Abstract
Background
Biannual distribution of azithromycin to children 1–59 months old reduced mortality by 14% in a cluster-randomized trial. The World Health Organization has proposed targeting this intervention to the subgroup of children 1–11 months old to reduce selection for antimicrobial resistance. Here, we describe a trial designed to determine the impact of age-based targeting of biannual azithromycin on mortality and antimicrobial resistance.
Methods
AVENIR is a cluster-randomized, placebo-controlled, double-masked, response-adaptive large simple trial in Niger. During the 2.5-year study period, 3350 communities are targeted for enrollment. In the first year, communities in the Dosso region will be randomized 1:1:1 to 1) azithromycin 1–11: biannual azithromycin to children 1–11 months old with placebo to children 12–59 months old, 2) azithromycin 1–59: biannual azithromycin to children 1–59 months old, or 3) placebo: biannual placebo to children 1–59 months old. Regions enrolled after the first year will be randomized with an updated allocation based on the probability of mortality in children 1–59 months in each arm during the preceding study period. A biannual door-to-door census will be conducted to enumerate the population, distribute azithromycin and placebo, and monitor vital status. Primary mortality outcomes are defined as all-cause mortality rate (deaths per 1000 person-years) after 2.5 years from the first enrollment in 1) children 1–59 months old comparing the azithromycin 1–59 and placebo arms, 2) children 1–11 months old comparing the azithromycin 1–11 and placebo arm, and 3) children 12–59 months in the azithromycin 1–11 and azithromycin 1–59 arms. In the Dosso region, 50 communities from each arm will be followed to monitor antimicrobial resistance. Primary resistance outcomes will be assessed after 2 years of distributions and include 1) prevalence of genetic determinants of macrolide resistance in nasopharyngeal samples from children 1–59 months old, and 2) load of genetic determinants of macrolide resistance in rectal samples from children 1–59 months old.
Discussion
As high-mortality settings consider this intervention, the results of this trial will provide evidence to support programmatic and policy decision-making on age-based strategies for azithromycin distribution to promote child survival.
Trial registration
This trial was registered on January 13, 2020 (clinicaltrials.gov: NCT04224987).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-021-10824-7.
Keywords: Azithromycin, Mortality, Cluster-randomized trial, Adaptive trial, Mass drug administration
Background
Despite an overall decrease in the burden of childhood mortality in sub-Saharan Africa, as many as 10% of children do not survive to the age of 5 years in some areas [1, 2]. To achieve the Sustainable Development Goal (SDG) to end preventable under-5 mortality by 2030, parts of western and central Africa must achieve unprecedented rates of mortality reduction [2, 3]. With estimates at 108 deaths per 1000 livebirths [1], under-5 mortality in Niger is more than 4 times the SDG target of 25 deaths per 1000 livebirths [3]. In such high mortality settings, effective and feasible interventions are urgently needed.
Azithromycin distribution has been proposed as a stop-gap intervention to reduce the burden of preventable deaths in high mortality settings while health systems are strengthened. Trachoma programs have provided oral azithromycin to entire communities for decades to prevent blindness [4], with more than 900 million doses distributed globally [5]. The potential for azithromycin distribution to reduce child mortality was discovered in randomized controlled trials conducted for trachoma [6–8]. The Macrolides Oraux pour Réduire les Décès avec un Œil sur la Résistance trial (MORDOR) later demonstrated that biannual distribution of oral azithromycin to children 1–59 months of age reduced mortality in this age group by 13.5% in Malawi, Niger, and Tanzania (95% CI 6.7 to 19.8) [9]. The greatest mortality reductions were observed in Niger (18.1% reduction, 95% CI 10.0 to 25.5) and among the youngest children (24.9% reduction in children 1–5 months old, 95% CI 10.6 to 37.0), subgroups which also had the highest baseline mortality rates [9]. Although the exact mechanism of effect remains unclear, evidence suggests that azithromycin distributions reduce the burden of respiratory infections, diarrhea, and malaria [10], common causes of under-5 mortality in sub-Saharan Africa [1].
Despite the potential for this intervention to improve child survival, concern about selection for antimicrobial resistance (AMR) underpins caution in implementation. Evidence from trachoma studies and the MORDOR trial indicate that azithromycin distribution increases the community prevalence of macrolide resistance in respiratory and enteric organisms [11, 12]. An increase in AMR could decrease the efficacy of macrolides or other antibiotics against target pathogens, although longer-term evidence from MORDOR did not find evidence of waning efficacy against all-cause mortality, even with an increase in genetic determinants of AMR [13]. Trachoma programs, which distribute azithromycin to everyone in a community as opposed to just children, suggest that resistance decreases once distributions are discontinued [12].
The World Health Organization (WHO) released conditional guidelines that specify targeting azithromycin distributions to children 1–11 months of age in high mortality settings [14]. The rationale for targeting 1–11 months is that the youngest children experience the highest risk of mortality and limiting distributions to a subgroup of children could reduce selection for antimicrobial resistance. However, distributing azithromycin to children 1–11 months alone remains untested, since MORDOR I treated children 1–59 months of age. A crude examination of relative mortality reductions by age group suggested a stronger effect in the younger age groups, but MORDOR was unable to demonstrate a differential effect by age group statistically [9]. Moreover, a herd effect could have contributed to the apparent larger effect sizes in the younger groups; treating children 12–59 months may protect children 1–11 months by reducing their exposure to infectious diseases causing mortality. Finally, in MORDOR-Niger there were 5 times as many children aged 12–59 months as children 1–11 months, resulting in nearly twice as many deaths averted in the older age group [15]. The greatest societal gains may thus come from intervening on a population with a broader risk spectrum instead of targeting high-risk subgroups [16, 17]. More evidence is needed to determine the magnitude of and difference in survival benefit when targeting different age groups, and of the magnitude of AMR selection when distributing to different age groups.
The AVENIR trial (Azithromycine pour la Vie des Enfants au Niger: Implementation et Recherche) was designed to test the effect of age-based targeting on mortality and resistance. Several design features support these objectives. First, as mortality is a rare event even in high mortality settings, a large sample size is required to detect modest intervention effects [18]. AVENIR thus uses a large simple trial design, with a simple intervention, minimal data collection, and simple outcome assessment allowing for feasible implementation in a very large population. Second, AVENIR is cluster randomized both because of the community-based nature of the intervention and to address potential contamination within communities [19]. As the mechanism of azithromycin’s effect is likely through its impact on infectious disease, community-level randomization prevents an individual’s treatment assignment from affecting the outcomes of individuals with different assignments within a community. Finally, AVENIR will use response-adaptive allocation to achieve several aims. This approach allows for more ethical allocation, as communities will have a higher probability of receiving the intervention that reduces mortality the most. By maintaining an allocation of at least 10% of communities to placebo, continued monitoring of efficacy is still possible. This includes the detection of waning effects over time which might indicate the appropriate duration of the intervention. The adaptive design also allows for greater flexibility, enabling the existing trial infrastructure to be used as a platform to evaluate other interventions [20]. The study can adapt to changing guidelines by randomizing in new intervention arms, for example, rather than being constrained by the original treatments which could become irrelevant during the course of the trial due to a change in policy recommendations.
Methods/design
Study design overview
AVENIR is a double-masked cluster-randomized placebo-controlled response-adaptive large simple trial in Niger. During a one-year run-in period, communities in the Dosso region in Niger will be randomized 1:1:1 to 1) azithromycin 1–11: biannual azithromycin to children 1–11 months old with placebo to children 12–59 months old, 2) azithromycin 1–59: biannual azithromycin to children 1–59 months old, or 3) placebo: biannual placebo to children 1–59 months old. Regions enrolled after the run-in period will be randomized with an allocation updated based on the probability of mortality in children 1–59 months in each arm during the preceding study period. Communities will retain their allocation for 4 distributions, after which they will be re-randomized with an updated allocation. All-cause mortality will be compared by arm at 2.5 years from the first enrollment. In a substudy, 50 randomly selected communities from each arm in the Dosso region will be followed to monitor antimicrobial resistance. Figure 1 summarizes the mortality and resistance trial designs for the Dosso and Tahoua regions.
Fig. 1.
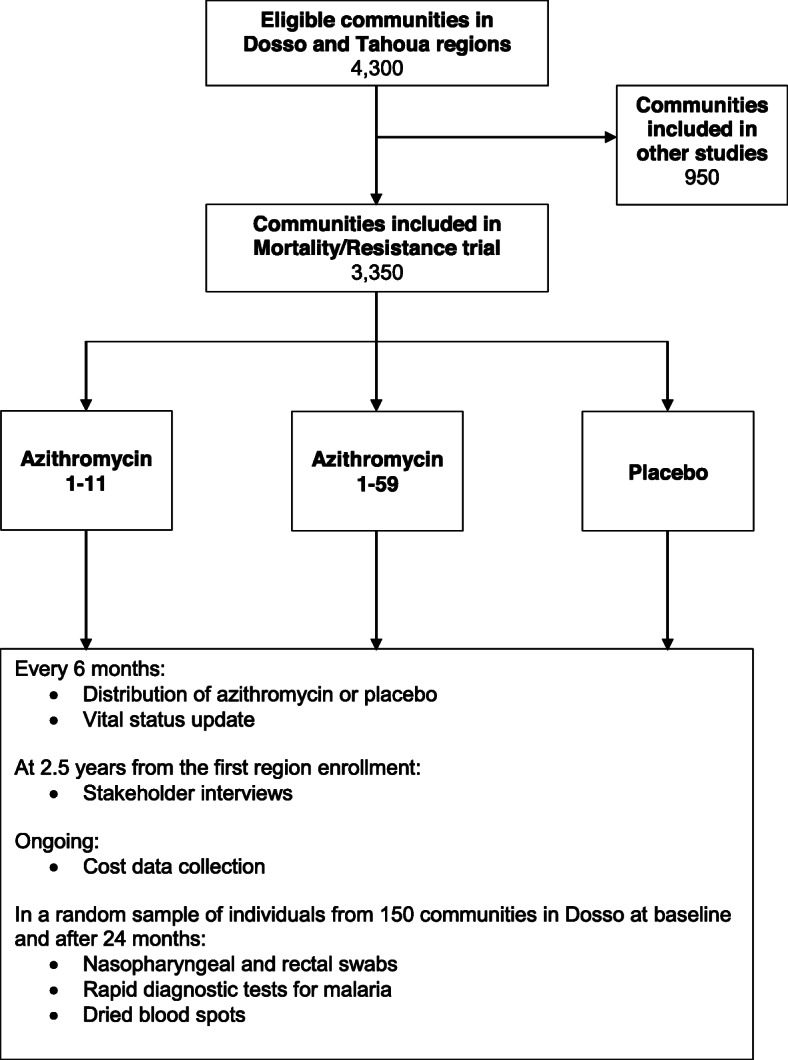
AVENIR trial design summary for communities included in primary outcomes during Stage I. Communities randomized to the azithromycin 1–11 arm will receive biannual distribution of azithromycin to children 1–11 months of age with placebo to children 12–59 months of age. Communities randomized to the azithromycin 1–59 arm will receive biannual distribution of azithromycin to children 1–59 months of age. Children randomized to the placebo arm will receive biannual distribution of placebo to children 1–59 months of age
The trial began enrollment in November 2020 and recruitment is anticipated through May 2023. Stage I includes enrollment through the primary mortality analysis and Stage II includes potential continued enrollment after this time point. This protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Supplementary Table S1, [21]) and refers to version 13 of the study protocol as revised 31 January 2021.
Objectives and hypotheses
The objectives of this study are 1) to compare the efficacy of two age-based approaches to biannual mass azithromycin distribution to reduce all-cause mortality, 2) to compare selection for antimicrobial resistance across these strategies, and 3) to evaluate implementation-related outcomes, including the costs, feasibility, and acceptability of this intervention, and to compare these indicators across distribution strategies. Specifically, objective 1 has 3 aims: 1) to replicate and assess the generalizability of the MORDOR trial by comparing 1–59-month mortality in the azithromycin 1–59 and placebo arms, 2) to test the efficacy of targeting treatment to children 1–11 months old by comparing 1–11-month mortality in the azithromycin 1–11 and placebo arms, and 3) to determine the additional benefit of including older children by comparing 12–59-month mortality in the azithromycin 1–11 and azithromycin 1–59 arms. We hypothesize that mortality will be lower in both azithromycin arms compared to placebo, and lower in the azithromycin 1–59 arm compared to the azithromycin 1–11 arm at 2.5 years from the first enrollment. For objective 2, the aims are 1) to compare the community-level prevalence of genetic determinants of macrolide resistance in respiratory organisms across arms and 2) to compare the community-level load of genetic determinants of macrolide resistance in enteric organisms across arms. We hypothesize that, after 2 years of distributions, resistance will be higher in both azithromycin arms compared to placebo, and higher in the azithromycin 1–59 arm compared to azithromycin 1–11. Objective 3 involves the calculation of program costs for each strategy, analysis of the comparative cost-effectiveness, and the measurement of feasibility and acceptability as perceived by stakeholders at the community, implementer, and governmental levels. We hypothesize that the azithromycin 1–59 arm will be more cost-effective than the azithromycin 1–11 arm and that these strategies will be similarly feasible and acceptable.
Setting, participants, and eligibility
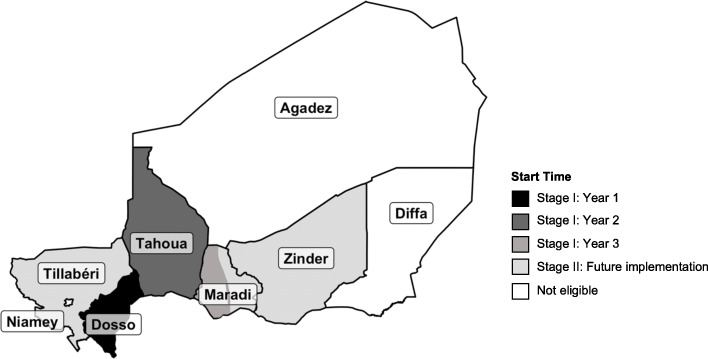
Full eligibility criteria at the community and individual levels are described in Table 1. This study will include secure and accessible rural and peri-urban communities in the 5 regions of Niger with the largest under-5 populations (Dosso, Tahoua, Maradi, Zinder, Tillabéri; Fig. 2, Table 2), with Stage I targeting the Dosso, Tahoua, and the first half of the Maradi regions for inclusion. Dosso and Tahoua regions will contribute to the primary outcomes in Stage I. The estimated 2017 regional under-5 mortality rates were 116 per 1000 live births in Dosso, 101 in Tahoua, 110 in Maradi, 120 in Zinder, and 116 in Tillabéri [22]. We estimate that these 5 regions include approximately 14,630 eligible communities encompassing approximately 2.3 million eligible children under 5 (Table 2) and 3350 of these are targeted for inclusion in Stage I.
Table 1.
Eligibility criteria at the community- and individual-levels for the AVENIR mortality and resistance Stage I trial in Niger
| Trial | Level | Eligibility Criteria |
|---|---|---|
| Mortality | Community |
• Location in Dosso or Tahoua region • Population between 250 and 2499a • Distance > 5 km from district headquarters town • Not designated as a “quartier” on national census • Accessible and safe for study team • Verbal consent from community leader(s) |
| Individual |
• Primary residence in a study community • Age 1–59 months • Weight ≥ 3.0 • No known allergy to macrolides • Verbal consent of guardian for study participation |
|
| Resistance | Community |
• Location in Dosso • Accessible and safe for study team • Randomly selected • Not included in MORDOR trials • Verbal consent of community leader(s) |
| Individual |
• Primary residence in a selected study community • 1–59 months old, 7–12 years old or guardian of child eligible for treatment • Randomly selected from census • Verbal consent from guardian |
a Population size as estimated from the most recent national census or projections
Fig. 2.
Eligible regions in Niger with and AVENIR study timeline. Stage I includes implementation in the Dosso, Tahoua, and first half of the Maradi regions, with communities from Dosso and Tahoua contributing to the primary outcomes described in this protocol. Continuation to Stage II depends on the primary outcome results as well as logistical, financial, and security concerns. Stage II would include implementation in the remaining half of Maradi as well as the Zinder and Tillabéri regions. The map was created by the authors in R (R Foundation for Statistical Computing, Vienna, Austria)
Table 2.
Estimated eligible population and study timeline by region in Stages I and II of the AVENIR trial
| Region | Estimated eligible under-5 populationc | Estimated eligible communitiesd | Stage Ia | Stage IIb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 3 | Year 4 | ||||||
| Round 1 | Round 2 | Round 3e | Round 4 | Round 5 | Round 6 | Round 7 | Round 8 | |||
| Dosso | 271,273 | 1800 | X* | X | X | X | X* | X | X | X |
| Tahoua (1st half) | 245,944 | 1250 | X | X | X | X | X | X | ||
| Tahoua (2nd half) | 245,944 | 1250 | X | X | X | X | X | |||
| Maradi (1st half) | 342,889 | 1700 | X | X | X | X | ||||
| Maradi (2nd half) | 342,890 | 1700 | X | X | X | |||||
| Zinder | 452,241 | 3860 | X | X | ||||||
| Tillabéri | 440,791 | 3070 | X | |||||||
| Total | 2,341,972 | 14,630 | ||||||||
Round = one distribution of azithromycin and/or placebo; x = census and treatment; x* = census, resistance assessments, treatment
a Stage I includes enrollment through the primary outcome analysis at 2.5 years from the first region enrollment
b Stage II is dependent upon primary outcome results, available resources, and security restrictions
c As estimated from projections by the Institute for Health Metrics and Evaluation multiplied by fraction of eligible grappes in each region
d Communities with total populations between 250 and 2499 not designated as urban quartiers according to the 2012 Niger national census. Dosso region estimates further exclude communities known to be located within 5 km of district headquarters town
Distance-based exclusions pending for the other 4 regions.
e Interim analysis to be conducted at 18 months from the first enrollment
Recruitment
AVENIR was designed in collaboration with local, national, and global stakeholders in child health. Sensitization campaigns will be conducted at the community, district, regional, and national levels before study activities begin and leaders at these levels will continue to be engaged by the study team throughout the course of the trial and in the dissemination of results. All households will be recruited for participation in the census, and all children 1–59 months of age will be recruited for the intervention.
Randomization and masking
The randomization unit will be the grappe, which is an administrative unit defined during the 2012 general population census in Niger, hereafter referred to as “community.” The randomization sequence will be generated by one trial biostatistician and data analyst in R (R Foundation for Statistical Computing, Vienna, Austria).
Implementation will be phased in by region (Fig. 2, Table 2). Communities in Dosso will be enrolled in the first year and randomized to the 3 arms in a 1:1:1 allocation. After 1 year, allocation probabilities for newly enrolled communities will adapt using the probability that each arm is superior with respect to the mortality rate in children 1–59 months, such that communities will have a higher probability of being allocated to the arm with the lowest mortality rate. From the pool of eligible communities in the Dosso region, a substudy will include a random sample of 50 communities per arm to monitor AMR outcomes.
After the primary outcome analysis at 2.5 years from the first enrollment, communities may be re-randomized with continued follow-up using the same adaptive allocation. To minimize the impact of a possible carry-over effect of treatment in the event that a community changes arms through adaptive allocation, each community will stay with its randomized assignment for 4 distributions (approximately 2 years). No community will be re-randomized by the time of the primary outcome analysis. Additional treatment arms may be added to the trial or arms may be dropped from the trial after the primary outcome analysis. With such changes, protocol details, power calculations, and analysis plans will be updated accordingly.
Masking will be facilitated by use of a matching placebo, which will be identical to azithromycin in appearance, smell, packaging, and distribution. Those masked to study arm allocation include participants, investigators, and most study personnel including census personnel administering treatment and collecting mortality outcomes and laboratory personnel processing samples for resistance outcomes. Unmasked personnel include the trial biostatistician and data analyst responsible for implementing the randomization sequence and key members of Pfizer staff responsible for implementation of the randomization sequence.
Interventions
Azithromycin and placebo will be administered biannually as a single, directly observed dose of 20 mg/kg (up to the maximum adult dose of 1 g) as oral suspension using dosing cups or syringes. For children 1–11 months of age and those unable to stand, dose will be determined by weight using a hanging scale. For children 12–59 months of age able to stand, dose will be determined by height approximation as currently performed by Niger’s trachoma program [23]. Azithromycin and placebo will be prepared by Pfizer, Inc. (New York, NY, USA) and shipped directly to the study sites.
Outcome assessments
Mortality
A door-to-door census will be conducted every 6 months to enumerate households, identify eligible children, administer azithromycin and placebo, and monitor vital status. At the household level, GPS coordinates will be taken and demographic data will be recorded for the head of household, caregivers, and children 1–59 months old. For eligible children, weight and/or dose will be recorded along with treatment adherence. At subsequent census periods, study teams will return to each household to update the vital status of children, indicating whether a child is alive, has died, has moved, or has an unknown status. New children and households will be added. The census will be updated at approximately 6, 12, 18, 24, 30, and 36 months from baseline.
The primary mortality outcome is the all-cause mortality rate (deaths per 1000 person-years at risk) at 2.5 years from the first enrollment, with a window of up to 3 months. Inter-census intervals will be used to determine mortality rates. Children will be included as died in the primary outcome if vital status is recorded as alive at one census and died at the subsequent census. Person-time at risk will be calculated as time alive and eligible for treatment while living in the study area, with children who died, moved, or have an unknown status contributing half of the person-time for their last inter-census interval. Three primary comparisons will be made: 1) mortality rate in children 1–59 months in the azithromycin 1–59 vs placebo arms, 2) mortality rate in children 1–11 months in the azithromycin 1–11 vs placebo arms, and 3) mortality rate in children 12–59 months in the azithromycin 1–11 vs azithromycin 1–59 arms.
Secondary mortality outcomes include mortality rate in children 12–59 months compared between the azithromycin 1–11 and placebo arms and mortality rate in children 1–11 months compared between the azithromycin 1–11 and azithromycin 1–59 arm. Subgroups of interest include region, number of past distributions, distribution timing and seasonality, compliance, anthropometric indicators, and presence of other interventions like seasonal malaria chemoprevention or trachoma distribution of azithromycin. Weight as measured to determine dosage will also be compared by arm over time.
Antimicrobial resistance
A random sample of 50 communities per arm will be selected from eligible communities in the Dosso region and followed to monitor antimicrobial resistance and other outcomes. In each of the selected communities, 30 children aged 1–59 months, 30 children aged 7–12 years, and 30 caregivers/guardians of eligible children will be randomly selected from the census for additional monitoring. Nasopharyngeal samples will be collected from all selected individuals. From children 1–59 months, rectal swabs, rapid diagnostic tests for malaria, and dried blood spots will also be collected. Children with positive RDT results will be referred for further care. Environmental samples will also be taken from selected communities and households, including community-level water sources and latrines, and household-level stored water, animal feces, and dirt. Samples will be collected at baseline before the first distribution and after 4 distributions (approximately 2 years). Genotypic methods will be used to process samples to determine the presence of genetic determinants of resistance to macrolides and other classes of antibiotics, including targeted PCR for the nasopharyngeal swabs and metagenomic deep sequencing for the rectal swabs at the community level. In the sample of children 1–59 months old, 10 children will be randomly selected for an additional nasopharyngeal swab which will be used for phenotypic testing of resistance in Streptococcus pneumoniae.
The primary AMR outcomes include 1) the prevalence of genetic determinants of macrolide resistance in children 1–59 months old from nasopharyngeal swabs, and 2) the load of genetic determinants of macrolide resistance in children 1–59 months old from rectal swabs.
Secondary outcomes from these collections include resistance to other classes of antibiotics among samples taken from children 1–59 months old, the proportion of macrolide-resistant pneumococcal isolates from nasopharyngeal swabs in children 1–59 months old, indirect effects of azithromycin distribution on resistance to macrolides and other classes of antibiotics among untreated individuals living in the same household as those eligible for treatment (children 7–12 years and adults), genetic determinants of antibiotic resistance in environmental samples, the prevalence of malaria in children 1–59 months old, and serological markers of pathogen infection.
Implementation outcomes
Program costs will be measured by macro-costing activities using routine administrative data collection and by focused micro-costing activities using both routine administrative data collection and detailed time and motion data collection. Cost-effectiveness analysis will assess the added net costs, added health outcomes, and the incremental cost-effectiveness ratio comparing cost per health outcome achieved between distribution strategies. These analyses will be assessed from different perspectives: households, community, health system, and society. Feasibility will be tracked using administrative and monitoring data collected on timeliness and fidelity of intervention rollout, training and supervision metrics, supply inventory and storage, and AMR monitoring. Acceptability of the program and the two distribution strategies will be assessed using semi-structured interviews on the perception of and satisfaction with the intervention, facilitators and barriers to participation and implementation, and relevance of the intervention to the interviewees. Interviews will be conducted with stakeholders at multiple levels, including participant, community leaders, implementers, and those involved in health system policy and programmatic decision-making.
Adverse events
At the time of treatment, caregivers will be instructed to report any adverse event experienced by the treated child within 28 days of treatment to the study team. Serious adverse events will be reported to UCSF and the Medical Monitors within 24 h of notification, and to Pfizer, the Data and Safety Monitoring Committee (DSMC), and Institutional Review Boards (IRBs) according to the requirements of each. Children will be referred for follow-up care on a case-by-case basis.
Data collection, management, and monitoring
All data will be collected electronically on mobile devices with a custom-designed mobile application (CommCare by Dimagi, Cambridge, MA, USA), and uploaded regularly to a secure, cloud-based server. All study personnel will attend intensive, two-day training sessions prior to conducting study procedures and collecting data. Data management teams in Niger and at UCSF will monitor data collection in real time daily. Investigators will visit study sites twice each year to monitor study implementation and ensure adherence to the study protocol.
Study participants will be assigned unique identification numbers that do not contain personal identifying information, which will be used to link data across databases and will be the only identifier shared in de-identified datasets. Upon completion of the trial, de-identified data will be made available upon request.
Study oversight
A Data and Safety Monitoring Committee (DSMC) will be empaneled before the study begins to provide independent oversight of data quality and participant safety. The DSMC will include independent experts with collective expertise in bioethics, biostatistics, antimicrobial resistance, and pediatric infectious disease. The DSMC will meet at least once per year to review study progress and adverse events, with ad hoc meetings convened as necessary. The DSMC will recommend modifications to the protocol as necessary. Major protocol changes will be reported to the DSMC and Institutional Review Board(s) as required.
Study investigators from UCSF and in Niger will monitor study conduct regularly through data monitoring as described above in addition to regular site visits to ensure adherence to the study protocol. Details of ethical approval and informed consent are included below. Two Medical Monitors with expertise in pediatrics and infectious disease will review the protocol before the trial begins and will review serious adverse events during the trial.
Statistical considerations
Adaptation
The adaptive allocation will use all outcome measurements collected by each time of adaptation. The algorithm involves fitting a negative binomial regression model with the count of deaths as the outcome, community-level person-time at risk during each inter-census interval as an offset, and indicators for each arm. The model will be used to estimate each arm’s log mortality rate and standard error. Then, 10,000 replicates of the log mortality rates will be drawn from the estimated distributions and the arm with the lowest mortality rate in each replicate will be determined. The probability that each arm has the lowest mortality rate will be estimated as the proportion of the 10,000 replicates in which the arm has the lowest mortality rate. To mitigate drastic swings in allocation due to change, a fourth-root transformation will be applied to the probabilities which was determined through simulation. To preserve the ability to make comparisons, no arm’s allocation probability will be allowed to fall below 10% [24].
Sample size and power
Power calculations were based on the primary mortality and resistance outcomes and the anticipated number of communities enrolled by 2.5 years (Table 2). In Stage I, 1400 communities will be randomly selected from the 1800 eligible communities in Dosso to participate for 2 full years (4 inter-census intervals), 975 communities in Tahoua will be selected from approximately 2500 eligible communities to participate for 1 full year (2 inter-census intervals), and another 975 communities in Tahoua will be selected from the eligible pool to participate for one half year (1 inter-census interval). In total, the primary outcome is expected to include 8525 inter-census intervals from 3350 communities (1116 per arm). Table 3 summarizes the detectable effect sizes and the assumptions used in the calculations.
Table 3.
Detectable effect sizes and assumptions used in sample size calculations for mortality and resistance primary outcomes for the AVENIR trial
| Trial | Outcome | Comparison | Power | Alpha | Baselinea,b | SD or ICCb | Number of communities per arm | Relative effect size | Absolute effect size |
|---|---|---|---|---|---|---|---|---|---|
| Mortalityc | Mortality in 1–59-month group | Azithromycin 1–59 vs placebo | 80% | 0.05 | 27 deaths per 1000 person-years | 0.019 | 1116 | 10% | 2.7 per 1000 person-years |
| Mortality in 1–11-month group | Azithromycin 1–11 vs placebo | 80% |
0.05 (if 1–59 group P < 0.05) |
45 deaths per 1000 person-years | 0.068 | 1116 | 19% | 8.8 deaths per 1000 person-years | |
| Mortality in the 12–59-month group | Azithromycin 1–11 vs azithromycin 1–59 | 80% |
0.05 (if 1–11 group P < 0.05) |
24 deaths per 1000 person-years | 0.018 | 1116 | 11% | 2.7 deaths per 1000 person-years | |
| Resistanced | Prevalence of genetic determinants, NP swabs in 1–59-month group |
Azithromycin 1–11 vs placebo Azithromycin 1–59 vs placebo Azithromycin 1–11 vs 1–59 |
80% | 0.05/2 | 30% | 0.045 | 20 | 43% | 13% |
| Load of genetic determinants, rectal swabs in 1–59-month groupe |
Azithromycin 1–11 vs placebo Azithromycin 1–59 vs placebo Azithromycin 1–11 vs 1–59 |
80% | 0.05/2 | 2.13 | 0.02 | 20 | 70% | 0.74 |
ICC intra-class correlation coefficient, SD standard deviation
a Refers to baseline mortality rate for mortality outcomes, and baseline prevalence or load of genetic determinants of macrolide
resistance for resistance outcomes.
b Estimated using data from the MORDOR Niger trial, SD reported for mortality rates and ICC for resistance
c Calculations based on standard Z-test formula for power
d Calculations based on Eqs. 7.11 and 7.12 for prevalence and load, respectively [19].
e Load of genetic determinants of resistance presented on the log2 scale
Sensitivity analyses were conducted to estimate power based on reduced enrollment caused by security or logistical concerns. If only the first half of Tahoua communities are enrolled by 2.5 years, resulting in 30% fewer communities overall (791 communities per arm), we would still have 80% power to detect the following relative effect sizes: 12, 23, and 13% for 1–59 months, 1–11 months, and 12–59 months, respectively.
Analysis of primary outcomes
The primary analysis for the mortality outcomes will be negative binomial regression with count of deaths in each community as the outcome and community-level person-time at risk during each inter-census interval as an offset. The models will include indicator variables for treatment arm and inter-census interval. The adaptive allocation will be accounted for using pooled regression, with each inter-census interval a stratum with its own allocation probabilities [24]. For each of the three primary mortality outcomes, the analysis will be subset to include the target age group. The parameter of interest estimated from each model is the incidence rate ratio (IRR), with 95% confidence intervals (Cis) estimated using bootstrap resampling at the community level. A fixed-sequence, hierarchical testing approach will be used for the three primary mortality outcomes [25], such that the 1–59-month mortality will be compared first. If that comparison between the azithromycin 1–59 and placebo arms is statistically significant at an alpha of 0.05, then testing proceeds to the second outcome, 1–11-month mortality. Similarly, if the comparison between azithromycin 1–11 and placebo arms is statistically significant at an alpha of 0.05, then testing proceeds to the third outcome. Regardless of hypothesis testing results, we will report 95% confidence intervals around estimates of the incidence rate ratio for each comparison. Effect heterogeneity will be assessed for pre-specified subgroups on the additive and multiplicative scales [26, 27].
The primary analysis for the AMR outcomes will be ANOVA to compare the community-level proportion or load of genetic determinants of resistance to macrolides across the three arms. P-values for all AMR analyses will be computed using a permutation test, with a step down minP procedure to control for three pairwise comparisons in each primary macrolide resistance outcome at an alpha of 0.05 [28, 29], and using a Benjamini-Hochberg correction to control the false discovery rate at 5% across additional (non-macrolide) endpoints [30]. For all AMR endpoints, we will additionally report unadjusted P-values, which will be less conservative to detect undesirable outcomes resulting from mass distribution of azithromycin.
All primary analyses will be intention-to-treat and hypothesis tests will be two-sided superiority tests. Permutation P-values will be estimated for each comparison [31]. Ongoing analyses after the primary endpoint will be conducted using a Bayesian perspective.
Interim analysis
An interim analysis will be conducted at 1.5 years after the first enrollment based on the Haybittle-Peto boundary, which results in an interim alpha of 0.001 and retains an alpha of 0.05 for the primary analysis [32]. Testing will proceed according to the hierarchical approach proposed for the primary analysis and will be conducted by the unmasked members to the data team. The DSMC will be notified of the results. If P < 0.001 for any comparison, the DSMC may consult with one of the investigators to determine a course of action while keeping the rest of the team masked.
Discussion
Targeted approaches to azithromycin distribution have been proposed to focus benefits on subgroups at the highest risk of mortality while limiting the amount of antibiotics distributed, theoretically reducing the risks posed by the intervention. Based on the results of the MORDOR trial [9], the WHO released conditional guidelines supporting azithromycin distribution to children 1–11 months of age to promote child survival in high mortality settings in sub-Saharan Africa [14]. As targeting this age group alone has not been tested, the AVENIR trial aims to compare the effect of distributing azithromycin to children 1–11 months old versus children 1–59 months old on mortality, resistance, and implementation outcomes in a cluster-randomized placebo-controlled large simple trial with response-adaptive allocation in Niger.
AVENIR is designed to provide evidence to support decision-making on age-based strategies for distributing azithromycin to support child survival. The large simple trial design ensures adequate power to detect a modest intervention effect on mortality [18], which is a rare outcome even in this high mortality setting. To ensure feasibility of conducting a study on such a large scale, this design requires minimal data collection, which limits the ability to describe the study population in detail as well as to define subgroups. In addition, this design lacks the power to detect differential effects of the intervention on mortality outcomes among subgroups. The use of response-adaptive randomization allows for ethical allocation of interventions and the ability to use the trial platform to evaluate other interventions as well. AVENIR’s cluster-randomized design reduces the contamination likely with an individual randomized design in this setting while aligning with the community-based nature of azithromycin distribution [19]. The main sets of outcomes included in this trial allow for a comparison of the potential reduction in mortality across strategies, the risk of increasing antimicrobial resistance, and the feasibility and acceptability of implementation.
Equipoise is among the implications of the ethical complexity involved in balancing the potential benefit against the risks of this intervention [33]. Although MORDOR provided evidence of the efficacy of azithromycin for child survival in one setting, the risk of antimicrobial resistance warrants caution before widespread implementation is adopted. Additionally, MORDOR suggested that the mortality effect is dependent on baseline mortality rates [9, 34], so intervention efficacy could change over time with secular trends in mortality. Similarly, another trial was unable to demonstrate an effect of azithromycin distribution on mortality when administered alongside Seasonal Malaria Chemoprevention [35], indicating the effect may depend on the presence or absence of other child survival interventions. Further evidence of efficacy in other settings along with continued monitoring of resistance would support programmatic decision-making. Placebo-controlled trials are the most rigorous approach to assess the effects of this intervention on both mortality and antimicrobial resistance, as the potential for bias is great without a proper comparison group, particularly for mortality.
In conclusion, AVENIR will provide evidence on mortality, AMR, and implementation outcomes after different age-based azithromycin distribution strategies. These results will support policy and programmatic decision-making as high mortality settings consider implementation of this intervention to promote child survival. The trial infrastructure will further provide a platform from which to continue to evaluate community-based interventions on a large scale.
Supplementary Information
Acknowledgements
The authors would like to acknowledge the AVENIR study group for their many contributions to this work: Bluesquare, Brussels, Belgium – Nicolas De Borman, Stephane Dresse, Romain Olekhnovitch, Grégoire Lurton, Léa Monchy, Martin De Wulf;
Bill & Melinda Gates Foundation, Seattle, WA, USA – Rebecca Brander, Dennis Chao, James Heine, Rasa Izadnegahdar, Laura Lamberti, Assaf Oron, Surabhi Rajaram, Matthew Steele; Centre de mère et d’enfant, Dosso, Niger – Amina Seyfoule; Centre de recherche et interventions en santé publique, Niamey, Niger – Ahmed Arzika, Fati Bello, Diallo Boubacar, Amadou Harouna, Alio Karamba, Ramatou Maliki, Farissat Nomao, Abraham Omar; Centre de recherche médical et sanitaire, Niamey, Niger – Ibrahima Issa, Ronan Jambou,
Rabiou Labbo, S’Hooshim N. Lamine, Sani Ousmane, Boubakar Rakia, Maikano Sadikou; ClinEpiDB, Philadelphia, PA, USA –Brianna Lindsay, Nupur Kittur, David Roos, Sheena Tomko; Comité national éthique pour la recherhce en santé, Niamey, Niger – Issa Adji, Djibo Ali, Souleymane Alzouma, Maidanda Boubacar, Diegou Boureima, Cheik Boureima Daouda, Idi Moussa Djatao, Ibrahim Jean Etienne, El Hadji Boubakar H Maiga, Amadou Oumarou, Ocquet Sakina, Sanoussi Samuila; Data and Safety Monitoring Committee – Emory University, Task Force for Global Health – David Addiss; Niger Ministry of Health – Mourtala Assao; St. George’s University of London – Julia Bielicki, Retired from CDC – Allen Hightower, Children’s Hospital of Colorado – Brian Jackson, University of Melbourne – Fiona Russell; Global Health Strategies, New York, NY, USA – Frances Hocking, Zied Mhirsi, Dan Pawson; Johns Hopkins University, Baltimore, MA, USA – Agbessi Amouzou, Robert Black; Pfizer, New York, NY, USA – Waqas Ahmed, Todd D. Hatajik, Julie Jenson, Chuck Knirsch, Chiao-Chin Lin; Programme national de santé oculaire, Niamey, Niger – Amza Abdou, Nassirou Beido, Boubacar Kadri, Boubacar Maïdanda; Speak Up Africa, Dakar, Senegal – Maelle Ba, Yaye Sophiétou Diop, Yacine Djibo, Gráinne Hutton, Fara Ndiaye; University of California, San Francisco, San Francisco, CA, USA – Benjamin Arnold, Cindi Chen, Emily Colby, Catherine A Cook, Thuy Doan, Jeremy D Keenan, Victoria Le, Elodie Lebas, Thomas M Lietman, Zijun Liu, William Nguyen, Kieran S O’Brien, Catherine E Oldenburg, Travis C Porco, Kevin Ruder, George W Rutherford, Lina Zhong, Zhaoxia Zhou; UCSF-UC Berkeley Center for Global Health Development, Diplomacy, and Economics, CA, USA – Stefano M. Bertozzi, Jim G. Khan, Elliot Marseille; University of Southampton, Southampton, United Kingdom – Gianluca Boo, Edith Darin, Andy Tatem.
Abbreviations
- AMR
Antimicrobial Resistance
- AVENIR
Azithromycine pour la Vie des Enfants au Niger: Implementation et Recherche
- DSMC
Data and Safety Monitoring Committee
- MORDOR
Macrolides Oraux pour Réduire les Décès avec un Œil sur la Résistance
- SDG
Sustainable Development Goal
- UCSF
University of California, San Francisco
- WHO
World Health Organization
Authors’ contributions
All authors contributed to the design of the trial. KSO, EC, ZL, BFA, and TML prepared the initial draft of the manuscript, provided critical review, and contributed to revisions of the manuscript, and AMA, AA, RM, SO, BK, BN, AKM, ANH, EL, VL, WN, JDK, CEO, TCP, TD, BFA, TML provided critical review and revisions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Bill & Melinda Gates Foundation grants OPP1210548 and INV-002454, the Peierls Foundation, Research to Prevent Blindness, and That Man May See. Pfizer donated azithromycin and placebo for the trial. The Bill & Melinda Gates Foundation reviewed and approved the study design. The funders had no role in study implementation, data collection, analysis, or preparation of this manuscript.
Availability of data and materials
Trial results will be presented at local, national and international meetings and submitted to peer-reviewed journals for publication. De-identified trial data will be made publicly available after publication of primary outcomes.
Declarations
Ethics approval and consent to participate
The University of California San Francisco Institutional Review Board has given ethical approval for the trial and will review the trial annually (19–28287). The Comité National Éthique pour la Recherche en Santé in Niger granted ethical approval before study activities began (DELIBERATION No. 041/2020/CNERS).
Verbal consent will be obtained from the head of household for participation in the census and from guardians for treatment administration. For children aged 30 to 42 days old, written consent will be obtained via caregiver thumbprint, as requested by the UCSF IRB given the possible risk of infantile hypertrophic pyloric stenosis with macrolides in this age group [36]. Verbal consent will be obtained from regional, district and community leaders before recruiting any participants into the study. For specimen collections, additional written informed consent will be obtained from participants and/or caregivers before data collection. There is no financial cost to the participant and there is no reimbursement for overall participation in this study.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas M. Lietman, Email: tom.lietman@ucsf.edu
the AVENIR Study Group:
Nicolas De Borman, Stephane Dresse, Romain Olekhnovitch, Grégoire Lurton, Léa Monchy, Martin De Wulf, Rebecca Brander, Dennis Chao, James Heine, Rasa Izadnegahdar, Laura Lamberti, Assaf Oron, Surabhi Rajaram, Matthew Steele, Amina Seyfoule, Ahmed Arzika, Fati Bello, Diallo Boubacar, Amadou Harouna, Alio Karamba, Ramatou Maliki, Farissat Nomao, Abraham Omar, Ibrahima Issa, Ronan Jambou, Rabiou Labbo, S’ Hooshim N. Lamine, Sani Ousmane, Boubakar Rakia, Maikano Sadikou, Brianna Lindsay, Nupur Kittur, David Roos, Sheena Tomko, Issa Adji, Djibo Ali, Souleymane Alzouma, Maidanda Boubacar, Diegou Boureima, Cheik Boureima Daouda, Idi Moussa Djatao, Ibrahim Jean Etienne, El Hadji Boubakar H. Maiga, Amadou Oumarou, Ocquet Sakina, Sanoussi Samuila, David Addiss, Mourtala Assao, Julia Bielicki, Allen Hightower, Brian Jackson, Fiona Russell, Frances Hocking, Zied Mhirsi, Dan Pawson, Agbessi Amouzou, Robert Black, Waqas Ahmed, Todd D. Hatajik, Julie Jenson, Chuck Knirsch, Chiao-Chin Lin, Amza Abdou, Nassirou Beido, Boubacar Kadri, Boubacar Maïdanda, Maelle Ba, Yaye Sophiétou Diop, Yacine Djibo, Gráinne Hutton, Fara Ndiaye, Benjamin Arnold, Cindi Chen, Emily Colby, Catherine A. Cook, Thuy Doan, Jeremy D. Keenan, Victoria Le, Elodie Lebas, Thomas M. Lietman, Zijun Liu, William Nguyen, Kieran S. O’Brien, Catherine E. Oldenburg, Travis C. Porco, Kevin Ruder, George W. Rutherford, Lina Zhong, Zhaoxia Zhou, Stefano M. Bertozzi, Jim G. Khan, Elliot Marseille, Gianluca Boo, Edith Darin, and Andy Tatem
References
- 1.GBD 2016 Child Mortality Collaborators Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1084–1150. doi: 10.1016/S0140-6736(17)31833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golding N, Burstein R, Longbottom J, Browne AJ, Fullman N, Osgood-Zimmerman A, et al. Mapping under-5 and neonatal mortality in Africa, 2000-15: a baseline analysis for the Sustainable Development Goals. Lancet. 2017;390(10108):2171–2182. doi: 10.1016/S0140-6736(17)31758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations Transforming our world . The 2030 agenda for sustainable development. New York: United Nations; 2015. [Google Scholar]
- 4.Emerson PM, Hooper PJ, Sarah V. Progress and projections in the program to eliminate trachoma. PLoS Negl Trop Dis. 2017;11(4):e0005402. doi: 10.1371/journal.pntd.0005402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Trachoma Initiative Zithromax Shipments: Cumulative Treatments Shipped 2020 [Available from: https://www.trachoma.org/.
- 6.Porco TC, Gebre T, Ayele B, House J. Keenan J, Zhou Z, et al. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA. 2009;302(9):962–968. doi: 10.1001/jama.2009.1266. [DOI] [PubMed] [Google Scholar]
- 7.Keenan JD, Ayele B, Gebre T, Zerihun M, Zhou Z, House JI et al. Childhood mortality in a cohort treated with mass azithromycin for trachoma. Clin Infect Dis. 2011;52(7):883–888. doi: 10.1093/cid/cir069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien KS, Cotter SY, Amza A, Kadri B, Nassirou B, Stoller NE, et al. Childhood mortality after mass distribution of azithromycin: a secondary analysis of the PRET cluster-randomized trial in Niger. Pediatr Infect Dis J. 2018;37(11):1082–1086. doi: 10.1097/INF.0000000000001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keenan JD, Bailey RL, West SK, Arzika AM, Hart J, Weaver J, et al. Azithromycin to reduce childhood mortality in sub-Saharan Africa. N Engl J Med. 2018;378(17):1583–1592. doi: 10.1056/NEJMoa1715474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan JD, Arzika AM, Maliki R, Elh Adamou S, Ibrahim F, Kiemago M, et al. Cause-specific mortality of children younger than 5 years in communities receiving biannual mass azithromycin treatment in Niger: verbal autopsy results from a cluster-randomised controlled trial. Lancet Glob Health. 2020;8(2):e288–ee95. doi: 10.1016/S2214-109X(19)30540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doan T, Arzika AM, Hinterwirth A, Maliki R, Zhong L, Cummings S, et al. Macrolide resistance in MORDOR I - a cluster-randomized trial in Niger. N Engl J Med. 2019;380(23):2271–2273. doi: 10.1056/NEJMc1901535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien KS, Emerson P, Hooper PJ, Reingold AL, Dennis EG, Keenan JD, et al. Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review. Lancet Infect Dis. 2019;19(1):e14–e25. doi: 10.1016/S1473-3099(18)30444-4. [DOI] [PubMed] [Google Scholar]
- 13.Keenan JD, Arzika AM, Maliki R, Boubacar N, Elh Adamou S, Moussa Ali M, et al. Longer-term assessment of azithromycin for reducing childhood mortality in Africa. N Engl J Med. 2019;380(23):2207–2214. doi: 10.1056/NEJMoa1817213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . WHO guideline on mass drug administration of azithromycin to children under five years of age to promote child survival. Geneva: World Health Organization; 2020. [PubMed] [Google Scholar]
- 15.Oldenburg CE, Arzika AM, Maliki R, Lin Y, O'Brien KS, Keenan JD, et al. Optimizing the number of child deaths averted with mass azithromycin distribution. Am J Trop Med Hyg. 2020;103(3):1308–1310. doi: 10.4269/ajtmh.19-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowkwanyun M, Bayer R, Galea S. “Precision” public health — between novelty and hype. N Engl J Med. 2018;379(15):1398–1400. doi: 10.1056/NEJMp1806634. [DOI] [PubMed] [Google Scholar]
- 17.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14(1):32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Collins R, Peto R. Why do we need large, simple randomized trials? Stat Med. 1984;3(4):409–422. doi: 10.1002/sim.4780030421. [DOI] [PubMed] [Google Scholar]
- 19.Hayes RJ, Moulton LH. Cluster Randomized Trials. Boca Raton: CRC/Taylor and Francis; 2009. [Google Scholar]
- 20.Angus DC, Alexander BM, Berry S, Buxton M, Lewis R, Paoloni M, et al. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- 21.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burstein R, Henry NJ, Collison ML, Marczak LB, Sligar A, Watson S, et al. Mapping 123 million neonatal, infant and child deaths between 2000 and 2017. Nature. 2019;574(7778):353–358. doi: 10.1038/s41586-019-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muñoz B, Solomon AW, Zingeser J, Barwick R, Burton M, Bailey R, et al. Antibiotic dosage in trachoma control programs: height as a surrogate for weight in children. Invest Ophthalmol Vis Sci. 2003;44(4):1464–1469. doi: 10.1167/iovs.02-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villar SS, Bowden J, Wason J. Response-adaptive designs for binary responses: How to offer patient benefit while being robust to time trends? Pharm Stat. 2018;17(2):182–197. doi: 10.1002/pst.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dmitrienko A, D'Agostino RB., Sr Multiplicity considerations in clinical trials. N Engl J Med. 2018;378(22):2115–22. doi: 10.1056/NEJMra1709701. [DOI] [PubMed] [Google Scholar]
- 26.Knol MJ, VanderWeele TJ, Groenwold RH, Klungel OH, Rovers MM, Grobbee DE. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26(6):433–438. doi: 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 28.Westfall PH, Young SS. Resampling-based multiple testing: examples and methods for P-value adjustment. New York: Wiley; 1993. [Google Scholar]
- 29.Ge Y, Dudoit S, Speed TP. Resampling-based multiple testing for microarray data analysis. Test. 2003;12(1):1–77. doi: 10.1007/BF02595811. [DOI] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57:289–300. [Google Scholar]
- 31.Simon R, Simon NR. Using randomization tests to preserve type I error with response-adaptive and covariate-adaptive randomization. Stat Probab Lett. 2011;81(7):767–772. doi: 10.1016/j.spl.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44(526):793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 33.Tam CC, Offeddu V, Lim JM, Voo TC. One drug to treat them all: ethical implications of the MORDOR trial of mass antibiotic administration to reduce child mortality. J Glob Health. 2019;9(1):010305. doi: 10.7189/jogh.09.010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oron AP, Burstein R, Mercer LD, Arzika AM, Kalua K, Mrango Z, et al. Effect modification by baseline mortality in the MORDOR azithromycin trial. Am J Trop Med Hyg. 2019;103(3):1295–1300. doi: 10.4269/ajtmh.18-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandramohan D, Dicko A, Zongo I, Sagara I, Cairns M, Kuepfer I, et al. Effect of adding azithromycin to seasonal malaria chemoprevention. N Engl J Med. 2019;380(23):2197–2206. doi: 10.1056/NEJMoa1811400. [DOI] [PubMed] [Google Scholar]
- 36.Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics. 2015;135(3):483–488. doi: 10.1542/peds.2014-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Trial results will be presented at local, national and international meetings and submitted to peer-reviewed journals for publication. De-identified trial data will be made publicly available after publication of primary outcomes.