methods
Case selection for study batch
Total thyroidectomy was performed during serial autopsies of 526 cases in an interval of 3 years (January 2017-February 2020) and macroscopically examined in search for small undetectable thyroid nodules. The study was performed at the forensic department of Brăila County Emergency Hospital (Braila, Romania).
The study batch comprised 416 males (79%) and 110 females (sex ratio=3.78), with an age range of 10-94 years (m=60.32, SD=±15.42). The highest incidence was found in patients aged between 60 and 70 years. None of the subjects included in the study had evidence of thyroid disease. The urban/rural distribution encountered in patients with neoplasms was approximately equal.
A complex database was compiled including variables such as age, sex, environmental factors and clinical factors related to the risk of thyroid cancer. Possible association with Hashimoto's thyroiditis or lymphocytic thyroiditis, along with overweight and cardiovascular diseases was also considered.
The study was conducted according to the World Medical Association Declaration of Helsinki, using a protocol approved by the local Bioethics Committee from Brăila Emergency County Hospital (Braila). All patients previously signed an informed written consent regarding hospitalization, treatment and a possible future publication of data.
Tissue sampling and stains
The thyroids were dissected and carefully separated from the soft tissues around the thyroid, fixed in 10% formalin and then weighed and measured. All macroscopic changes were noted, with special propensity for whitish star-shape areas and the scars suspected to be a carcinoma. Subsequently, tissue specimens were carefully harvested from different parts of both thyroid lobes for microscopicanalysis in order to perform a systematic study.
Multiple, serial sections were performed to cover a wide range of undetected pathologies in the macroscopic examination. The selected tissue samples were fixed in 10% neutral-buffered formalin (pH 7.0) for 24-48 hand paraffin embedded. Sections were cut at 5 µm and stained with standard hematoxylin-eosin (HE). Approximately 1,000 fragments were processed and microscopically examined.
Immunohistochemical analysis
Immunohistochemical analysis (IHC) was performed for a panel of 7 antibodies, using sections show non slides treated first with poly-L-lysine. The panel comprised the following antibodies: CK7 (mouse monolconal, clone: OV-TL12/20, ready to use-RTU, Cell Marque), TTF1 (mouse monolconal, clone: 8G7G3/1, RTU, Cell Marque), thyroglobulin (mouse monoclonal, clone: 2H11+6E1, RTU, Cell Marque), EMA (mouse monoclonal, clone: E29, RTU Cell Marque), vimentin (mouse monoclonal, clone: V9, RTU, Cell Marque), PCNA (mouse monoclonal, clone PC10, 1:200, Dako; Agilent Technologies), and p53 (mouse monoclonal, clone: SP5, RTU, Cell Marque). IHC was performed on 3 µm thick sections from formalin-fixed paraffin-embedded specimens.
The method used was an indirect tristadial Avidin-Biotin-Complex technique, with a NovoLink Polymer detection system which utilizes a novel control polymerization technology to prepare polymeric HRP-linker antibody conjugates, according to the manufacturer's specifications (Novocastra). Antigen retrieval technique (enzymatic pre-treatment) was carried out, according to the producer's specifications.
All slides were examined and photographed on a Nikon Eclipse Ci. Digital images captured with Digital Microscope Camera program were processed and analyzed with Photos App, running under Windows 10.
The criteria of the World Health Organization (WHO) were used to diagnose and classify histological subtypes of thyroid tumors. The Turin criteria for poor differentiated thyroid carcinoma include: i) presence of a solid/trabecular/insular pattern of growth, ii) absence of the conventional nuclear features of papillary carcinoma, and iii) presence of at least one of the following features: Convoluted nuclei; mitotic activity > or =3x10 HPF (high power field) and tumor necrosis (16).
Results
Tumor distribution areas
From 526 cases, 153 cases had thyroid nodules, out of which 135 had multinodular goiters and 18 uninodular goiter; 51 cases had malignant nodules and 322 had colloid nodules. The average weight of the thyroid with goiter was 88 g, with variations between 25 and 500 g.
A total of 39 patients with goiter had associated diseases: 35 cases had associated lymphocytic thyroiditis and 4 cases had focal lymphocytic thyroiditis associated with neoplasia. A total of 39 thyroid glands showed thyroiditis, of which focal lymphocytic thyroiditis occurred in 35 cases associated with goiter, and in 2 cases associated with neoplasia. One case of Hashimoto's thyroiditis was found concurrently with papillary carcinoma.
Thyroid pathology
The incidence of thyroid pathology was notedin association with cardiovascular diseases. In the present study, the associated cardiovascular diseases were divided into three distinct categories: 67.3% had chronic cardiovascular diseases (especially myocardofibrosis), 5.3% had chronic cardiovascular diseases associated with acute myocardial ischemia, and 7.8% had acute heart diseases. A causal relationship between obesity and thyroid pathology was found in our cases, as follows: 33.3% had obesity, 19.5% had cachexia and 52% were normostenic.
Histopathological diagnosis of malignancy was found in 51 cases of the analyzed thyroid glands. The youngest patient was 40 years of age and the oldest 94 years of age, the highest incidence being found in patients aged between 45 and 60 years.
Thyroid neoplasia was noted in 51 cases (33.33%), 3-fold higher in men than in women: Micropapillary carcinomas in 47 cases, undifferentiated thyroid carcinoma in 3 cases and squamous cell thyroid carcinoma in 1 case. The association with another thyroid pathology was seen in 8 cases: 5 goiters associated with papillary carcinomas, and 3 thyroiditis associated with papillary carcinomas.
In 54.1% of cases, the malignant nodules were distributed in the right thyroid lobe 43.1% in the left thyroid lobe. In some cases (9%), the tumor areas were multiple, considered multifocal, disseminated in both thyroid lobes (Fig. 1).
Figure 1.
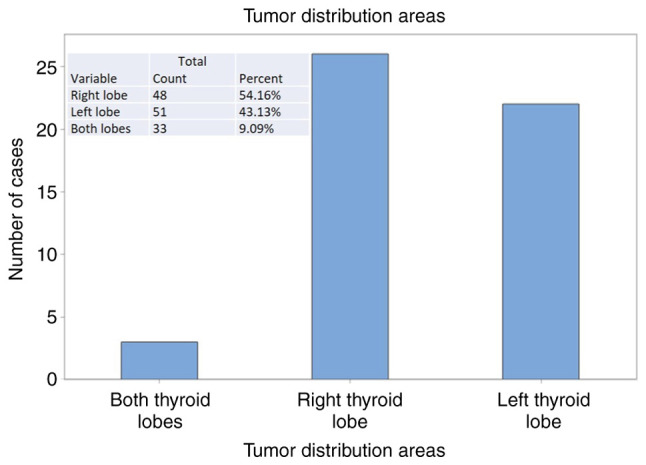
Localization of tumor nodules in the thyroid gland.
Approximately 92% of thyroid carcinomas were of papillary type. Grossly, most papillary tumors were white-grayish with infiltrative borders and firm surface (Fig. 2).
Figure 2.
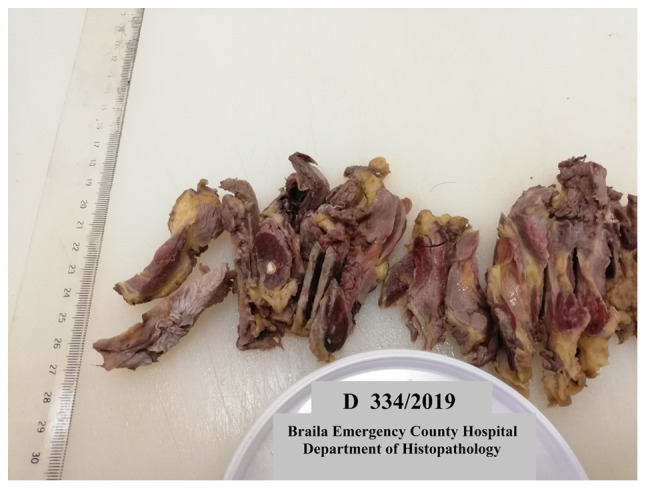
Infiltrative thyroid carcinoma of papillary type, with cystic nodular areas, cut section.
The microscopicarchitectural patterns were micro-papillary, trabecular, solid and cystic types. The stroma around the tumor was sometimes fibrotic and sclerotic.
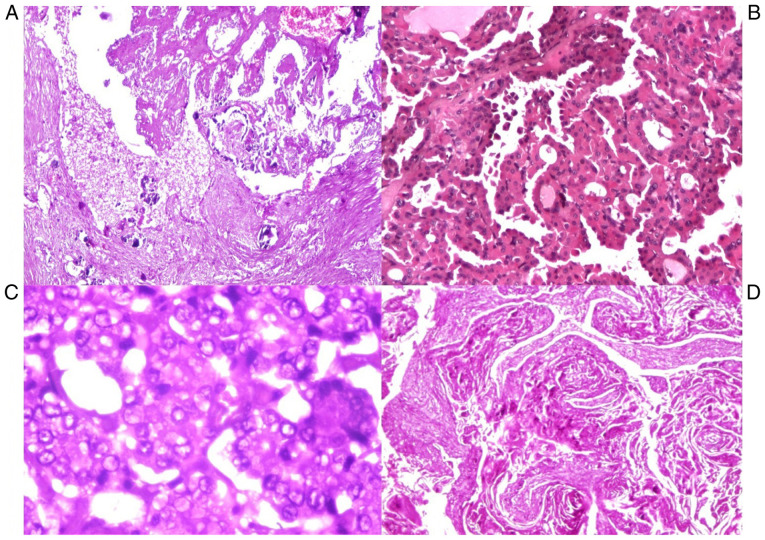
Another typical, but not pathognomonic feature, was the presence of psammoma bodies (5 cases). Distinct nuclear features were crowded vesicular nuclei, optically clear (‘Orphan Annie eyes’), irregular nuclear contours, nuclear grooves and intranuclear cytoplasmic inclusions, marked crowding with overlapping of adjacent nuclei, pale and clear chromatin (Fig. 3). The carcinomas were <1 cm, so-called papillary microcarcinoma (in the present study the dimensions were between 0.2 and 1 cm).
Figure 3.
(A) Thyroid carcinoma: micro-papillary type with psammoma bodies (HE, x40). (B) Papillary carcinoma type with follicular areas (HE, x100). (C) Papillary carcinoma, detail with optical clear, vesicular nuclei (‘Orphan Annie eyes’) (HE, x200). (D) Squamous cell carcinoma (HE, x100). HE, hematoxylin-eosin.
Squamous cell carcinoma involved both lobes
The gross appearance was a firm consistency and grayish-white color with areas of necrosis and extensive infiltration of peri-thyroid soft tissue, vascular and peri-neural invasion. Microscopically these tumors consisted of nests and sheets of cells with squamous differentiation, with keratin ‘pearl’ formation.
Poorly differentiated carcinoma showed evidence of follicular differentiation, fitting morphologically between well-differentiated and undifferentiated thyroid carcinoma, but WHO recognizes it as a separate entity (6). In the present study, we classified them as undifferentiated thyroid carcinoma after Turin criteria and applied IHC.
Grossly, these tumors presented as fleshy, large, solid grey to white nodules with areas of hemorrhage and necrosis. Microscopically, they were composed of a variable admixture of spindle cells. The cells had eosinophilic cytoplasm, brisk mitotic activity with abundant apoptosis.
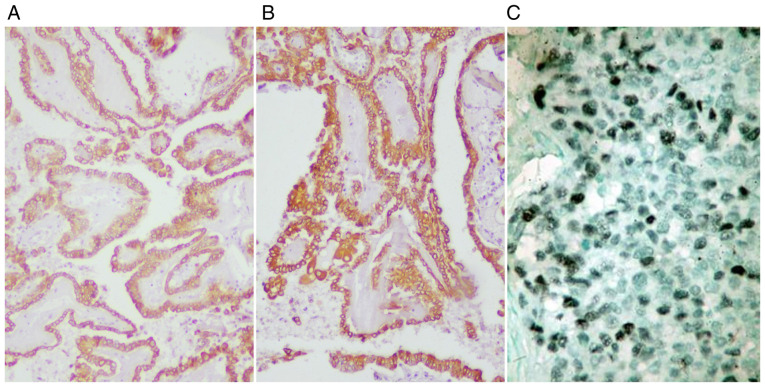
Overall, CK7 was diffusely expressed in papillary carcinomas and focally expressed in squamous cell tumors and undifferentiated tumors. EMA was diffusely positive in all thyroid carcinomas, while TTF1 was negative in most of the cases (Fig. 4). Thyroglobulin was positive in all cases, with variable expression in the cytoplasm of tumor cells.
Figure 4.
Thyroid carcinoma: (A) EMA diffusely positive (IHC, x100), (B) CK7 diffusely positive (IHC, x100), (C) PCNA positive in ~10-15% of tumor cells nuclei (IHC, x200). IHC, immunohistochemistry.
PCNA showed positive expression in ~7-8% of tumor cells nuclei in undifferentiated carcinomas, in ~3-5% of tumor cells nuclei in papillary carcinomas and ~20-25% of tumor cells nuclei in squamous cell carcinoma. The PCNA proliferation index was expressed in tumor cell nuclei, in 25% of well-differentiated squamous tumor cells, both basal and parabasal; in the areas with keratin and para-keratin formation, no expression was observed.
P53 was negative in all cases, including undifferentiated and squamous cell carcinomas. Nuclear antibodies were affected by autolysis and necrosis, therefore the antigen-antibody reaction in the nucleus lost its expression. Vimentin was negative in tumor cells, with expression only in stroma and blood vessels.
Discussion
Thyroid carcinomas had an incidence in the present study of 10%, which is consistent with the percentage reported by studies in the literature (17,18). Normal thyroid appeared in 40% of our cases, an incidence close to that reported in the literature.
According to the WHO, the normal weight of the thyroid has an upper limit of 40 g, anything exceeding this limit is considered to be goiter. Our study showed a thyroid weight of 25-40 g, considered to be within normal limits.
Focal lymphocytic thyroiditis has been found in 39 cases. It is seen by some authors as a nonspecific inflammation associated with etiological factors such as acute infections, local trauma, chemicals, radiation, immune and with an uncertain association with papillary thyroid cancer (19). In the current study, Hashimoto's thyroiditis was associated with neoplasia in 1 case. Squamous metaplasia had an incidence of 7.3% in our cases, with an association of 20% of cases with thyroiditis. The incidence of thyroid disease was not closely associated between sex, age and pathological background.
Immunohistochemistry for CK7 and EMA was positive in all three cases of undifferentiated carcinoma. These stains are useful for diagnosis of tumors and to confirm that the neoplasm is a carcinoma rather than a high-grade sarcoma (20). TTF-1 and thyreoglobulin are typically negative in undifferentiated carcinoma. In the present study, all 3 cases were also negative for TTF1.
Nuclear reactivity for PCNA has been reported in ~75-80% of anaplastic carcinoma (21). In our study, there were 2 cases of anaplastic carcinoma, with 5-10% positivity in the tumor cell nuclei and one case was negative.
Systematic detailed studies have demonstrated that a thyroid, which is apparently normal on macroscopic examination, can be the site of pathological manifestations, and sometimes carcinomatous (22).
Thyroid nodules are quite common in the general population, most often they are benign, about 10% of the detected solitary nodules are malignant. Thyroid nodules detected in polynodular goiter are generally colloidal, the risk of malignancy being reduced (23).
In cases of thyroid carcinomas, the highest frequency is occupied by papillary microcarcinomas, which have a long evolution, these being detected incidentally during autopsies. Papillary microcarcinoma is an extremely common incidental finding and the vast majority of these tumors pursue a benign course (24).
Regarding the association with the risk of thyroid cancer in patients with pre-existing thyroid disease or cardiovascular diseases (25), as well as the association with overweight (26), our study did not show a direct correlation.
The rise in incidence of papillary micro-carcinomas creates management dilemmas. Therefore, the decision in the management of these type of tumors begins at the time of surgery and is associated with a more aggressive course. Younger age may be predictive for radioactive iodine administration in the lowest-risk patients (27,28).
In a cross-sectional study over an historical cohort during a decade, conducted in Brazil, it was found that there was an increase in the proportion of cases with malignant cytological results among microcarcinomas, related to an enhancement in preoperative diagnostic methods (29-31).
In another exhaustive retrospective study, performed on more than 2,000 patients in an interval of 8 years, conducted in Japan, it was also found that the oncological outcomes of the immediate surgery and active surveillance groups were similarly excellent, but the incidences of unfavorable events were higher in the immediate surgery group (32).
In summary, papillary thyroid carcinomas are a compact group with a heterogenous morpho-phenotypical and immune-biological expression and behavior that are sometimes overlooked (particularly the small size tumors, such as micro-carcinomas). The majority of these carcinomas are incidentally identified during a routine check or at autopsy.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ISM and MC performed the histological examinations and IHC, and substantially contributed to the writing of the manuscript. ZC and VM analyzed and interpreted the patient data. BS, DS and AT searched the literature for similar work and articles and contributed to writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted according to the World Medical Association Declaration of Helsinki, using a protocol approved by the local Bioethics Committee from Brăila Emergency County Hospital (Braila). All patients previously signed an informed written consent about hospitalization, treatment and a possible future publication of data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict or competing interests.
References
- 1.Pezzolla A, Lattarulo S, Milella M, Barile G, Pascazio B, Ciampolillo A, Fabiano G, Palasciano N. Incidental carcinoma in thyroid pathology: Our experience and review of the literature. Ann Ital Chir. 2010;81:165–169. [PubMed] [Google Scholar]
- 2.Hurtado-López LM, Basurto-Kuba E, Montes de Oca-Durán ER, Pulido-Cejudo A, Vázquez-Ortega R, Athié-Gutiérrez C. Prevalence of thyroid nodules in the valley of Mexico. Cir Cir. 2011;79:114–117. [PubMed] [Google Scholar]
- 3.Ramirez-Gonzalez LR, Sevilla-Vizcaino R, Monge-Reyes P, Aldaz-Dorantes JE, Márquez-Valdez AR, García-Martínez D, González-Ojeda A, Fuentes-Orozco C. Findings of thyroid nodules in autopsies in western Mexico. Rev Med Inst Mex Seguro Soc. 2017;55:594–598. (In Spanish) [PubMed] [Google Scholar]
- 4.Yu GP, Li JCL, Branovan D, McCormick S, Schantz SP. Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid. 2010;20:465–473. doi: 10.1089/thy.2008.0281. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Jung YS, Ryu CH, Lee CY, Lee YJ, Lee EK, Kim SK, Kim TS, Kim TH, Jang J, et al. Identification of occult tumors by whole-specimen mapping in solitary papillary thyroid carcinoma. Endocr Relat Cancer. 2015;22:679–686. doi: 10.1530/ERC-15-0152. [DOI] [PubMed] [Google Scholar]
- 6.Pinchera A. Thyroid incidentalomas. Horm Res. 2007;68 (Suppl 5):S199–S201. doi: 10.1159/000110625. [DOI] [PubMed] [Google Scholar]
- 7.Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: Why is incidence increasing? Curr Opin Oncol. 2015;27:1–7. doi: 10.1097/CCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 8.Vaideeswar P, Singaravel S, Gupte P. The thyroid in ischemic heart disease: An autopsy study. Indian Heart J. 2018;70 (Suppl 3):S489–S491. doi: 10.1016/j.ihj.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies L, Morris LGT, Haymart M, Chen AY, Goldenberg D, Morris J, Ogilvie JB, Terris DJ, Netterville J, Wong RJ, Randolph G. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: The increasing incidence of thyroid cancer. Endocr Pract. 2015;21:686–696. doi: 10.4158/EP14466.DSCR. AACE Endocrine Surgery Scientific Committee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off J Am Thyroid Association. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. [DOI] [PubMed] [Google Scholar]
- 11.Lee YS, Lim H, Chang HS, Park CS. Papillary thyroid microcarcinomas are different from latent papillary thyroid carcinomas at autopsy. J Korean Med Sci. 2014;29:676–679. doi: 10.3346/jkms.2014.29.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janovsky CCPS, Bittencourt MS, de Novais MAP, Maciel RMB, Biscolla RPM, Zucchi P. Thyroid cancer burden and economic impact on the Brazilian public health system. Arch Endocrinol Metab. 2018;62:537–544. doi: 10.20945/2359-3997000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaliszewski K, Zubkiewicz-Kucharska A, Kiełb P, Maksymowicz J, Krawczyk A, Krawiec O. Comparison of the prevalence of incidental and non-incidental papillary thyroid microcarcinoma during 2008-2016: A single-center experience. World J Surg Oncol. 2018;16(202) doi: 10.1186/s12957-018-1501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slijepcevic N, Zivaljevic V, Marinkovic J, Sipetic S, Diklic A, Paunovic I. Retrospective evaluation of the incidental finding of 403 papillary thyroid microcarcinomas in 2466 patients undergoing thyroid surgery for presumed benign thyroid disease. BMC Cancer. 2015;15(330) doi: 10.1186/s12885-015-1352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyauchi A, Ito Y, Oda H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid. 2018;28:23–31. doi: 10.1089/thy.2017.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M, Sobrinho-Simoes M, et al. Poorly differentiated thyroid carcinoma: The turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31:1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 17.Shah JP. Thyroid carcinoma: Epidemiology, histology, and diagnosis. Clin Adv Hematol Oncol. 2015;13 (Suppl 4):S3–S6. [PMC free article] [PubMed] [Google Scholar]
- 18.Olson E, Wintheiser G, Wolfe KM, Droessler J, Silberstein PT. Epidemiology of thyroid cancer: A review of the national cancer database, 2000-2013. Cureus. 2019;11(e4127) doi: 10.7759/cureus.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee I, Kim HK, Soh EY, Lee J. The association between chronic lymphocytic thyroiditis and the progress of papillary thyroid cancer. World J Surg. 2020;44:1506–1513. doi: 10.1007/s00268-019-05337-9. [DOI] [PubMed] [Google Scholar]
- 20.Shvero J, Koren R, Shpitzer T, Feinmesser R, Segal K. Immunohistochemical profile and treatment of uncommon types of thyroid carcinomas. Oncol Rep. 2003;10:2075–2078. doi: 10.3892/or.10.6.2075. [DOI] [PubMed] [Google Scholar]
- 21.Cvejic D, Savin S, Petrovic I, Selemetjev S, Paunovic I, Tatic S, Havelka M. Galectin-3 and proliferating cell nuclear antigen (PCNA) expression in papillary thyroid carcinoma. Exp Oncol. 2005;27:210–214. [PubMed] [Google Scholar]
- 22.Brigante G, Monzani ML, Locaso M, Gnarini VL, Graziadei L, Kaleci S, De Santis MC, Tagliavini S, Simoni M, Rochira V, Madeo B. De novo lesions frequently develop in adult normal thyroid over almost six years. Front Endocrinol (Lausanne) 2020;11(18) doi: 10.3389/fendo.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901–911. doi: 10.1016/j.beem.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur J Surg Oncol. 2018;44:307–315. doi: 10.1016/j.ejso.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Gaman MA, Dobrica EC, Pascu EG, Cozma MA, Epingeac ME, Gaman AM, Pantea Stoian A, Bratu OG, Diaconu CC. Cardio metabolic risk factors for atrial fibrillation in type 2 diabetes mellitus: Focus on hypertension, metabolic syndrome and obesity. J Mind Med Sci. 2019;6:157–161. [Google Scholar]
- 26.Epingeac ME, Gaman MA, Diaconu C, Gad M, Gaman AM. The evaluation of oxidative stress levels in obesity. Rev Chim (Bucharest) 2019;70:2241–2244. [Google Scholar]
- 27.Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: A comprehensive review of animal and human studies. Thyroid Res. 2015;8(8) doi: 10.1186/s13044-015-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haymart MR, Cayo M, Chen H. Papillary thyroid microcarcinomas: Big decisions for a small tumor. Ann Surg Oncol. 2009;16:3132–3139. doi: 10.1245/s10434-009-0647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girardi FM, Barra MB, Zettler CG. Analysis of pattern of occurrence of thyroid carcinoma between 2001 and 2010. Braz J Otorhinolaryngol. 2015;81:541–548. doi: 10.1016/j.bjorl.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai SA. The Washington manual of surgical pathology. J Clin Pathol. 2009;62:766–767. [Google Scholar]
- 31.Dumitru N, Cocolos A, Caragheorgheopol A, Dumitrache C, Bratu OG, Neagu TP, Diaconu CC, Ghemigian A. Collagen-the ultrastructural element of the bone matrix. Rev Chim (Bucharest) 2018;69:1706–1709. [Google Scholar]
- 32.Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, Masuoka H, Yabuta T, Fukushima M, Higashiyama T, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26:150–155. doi: 10.1089/thy.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.