SUMMARY
Background:
The UNAIDS recommends integrating methadone or buprenorphine treatment of opioid use disorder (OUD) with HIV care to improve HIV outcomes, but buprenorphine adoption remains limited in many countries. The study aimed to assess whether HIV clinic-based buprenorphine-naloxone treatment (BUP) for OUD was non-inferior to referral for methadone maintenance therapy (MMT) in achieving HIV viral suppression in Vietnam.
Methods:
We conducted a non-blinded, non-inferiority trial randomizing people with HIV and OUD to BUP (n=141) versus MMT referral (n=140) in six Vietnam HIV clinics. Randomization was conducted by computer generated random number sequence in blocks of 10 and by site. Research staff actively queried treatment-emergent adverse events during quarterly study visits and passively collected adverse events reported during HIV clinic visits. The primary outcome was HIV viral suppression at 12 months (HIV-1 PCR ≤ 200 copies/mL) by intention-to-treat (absolute risk difference (RD) margin ≤ 13%). Generalized estimating equations compared outcomes (NCT01936857; status: completed).
Findings:
At baseline, 272 of 281 (97.8%) participants were male, mean age was 38.3 (SD 6.1) years, and mean CD4 count 405 (SD 224) cells per μL. Viral suppression at 12 months improved for both BUP (97/140 [69.3%] to 74/91 [81.3%]) and MMT (92/140 [65.7%] to 99/107 [92.5%]). BUP did not demonstrate non-inferiority to MMT in achieving viral suppression at 12 months (RD=−0.11; 95%CI −0.20, −0.02). Medication retention at 12 months was lower for BUP than MMT (40.4% versus 65%, RD= −0.53; 95% CI −0.75, −0.31). Participants assigned to BUP more frequently experienced serious adverse events (10 [7.1%] of 141 BUP versus 4 of 140 [2.9%] MMT) and deaths (7 of 141 [5.0%] BUP versus 3 of 141 [2.1%] MMT). SAEs and deaths typically occurred in people no longer taking ART or OUD medications.
Interpretation:
While integrated buprenorphine and HIV care may potentially increase OUD treatment access, scale-up in middle-income countries may require enhanced support for buprenorphine adherence to improve HIV viral suppression . The study’s strength as a multi-site randomized trial was offset by limited buprenorphine retention.
Funding:
National Institute of Health, National Institute on Drug Abuse
Keywords: HIV, buprenorphine, methadone, Vietnam, randomized controlled trials, opioid related disorders
Introduction
Heroin injection drug use remains a main driver of HIV transmission in Vietnam and a recalcitrant source of new HIV infections worldwide.1,2 Opioid use can further amplify transmission due to lack of HIV viral suppression among those who do not fully access HIV care. Injection drug use adversely impacts engagement across the continuum of HIV care: timely HIV diagnosis, linking to HIV care, receiving and adhering to antiretroviral therapy (ART), and staying in HIV care.3 Successful engagement throughout this continuum is required to achieve sustained HIV viral suppression.4 Interventions that close gaps in engagement in the HIV care continuum are urgently needed worldwide.
Engagement in methadone maintenance therapy (MMT) is effective for the treatment of OUD,5 decreases HIV transmission 6, and improves HIV care engagement7 and mortality.8 Yet MMT programs require strict monitoring, limiting access for many people living with HIV, and drug-drug interactions with antiretrovirals complicate dosing.9
Vietnam has expanded ART treatment since 2004 with support from the Global Fund and the President’s Emergency Plan for AIDS Relief (PEPFAR), treating over 150,000 patients in 2019. Ministry of Health HIV treatment guidelines recommend three-drug ART treatment (typically as once-daily treatment with two NRTIs and one NNRTI as first line treatment) regardless of CD4 count. MMT programs were introduced in 2008 and have now expanded to more than 250 treatment centers, but compulsory 06 center detention remains a common treatment approach and does not decrease opioid use or improve HIV care engagement in Vietnam.10 In 2010, the government of Vietnam issued guidance for integrating MMT and ART treatment with referral for MMT remaining the dominant approach to OUD treatment, where available. As in other countries, many people who inject drugs and have HIV in Vietnam experience difficulty engaging in MMT.11,12
Like methadone, systematic reviews demonstrate that buprenorphine, a partial opioid agonist, is effective for treatment of OUD5, reduces HIV transmission6, and lowers overdose and all-cause mortality.8 Training HIV providers to manage OUD with buprenorphine during HIV clinic visits has the potential to expand access to treatment while increasing engagement in HIV care. Buprenorphine has few drug-drug interactions with HIV or tuberculosis medications, low overdose risk, capacity for flexible dosing, and avoids the administrative requirements of MMT, making it feasible for use in busy HIV clinics.13,14 Two U.S. pilot studies of HIV clinic-based buprenorphine demonstrated it was safe, feasible and reduced opioid use, but were limited by lack of a comparison group13,14 or small sample size.15 A third observational study found buprenorphine improved HIV viral suppression following release from incarceration.16 The WHO and the Joint United Nations Programme on HIV/AIDS (UNAIDS) recommend integrating methadone or buprenorphine treatment of OUD with HIV care to improve HIV outcomes17, but no multisite, randomized trials have compared the effectiveness of HIV clinic-based buprenorphine (BUP) and MMT referral models of care for improving HIV outcomes.
In 2015, Vietnam approved buprenorphine for use in the current research trial in Hanoi and a second pilot study in Ho Chi Minh City. National treatment guidelines requiring directly observed treatment, were issued for other settings, but access to buprenorphine remains limited. The Ministry of Health approved scale-up of buprenorphine scale-up in select mountainous provinces in 2019. A preliminary study of people with OUD in Vietnam HIV clinics indicated that patients strongly preferred receiving OUD treatment in their HIV clinic.12
The objective of this study was to assess whether an integrated BUP treatment strategy for OUD was non-inferior to referral for MMT in achieving HIV viral suppression. We hypothesized that the integrated buprenorphine-HIV care treatment model would be non-inferior to the MMT referral model for facilitating HIV viral suppression at 12 months. Secondary study objectives compared BUP versus MMT referral for receiving ART, improving ART adherence, retaining on OUD treatment medication, and decreasing heroin use at 12 months.
Methods
Study design, setting, and participants.
The Buprenorphine to Improve HIV Care Engagement and Outcomes (BRAVO) Randomized Trial (ClinicalTrials.gov NCT01936857) is a non-blinded, non-inferiority randomized trial that compared the effectiveness of two models of OUD treatment on HIV and opioid use outcomes at 12 months. Recruitment began July 27, 2015 and the final follow-up assessment occurred on January 31, 2019. Participants were recruited from six HIV clinics in northern Vietnam (four HIV clinics in Hanoi, one in Thanh Hoa Province, and one in Bac Giang Province). Study HIV clinics were selected with the support of local public health authorities based on high prevalence of untreated OUD among community members with HIV, likelihood of meeting enrollment targets, and availability of MMT referral. Participants completed written informed consent including and a consent comprehension quiz prior to enrollment.
Methadone was dispensed at local MMTs daily by directly observed therapy, per Ministry of Health guidelines, which also include requirements for counseling. MMT programs were within walking distance of HIV clinics, or co-located with the HIV clinic pharmacy in some clinics. HIV clinic providers were trained in the use of buprenorphine for the management of OUD prior to study initiation. They received ongoing, on-site technical assistance several times per year from addiction medicine specialists who reviewed clinical charts of participants enrolled in BUP. Clinic pharmacy staff dispensed buprenorphine-naloxone tablets daily with directly observed treatment similar to MMT, per Vietnam Ministry of Health requirements, throughout the 12 months of study participation. After taking a stable dose of buprenorphine-naloxone for one month without evidence of heroin use, participants were offered three- or four-day per week directly observed buprenorphine-naloxone dosing. Participants who completed 12 months of buprenorphine/naloxone were switched to methadone unless they declined MMT, in which case buprenorphine/naloxone was slowly tapered off. HIV clinics offered antiretroviral therapy (ART) and other usual medical care regardless of treatment assignment.
This study was conducted in partnership with Hanoi Medical University, the Provincial AIDS Control authorities of Hanoi, Thanh Hoa and Bac Giang, the Vietnamese National Institute of Mental Health, and Oregon Health & Science University in Portland, Oregon. The study was approved by institutional review boards at Oregon Health & Science University (IRB00000471), Hanoi Medical University (IRB00003121), and the Socialist Republic of Vietnam’s Ministry of Health Ethics Review Committee (IORG0006396).
Participants were eligible for inclusion if they were HIV-infected, age 18 or older, had current moderate-to-severe DSM-5 OUD, a positive urine drug screen for opioids, interest in receiving treatment for OUD, and were willing to practice birth control, if female. Study research staff prioritized patients new to HIV care or registered for care but not receiving ART, though participants already receiving ART were allowed to enroll. There was no HIV viral load requirement for participation. Participants were ineligible if they had a known hypersensitivity to buprenorphine or naloxone, an aspartate aminotransferase or alanine aminotransferase greater than five times the upper limit of normal, a serious medical or psychiatric illness in the past 30 days that precluded safe participation in the opinion of the study physician, received MMT within the past 30 days, or were currently pregnant or breastfeeding.
Randomisation and Masking
Participants were randomized to receive one of two models of OUD treatment: referral to MMT versus BUP. Randomization was conducted by computer generated random number sequence in blocks of 10 and by site at Oregon Health & Science University. Allocation assignments were maintained in sealed, sequentially numbered, opaque envelopes at the research coordinating center at Hanoi Medical University. Research assistants called the coordinating center for allocation assignment after a participant met all criteria for study participation. The study was non-blinded.
Procedures
Research assistants administered surveys assessing self-reported demographics (sex, marital status, level of education completed, and employment status), smoking frequency, history of arrest and compulsory rehabilitation detention (“06 center”), years since HIV diagnosis, mental health symptoms (Depression Anxiety and Stress Scale, where a score > 33 is consistent with severe depression or anxiety18), and HIV care at baseline, 3, 6, 9, and 12 months in confidential settings using secured, web-based electronic data entry. All survey instruments were administered in Vietnamese and adapted for regional dialect where needed. Urine drug screens (UDS) for morphine, amphetamines, methamphetamines, methadone, and buprenorphine were collected during screening and quarterly follow up visits. Blood specimens for HIV viral load and CD4 count were collected at HIV clinics and sent to the National Hospital for Tropical Diseases in Hanoi, Vietnam, for testing at baseline, six, and 12 months. Review of MMT records documented methadone dosing at 12 months. Review of HIV clinic records documented buprenorphine dosing, ART initiation, and HIV clinic visits at 12 months.
Outcomes
The primary outcome was HIV viral suppression at 12 months, defined as HIV-1 PCR ≤ 200 copies/mL. HIV-1 PCR testing was performed using the Abbott m2000rt RealTime HIV-1 PCR amplification assay. Secondary outcomes included ART initiation and HIV clinic attendance for HIV care (at least 1 visit in past 90 days) per medical record abstraction, self-reported ART adherence on a scale of 0 to 100% of doses taken in the past week19 (dichotomized as 100% adherence versus less than 100% adherence), and heroin use (defined both as UDS positive for morphine by point-of-care rapid immunoassay and also by self-reported use in the last 30 days on the Addiction Severity Index-lite20). We abstracted retention on medication from buprenorphine and methadone dosing logs, defined as receiving at least one dose in the 90 days prior to their 3, 6, 9, and 12 month study visits. Research staff actively queried treatment-emergent adverse events and serious adverse events during quarterly study visits and passively collected adverse events reported during HIV clinic visits.
Statistical analysis
We estimated that 340 participants would be required to achieve 80% power for assessing a pre-specified non-inferiority margin of ≤ 13% in the absolute value of the risk difference (RD) comparing BUP to MMT referral for HIV viral suppression, assuming an HIV viral suppression rate of 50% in the reference group. We based the noninferiority margin on ART noninferiority trials with HIV viral suppression as the primary outcome that used margins ranging from 10% to 15% 21-23. We affirmed the clinical relevance of a 13% margin by querying national HIV clinician scientists.
We used a generalized estimating equation (GEE) model with a binomial distribution, logit link, and independent working covariate structure to compare BUP versus MMT referral on the primary outcome of viral suppression. The optimal covariance structure was selecting using quasi information criteria. Combinations of model parameters were back-transformed to the risk scale to obtain a risk difference at 12 months. The delta method was used with the robust sandwich-type variance-covariance matrix estimated by the GEE model to obtain a 95% confidence interval on the risk difference. 24,25
Generalized linear mixed models were used to assess secondary outcomes of ART initiation, ART adherence, heroin use, and retention on study medication at 12 months under intention-to-treat (ITT) principles. Superiority analyses were conducted for all secondary outcomes.. All analyses included subject-level random intercepts to account for repeated measurements within individuals. Data cleaning and analyses were conducted in Stata version 15 and SAS software 9.4.
We conducted four sensitivity analyses. First, we reanalyzed the primary outcome analysis using a generalized linear mixed model. The mixed model approach is a recommended strategy for handling missing data in clinical trials, under the assumption that the data are missing at random.26 Second, we used single imputation of “worst-case” scenarios for missing data (imputing “no” for missing viral suppression, ART initiation, ART adherence, and study medication, and “yes” for missing heroin use) in all generalized linear mixed models. Third, we used multiple imputation with Markov-Chain Monte Carlo methods and “auxiliary variables” (i.e., covariates that are not part of the main analysis, but may help predict data missingness27) to address missing data. We considered baseline-only covariates (gender, employment, age, having at least a ninth-grade education, marital status) as well as covariates we collected as time-varying (ART initiation, ART adherence, methamphetamine use, heroin use, Depression Anxiety Stress Scale scores,18 receipt of study medication). If covariates were associated with missingness at time-points (using a relaxed p-value of 0.2), we included them in our multiple imputation model, ordering variables for imputation by time. Finally, we conducted a per-protocol analysis among participants who received at least one dose of study medication in the 90 days before each visit. In this analysis, retention on study medication was included as a time-varying covariate in the models, and risk differences were estimated among the subset of participants on medication at each time point. If sensitivity analysis results changed the 12-month study outcomes, we planned to report the results alongside our main analysis.
Role of the Funding Source
This work was funded by the U.S. National Institutes of Health National Institute on Drug Abuse (R01DA037441). The study received donated buprenorphine/naloxone tablets from Indivior, which played no role in study design, analysis, manuscript development, or publication decisions. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
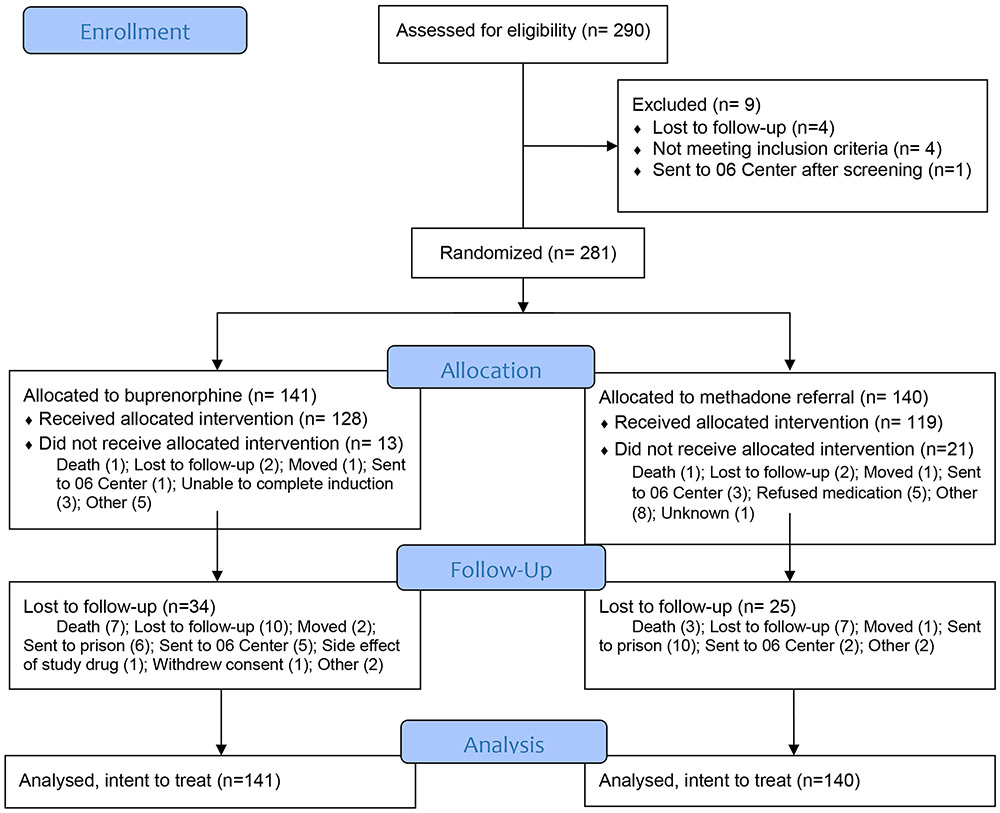
Research staff screened 290 participants to enroll 281 for study participation. Of 141 participants allocated to the BUP treatment strategy, 128 (91%) initiated buprenorphine-naloxone and 107 (76%) completed follow-up assessments. Of 140 participants allocated to the MMT referral treatment strategy, 119 (85%) initiated methadone and 115 (82%) completed follow-up assessments (Figure 1). We enrolled 281 (82.6%) of the targeted 340 sample size due to difficulty recruiting community-based participants toward the end of the trial, as Vietnam expanded MMT access during the study and fewer people with OUD willing to engage in HIV care were available to recruit. All randomized participants were included in the intention-to-treat primary analysis.
Figure 1.
BRAVO CONSORT Flow Diagram
At baseline, 272 of 281 (97.8%) of participants were male and mean age was 38.3 (standard deviation [SD] 6.1) years (Table 1). Participants reported an average 7.5 (SD 5.7) years since their HIV diagnosis, had an average CD4 count of 405 (SD 224) cells per μL, and 191 (68.0%) had initiated ART prior to study enrollment. Criminal justice involvement was common, with 234 (84%) reporting prior arrest and 172 (61%) previously enrolled in compulsory 06 center rehabilitation. All participants were positive for opioids at baseline, as required for trial eligibility; 53 (18.9%) also had UDS positive for amphetamine/methamphetamines and 230 (81.9%) reported using tobacco products daily. Participant characteristics were comparable by treatment assignment.
Table 1.
Baseline BRAVO participant characteristics, overall, and by treatment assignment.
| All participants (n=281) |
HIV Clinic-Based Buprenorphine (n=141) |
MMT Referral (n=140) |
|
|---|---|---|---|
| Mean Age (SD) | 38.3 (6.1) | 38.0 (5.8) | 38.6 (6.4) |
| Gender | |||
| Cis-male | 272 (96.8%) | 136 (96.5%) | 136 (97.1%) |
| Cis-female | 9 (3.3%) | 5 (3.5%) | 4 (2.9%) |
| Married | 109 (38.8%) | 52 (36.9%) | 57 (40.7%) |
| Ninth grade education | 114 (40.6%) | 65 (46.0%) | 49 (35.0%) |
| Employed | 129 (45.9%) | 62 (44.0%) | 67 (47.9%) |
| Every day smoker | 230 (81.9%) | 116 (82.2%) | 114 (81.4%) |
| Ever arrested | 234 (83.6%) | 118 (83.7%) | 116 (82.9%) |
| Ever 06 Center | 172 (61.2%) | 89 (63.1%) | 83 (59.3%) |
| Mean years of heroin use (SD) | 12.8 (6.0) | 12.4 (6.3) | 13.2 (5.7) |
| UDS positive for Amphetamine/Methamphetamine | 53 (18.9%) | 33 (23.4%) | 20 (14.3%) |
| Mean Years since HIV diagnosis (SD) | 7.5 (5.7) | 7.3 (5.6) | 7.6 (5.8) |
| Mean CD4 count (cells per μL ) (SD) | 405 (224.1) | 412 (222.7) | 397 (226.2) |
| Mean Depression Anxiety Stress Score (SD) | 16.0 (23.4) | 14.4 (18.3) | 17.4 (27.6) |
SD = Standard Deviation
Among those who engaged with medication treatment at each time point, the average dose of buprenorphine was 16.9mg (SD 6.5) at three months, 16.2mg (SD 6.3) at six months, 16.3mg (SD 6.3) at nine months, and 15.9mg (SD 6.1) at 12 months. The average dose of methadone was 104.7mg (SD 57.1) at three months, 126.4mg (SD 75.5) at six months, 131.4mg (SD 80.6) at nine months, and 132.7mg (SD 83.2) at 12 months.
The observed proportion of participants with HIV viral suppression improved from 97 of 140 (69.3%) participants at baseline to 74 of 91 (81.3%) at 12 months in the HIV clinic-based buprenorphine group and from 92 of 140 (65.7%) participants to 99 of 107 (92.5%) in the MMT referral group (Table 2). This corresponded to similar 12-month improvements in the observed proportion of participants on ART from 99 of 136 (72.8%) at baseline to 108 of 128 (84.4%) at 12 months for BUP and from 92 of 135 (68.1%) at baseline to 116 of 131 (88.5%) at 12 months for MMT referral). Nearly all participants treated with ART during the trial (249 of 252; 98.8%) received NNRTI-based ART, typically with once-daily lamivudine-tenofovir-efavirenz (233 of 252, 92.5%). Forty-six of 141 (32.6%) participants in the BUP arm and 66 of 140 (47.1%) of participants in the MMT arm had a suppressed HIV viral load at every time point. ART adherence among those on ART and HIV clinic visit engagement changed little over time in either group.
Table 2.
Observed proportions experiencing study outcomes, with general estimating equation risk differences (viral suppression) and generalized linear model risk differences (secondary outcomes).
| HIV Clinic-Based Buprenorphine |
MMT Referral | Risk Difference RD (95% CI) |
|
|---|---|---|---|
| Viral suppression | |||
| 0 months | 97/140 (69.3%) | 92/140 (65.7%) | - |
| 6 months | 73/92 (79.3%) | 100/110 (90.9%) | −0.12 (−0.21, −0.02) |
| 12 months | 74/91 (81.3%) | 99/107 (92.5%) | –0.11 (−0.21, −0.02) |
| ART receipt | |||
| 0 months | 99/136 (72.8%) | 92/135 (68.1%) | - |
| 3 months | 116/134 (86.6%) | 122/134 (91.0%) | −0.01 (−0.03, 0.01) |
| 6 months | 99/133 (74.4%) | 115/131 (87.8%) | −0.08 (−0.15, −0.01) |
| 9 months | 103/131 (78.6%) | 114/131 (87.0%) | −0.05 (−0.10, 0.01) |
| 12 months | 108/128 (84.4%) | 116/131 (88.5%) | −0.02 (−0.05, 0.01) |
| ART adherence | |||
| 0 months | 26/96 (27.1%) | 25/95 (26.3%) | - |
| 3 months | 29/101 (28.7%) | 27/106 (25.5%) | 0.003 (−0.13, 0.13) |
| 6 months | 33/92 (35.9%) | 33/112 (29.5%) | 0.05 (−0.11, 0.21) |
| 9 months | 29/92 (31.5%) | 32/118 (27.1%) | 0.04 (−0.10, 0.18) |
| 12 months | 29/104 (27.9%) | 31/114 (27.2%) | 0.0001 (−0.13, 0.13) |
| HIV Clinic Visit in past 3 months | |||
| 0 months | - | - | - |
| 3 months | 121/140 (86.4%) | 123/141 (87.2%) | −0.0001 (−0.01, 0.01) |
| 6 months | 114/140 (81.4%) | 107/141 (75.9%) | −0.02 (−0.07, 0.02) |
| 9 months | 116/140 (82.9%) | 100/141(70.9%) | −0.07 (−0.15, 0.01) |
| 12 months | 118/140 (84.3%) | 111/141 (78.7%) | −0.02 (−0.05, 0.01) |
| Retention on medication | |||
| 0 months | - | - | |
| 3 months | 127/141 (90.1%) | 101/140 (72.1%) | 0.06 (0.003, 0.11) |
| 6 months | 85/141 (60.3%) | 94/140 (67.1%) | −0.10 (−0.26, 0.06) |
| 9 months | 63/141 (44.7%) | 92/140 (65.7%) | −0.43 (−0.66, −0.20) |
| 12 months | 57/141 (40.4%) | 91/140 (65.0%) | −0.53 (−0.75, −0.31) |
| Heroin use: UDS | |||
| 0 months | 141/141 (100%) | 140/140 (100%) | - |
| 3 months | 81/117 (69.2%) | 106/122 (86.9%) | −0.14 (−0.25, −0.04) |
| 6 months | 62/101 (61.4%) | 81/114 (71.1%) | −0.12 (−0.29, 0.06) |
| 9 months | 60/97 (61.9%) | 88/117 (75.2%) | −0.13 (−0.29, 0.03) |
| 12 months | 66/98 (67.3%) | 72/110 (65.5%) | 0.05 (−0.12, 0.21) |
| Heroin use: ASI Self Report | |||
| 0 months | 141/141 (100%) | 140/140 (100%) | - |
| 3 months | 100/124 (80.6%) | 80/117 (68.4%) | −0.12 (−0.24, −0.005) |
| 6 months | 85/118 (72.0%) | 65/103 (63.1%) | –0.12 (−0.29, 0.05) |
| 9 months | 86/119 (72.3%) | 58/100 (58.0%) | −0.18 (−0.36, 0.001) |
| 12 months | 67/115 (58.3%) | 64/107 (59.8%) | 0.05 (−0.16, 0.26) |
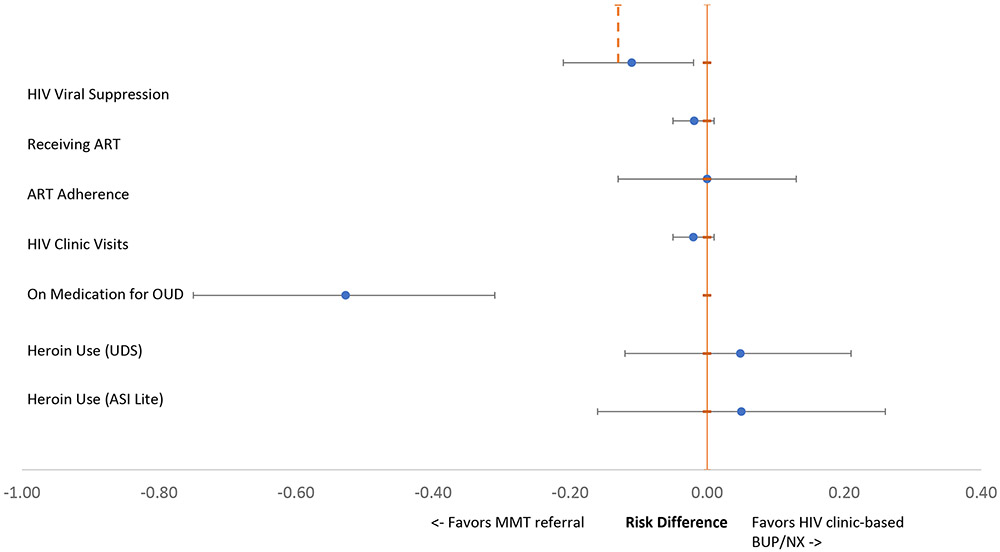
In intention-to-treat mixed model analyses, the BUP treatment strategy failed to demonstrate non-inferiority to the MMT referral strategy for achieving HIV viral suppression at 12 months (Risk difference [RD] = −0.11; 95% CI −0.21, −0.02), overall (Table 2 and Figure 2). HIV viral suppression patterns diverged when stratified by baseline ART status. Among those already established as patients in the HIV clinic (i.e. on ART at baseline), BUP was non-inferior to MMT referral (RD = −0.04; 95% CI −0.10, 0.01 at 6 months; RD = −0.05; 95% CI −0.10, 0.01 at 12 months), in contrast to the main analysis. Among those recruited from outside of HIV clinical care (i.e. not on ART at baseline), however, the risk difference for HIV viral suppression favored MMT (RD = −0.24; 95% CI −0.54, 0.06 at 6 months; RD = −0.15; 95% CI −0.42, 0.12) at 12 months) and noninferiority criteria were not met. BUP did not increase receipt of ART (RD = −0.02; 95% CI −0.05, 0.01), ART adherence (RD = 0.0001; 95% CI −0.13, 0.13), or HIV clinic visit attendance for HIV care (RD = −0.02; 95% CI −0.05, 0.01) compared with MMT referral.
Figure 2.
Twelve-Month HIV Care Continuum and Heroin Use Outcome Risk Differences, by Treatment Strategy.
At baseline, all participants (n=281) used heroin. The observed proportion of participants with heroin use at 12 months decreased from 100% at baseline to 66 of 98 (67.3%) by UDS, and to 67 of 115 (58.3%) by self-report for those assigned to BUP, and from 100% at baseline to 72 of 110 (65.5%) by UDS and to 64 of 107 (59.8%) by self-report for those referred to MMT (Table 2 and Figure 2). The mean number of days of self-reported past 30 day heroin use at baseline and 12 months decreased from 28.4 (SD 5.1) to 11.1 (SD 13.1) for those assigned to HIV clinic-based buprenorphine and from 28.7 (SD 4.5) to 8.3 (SD 11.2) for those assigned to MMT referral.
In intention-to-treat mixed model analyses, BUP was comparable to MMT referral at 12 months for reducing UDS heroin positivity (RD = 0.05; 95% CI −0.12, 0.21) or self-reported past 30 day heroin use (RD = 0.05, 95% CI −0.16, 0.26). In contrast, retention on medications for OUD varied widely: 57 of 141 (40.4%) participants assigned to BUP and 91 of 140 (65%) participants referred to MMT were retained on study medication at 12 months (RD= −0.53; 95% CI −0.75, −0.31) (Table 2). Sensitivity analyses used the number of participants who initiated medications as the denominator for the per protocol (i.e., “on treatment”) analysis, and assessed two imputation strategies on data from the full sample. Reanalysis using a generalized linear mixed model for the primary outcome, and all per-protocol analyses, worst-case single imputation analyses, and multiple imputation models did not change main analysis study outcomes. (Appendix 1).
Overall, 26 of 281 (9.2%) of participants reported 32 mild or moderate adverse events and 14 (5.0%) experienced a total of 20 severe adverse events, including 10 deaths (7 of 141 (5.0%) of BUP participants and 3 of 140 (2.1%) MMT participants). Deaths typically occurred in people no longer taking ART or OUD medications, including 3 heroin overdose deaths and 3 AIDS-related deaths, related to decreased medication retention in the BUP arm. Participants assigned to BUP more frequently reported adverse events (16.3% versus 2.1%) and serious adverse events (7.1% versus 2.9%) compared to those referred to MMT (Table 3). No serious adverse events were attributable to buprenorphine-naloxone or methadone.
Table 3.
BRAVO adverse events and serious adverse events
| HIV Clinic-Based Buprenorphine (n=141) |
MMT Referral (n=140) |
|
|---|---|---|
| Mild (Grade 1) or moderate (Grade 2) adverse events | ||
| Participants with one or more adverse events | 23 (16.3%, 95% CI= (10.6%, 23.5%)) | 3 (2.1%, 95% CI= (0.4%, 6.1%)) |
| Number of adverse events | 29 | 3 |
| Type of adverse event | ||
| Unintentional injury | 12 | 2 |
| Abnormal lab result | 1 | 0 |
| Neuropathy | 1 | 1 |
| Suspected infectious | 5 | 0 |
| Vascular | 1 | 0 |
| Cardiac | 1 | 0 |
| Gastrointestinal | 4 | 0 |
| Headache | 2 | 0 |
| Allergic reaction (non-study medication) | 1 | 0 |
| Malaise | 1 | 0 |
| Serious (Grade 3 or higher) adverse events, including deaths | ||
| Participants with one or more serious adverse events | 10 (7.1%, 95% CI=(3.5%, 12.7%)) | 4 (2.9%, 95% CI= (0.8%, 7.2%)) |
| Number of serious adverse events | 16 | 4 |
| Type of serious adverse event | ||
| Unintentional injury | 5 | 0 |
| AIDS-related | 3 | 2 |
| Nephrogenic | 1 | 0 |
| Respiratory | 1 | 0 |
| Psychiatric | 1 | 0 |
| Overdose | 2 | 1 |
| Unknown | 1 | 1 |
| Malaise | 1 | 0 |
| Mortality events | ||
| All deaths | 7 (4.9%, 95% CI= (2.0%, 10.0%)) | 3 (2.1%, 95% CI= (0.4%, 6.1%)) |
| Overdose deaths | 2 | 1 |
| AIDS-related deaths | 2 | 1 |
| Other/Unknown | 3 | 1 |
Discussion
The BRAVO trial was unable to demonstrate non-inferiority of BUP compared to MMT referral for achieving the primary outcome of HIV viral suppression, though other HIV care continuum outcomes and heroin use did not differ by treatment model assignment. This is in contrast to a small (n=93), single-center randomized trial of BUP versus MMT referral, which demonstrated no difference in change in HIV viral suppression at 12 months.16 Lower retention on buprenorphine/naloxone compared with methadone referral may have influenced study findings and suggests the need for interventions to support buprenorphine adherence as governments seek to expand access to OUD treatment with integrated treatment models.
Overall, both BUP and MMT referral strategies for treatment of OUD reduced heroin use and increased receipt of ART and HIV viral suppression among people with OUDand HIV in Vietnam. The observed overall improvements in HIV care continuum outcomes, regardless of treatment model assignment, are consistent with previous literature supporting the role of integrated HIV and OUD treatment. A systematic review of 32 mostly observational studies assessing the effect of medications for OUD treatment on HIV outcomes reported a 54% increase in ART coverage and 45% increase in HIV viral suppression.7 Similarly, in serial cross-sectional surveys of people living with HIV who inject drugs in Ukraine, opioid agonist treatment was associated with improvements in each stage of the HIV care continuum.28 Continued support for expanding access to opioid agonist treatment with buprenorphine or methadone may advance progress in Vietnam and elsewhere toward UNAIDS goals of achieving 95% diagnosis, 95% ART coverage and 95% HIV viral suppression by 2030.29
Comparable advances in other stages of the HIV care continuum in both treatment models, however, did not translate to comparable rates of HIV viral suppression. This may have been related to lower levels of adherence to buprenorphine compared with methadone, with subsequent loss to follow up. MMT programs typically provide more structured behavioral support than is available in HIV clinic-based settings, which results in better support for retention on methadone. Further research is required to understand participant reasons for not taking buprenorphine. We hypothesize that HIV stigma may have also played a role, with research assistants reporting that some HIV treatment-naive participants recruited from community outreach were comfortable receiving care in an MMT clinic, but feared disclosing their HIV status by attending an HIV clinic-based BUP program. This may explain the finding of BUP noninferiority to MMT for HIV viral suppression among those already receiving ART at baseline, and the lack of noninferiority favoring MMT among those who were not already engaged in ART treatment at baseline. Outreach interventions that overcome HIV stigma in engaging people in HIV treatment are urgently needed.
While heroin use decreased with both treatment strategies, fewer participants were retained on buprenorphine/naloxone than on methadone. This is consistent with a systematic review of 31 trials that demonstrated lower retention rates for buprenorphine than for methadone (788 participants, RR 0.83, 95% CI 0.72, 0.95).5 In the same systematic review, there was no difference in UDS measured opioid use for those retained in buprenorphine versus methadone treatment. The adverse consequences of lower buprenorphine retention rates observed in other countries may be offset by its broader reach. Methadone maintenance treatment programs require careful monitoring and infrastructure that is unavailable in many rural and other communities. Buprenorphine’s lower overdose risk and flexible dosing options make it safe for integrating into primary care and community health settings, efficiently expanding access to convenient treatment for people with OUD. Take-home dosing options have further increased use of buprenorphine in other countries. In the current study, participants were not allowed to receive buprenorphine take-home doses, which may have limited uptake. Easing Vietnam’s current requirements for directly observed buprenorphine dosing to allow some form of take-home doses may improve its uptake and retention in community-based settings.
Participants assigned to BUP reported a greater numbers of mild-to-moderate and serious adverse events, compared to those assigned to MMT referral. The greater number of adverse events may have partly stemmed from the nature of clinic-based versus off-site referral intervention strategies, in which BUP treatment arm participants had more opportunities to record adverse events than those in the MMT arm. It is also likely that lower ART and buprenorphine retention among those randomized to BUP may have led to greater morbidity from untreated HIV and OUD, including death. Treatment for many participants was interrupted by incarceration, which carries an increased risk of death upon release. 30,31 The greater number of overdose and AIDS deaths in the BUP arm suggests that potential benefits of buprenorphine cannot be fully realized without optimizing buprenorphine retention.
Our findings highlight the need for interventions to improve retention on buprenorphine as essential for scaling up BUP treatment programs in Vietnam and other countries. Retention on medications for OUD treatment is associated with improved HIV viral suppression 7,16,32. In addition to allowing take-home doses, countries seeking to expand access to integrated buprenorphine treatment models in HIV and other healthcare settings might tailor candidate interventions for improving buprenorphine retention to local strengths and context. For example, the majority of BRAVO participants were married, lived with family members, or both, often in multi-generational households. Family support interventions might facilitate engagement and retention in BUP programs in countries such as Vietnam where the family plays a central role in all aspects of daily life. Similarly, Vietnam’s healthcare system benefits from an extensive network of community health workers, who might be enlisted to support buprenorphine scale-up and improve buprenorphine retention. As extended release formulations of buprenorphine and ART (e.g. cabotegravir) become available, these might also improve medication retention and HIV viral suppression in Vietnam.
Our study findings should be interpreted in view of several potential limitations. First, we did not exclude participants with OUD who were already taking ART at baseline so that two-thirds of participants had HIV viral suppression prior to initiating buprenorphine or methadone. The relationship between OUD treatment strategies and HIV viral suppression may have been different in treatment naïve patients. We chose, instead, to include participants regardless of treatment status to maximize generalizability of BUP treatment to real-world HIV clinic populations who are at risk for opioid use-related mortality regardless of HIV viral suppression. Second, though overall follow-up rates were reasonable, the most common reason for missing follow-up data was detention (combined incarceration or compulsory 06 center rehabilitation).10 Many countries rely on criminal justice systems for addressing drug use disorders, which are ineffective for decreasing drug use, impair engagement in HIV treatment, and increase the risk of overdose following release 10,31. Initiating medications for OUD treatment prior to release reduces mortality following release 30. UNAIDS has consequently called for adoption of evidence-based prevention and treatment interventions, including buprenorphine and methadone, without detention as a means of decreasing HIV transmission and improving the lives of people who use drugs.33 Third, the lack of blinding might have overestimated BUP treatment effects for patients and providers favoring buprenorphine, and underestimated treatment effects among those favoring MMT. Fourth, the study achieved 82.6% of its projected recruitment target, a shortcoming potentially offset by the conservative approach of ignoring repeated HIV viral load measures in the sample size analysis. Finally, we conducted the study in a single country in Southeast Asia whose unique social-cultural context and health system may be important confounders. Still, we believe many lessons may be generalizable to other countries seeking to implement integrated BUP treatment programs, who share common challenges.
As the world faces continued growth of OUD and its consequences, efficient pathways for expanding access to treatment that saves lives and decreases HIV transmission are urgently needed. The BRAVO study suggests that HIV clinic-based buprenorphine and methadone maintenance therapy referral can both improve uptake of ART and HIV viral suppression. Expanding treatment access by integrating buprenorphine in HIV clinic settings may benefit from culturally tailored interventions that improve buprenorphine adherence.
Supplementary Material
Research in Context Panel.
Evidence before this study:
We conducted a review of literature evaluating buprenorphine in supporting HIV care continuum outcomes (antiretroviral therapy (ART) initiation, adherence, and viral suppression) and treatment engagement and cessation of illicit opioids among those with opioid use disorder (retention on study medication and heroin use). We searched PubMed and Cochrane databases for systematic reviews published through April 23rd, 2020 using the following search strategy: “buprenorphine” AND “HIV”; “buprenorphine” AND “HIV” AND “systematic review.” A systematic review evaluating use of medications for opioid use disorder (MOUD) on antiretroviral therapy outcomes found that MOUD was associated with increased ART initiation (HR=1.69, 95% CI=(1.32, 2.15)), increased ART adherence (OR=2.15, 95% CI=(2.41, 3.26)), and decreased drop out (OR=0.77, 95% CI=(1.21, 1.73)). Similarly, MOUD increased the odds of HIV viral suppression (OR=1.45, 95% CI=(1.21, 1.73)). Of the 32 studies included, only 7 included buprenorphine as a study medication and nearly all were observational studies (moderate risk of bias). A Cochrane review of buprenorphine versus methadone maintenance therapy for opioid dependence found high quality evidence that buprenorphine was superior to placebo, but less effective than methadone, at retaining trial participants. The review cited only moderate quality evidence, finding that only high doses of buprenorphine effectively decreased illicit opioid use; in some studies, opioid use was not decreased.
Added value of this study:
To our knowledge, this is the first multi-site randomized trial evaluating the non-inferiority of HIV clinic-based buprenorphine versus referral for methadone maintenance therapy for HIV viral suppression among patients with both opioid use disorder (OUD) and HIV. We evaluated critical outcomes for both OUD and HIV, including retention on study medication, heroin use, ART initiation, adherence, and HIV viral suppression.
Implications of all the available evidence:
Access to buprenorphine is an essential option for countries that seek to expand access to treatment for OUD, particularly among patients with both OUD and HIV. Retention on medication and HIV viral suppression may be lower for HIV-clinic-based buprenorphine than for methadone maintenance referral and may benefit from additional retention support interventions.
Acknowledgements
The authors wish to thank Dr. Hoang Dinh Canh, MD of the Vietnam Ministry of Health for supporting study implementation approval, the BRAVO Data Safety and Monitoring Board members Walter Ling, MD, Dennis McCarty, PhD, Rick Rawson, PhD, and Kevin Mulvey, PhD for their service, and the HIV clinic staff and patients who contributed the study’s success.
Source of Funding
This research was supported through grants from the National Institutes of Health National Institute on Drug Abuse (R01DA037441, UG1DA015815). Grant UL1TR002369 provided support of REDCap, the web application this study used for data collection.
Footnotes
Declaration of Interests
Dr. Korthuis reports grants from NIH National Institute on Drug Abuse, during the conduct of the study; and Dr. Korthuis serves as principal investigator for NIH-funded studies that accept donated study medication from Indivior (buprenorphine) and Alkermes (extended-release naltrexone). Indivior donated buprenorphine/naloxone tablets for BRAVO study participants. Dr. Bart reports grants from National Institute on Drug Abuse during the conduct of the study; grants from National Institute on Drug Abuse, grants from National Institute of Diabetes and Digestive and Kidney Diseases, grants from Substance Abuse and Mental Health Services Administration, outside the submitted work. Dr. Cook reports grants from NIDA during the conduct of the study; grants from NIDA, outside the submitted work. Ms. King reports grants from NIDA during the conduct of the study; grants from NIDA outside the submitted work; Other authors report no conflicts of interest.
Data Sharing
Following publication, deidentified data, a data dictionary, and study protocols will be shared with outside investigators upon approval of their proposal. A team of lead investigators will review proposals to ensure they do not overlap with previously approved proposals or manuscripts in progress. Upon approval, investigators will be invited to access the materials on Oregon Health and Science’s secure, password protected, cloud-based data sharing platform, Box.com.
REFERENCES
- 1.Nguyen VT, Scannapieco M. Drug abuse in Vietnam: a critical review of the literature and implications for future research. Addiction. 2008;103(4):535–543. [DOI] [PubMed] [Google Scholar]
- 2.Strathdee SA, Hallett TB, Bobrova N, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300(5):550–554. [DOI] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014(2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low AJ, Mburu G, Welton NJ, et al. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016;63(8):1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCance-Katz EF, Rainey PM, Smith P, et al. Drug interactions between opioids and antiretroviral medications: interaction between methadone, LAAM, and nelfinavir. Am J Addict. 2004;13(2):163–180. [DOI] [PubMed] [Google Scholar]
- 10.Vuong T, Ritter A, Shanahan M, et al. Outcomes of compulsory detention compared to community-based voluntary methadone maintenance treatment in Vietnam. J Subst Abuse Treat. 2018;87:9–15. [DOI] [PubMed] [Google Scholar]
- 11.Go VF, Morales GJ, Mai NT, Brownson RC, Ha TV, Miller WC. Finding what works: identification of implementation strategies for the integration of methadone maintenance therapy and HIV services in Vietnam. Implement Sci. 2016;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen Bich D, Korthuis PT, Nguyen Thu T, Van Dinh H, Le Minh G. HIV Patients' Preference for Integrated Models of Addiction and HIV Treatment in Vietnam. J Subst Abuse Treat. 2016;69:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altice FL, Bruce RD, Lucas GM, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiellin DA, Weiss L, Botsko M, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas GM, Chaudhry A, Hsu J, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Ann Intern Med. 2010;152(11):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS ONE. 2012;7(5):e38335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran TD, Tran T, Fisher J. Validation of the depression anxiety stress scales (DASS) 21 as a screening instrument for depression and anxiety in a rural community-based cohort of northern Vietnamese women. BMC Psychiatry. 2013;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–266. [DOI] [PubMed] [Google Scholar]
- 20.Cacciola JS, Alterman AI, McLellan A, Lin Y-T, Lynch KG. Initial evidence for the reliability and validity of a "Lite" version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87(2-3):297–302. [DOI] [PubMed] [Google Scholar]
- 21.De Castro N, Braun J, Charreau I, et al. Switch from enfuvirtide to raltegravir in virologically suppressed multidrug-resistant HIV-1-infected patients: a randomized open-label trial. Clin Infect Dis. 2009;49(8):1259–1267. [DOI] [PubMed] [Google Scholar]
- 22.Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-Lamivudine: a randomized, 96-week trial. Clin Infect Dis. 2009;49(10):1591–1601. [DOI] [PubMed] [Google Scholar]
- 23.Moyle GJ, DeJesus E, Cahn P, et al. Abacavir once or twice daily combined with once-daily lamivudine and efavirenz for the treatment of antiretroviral-naive HIV-infected adults: results of the Ziagen Once Daily in Antiretroviral Combination Study. J Acquir Immune Defic Syndr. 2005;38(4):417–425. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol. 2010;63(1):2–6. [DOI] [PubMed] [Google Scholar]
- 25.Højsgaard S, Halekoh U, Yan J. The R Package geepack for Generalized Estimating Equations. 2005. 2004;15(2):11. [Google Scholar]
- 26.Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple imputation after 18+ years. Journal of the American Statistical Association. 1996;91(434):473–489. [Google Scholar]
- 28.Mazhnaya A, Marcus R, Bojko MJ, et al. Opioid Agonist Treatment and Improved Outcomes at Each Stage of the HIV Treatment Cascade in People Who Inject Drugs in Ukraine. J Acquir Immune Defic Syndr. 2018;79(3):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United Nations General Assembly J. Resolution No A/RES/70/266, Political Declaration on HIV and AIDS: On the Fast-Track to Accelerate the Fight against HIV and to End the AIDS Epidemic by 2030. Available at http://www.unaids.org/sites/default/files/media_asset/2016-political-declaration-HIV-AIDS_en.pdf. Accessed April 30, 2020. 2016.
- 30.Marsden J, Stillwell G, Jones H, et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction. 2017;112(8):1408–1418. [DOI] [PubMed] [Google Scholar]
- 31.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer SA, Di Paola A, Barbour R, Azar MM, Altice FL. Extended-release Naltrexone Improves Viral Suppression Among Incarcerated Persons Living with HIV and Alcohol use Disorders Transitioning to the Community: Results From a Double-Blind, Placebo-Controlled Trial. J Acquir Immune Defic Syndr. 2018;79(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joint United Nations Programme on HIV/AIDS. Health, Rights, and Drugs: Harm Reduction, Decriminalization, and Zero Discrimination for People Who Use Drugs. Available at: https://www.unaids.org/sites/default/files/media_asset/JC2954_UNAIDS_drugs_report_2019_en.pdf Accessed May 4, 2020. In. Geneva: Joint United Nations Programme on HIV/AIDS; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.