Abstract
Parents of young children with type 1 diabetes (T1D) experience unique, developmental challenges in managing their child’s T1D, resulting in psychosocial distress. Only a small portion of young children reach glucose goals and adherence to diabetes devices that help improve T1D management have historically been low in this population. The purpose of this study is to test four interventions that couple developmentally tailored behavioral supports with education to optimize use of diabetes devices and reduce psychosocial distress for parents of young children with T1D. The study team designed four behavioral interventions, two aimed at improving glucose control and two aimed at optimizing use of diabetes devices. The goal of this paper is to describe the behavioral interventions developed for this study, including the results of a pilot test, and describe the methods and analysis plan to test this intervention strategy with ninety participants in a large-scale, randomized trial using a SMART design. A SMART design will permit a clinically relevant evaluation of the intervention strategy, as it allows multiple randomizations based on individualized assessments throughout the study instead of a fixed intervention dose seen in most traditional randomized controlled trials.
1. Introduction
Type 1 diabetes (T1D) is a common chronic medical condition in childhood, affecting 1 in 400 youth in the U.S. under the age of 20 (1), many of whom are diagnosed with T1D at a very young age (i.e. < 6 years old)(2). According to recent data from a large diabetes registry, only 23% of children ages 2-5 years old meet glucose targets set by the American Diabetes Association (i.e., A1C <7.5%) (3), despite technological advances in insulin pumps and continuous glucose monitoring (CGM) that permit precision and flexibility in insulin dosing and rapid detection of glucose fluctuations. However, regular use (i.e., adherence) of CGM therapy has been low in young children (4-6). Therefore, the purpose of the current study was to test strategies to improve T1D management in young children by optimizing use of diabetes technologies to improve T1D health outcomes.
Young children with T1D have unique challenges for T1D management resulting in significant psychosocial burden for parents (7, 8). Young children have unpredictable eating habits, making precise meal-time insulin dosing difficult and they often have erratic activity patterns causing greater glycemic variability (9). In addition, young children are unable to recognize and articulate symptoms of hypo- and hyperglycemia often resulting in parental hyper-vigilance to detect and correct glycemic excursions in their children. Hypoglycemia can cause seizures and parents often awaken several times per night to check their child’s glucose levels (7, 10, 11). During the day, parents must rely on other, often inexperienced caregivers, such as school or daycare personnel, to manage their child’s T1D, which is an additional source of stress and worry (12). Parents of young children are often fearful of hypoglycemia and are at greater risk for depression and anxiety compared to parents of older children with T1D (13). Parents who are fearful of hypoglycemia may maintain high blood glucose in their young children to allay their worries about hypoglycemia (14).
Despite the unique developmental and psychosocial challenges that may negatively influence glucose control in young children with T1D, there is limited research on behavioral interventions to address T1D management challenges while addressing the psychosocial needs of parents (7). Further, there are no interventions to improve the use of diabetes technologies in young children and their parents. Therefore, we designed a study to test an intervention targeting parents of young children with T1D that couples developmentally tailored behavioral supports with education to optimize use of CGM to improve glycemic control and reduce the psychosocial burdens of T1D management. The purpose of this paper is to: 1) describe the behavioral interventions developed for this study, including the results of a pilot study; and 2) describe the methods and analysis plan for a large scale, adaptive randomized clinical trial using a sequential multiple assignment randomized trial (SMART) design.
2. Behavioral interventions and pilot study
The first step in designing this clinical trial was to create behavioral interventions designed to increase adherence to diabetes devices and improve glucose control for young children with T1D. After intervention development was complete, a pilot study was conducted to gain experience implementing the interventions and to test and refine the interventions in preparation for the main trial. An additional goal of the pilot study was to assess the feasibility of delivering the behavioral interventions remotely, via web-based conference software with video capability.
2.1. Behavioral interventions
A multidisciplinary team of pediatric endocrinologists, psychologists, nurses, and certified diabetes educators collaborated to develop four behavioral interventions to address low adherence to CGM and suboptimal glucose control. Intervention content was derived from the clinical experience of the research team in addition to a review of the scientific literature. Specifically, the goals of the four interventions were to: 1) provide technical education on device use and age-appropriate strategies for improving glycemic control; 2) reduce distress and fear of hypoglycemia; and 3) teach problem-solving skills to overcome barriers to device use. Distress Reduction and Developmental Demands interventions were designed to increase adherence to CGM, referred to as the ‘Optimize Adherence’ interventions. Fear of Hypoglycemia and Dual Wave interventions were designed to improve blood glucose control, referred to as the ‘Glucose Target’ interventions.
Optimize Adherence Interventions
The Developmental Demands intervention focused on increasing parents’ comfort with using technology with their young children. In session 1, Technical aspects of using CGM were reviewed as well as benefits of diabetes technology, including remote monitoring of glucose levels and trends while the child was out of parental care (e.g., at school, with another relative or caregiver). Parents were taught problem-solving skills to identify barriers to optimal utilization of diabetes technology with their young children and to develop age-appropriate solutions to overcome these barriers. Common barriers to technology use included insurance coverage, struggles to keep devices attached to the child, handling questions from others about the devices, training other caregivers to use the technology, and feeling anxious that the technology might fail. In session 2, progress since the last session was discussed, and parents were again engaged in problem solving to develop solutions to both previously identified and newly identified barriers. Parents were provided with the opportunity to role-play solutions to identified barriers, and to discuss potential barriers that could develop in the future. Finally, parents were provided with suggestions for resources if problems overcoming barriers should arise in the future.
The Distress Reduction intervention focused on reducing parental stress related to their child’s T1D and other parenting stressors. Emphasis was placed on identification of worries and sources of stress, as well as utilization of stress reduction strategies such as problem- solving, use of balanced/reality-based self-talk, relaxation exercises, and obtaining social support. The interventionist provided global psychoeducation and support and tailored the intervention to specific areas of distress raised by parents. In session 1, interventionists assisted parents to identify their most prominent fears, worries, and sources of distress related to their child’s T1D, and discussed strategies for stress management. Interventionists educated parents on the stress management strategies and encouraged parents to practice these before the next session. In session 2, progress since the last session was reviewed, and the parents were guided in discussion of whether additional strategies were needed to address already identified worries and sources of stress, or if new worries or sources of stress had emerged. Parents were provided with the opportunity to practice skills and/or role-play scenarios as appropriate. Finally, interventionists assisted parents in problems solving any barriers that arose in implementing strategies to reduce worry and distress.
Glucose Target Interventions
Maintaining high blood glucose levels is a common response to worries about hypoglycemia and may include behaviors such as treating hypoglycemia with too many carbohydrates (i.e., over treating hypoglycemia), under-dosing insulin to maintain a higher than recommended blood glucose level, or initiating hypoglycemia treatment when glucose levels are above the hypoglycemic range (i.e., 70 mg/dl). The Fear of Hypoglycemia intervention incorporated principles of exposure-based cognitive behavioral therapy into hypoglycemia management (15) and reinforced appropriate hypoglycemia treatment and prevention strategies. The interventionist guided parents to gradually reduce blood glucose levels to the medically recommended range, with blood glucose monitoring prior to meals, at bedtime and as needed for symptoms of hypo or hyperglycemia, along with anxiety management strategies.
The Dual Wave intervention focused on reducing post-meal hyperglycemia by using the dual wave bolus feature of the insulin pump, when parents were unsure of what their child would eat. A dual wave bolus provides a portion of the meal bolus as a standard bolus at the time of the meal, and extends a second portion of the meal bolus for a variable numbers of hours, as programmed by the user (usually 1-3 hours). Parents were guided to calculate the immediate portion of the dual wave bolus based on the minimum number of carbohydrates they confident their child would eat, and to extend the remaining amount of insulin to cover the rest of the carbohydrates in the planned meal as the extended portion of the bolus. The extended portion of the bolus could be cancelled if the child failed to eat their entire meal. Session 1 focused on education regarding these procedures as well as answering any questions from parents. Session 2 focused on refining parents’ techniques after they had some experience utilizing the approach.
2.2. Pilot study
Participants and pilot study design
Nineteen parents of children < 6 years old using both CGM and insulin pumps for T1D management enrolled in a 6-week, pilot feasibility study to test and refine the interventions in preparation for the larger, randomized clinical trial. The children of the participating parents were 4.2 ±1.3 years of age (range= 1.6 – 5.9 yrs of age) and had type 1 diabetes for an average of 2.3 ± 1.0 years (range= 0.6-4.8 yrs). Fifty-three percent of children had been using insulin pumps to manage T1D for ≥ 1 year and 42% had been using CGM for ≥ 1 year. The remaining participants used insulin pumps and CGM for < 1 year. One parent withdrew from the study prior to completing any interventions, resulting in a final sample size of 18 parents. The study took place at the University of Colorado Barbara Davis Center, Stanford University, and Indiana University, and IRB approval was obtained at each study site. Stanford served as the coordinating center for devices and data management. Informed consent was obtained from parent participants.
Nine families were assigned to complete the ‘Optimizing Adherence’ interventions (consisting of Developmental Demands and Distress Reduction) and nine families were assigned to complete the ‘Glucose Control’ interventions (consisting of the Fear of Hypoglycemia and Dual Wave). Each intervention consisted of two, 60-90 minute sessions with a certified diabetes educator, physician, or psychologist completed 1 week apart, for a total of four intervention sessions completed in a 4-6 week period. Interventionists followed an intervention guide to ensure the delivery of all intervention components. All intervention sessions were completed remotely from the participants’ home, via web-based, HIPPA-secure video conferencing software (e.g. Blue Jeans, Vidyo or Google Hangouts).
Measures
Participants completed three questionnaires at the beginning and end of the pilot study: 1) Hypoglycemia Fear Survey- worry subscale (16), a self-report measure of worry related to hypoglycemia and its negative consequences; 2) Diabetes Distress scale (17), a self-report measure of emotional distress related to diabetes and its management; and 3) the CGM satisfaction scale, which is a self-report measure of treatment satisfaction with continuous glucose monitoring (18). To evaluate the impact on glycemic outcomes, parents downloaded their child’s CGM 1 week prior to the first intervention session and again 1 week after completing all assigned intervention sessions to calculate the percent time sensor glucose values were in target range (70-180 mg/dl).
Results
Sixteen of the 18 families completed at least 3 of the 4 intervention sessions (89%). There were minimal technical difficulties, parents, and interventionists found the web-based program convenient and easy to use. Paired t-tests showed a statistically significant decrease in fear of hypoglycemia scores and a significant increase in CGM satisfaction for the 16 families who completed at least 3 intervention sessions (p=0.04 for both), with moderate effect sizes (Cohen’s d=0.59 and 0.55 respectively). A decrease in diabetes distress scores was also observed, however, the effect size was small (Cohen’s d=0.32) and it was not statistically significant (p= 0.23). Analyzable CGM data were available for 10 of the 16 completing families. Typically, children in this age group have sensor values within target range (70-180 mg/dl) 40-50% of the time (19). In this study, we observed a 9% increase in glucose time in range, from 45% at study enrollment to 54% following the interventions. Data were missing from six participants due to parents’ not uploading CGM data as requested or errors in the uploaded CGM files making calculation of percent time in target range impossible (table 1).
Table 1.
Changes from pre- to post-intervention on parent measures for completing families in the pilot study
| mean (SD) | mean comparisons | |||||
|---|---|---|---|---|---|---|
| Parent Measures | N | pre- | post- | t | P | Cohen’ sd |
| Hypoglycemia fear | 16 | 22.3 (7.3) | 18.8 (5.2) | 2.26 | 0.04 | 0.59 |
| Glucose monitoring satisfaction | 16 | 20.9(3.2) | 23.0 (3.1) | 2.24 | 0.04 | 0.55 |
| Diabetes distress | 16 | 25.3 (6.6) | 23.9 (5.7) | 1.26 | 0.23 | 0.32 |
| % time in range | 10 | 45.2 (17.9) | 53.7(11.6) | 1.75 | 0.11 | 0.60 |
Summary of Pilot Results
The high completion rate and positive feedback from interventionists confirmed that completing the interventions remotely was effective. The pilot results from this small sample population suggest that the interventions have potential to improve some psychosocial aspects of care, including reducing fear of hypoglycemia, increasing satisfaction with CGM, and increasing sensor glucose time in target range. The interventions had minimal impact on diabetes distress, which could be due to the short duration of the pilot and that diabetes distress is a global stressor related to many facets of diabetes, not just technology use. It was difficult to get all participants to complete uploads of their CGM devices at home. Therefore, we chose to use a cloud-based system that did not require parents to upload the CGM device, to collect glucose data for the larger scale, clinical trial and reduce the incidence of missing data.
2.3. Intervention Refinement
The behavioral interventions were refined based on the pilot study results and feedback from study participants and interventionists. For the larger adaptive, randomized clinical trial, interventions were also refined to be specific to CGM use, which permitted enrollment of participants using multiple daily injections (MDI) or insulin pump therapy. The primary change made to the interventions following the pilot study was to replace the ‘Dual Wave’ intervention, which was only applicable to insulin pump users, with a ‘Remote Monitoring’ intervention, specific to CGM use.
The Remote Monitoring intervention focused on using the remote monitoring feature on the Dexcom G5 CGM system (i.e. ‘Share’), the device used in this study, which allows parents to view their child’s glucose data directly on their smart phone, even when they are away from their child. Thus, the Remote Monitoring intervention also focused on overcoming challenges to remotely monitoring their child’s glucose levels, in addition to strategies to use remote monitoring to reduce worries about hypoglycemia, increase safety, and facilitate communication with other caregivers, including school personnel. Finally, participants were also taught to identify patterns of high and low blood glucose by reviewing their child’s CGM data and making therapy adjustments in response to the identified patterns. Only minor adjustments were made to the other three interventions. See Table 2 for a description of the final behavioral interventions used in the larger scale, adaptive, randomized clinical trial.
Table 2:
Description of the behavioral interventions developed for the main clinical trial
| Intervention Name |
Intervention Goal |
|---|---|
| Developmental Demands | Provide education on using diabetes technology in various settings and formats and increase problem-solving skills. |
| Distress Reduction | Identify and reduce parent distress and worries. Provide strategies for obtaining social support. |
| Fear of Hypoglycemia | Decrease fear of hypoglycemia by decreasing maladaptive cognitions and decreasing behaviors that maintain high blood glucose levels |
| Remote Monitoring | Optimize use of remote monitoring (i.e., Share) by focusing on situational demands and problem solving. |
Each intervention consists of two, 45-60 minute sessions delivered over a two-week period.
Interventions are delivered by a Certified Diabetes Educator (CDE), medical personnel or psychologist with advanced training and experience in T1D care with young children. Distress Reduction intervention is delivered by a PhD psychologist with advanced training and experience in diabetes.
The main barriers to intervention completion were scheduling difficulties and time constraints. Therefore, to reduce scheduling difficulties, intervention time was reduced from 60-90 min to 45-60 min. Additionally, more flexibility will be employed when scheduling intervention sessions (i.e., weekends, evenings) for the large-scale adaptive, randomized clinical trial.
3. Sequential multiple assignment randomization trial (SMART) methods
3.1. Study Aims and Hypothesis
The primary aim of the SMART design is to leverage CGM and behavioral supports for parents to improve glycemic and psychosocial outcomes. We hypothesize that the exposure to the interventions will increase the family’s adherence to CGM use (i.e., increase the number of days used during the week) and increase time in the target glucose range of 70-180mg/dl, our primary outcome. Additionally, we hypothesize that exposure to the interventions will improve psychosocial indicators of quality of life for parents of young children with type 1 diabetes, our secondary outcomes.
3.2. SMART design rationale
This study uses a SMART design, which is an adaptive study design that allows for multiple assessments and subsequent randomizations based on the result of each assessment. A traditional randomized controlled trial, which tests the effect of a set intervention dose, does not align well with the reality of clinical practice where intervention strategies and dose are adapted based on individual patient needs and response to intervention (20, 21). Typically, in T1D clinical practice, a patient begins using a diabetes management device with an initial dose of education and if problems arise, the clinical team offers further assistance (which may include more education, help with problem-solving challenges, etc.) to optimize use of the device. A SMART design allows for the testing of the multiple intervention strategies developed for this trial (Table 2), which are employed based on individual patient outcomes and response to previous interventions during the course of the study. This approach tests which interventions are best to address the unique challenges parents of young children experience in using diabetes devices and achieving glucose targets.
3.3. SMART Trial Study Design
Participants
Ninety parents with children 2- 6 years old with T1D for > 6 months will be recruited from four academic clinical centers that specialize in pediatric T1D care: University of Colorado Barbara Davis Center for Diabetes, Stanford University, Indiana University, and University of South Florida. Children using CGM must have an A1C >7.5% to be eligible for enrollment. If children are not current CGM users, there is no A1C eligibility requirement. All children will use the Dexcom G5 CGM system with initial education that is consistent with practice standards for starting CGM systems.
Study Procedures
All caregivers will complete a “checkpoint” visit every 4 weeks while they are enrolled in the 6-month study. The Dexcom G5 CGM system uses a mobile application that automatically uploads glucose data to the cloud when connected to Wi-Fi or cellular data, permitting easy access to CGM data for study staff At each monthly checkpoint, study staff will review the CGM data from the previous 4 weeks to assess for CGM adherence or glucose control targets. The CGM adherence target is sensor wear at least 6 of 7 days (on average) during a week; or ≤ 4 missed days in the previous 4 weeks. The glucose target is sensor glucose values in the target range (70-180mg/dl) at least 60% of the time.
At each checkpoint, if the defined glucose and/or adherence targets is not met, a randomization occurs. For each randomization pathway (Optimize Adherence and Glucose Target), there are 3 conditions; 2 active interventions and 1 no-intervention/control. Randomization will occur by order and the sequence of interventions will depend on whether a previous intervention was completed (i.e., a participant can only be randomized to each intervention once). Participants can receive a minimum of zero interventions or a maximum of 4 interventions (a maximum of 2 Optimize Adherence interventions and 2 Glucose Target interventions) during the 6-month period.
Figure 1 shows the randomization strategy that will be used in this study. If the participant does not meet the adherence target, he/she will enter the adherence pathway and be randomized to 1 of 3 groups: 1) no intervention; 2) Developmental Demands; or 3) Distress Reduction. If the participant does not meet the glucose target, he/she will enter the glucose control pathway and be randomized to one of 3 groups: 1) no intervention; 2) Fear of Hypoglycemia; or 3) Remote Monitoring. Finally, if a participant does not meet both the glucose and adherence targets at the same checkpoint, she/he will enter the adherence randomization pathway first. Using the CGM is critical to improving glucose control. Therefore, CGM adherence interventions precede glucose control interventions in these cases. At each monthly checkpoint, if the participant meets both the glucose and adherence targets, no randomization occurs.
Figure 1:
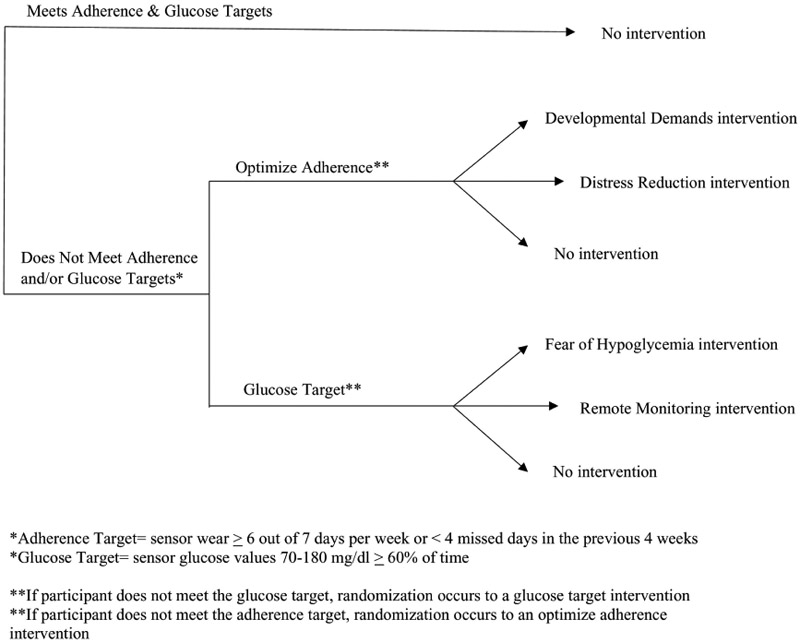
Randomization strategy at each monthly checkpoint
Measures
Data will be collected either remotely (via RedCap) or in clinic at baseline (week 0), at each monthly checkpoint visit (weeks 4, 8, 12, 16, 20) and at study completion (week 24). Demographic data, including socioeconomic status, racial and ethnic background, and diabetes device use history will be collected at study enrollment. The two primary outcomes will be glycemic control and quality of life. Glycemic control variables include change in time spent in target range (70-180 mg/dl) from baseline to study completion; and change in HbA1c. Psychosocial variables to assess quality of life include health related quality of life, parents’ general depression and anxiety symptoms, T1D-related distress, and hypoglycemia worries. T1D-related technology attitudes will also be assessed and include CGM satisfaction and comfort with use (See Table 3). The time in target blood glucose range and adherence will be collected from CGM data, whereas psychosocial and T1D technology attitude variables will be collected via questionnaire completion via RedCap (22). At baseline (week 0) and study completion (week 24) a capillary blood sample will be obtained to measure A1c. If the participant has a clinic visit at 12 weeks (+/− 2 weeks), an A1c value will be captured from the electronic medical chart. At baseline (week 0) and study completion (week 24), parents complete the full psychosocial assessment battery and at each monthly checkpoint visit, parents complete only select questionnaires.
Table 3:
Psychosocial & T1D-Technology Attitudes Assessment Battery
| Measure | Construct | Items and Scoring |
Psychometric properties |
|---|---|---|---|
| *Problem Areas in Diabetes, Parent Version (Paid-PR) | Parental distress related to child’sT1D | 18 items | α= 0.87(23) |
| *Patient Health Questionnaire (PHQ-8) | Depressive symptoms | 8 items Prevalence of current depression if score ≥10 | Sensitivity=0.80 Specificity=0.92 (24) [article is discussing PHQ9] |
| * State-Trait Anxiety Inventory (STAI) | Anxiety symptoms. | 40 items | α= 0.86-0.95(25) |
| Pittsburgh Sleep Quality Index (abbreviated) | Self-reported sleep quality and quantity. | 8 items | α= 0.83(26) |
| Hypoglycemic Fear Survey-Parents; worry subscale (HFS-P) | Parents’ worry related to hypoglycemia | 18 items | α= 0.89(16) |
| Hypoglycemic Confidence Questionnaire (parent) | Parents’ self-assurance in managing hypoglycemia. | 8 items | α= 0.87(27) |
| Pediatric Quality of Life Inventory (PedsQL) | Health related quality of life | 23 items | α =0.90(28) |
| T1D Technology Attitudes | |||
| Measure | Construct | Items and Scoring |
Psychometric Properties |
| Glucose Monitoring System Satisfaction Survey (GMSS-T1D) | Self-report of treatment satisfaction with glucose monitoring devices and its impact on quality of life | 15 items | α =0.90(18) |
| Use and comfort with technology | Objective questions capturing the frequency and variability of technology use for both general and diabetes-specific devices. | 22 items | N/A |
indicates the questionnaire is completed at each monthly checkpoint visit in addition to baseline and study completion
4. Statistical Analysis Plan
After data integrity checks are completed and the data are determined to be complete, descriptive statistics will be carried out to characterize the sample and note baseline scores on glycemic and psychosocial measures as well as rates of meeting criteria for randomizations at each checkpoint. Our primary and secondary outcomes will be tested in two ways. First, by using latent growth curve models to compare change in response to treatment strategies, we can examine outcomes from checkpoint to checkpoint. For example, we can test percent change in time spent in range from month 2 to 3 and determine which intervention is associated with promotion of change during that time period. Because more than three time points are available in this study, nonlinear trajectories will also be examined. Second, we will also examine the treatment effect using separate individual trajectory models for both primary outcomes across the entire 6 months of the study. Following this examination, we will investigate moderators of the treatment effects to determine for whom the interventions are most or least effective by including the baseline measure in a three-way interaction with the treatment by time interaction terms within the individual trajectory models. These analyses will inform more precise sample size considerations based on observed effect sizes for a larger efficacy trial conducted after this research is completed.
5. Discussion
Diabetes technologies, such as CGM, are valuable tools to improve glycemic control. However, individuals have to use the technology consistently to realize these benefits and they need sufficient education and support to optimize use. This is especially true for parents of young children with type 1 diabetes, who experience unique challenges in diabetes management related to their child’s developmental stage. This trial aims to test a series of interventions that were strategically developed for this unique population and pilot tested.
There is no “one-size fits all” approach to improving diabetes care for young children and their families. The novel SMART design being used in this trial is more clinically relevant than a traditional randomized controlled trial, as it permits individualized assessment, similar to how a clinician would approach care. Further, integration of psychosocial support with diabetes education is paramount to improving outcomes for this vulnerable population, and the interventions being tested here attempt to provide this holistic approach. The results of this trial will add valuable knowledge to the field about what types of interventions may be most helpful in increasing use of CGM technology and improving glycemic control for young children with T1D.
Acknowledgments
This work was supported by the National Institutes of Health grant number 1DP3DK104059
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37(2):402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;377(3):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8. [DOI] [PubMed] [Google Scholar]
- 4.Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2012;35(2):204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. [DOI] [PubMed] [Google Scholar]
- 6.Tansey M, Weinzimer S, Beck R, Ruedy K, Cheng P, Tamborlane W, et al. Extended 6-month follow-up of a randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2013;36(5):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep. 2014;14(9):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington KR, Boyle CT, Miller KM, Hilliard ME, Anderson BJ, Van Name M, et al. Management and Family Burdens Endorsed by Parents of Youth <7 Years Old With Type 1 Diabetes. J Diabetes Sci Technol. 2017;11(5):980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monaghan M, Herbert LJ, Wang J, Holmes C, Cogen FR, Streisand R. Mealtime behavior and diabetes-specific parent functioning in young children with type 1 diabetes. Health Psychol. 2015;34(8):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaser SS, Lord JH, Simmons JH, Malow BA. Brief report: Sleep disturbances in young children with type 1 diabetes. Diabetes Res Clin Pract. 2016;120:232–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Name MA, Hilliard ME, Boyle CT, Miller KM, DeSalvo DJ, Anderson BJ, et al. Nighttime is the worst time: Parental fear of hypoglycemia in young children with type 1 diabetes. Pediatr Diabetes. 2018; 19(1):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert LJ, Clary L, Owen V, Monaghan M, Alvarez V, Streisand R. Relations among school/daycare functioning, fear of hypoglycaemia and quality of life in parents of young children with type 1 diabetes. J Clin Nurs. 2015;24(9-10):1199–209. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of Hypoglycemia in Children and Adolescents and Their Parents with Type 1 Diabetes. Curr Diab Rep. 2016;16(8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with Type 1 diabetes and their parents. Diabet Med. 2013;30(9):1126–31. [DOI] [PubMed] [Google Scholar]
- 15.Vallis M, Jones A, Pouwer F. Managing hypoglycemia in diabetes may be more fear management than glucose management: a practical guide for diabetes care providers. Curr Diabetes Rev. 2014; 10(6):364–70. [DOI] [PubMed] [Google Scholar]
- 16.Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with Type 1 diabetes and their parents. Diabetes Manag (Lond). 2011;1(6):627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–31. [DOI] [PubMed] [Google Scholar]
- 18.Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010;12(9):679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tansey M, Beck R, Ruedy K, Tamborlane W, Cheng P, Kollman C, et al. Persistently high glucose levels in young children with type 1 diabetes. Pediatr Diabetes. 2016;17(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014;4(3):260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelleher SA, Dorfman CS, Plumb Vilardaga JC, Majestic C, Winger J, Gandhi V, et al. Optimizing delivery of a behavioral pain intervention in cancer patients using a sequential multiple assignment randomized trial SMART. Contemp Clin Trials. 2017;57:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio J, Anderson BJ, Laffel LM. Reexamining a measure of diabetes-related burden in parents of young people with Type 1 diabetes: the Problem Areas in Diabetes Survey - Parent Revised version (PAID-PR). Diabet Med. 2012;29(4):526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22(11):1596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 27.Polonsky WH, Fisher L, Hessler D, Edelman SV. Investigating Hypoglycemic Confidence in Type 1 and Type 2 Diabetes. Diabetes Technol Ther. 2017;19(2):131–6. [DOI] [PubMed] [Google Scholar]
- 28.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and Validity of hte Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in Healthy and Patient Populations. Med Care. 2001;39(8):800–12. [DOI] [PubMed] [Google Scholar]


