Abstract
Neisseria meningitidis, the causative agent of invasive meningococcal disease (IMD), is classified into different serogroups defined by their polysaccharide capsules. Meningococcal serogroups A, B, C, W, and Y are responsible for most IMD cases, with serogroup B (MenB) causing a substantial percentage of IMD cases in many regions. Vaccines using capsular polysaccharides conjugated to carrier proteins have been successfully developed for serogroups A, C, W, and Y. However, because the MenB capsular polysaccharide is poorly immunogenic, MenB vaccine development has focused on alternative antigens.
The 2 currently available MenB vaccines (MenB-4C and MenB-FHbp) both include factor H binding protein (FHbp), a surface-exposed protein harboured by nearly all meningococcal isolates that is important for survival of the bacteria in human blood. MenB-4C contains a nonlipidated FHbp from subfamily B in addition to other antigens, including Neisserial Heparin Binding Antigen, Neisserial adhesin A, and outer membrane vesicles, whereas MenB-FHbp contains a lipidated FHbp from each subfamily (A and B). FHbp is highly immunogenic and a main target of bactericidal activity of antibodies elicited by both licensed MenB vaccines. FHbp is also an important vaccine component, in contrast to some other meningococcal antigens that may have limited cross-protection across strains, as FHbp-specific antibodies provide broad cross-protection within each subfamily. Limited cross-protection between subfamilies necessitates the inclusion of FHbp variants from both subfamilies to achieve broad FHbp-based vaccine coverage. Additionally, immune responses to the lipidated form of FHbp have a superior cross-reactive profile to those elicited by the nonlipidated form. Taken together, the inclusion of lipidated FHbp variants from both FHbp subfamilies is expected to provide broad protection against the diverse disease-causing meningococcal strains expressing a wide range of FHbp sequence variants. This review describes the development of vaccines for MenB disease prevention, with a focus on the FHbp antigen.
Keywords: factor H binding protein, meningococcal serogroup B vaccine, Neisseria meningitidis, immune selection
Introduction
Neisseria meningitidis strains are classified into different serogroups based on their capsular polysaccharide structures, with most invasive meningococcal disease (IMD) cases caused by serogroups A, B, C, W, and Y [1,2]. The predominant disease-causing serogroups vary by location, over time, and by age-based population [1–3], with meningococcal serogroup B (MenB) in particular responsible for a substantial percentage of IMD cases in diverse global regions [2]. In 2017, MenB caused 38% and 51% of cases in the United States and European Union, respectively [3,4]. Among older adolescents and young adults (age 16–23 years), MenB strains caused 70% of US cases in 2017, compared with 38% in the overall population; incidence rates were also elevated in adolescents and young adults [4].
Vaccination is the preferred strategy to control IMD because of the nonspecific initial presentation, rapid progression, and considerable potential for devastating or fatal sequelae [5]. Purified polysaccharide and polysaccharide protein conjugate vaccines have been developed and successfully used to prevent disease caused by meningococcal serogroups A, C, W, and Y [5,6]. Unlike these serogroups, the MenB polysaccharide capsule is poorly immunogenic [7], likely because it resembles a polysialylated protein present on human neural cells [8]. Consequently, efforts to develop a broadly protective MenB vaccine have focused on surface protein antigens [9]. These antigens are often extremely diverse, with some exhibiting >1000 allelic variants [10]; as such, it is critical that the antigens included in a MenB vaccine induce immune responses that are protective against the diversity of disease-causing strains.
Currently licensed vaccines for MenB prevention include MenB-4C (Bexsero®, 4CMenB; GSK Vaccines Srl, Sovicille, Italy) [11] and MenB-FHbp (Trumenba®, bivalent rLP2086; Pfizer Inc, Philadelphia, PA) [12] (Table 1). MenB-4C contains 3 main recombinant protein antigens, 2 of which are formulated as fusion proteins [11]. These include a nonlipidated variant of a subfamily B factor H binding protein (FHbp), Neisserial Heparin Binding Antigen (NHBA), and Neisserial adhesion A (NadA), in addition to outer membrane vesicles (OMVs). MenB-4C has been approved in several countries and regions, including Argentina, Australia, Brazil, Canada, Chile, the European Union, Israel, New Zealand, the United States, and Uruguay [11,13–21]. MenB-4C can be administered as early as age 2 months in all of these countries/regions except the United States; most countries recommend a specific 2- or 3-dose schedule with a booster dose, depending on age group. The other licensed MenB vaccine, MenB-FHbp, includes 2 lipidated variants of recombinant FHbp, 1 from each of the 2 FHbp phylogenetic subfamilies (termed A and B [22]) [12]. MenB-FHbp is licensed in several countries and regions, including Australia, Canada, Chile, the European Union, and the United States; it is generally indicated for use in individuals >10 or 10–25 years of age under either a 2- or 3-dose schedule [12,23–26].
Table 1.
Currently Available Vaccines for the Prevention of Serogroup B IMD
| Name | Manufacturer | Antigens Included | Global Licensed Usage | ||
|---|---|---|---|---|---|
| Country/Region | Licensed Age Group | Recommended Posology | |||
| MenB-4C | GSK Vaccines Srl; Sovicille, Italy | Nonlipidated FHbp subfamily B (fusion protein), NHBA (fusion protein), NadA, and OMVs [11] | Argentina [17] | ≥2 mo | Children aged 2–5 mo: 3-dose schedule ≥1 mo apart with booster at age 12–23 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster ≥2 mo from last primary dose at 12–24 mo; children aged 1–10 y: 2-dose schedule ≥2 mo apart with no booster; children aged ≥11 y and adults: 2-dose schedule ≥1 mo apart with no booster |
| Australia [19] | ≥2 mo | Children aged 2–5 mo: 2-dose schedule ≥2 mo apart or 3-dose ≥1 mo apart with booster at ≥12 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster at ≥12 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with no booster; children aged ≥2 y and adults: 2-dose schedule ≥1 mo apart with no booster | |||
| Brazil [13] | ≥2 mo | Children aged 2–5 mo: 3-dose schedule ≥1 mo apart with booster ≥6 mo from last primary dose at 12–15 mo; children aged 3–5 mo: 2-dose schedule ≥2 mo apart with booster ≥6 mo from last primary dose at 12–15 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster ≥2 mo from last primary dose at 12–24 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with booster at 12–23 mo from last primary dose; children aged ≥2 y and adults: 2-dose schedule ≥1 mo apart with no booster | |||
| Canada [20] | 2 mo–25 y | Children aged 2–5 mo: 2-dose schedule ≥2 mo apart or 3-dose ≥1 mo apart with booster at ≥12 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster at ≥12 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with no booster; children aged ≥2 y and adults: 2-dose schedule ≥1 mo apart with no booster | |||
| Chile [18] | ≥2 mo | Children aged 2–5 mo: 3-dose schedule ≥1 mo apart with booster at 12–23 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster ≥2 mo from last primary dose at 12–24 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with booster 12–23 mo after primary dose; children aged 2–10 y: 2-dose schedule ≥2 mo apart with no booster; children aged ≥11 y and adults: 2-dose schedule ≥1 mo apart with no booster | |||
| European Union [14] | ≥2 mo | Children aged 2–5 mo: 3-dose schedule ≥1 mo apart with booster ≥6 mo from last primary dose at 12–15 mo; children aged 3–5 mo: 2-dose schedule ≥2 mo apart with booster ≥6 mo from last primary dose at 12–15 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster ≥2 mo from last primary dose at 12–24 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with booster at 12–23 mo from last primary dose; children aged ≥2 y and adults: 2-dose schedule ≥1 mo apart with potential booster | |||
| Israel [16] | ≥2 mo | Children aged 2–5 mo: 3-dose schedule ≥1 mo apart with booster at 12–15 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster ≥2 mo from last primary dose at 12–24 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with booster 12–23 mo after last primary dose; children aged 2–10 y: 2-dose schedule ≥2 mo apart with no booster; children aged ≥11 y and adults: 2-dose schedule ≥1 mo apart with no booster | |||
| New Zealand [21] | ≥2 mo | Children aged 2–5 mo: 2-dose schedule ≥2 mo apart or 3-dose ≥1 mo apart with booster ≥12 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster ≥12 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with no booster; children aged ≥2 y and adults: 2-dose schedule ≥1 mo apart with no booster | |||
| United States [11] | 10–25 y | 2-dose schedule ≥1 mo apart | |||
| Uruguay [15] | ≥2 mo | Children aged 2–5 mo: 3-dose schedule ≥1 mo apart with booster at 12–15 mo; children aged 6–11 mo: 2-dose schedule ≥2 mo apart with booster at 12–24 mo; children aged 12–23 mo: 2-dose schedule ≥2 mo apart with booster 12–23 mo after primary dose; children aged 2–10 y: 2-dose schedule ≥2 mo apart with no booster; children aged ≥11 y and adults: 2-dose schedule ≥1 mo apart with no booster | |||
| MenB-FHbp | Pfizer Inc, Philadelphia, PA, USA | Lipidated FHbp subfamily A and lipidated FHbp subfamily B [12] | Australia [25] | ≥10 y | 2-dose schedule administered at 0 and 6 mo; for higher risk individuals, 3-dose schedule with 2 doses administered ≥1 mo apart, and third dose ≥4 mo after second dose |
| Canada [24] | 10–25 y | 2-dose schedule at 0 and 6 mo | |||
| Chile [26] | 10–25 y | 2-dose schedule administered at 0 and 6 mo; for higher risk individuals, 3-dose schedule with 2 doses administered ≥1 mo apart, and third dose ≥4 mo after second dose | |||
| European Union [23] | ≥10 y | 2-dose schedule at 6-mo intervals or 3-dose schedule with 2 doses administered ≥1 mo apart, and third dose ≥4 mo after second dose | |||
| United States [12] | 10–25 y | 2-dose schedule at 0 and 6 mo or 3-dose schedule at 0, 1–2, and 6 mo | |||
FHbp=factor H binding protein; IMD=invasive meningococcal disease; MenB-4C=Bexsero®, 4CMenB; MenB-FHbp=Trumenba®, bivalent rLP2086; NadA=Neisserial adhesion A; NHBA=Neisserial Heparin Binding Antigen; OMV=outer membrane vesicle.
This review provides historical and scientific context to the development of vaccines for the prevention of MenB IMD with a focus on FHbp, an important component and a main target of bactericidal activity of both licensed MenB vaccines [11,12,27]. The presentation and formulation of FHbp included in each vaccine differs, in turn affecting the breadth of protection afforded by this protein antigen against diverse, circulating, disease-causing MenB strains.
Early MenB Vaccines and the Quest for Broad Coverage
Meningococcal serogroups generally comprise a wide diversity of disease-causing strains [28]. Vaccines for preventing disease caused by a particular meningococcal serogroup should ideally provide complete coverage, ie, induce a protective response against all strains within that serogroup [29]. For meningococcal serogroups A, C, W, and Y, safe and effective vaccines using the capsular polysaccharide as the vaccine antigen have been developed [5]; this approach has been successful because the same capsular polysaccharide is present in all strains within a given serogroup [29], and vaccine-induced antibodies targeting the capsular polysaccharide thus provide protection against all strains of that particular serogroup. Recognition that the MenB capsular polysaccharide is poorly immunogenic and has the potential to induce autoimmune responses [7,8] led to proposals for using surface protein antigens in vaccines for the prevention of MenB IMD [9]. The ideal MenB vaccine antigen would be similar to the capsular polysaccharide for meningococcal serogroup A, C, W, and Y vaccines in that it would be abundant in all circulating, disease-causing, MenB strains and would either be completely conserved across strains or induce functional antibodies that are cross-protective against all antigenic variants [9,29].
Early MenB vaccines included monovalent OMV vaccines that contain the immunodominant outer membrane protein, porin A (PorA) [6,30]; these were successfully used in response to national MenB epidemics dominated by a single bacterial clone, and hence PorA serosubtype, in Norway [31], Cuba [32], and New Zealand [33]. However, these monovalent OMV vaccines had little utility in other geographic areas [6,30]. This is because OMV-induced responses are primarily directed against the immunodominant PorA, which has a high degree of sequence diversity among strains in its surface-exposed loops, and antibodies raised against one PorA serosubtype have very limited cross-reactivity with other PorA subtypes. Polyvalent OMV vaccines containing multiple PorA variants were subsequently developed to broaden coverage; these have included bivalent [34], hexavalent [35], and nonavalent [36] vaccines. However, none have progressed beyond clinical development to real-world use [30].
Given the limitations of OMV vaccines in providing broad coverage of diverse MenB strains, there was considerable interest in identifying an immunogenic vaccine antigen that was surface-exposed, conserved, and widely expressed across MenB strains [9,29].
Use of FHbp as a MenB Vaccine Antigen
Factor H binding protein was independently identified as a potential MenB vaccine antigen in the development of both MenB-4C and MenB-FHbp [22,37]. In early MenB-4C studies, FHbp was identified via genomic mining and termed Genome-derived Neisserial Antigen (GNA) 1870 [37]. During initial MenB-FHbp studies, FHbp was identified using a combined biochemical and immunological screening approach and referred to as lipoprotein 2086 (LP2086) [22,38]. FHbp is a surface-exposed protein harboured by >99% of meningococcal isolates; each strain codes for a single FHbp sequence variant [22,37–39]. Expressed as a precursor protein, the initial FHbp is processed prior to localization on the bacterial surface [40]. In a manner that now appears to be dependent upon the FHbp signal peptide sequence, the protein on the bacterial surface is lipidated in certain MenB strains and nonlipidated in others [37,40].
The near ubiquity of FHbp is potentially explained by its role in binding human factor H (FH), a protein that downregulates the alternative complement pathway, in turn leading to evasion of complement-mediated bacterial lysis [41,42]. FHbp, or an alternative protein that binds FH, thus plays an important role in meningococcal survival during systemic spread and, presumably, mucosal colonization [43–45]. For FHbp, this was demonstrated in an ex vivo human whole blood model of meningococcal septicemia in which deletion of the fhbp gene resulted in a dramatic decrease in meningococcal survival; similar results were observed in serum bactericidal antibody (SBA) assays [43,46].
Several FHbp characteristics have implications for its use as a vaccine antigen. Despite the widespread expression of FHbp across the vast majority of strains, a few invasive MenB strains have been identified that either carry a frameshift mutation in the fhbp gene, as found in some clonal complex 11 (cc11) sequence type 11 (ST-11) isolates, or have lost the gene entirely, leading to deficient or nonexistent FHbp expression [47,48]. Such strains will therefore not be covered by an FHbp-based vaccine. Additionally, although FHbp is extremely diverse, with 1241 known allelic variants as of September 2019 [10], variants are grouped into 2 subfamilies (A and B; Figure 1) [22]; prevalence of subfamilies and individual strains varies by region (Table 2) [39]. Importantly, FHbp sequence identity is relatively high within a subfamily (>84%) but lower between subfamilies (~60%–75%). The FHbp expression level can also vary substantially among isolates and may therefore influence whether anti-FHbp antibodies are able to confer protection in cases of low expression [37,49]. Finally, it should be noted that because FHbp is a meningococcal antigen that is not exclusive to serogroup B [22], an FHbp-based vaccine could potentially provide protection against non–MenB strains. These attributes will be described in greater detail in the following sections.
Figure 1.
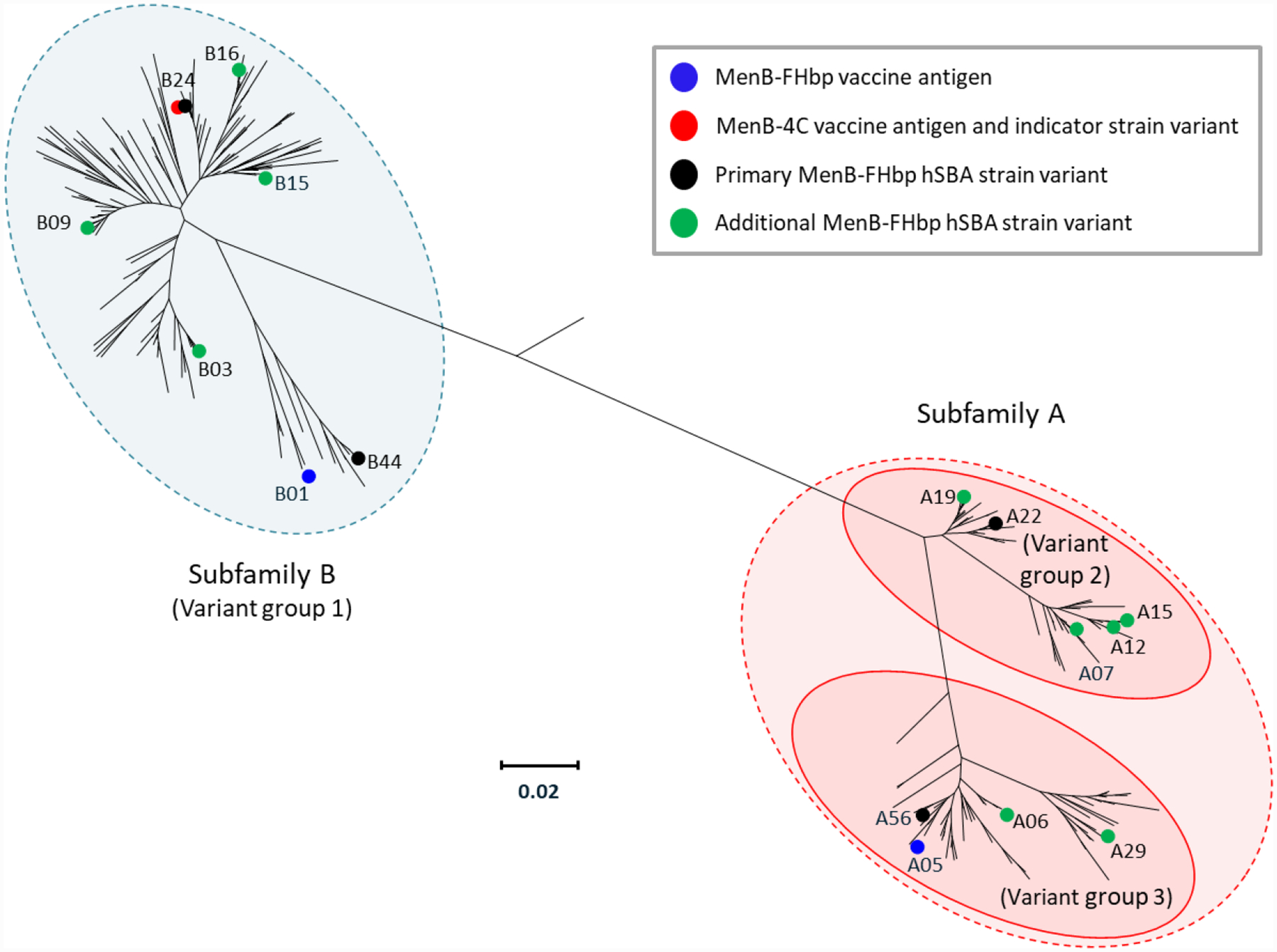
Phylogenetic tree for FHbp, adapted with permission from Ostergaard et al. N Engl J Med. 2017;377:2349–2362 [76]. The grouping of variants into subfamilies A and B [22] is indicated; an alternative classification scheme involving 3 variant groups [37] is also depicted. Coloured circles indicate FHbp antigens included in MenB-4C [11] and MenB-FHbp [12] as well as strains used to evaluate immune responses to both vaccines in clinical studies [58,75]. For MenB-4C, the indicator strain used to evaluate FHbp-mediated bactericidal immune responses expresses FHbp variant B24 and is thus homologous to the vaccine antigen for FHbp [58]. The 4 primary and 10 additional strains used to measure the immune response to MenB-FHbp express sequence-diverse FHbp variants that are different from the vaccine antigens [9,74]. The scale bar indicates phylogenetic distance using protein sequence. FHbp=factor H binding proteins; hSBA=serum bactericidal antibody assay using human complement; MenB-4C=Bexsero®, 4CMenB; MenB-FHbp=Trumenba®, bivalent rLP2086.
Table 2.
FHbp Variants Expressed by Primary and Additional MenB-FHbp hSBA Test Strains and Prevalence Among MenB Disease-Causing Isolates from the United States and European Union
| FHbp Varianta | FHbp Variant Rank in USb | US Variant Prevalence, %b | FHbp Variant Rank in Europec | EU Variant Prevalence, %c | FHbp Subgroup | Identity to Vaccine Component from Same Subfamily, % |
|---|---|---|---|---|---|---|
| B24 | 1 | 42.6 | 1 | 16.7 | N6 | 86.2 |
| A22 | 2 | 10.4 | 5 | 10.0 | N2C2 | 88.9 |
| A12 | 3 | 6.3 | 13 | 1.5 | N2C1 | 85.4 |
| B16 | 4 | 5.1 | 2 | 12.2 | N6 | 86.2 |
| B09 | 5 | 3.9 | 6 | 6.4 | N6 | 88.1 |
| A19 | 6 | 3.5 | 7 | 3.1 | N2C2 | 88.1 |
| B03 | 7 | 3.2 | 3 | 11.1 | N6 | 90.8 |
| A07 | 8 | 3.0 | 12 | 1.6 | N2C1 | 85.4 |
| B15 | 9 | 2.3 | 49 (tie) | 0.1 | N6 | 86.5 |
| A15 | 10 | 1.9 | 16 (tie) | 0.5 | N2C1 | 85.1 |
| A29 | 13 (tie) | 0.7 | 22 (tie) | 0.3 | N1C1 | 93.1 |
| B44 | 27 (tie) | 0.2 | 4 | 10.8 | N4/N5 | 91.6 |
| A06 | 27 (tie) | 0.2 | 9 (tie) | 2.5 | N1C2 | 96.2 |
| A56 | N/A | 0.0 | 49 (tie) | 0.1 | N1C2 | 98.1 |
FHbp=factor H binding protein; MenB=meningococcal serogroup B; MenB-FHbp=Trumenba®, bivalent rLP2086; hSBA=serum bactericidal antibody assay using human complement.
Primary strain variants are in bold font; additional strain variants are in unbolded font.
US strain data based on N=1263 MenB SBA strain pool that included US isolates collected during 2000–2005.
European strain data based on N=1814 extended MenB SBA strain pool that included EU isolates collected during 2001–2006.
Factor H binding protein is thus similar to the “holy grail” antigen in that it is surface-expressed, induces functional antibodies, and is harboured by almost all disease-causing strains [22,39]; however, FHbp falls short in that low expression in some strains may reduce susceptibility to antibodies [49]. In rare cases, strains lacking FHbp may use alternative proteins such as Porin B (PorB) or Neisserial surface protein A (NspA) to bind FH [44,45,47,50]; however, one analysis indicated that both PorB and NspA were required for sufficient resistance of complement-mediated bacterial lysis [50]. Additionally, FHbp protein sequences are not conserved across all strains; however, this limitation can be mitigated by inclusion of variants from each of the 2 subfamilies exhibiting high intrafamily sequence homology, enabling potential cross-protection within subfamilies and ultimately breadth of coverage [22,49]. Breadth of coverage therefore depends on immunogenicity of the FHbp antigen, which is driven by antigenic presentation and overall formulation of individual vaccines.
MenB-4C
The FHbp antigen included in MenB-4C is a recombinant, nonlipidated version of a subfamily B variant (Figure 1) [11,30]. In early MenB-4C evaluations, use of recombinant FHbp alone failed to induce functional antibodies to strains expressing FHbp subfamily A variants [37]. Later studies preceding the final MenB-4C formulation indicated that fusing the nonlipidated FHbp to another protein (GNA2091) increased immunogenicity compared with FHbp alone; however, this increase only manifested with 2 of the 3 strains evaluated, one of which only exhibited a 2-fold titre difference compared with FHbp alone [51]. Early clinical studies in infants indicated that immune responses to a vaccine containing the nonlipidated FHbp fusion protein as well as NHBA (also formulated as a fusion protein) and NadA failed to induce robust immune responses against certain MenB strains, particularly those with vaccine-heterologous FHbp variants that also had low or nonexistent NadA expression; thus, neither FHbp nor the other vaccine antigens induced protective antibodies against these strains [52,53].
An alternative vaccine formulation that also included OMVs from the NZ98/254 strain increased immunogenicity against most MenB strains evaluated, beyond those matched for PorA (Figure 2), and was selected as the MenB-4C final formulation [52–54]. The addition of OMVs to the final MenB-4C formulation does not broaden responses to FHbp because detergent extraction of the OMV removes FHbp [55]; as such, immune responses to FHbp are expected to remain unchanged for this formulation. Moreover, more recent studies using the meningococcal antigen typing system (MATS; discussed in detail below) have predicted little to no FHbp-mediated coverage of subfamily A strains by MenB-4C, although other antigens may provide protection against these isolates [56,57]. This formulation was demonstrated to exhibit an acceptable safety profile across many studies [58].
Figure 2.
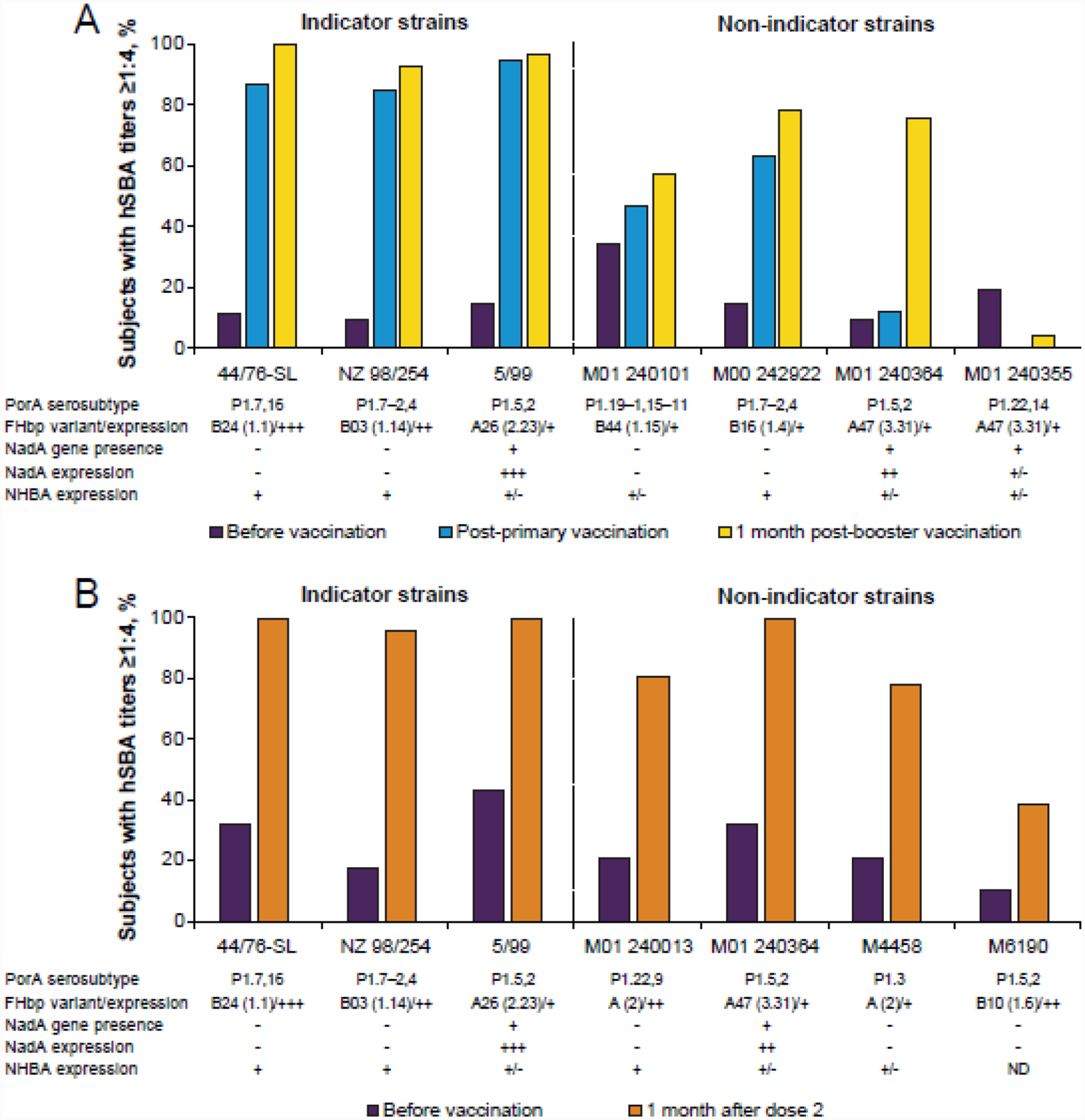
Percentages of subjects with hSBA titres ≥1:4 before and after MenB-4C vaccination in infants and toddlers [52] (A) and adults (B) [54] across MenB indicator and diverse strains. In the infant/toddler study presented in panel A, infants received MenB-4C at 2, 4, 6, and 12 months of age; serum samples were taken at 2, 5, 7, 12, and 13 months of age. In the adult study presented in panel B, participants received 3 doses of MenB-4C, each spaced 1 month apart. Indicator strains 44/76-SL, NZ98/254, and 5/99 are intended to highlight responses against FHbp, PorA, and NadA, respectively [58]. Classification of the strains for each study in terms of the MenB-4C antigens is provided below the x-axis of each graph using data from the original studies, with – indicating low expression, +/− indicating medium expression, and +,++, and +++ indicating increasingly high expression. FHbp variants are indicated using the subfamily A/B classification scheme [22] as well as an alternative classification scheme involving 3 variant groups [37]. FHbp=factor H binding protein; hSBA=serum bactericidal antibody assay using human complement; MenB-4C= Bexsero®, 4CMenB; NadA=Neisserial adhesin A; ND=not determined; NHBA=Neisserial Heparin Binding Antigen; PorA=porin A.
Multiple studies have focused on testing the breadth of protection by antibodies induced by the FHbp component of MenB-4C. The manufacturer of MenB-4C used specific “indicator strains” to evaluate MenB-4C–induced SBA in assays using human complement (hSBA), with the goal of evaluating contributions of individual antigens to killing [58,59]. The FHbp indicator strain used in clinical studies, 44/76-SL, includes a vaccine-matched FHbp variant that is highly expressed [11,58,59]; use of this strain fails to provide data regarding breadth of coverage against strains with divergent FHbp sequences. Potential coverage of diverse strains was evaluated in the same MATS studies referenced previously, which do not predict 100% coverage of FHbp subfamily B strains by anti-FHbp antibodies induced by MenB-4C (coverage of strains expressing a particular subfamily B variant was predicted at 24% in one study) [56,57]. Additionally, other studies using isogenic strains expressing different FHbp subfamily B variants found decreasing titres in hSBA assays with increasing divergence from the MenB-4C variant; in humans, this was age-related and most pronounced in infants [60,61]. Similar findings regarding lack of anti-FHbp subfamily A protection and lack of cross-reactivity to all FHbp subfamily B–expressing strains have been demonstrated in clinical evaluations and directly contrast with results obtained for strain 44/76-SL (Figure 2) [52–54]. Despite the limit in breadth of protection across FHbp variants, it is important to note the potential of MenB-4C to provide protection against non–serogroup B meningococci, with 1 study demonstrating substantial hSBA activity against most strains comprising a serogroup X strain panel [62]; however, these responses may have been directed against antigens other than FHbp.
The additional antigens included in MenB-4C are intended to enhance the vaccine’s breadth of coverage [51]. However, understanding the prevalence of these antigens within disease-causing strains is important for predicting vaccine-induced protection. For example, the nadA gene was present in only 22% and 39% of invasive isolates collected in the European Union during 2007–2008 and the United States during 2000–2008, respectively [56,57]. Additionally, NadA variants segregate into 2 groups that do not induce cross-reactive functional immunity [63]; thus, the presence of the nadA gene in a given disease-causing strain may not guarantee protection against a NadA-expressing strain. Similarly, antibodies directed against PorA, the immunodominant antigen in the OMV component of MenB-4C, have limited cross-reactivity [30]; as such, MenB-4C strain coverage via the OMV component is restricted to strains harbouring vaccine-homologous PorA subtypes, which can be limited among disease-causing strains [56,57]. As with FHbp [49], expression levels of the additional MenB-4C antigens may also affect vaccine coverage; for NadA, only a small percentage of US and EU strains harbouring the nadA gene expressed the protein at protective levels [56,57]. Despite evidence of MenB-4C effectiveness against prevalent MenB strains [64], the use of multiple different antigens within a MenB vaccine thus does not necessarily afford protection against all MenB strains.
Although the hSBA assay is the only generally accepted surrogate measure of protection for MenB disease [65], the MenB-4C manufacturer developed an alternative assay, MATS, to predict breadth of strain coverage [66]. MATS was developed with the aim of improving understanding of the contributions of antibodies raised against individual antigens to overall vaccine coverage. Specifically, MATS uses an enzyme-linked immunosorbent assay (ELISA) to test individual MenB isolates and simultaneously measure the ability of MenB-4C–induced antibodies to recognise each of the 3 proteins (ie, FHbp, NadA, and NHBA) harboured by each isolate in conjunction with the amount of protein expressed. A particular strain is predicted to be covered by MenB-4C if ELISA reactivity for any of the 3 vaccine proteins expressed by that strain exceeds antigen-specific thresholds or if the PorA serosubtype or genosubtype of the strain matches that of the OMV vaccine component. Using MATS, studies have shown that, in some cases, protection afforded by MenB-4C against a given MenB strain is predicted to result from bactericidal activity induced by as many as 4 antigens [56,57]. This observation may be important because the bactericidal contributions of antibodies can vary depending on the antigen specificity, with maximum killing demonstrated when antibody populations to multiple antigens are able to act synergistically [67,68]; this can occur even when antibodies to individual antigens are not independently bactericidal [59]. It has been suggested that MATS underestimates strain coverage in comparison with hSBA, possibly as a result of such additive contributions [69]. Similarly, antibodies targeting multiple OMV proteins including minor antigens have also demonstrated additive bactericidal activity when tested in combination, despite having low killing activity when tested alone [70]. Importantly, despite the inclusion of multiple antigens in MenB-4C, MATS has predicted that 9%–22% of MenB strains in the United States and Europe will not be covered by the vaccine [56,57].
MenB-4C effectiveness against IMD has been evaluated following its addition to the UK national immunization program in September 2015. Recent data evaluating the first 3 years of the programme indicated that effectiveness of a 2-dose infant schedule was 52.7% [64], supporting the utility of noncapsular protein vaccines against IMD. However, effectiveness against strains predicted by MATS was 64.4%, highlighting limitations of using a secondary, in vitro assay rather than hSBA to predict breadth of coverage.
MenB-FHbp
The development of FHbp as an antigen in MenB-FHbp followed a different pathway than that of MenB-4C. In preclinical studies, lipidated FHbp was observed to induce bactericidal antibody titers that were higher than those induced by nonlipidated FHbp antigens [22]. Lipidated subfamily B FHbp variants induced SBA that was cross-reactive to other subfamily B variants tested and was also associated with some cross-reactivity against subfamily A variants [22,49]. However, responses were lower than those directed against subfamily B variants, indicating that a monovalent lipidated subfamily B antigen was not sufficient to provide broad coverage against strains harbouring subfamily A FHbps. Use of lipidated subfamily A variants also induced immune responses with high cross-reactivity within subfamily A and some cross-reactivity against subfamily B variants. Based on these data, the MenB-FHbp final formulation includes 2 lipidated FHbp variants, 1 from each subfamily (Figure 1), along with aluminium adjuvant [12]; the lipidated FHbp variants have been described as “self-adjuvanting” due to the enhanced immune responses they induce compared with non-lipidated formulations [71]. Thus, the MenB-FHbp formulation is expected to induce antibodies against nearly all invasive MenB isolates.
The considerations of antigen presence and expression relate differently to MenB-FHbp compared with MenB-4C. Unlike nadA, but similar to porA, the fhbp gene is harboured by nearly all meningococcal isolates [39,56,57,72], consistent with its important role in bacterial survival [43]. However, as mentioned previously, certain MenB strains with low or no FHbp expression have been identified [47–49], which could potentially limit the breadth of protection of an FHbp-based vaccine. Nonetheless, a study evaluating an extensive collection of invasive MenB isolates (N=1814) found that >91% were predicted to be susceptible to bactericidal killing by MenB-FHbp vaccine-induced antibodies [73].
Immunogenicity analyses for MenB-FHbp clinical studies evaluated hSBA activity against 4 primary and 10 additional vaccine-heterologous strains that were chosen to provide an estimate of the vaccine’s breath of coverage (Figure 1) [9,74]. Thus, in contrast to MenB-4C, MenB-FHbp breadth of coverage estimates rely directly on evaluations of hSBA activity, the accepted surrogate of MenB disease protection [65], using diverse strains; no secondary assay (eg, MATS) is used. The primary strains were randomly selected from a pool of isolates harbouring vaccine-heterologous FHbp variants that were representative of the diversity of MenB isolates, having low to medium FHbp surface expression, and associated with low baseline hSBA activity [9]. The FHbp variants expressed by the 4 primary test strains collectively were found in 42% of invasive MenB isolates from a 1263-strain pool of disease isolates from the United States and Europe (Table 2) [74]. Selection of the 10 additional test strains was subject to criteria similar to those for the primary strains; the FHbp variants included in the primary and additional test strains collectively represent FHbp variants harboured by 80.8% of strains within the strain pool described above.
Clinical data have suggested broad coverage by the final MenB-FHbp formulation. Phase 2 and 3 clinical MenB-FHbp studies in >20,000 adolescent and adult subjects found this formulation to have an acceptable safety profile [75]. Evaluation of sera collected 1 month after a 3-dose vaccine series in hSBA assays indicated that up to 94% of vaccinated subjects achieved protective titres and ≥4-fold rises in hSBA titre against the 4 primary MenB test strains [75]. High percentages (71.3%–99.3%) of immunized subjects aged 10–25 years in the pivotal MenB-FHbp phase 3 studies additionally achieved hSBA titres above protective levels against the 10 additional test strains (Figure 3) [76]. Additional studies using sera from adolescent and adult subjects demonstrated robust hSBA responses following MenB-FHbp vaccination against MenB strains harbouring diverse FHbp variants from both subfamilies, including a number of strains associated with outbreaks from various global regions [29,77–79]. Sera from adolescent subjects vaccinated with MenB-FHbp also induced substantial immune responses against strains from meningococcal serogroups C, W, X, and Y, with lower responses against a serogroup A strain [80]. Recent smaller-scale clinical studies have supported MenB-FHbp breadth of coverage in toddlers and young children using the 4 primary MenB test strains [81,82]; however, the vaccine is not currently licensed for these age groups[12].
Figure 3.
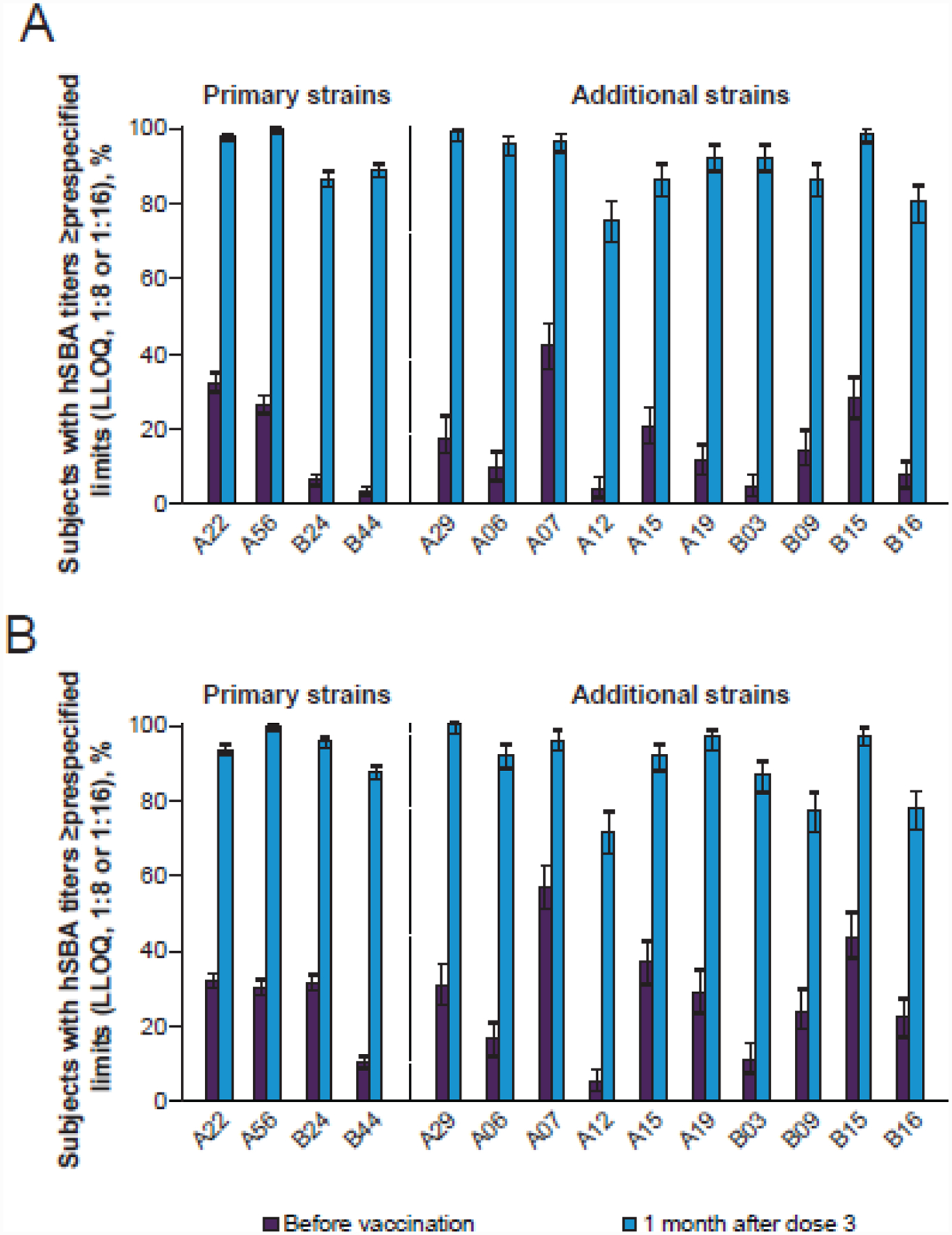
Percentages of adolescents (A) and young adults (B) with hSBA titres ≥LLOQ against the 4 primary and 10 additional MenB test strains following vaccination with MenB-FHbp, adapted with permission from Ostergaard et al. N Engl J Med. 2017;377:2349–2362 [76]. Strains are indicated by their corresponding FHbp sequence variants using Pfizer nomenclature (http://pubmlst.org/neisseria/fHbp). The LLOQ was 1:8 or 1:16 depending on test strain. FHbp=factor H binding protein; hSBA=serum bactericidal antibody assay using human complement; LLOQ=lower limit of quantitation; MenB=meningococcal serogroup B; MenB-FHbp= Trumenba®, bivalent rLP2086.
Clinical data thus provide evidence that MenB-FHbp is expected to provide protection across disease-causing MenB strains beyond the 14 test strains used in the phase 3 clinical studies. This demonstrated breadth of coverage can be attributed to the inclusion of FHbp variants from both subfamilies as well as the lipidated nature of the vaccine antigens.
Other FHbp-Containing Vaccines
Additional FHbp-based MenB vaccines are currently in development [83]. One approach uses native OMVs from meningococcal strains genetically engineered to overexpress FHbp variants from both subfamilies; this formulation elicited broad protection as measured by SBA responses in nonhuman primates against MenB strains from both FHbp subfamilies [84]. Another alternative FHbp-based vaccine formulation uses a mutant FHbp with decreased FH binding to reduce epitope masking and increase the functional activity of anti-FHbp antibodies [85]. More recently, these 2 approaches have been combined, with the resulting vaccine eliciting hSBA responses in primates that were superior to those induced by MenB-4C for strains expressing PorA variants heterologous to both vaccines [83]. Importantly, the strains used in the latter study expressed subfamily B FHbp variants, and it was noted that a vaccine using this approach should include antigens from both FHbp subfamilies to provide broad coverage.
A recently published murine study evaluated immunogenicity of a group of novel MenB antigens that used FHbp variant B24 as a molecular scaffold, with PorA surface loop epitopes integrated at different amino acid positions in FHbp [86]. SBA activity (using rabbit complement) against the FHbp-homologous strain H44/76 was detected for all antigens tested, with killing seemingly dominated by antibodies targeting FHbp.
Vaccine Pressure and Generation of Escape Mutants
Use of only FHbp within MenB-FHbp has led to concern about the potential generation of escape mutants with low FHbp expression levels or lacking fhbp entirely [47]. Vaccination could potentially place selective evolutionary pressures on meningococcal populations, which can lead to increased prevalence of strains lacking the protein(s) covered by a given vaccine [87].
When reviewing changing epidemiology, it can be difficult to separate the roles of vaccine pressure and temporal trends of a given disease-causing organism [88]. Temporal trends have yielded important influence on meningococcal disease epidemiology; for example, MenB disease incidence has decreased worldwide in recent years despite lack of widespread vaccination strategies [2], possibly because of immunologic factors and behavioural shifts in the population. However, MenB disease resurgence remains a possibility, as shown by recent outbreaks [79,89].
Following the widespread use of monovalent capsular polysaccharide conjugate meningococcal vaccines, there has not been an appreciable increase in IMD due to other serogroups driven by vaccine pressure. For example, in Africa, widespread use of a meningococcal serogroup A (MenA) conjugate vaccine has been associated with a dramatic decrease in MenA IMD incidence [90]. IMD cases due to other serogroups, such as serogroups C, W, and X, have increased in subsequent years in African countries that implemented MenA vaccination; however, outbreaks associated with these serogroups also occurred before MenA vaccine introduction, and overall IMD rates remain substantially lower than prevaccination rates [2,90]. Similarly, the decreases in meningococcal serogroup C (MenC) disease incidence following widespread MenC vaccination in countries such as England and the Netherlands was not accompanied by any significant increases in disease caused by other serogroups [91,92]. The number of meningococcal serogroup W (MenW) IMD cases in England (and many other countries throughout the world [93]) has dramatically increased in recent years, but overall IMD incidence rates remain much lower than before widespread MenC vaccination [91]. For monovalent OMV MenB vaccines, such as those used in Cuba and New Zealand, there was no evidence of vaccine-induced MenB strain replacement following mass vaccination campaigns [32,33]. This was despite high incidence rates before vaccination, although cross-protection against nonepidemic strains, albeit to a lesser extent, may have contributed. Thus, meningococcal vaccination campaigns have historically not been been associated with the generation of vaccine pressure and escape mutants.
On the other hand, it could be argued that vaccine escape mutants are most likely generated when selective vaccine pressure is placed on either a dispensable antigenic component or a limited number of antigenic variants of a diverse antigenic component. As has been observed for other pathogens, either situation could lead to an increased prevalence of strains expressing variants not covered by the vaccine [87]. For instance, there are now Bordatella pertussis strains lacking the vaccine antigen pertactin, resulting in decreased vaccine efficacy and a resurgence of pertussis in many countries [94]. For this reason, it is critical that even for a ubiquitous antigen, a given meningococcal vaccine should include antigens covering all variants expressed by targeted disease-causing strains (eg, both subfamilies A and B in the case of MenB-FHbp), including potentially emerging variants. Selective pressure could potentially be a more realistic concern for MenB-4C, in which lack of coverage by the FHbp antigen against strains harbouring subfamily A variants [56,57], and even some subfamily B variants [52,53], may lead to increases in strains with these non-covered FHbp variants. Increases in proportions of subfamily A strains have naturally occurred in some countries, such as Spain and the Netherlands [95–97].
For MenB-FHbp, however, this progression may be less likely to occur because of broad protection conferred by targeting FHbp, which is nearly ubiquitous across MenB strains [22,38,39,49] and due to the important role it plays in bacterial survival [43,46]. Of note, however, is that some strains have low or no FHbp expression and instead appear to rely on other FH ligands that permit survival in immunocompetent hosts in the presence of FH [44,45,47,50]. The near ubiquity of FHbp in MenB disease-causing strains contrasts with the frequent absence of some other antigens used in MenB vaccines [56,57].
Vaccine strategies should also consider the age group in which the vaccine is predominantly used and how this use contributes to herd protection. Limiting vaccination to infants may reduce vaccine pressure because infants and young children rarely carry meningococci [98] and are therefore unlikely to drive strain evolution [99]. By contrast, the MenC conjugate vaccine program in the United Kingdom offered vaccination to all individuals up to age 24 years and resulted in remarkable herd protection, with a reduction in MenC carriage without serogroup replacement [100,101]. Additionally, there was a greater effect against strains from the ST-11 clonal complex, which was the predominant disease-causing lineage when the vaccine was introduced, compared with other sequence types, with these strains exhibiting high capsule expression rates [101]. This raises the possibility that immune escape could happen with other antigens and, in contrast to the loss of capsule, which is essential for virulence, that these strains might still cause disease. Recent data have shown that MenB-4C does not reduce acquisition of meningococcal carriage or affect carriage density [102,103] and will therefore be unlikely to induce herd protection or result in vaccine pressure during asymptomatic carriage. As of yet, no large-scale studies have evaluated MenB-FHbp effects on carriage, although two smaller studies suggested that MenB-FHbp did not affect carriage at the population level [104,105]. However, the use of 2 different FHbp variants and different presentation (ie, as lipidated nonfusion proteins) may affect carriage differently compared with the FHbp, or other antigens, included in MenB-4C. An ongoing study in the United Kingdom evaluating the impacts of both MenB-4C and MenB-FHbp on meningococcal carriage [106] will provide further insight on this topic.
The importance of FHbp is supported by its presence in meningococcal strains irrespective of serogroup [22,107], and both MenB-4C and MenB-FHbp studies have indicated the potential for these vaccines to provide protection against non–serogroup B strains [62,80]. However, capsular polysaccharide vaccines for serogroups A, C, W, and Y are still ideal for preventing disease caused by each of these serogroups because the capsular polysaccharide is highly immunogenic and conserved across all strains within a given serogroup [6,29].
Conclusions
Several attributes distinguish FHbp as a potentially broadly protective vaccine antigen, including expression at the bacterial surface, role as a virulence factor for bacterial survival, ability to elicit a bactericidal response, and, although sequences are diverse, segregation of variants into 2 well-defined subfamilies.
The 2 currently licensed MenB vaccines, both of which have acceptable safety profiles and are currently administered in widespread global regions, use different strategies to induce humoral immune responses and to protect against IMD. MenB-4C includes a single nonlipidated subfamily B FHbp variant, which lacks protection against strains that express subfamily A variants and even limited cross-protection within subfamily B; the vaccine formulation includes other antigens for this reason. The dynamic nature of FHbp epidemiology, as demonstrated in countries such as Spain and the Netherlands, can render this strategy potentially subject to vaccine pressure, which may lead to increased prevalence of strains not covered by the MenB-4C FHbp component. For MenB-FHbp, early evaluations demonstrated that FHbp lipidation was critical for increased immunogenicity and led to the possibility of a broadly protective vaccine based on only 2 FHbp antigens (ie, a representative each from subfamily A and subfamily B). Due to the cross protection afforded by MenB-FHbp, vaccine pressure induced by this strategy is placed on FHbp as a whole rather than a specific subfamily sequence variant, and is not predicted to be affected by changing proportions of FHbp subfamilies among disease-causing variants. Furthermore, the important role of FHbp in evasion of complement-mediated bacterial lysis suggests that loss of FHbp expression among strains in response to use of either vaccine is unlikely.
Although vaccine pressure and the generation of escape mutants continue to be potential concerns, there have been few observations of strain replacement following mass meningococcal vaccinations. Nevertheless, the possibility remains for strong selective pressures to lead to increases in the prevalence of strains with vaccine antigen variants not covered by a particular vaccine. The expected broad protection provided by MenB-FHbp against the highly diverse range of disease-causing meningococci across both FHbp subfamilies stems concerns regarding strain replacement, particularly in comparison with limited cross-protection afforded by the MenB-4C FHbp component. Continued experience with both vaccines will further inform the practical implications of using FHbp as a vaccine antigen. Additional insights may also be provided by potential use of alternative FHbp-based vaccines currently under development.
Highlights.
MenB vaccines with FHbp variants can be highly immunogenic against diverse strains
Lipidated FHbp induces superior immune responses compared with nonlipidated FHbp
MenB vaccines differ in presentation and number of FHbp antigens included
Lipidated FHbps from both subfamilies A and B successfully provide broad protection
The critical role of FHbp limits the risk of vaccine escape and strain replacement
Acknowledgments
This work was supported by Pfizer Inc. Editorial/medical writing support was provided by Judith Kandel, PhD, of ICON plc (North Wales, PA, USA), and was funded by Pfizer Inc. This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria/) sited at the University of Oxford [108]; the development of this site has been funded by the Wellcome Trust and European Union. This publication also made use of the Meningitis Research Foundation Meningococcus Genome Library (http://www.meningitis.org/research/genome) developed by Public Health England, the Wellcome Trust Sanger Institute, and the University of Oxford as a collaboration; this project is funded by Meningitis Research Foundation.
Footnotes
Conflicts of Interest
JF, PL, and PB are employees of Pfizer and may hold Pfizer stock or stock options. CDB has or has had contract or collaborative interactions with GSK, Pfizer, Roche and Sanofi Pasteur. PTB is named as an inventor on patents relating to FHbp mutants with decreased binding of factor H, which have been assigned to the Children’s Hospital & Research Center at Oakland. RB performs contract research on behalf of Public Health England for GSK, Pfizer, and Sanofi Pasteur.
References
- [1].Purmohamad A, Abasi E, Azimi T, Hosseini S, Safari H, Nasiri MJ, et al. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog 2019;134:103571. 10.1016/j.micpath.2019.103571. [DOI] [PubMed] [Google Scholar]
- [2].Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines 2019;18:15–30. 10.1080/14760584.2019.1557520. [DOI] [PubMed] [Google Scholar]
- [3].European Centre for Disease Prevention and Control. Annual epidemiological report for 2017: invasive meningococcal disease, https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2017-invasive-meningococcal-disease.pdf; 2019. [accessed July 17, 2019].
- [4].Centers for Disease Control. Enhanced meningococcal disease surveillance report, 2017, https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf; 2017. [accessed May 10, 2019].
- [5].Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP), MMWR Recomm Rep, https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm; 2013. [accessed February 3, 2020]. [PubMed]
- [6].Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med 2010;362:1511–20. 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- [7].Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, Altieri PL, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis 1972;126:514–21. 10.1093/infdis/126.5.514 [DOI] [PubMed] [Google Scholar]
- [8].Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983;2:355–7. 10.1016/S0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- [9].Zlotnick GW, Jones TR, Liberator P, Hao L, Harris S, McNeil LK, et al. The discovery and development of a novel vaccine to protect against Neisseria meningitidis serogroup B disease. Hum Vaccin Immunother 2015;11:5–13. 10.4161/hv.34293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].PubMLST. Neisseria sequence typing home page, University of Oxford, http://pubmlst.org/neisseria/; 2019. [accessed December 16, 2019].
- [11].Bexsero®, GSK Vaccines, Srl, https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm431447.pdf; 2018. [accessed July 7, 2020].
- [12].Trumenba® (meningococcal group B vaccine), https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm421139.pdf; 2017. [accessed February 28, 2018].
- [13].GlaxoSmithKline Brasil Ltda. Modelo de texto de bula – Profissional de Saúde: Bexsero™, https://br.gsk.com/media/613334/l1310_bexsero_susp_inj_gds010.pdf; 2019. [accessed November 1, 2019].
- [14].European Medicines Agency. Annex I: Summary of product characteristics (Bexsero), https://www.ema.europa.eu/documents/product-information/bexsero-epar-product-information_en.pdf; [accessed November 7, 2018].
- [15].Ministerio de Salud (Uruguay). Vacunas antimeningocócicas en Uruguay, https://www.gub.uy/ministerio-salud-publica/sites/ministerio-salud-publica/files/documentos/noticias/Postura%20sobre%20vacunas%20antimeningocócicas%20en%20Uruguay%202310.pdf; [accessed November 1, 2019].
- [16].GlaxoSmithKline (Israel) Ltd. Bexsero, https://www.old.health.gov.il/units/pharmacy/trufot/alonim/Rishum_7_64465718.pdf; 2017. [accessed November 1, 2019].
- [17].Sociedad Argentina de Pediatria. Lo que el pediatra debe saber sobre vacuna para Neisseria meningitis B(4CMenB) ®Bexsero, https://www.sap.org.ar/novedades/194/lo-que-el-pediatra-debe-saber-sobre-vacuna-para-neisseria-meningitis-b4cmenb-bexsero-.html; [accessed November 1, 2019].
- [18].Knuf M, Szenborn L, Moro M, Petit C, Bermal N, Bernard L, et al. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Pediatr Infect Dis J 2009;28:S97–S108. 10.1097/INF.0b013e318199f61b. [DOI] [PubMed] [Google Scholar]
- [19].GlaxoSmithKline Australia Pty Ltd. Australian product information: Bexsero (multicomponent meningococcal group B vaccine) suspension for injection, https://au.gsk.com/media/404836/bexsero_pi_007.pdf; 2018. [accessed November 1, 2019].
- [20].Product Monograph: Bexsero, GlaxoSmithKline Inc, https://ca.gsk.com/media/1212390/bexsero.pdf; 2017. [accessed February 14, 2018].
- [21].New Zealand Data Sheet (Bexsero), https://www.medsafe.govt.nz/profs/Datasheet/b/bexseroinj.pdf; 2018. [accessed November 1, 2019].
- [22].Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 2004;72:2088–100. 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].European Medicines Agency. Annex I: Summary of product characteristics (Trumenba), https://www.ema.europa.eu/documents/product-information/trumenba-epar-product-information_en.pdf; [accessed November 7, 2018].
- [24].Pfizer Canada Inc. Product monograph: Trumenba, Pfizer Canada Inc, https://pdf.hres.ca/dpd_pm/00041515.PDF; 2017. [accessed October 11, 2018]. [Google Scholar]
- [25].Australian Government Department of Health. Product Information: Trumenba®, https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2017-PI-02674-1; 2019. [accessed July 18, 2019].
- [26].Folleto de información al profesional Trumenba suspensión inyectable (vacuna meningocócica recombinante, adsorbida (grupo B)), https://docplayer.es/90688351-Folleto-de-informacion-al-profesional-trumenba-suspension-inyectable-vacuna-meningococica-recombinante-adsorbida-grupo-b.html; [accessed November 1, 2019].
- [27].Rossi R, Beernink PT, Giuntini S, Granoff DM. Susceptibility of meningococcal strains responsible for two serogroup B outbreaks on U.S. university campuses to serum bactericidal activity elicited by the MenB-4C vaccine. Clin Vaccine Immunol 2015;22:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Caugant DA, Mocca LF, Frasch CE, Froholm LO, Zollinger WD, Selander RK. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol 1987;169:2781–92. 10.1128/jb.169.6.2781-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Donald RG, Hawkins JC, Hao L, Liberator P, Jones TR, Harris SL, et al. Meningococcal serogroup B vaccines: estimating breadth of coverage. Hum Vaccin Immunother 2017;13:255–65. 10.1080/21645515.2017.1264750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang NY, Pollard AJ. The next chapter for group B meningococcal vaccines. Crit Rev Microbiol 2018;44:95–111. 10.1080/1040841X.2017.1329276. [DOI] [PubMed] [Google Scholar]
- [31].Bjune G, Hoiby EA, Gronnesby JK, Arnesen O, Fredriksen JH, Halstensen A, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 1991;338:1093–6. 10.1016/0140-6736(91)91961-s [DOI] [PubMed] [Google Scholar]
- [32].Climent Y, Yero D, Martinez I, Martin A, Jolley KA, Sotolongo F, et al. Clonal distribution of disease-associated and healthy carrier isolates of Neisseria meningitidis between 1983 and 2005 in Cuba. J Clin Microbiol 2010;48:802–10. 10.1128/JCM.01653-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arnold R, Galloway Y, McNicholas A, O’Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine 2011;29:7100–6. 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- [34].Boutriau D, Poolman J, Borrow R, Findlow J, Domingo JD, Puig-Barbera J, et al. Immunogenicity and safety of three doses of a bivalent (B:4:p1.19,15 and B:4:p1.7–2,4) meningococcal outer membrane vesicle vaccine in healthy adolescents. Clin Vaccine Immunol 2007;14:65–73. 10.1128/cvi.00230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vermont CL, van Dijken HH, Kuipers AJ, van Limpt CJ, Keijzers WC, van der Ende A, et al. Cross-reactivity of antibodies against PorA after vaccination with a meningococcal B outer membrane vesicle vaccine. Infect Immun 2003;71:1650–5. 10.1128/iai.71.4.1650-1655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kaaijk P, van Straaten I, van de Waterbeemd B, Boot EP, Levels LM, van Dijken HH, et al. Preclinical safety and immunogenicity evaluation of a nonavalent PorA native outer membrane vesicle vaccine against serogroup B meningococcal disease. Vaccine 2013;31:1065–71. 10.1016/j.vaccine.2012.12.031. [DOI] [PubMed] [Google Scholar]
- [37].Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 2003;197:789–99. 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McNeil LK, Zagursky R, Shuo L, Murphy E, Zlotnick G, Hoiseth SK, et al. The role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol Mol Biol Rev 2013;77:234–52. 10.1128/MMBR.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 2009;200:379–89. 10.1086/600141. [DOI] [PubMed] [Google Scholar]
- [40].da Silva RAG, Karlyshev AV, Oldfield NJ, Wooldridge KG, Bayliss CD, Ryan A, et al. Variant signal peptides of vaccine antigen, FHbp, impair processing affecting surface localization and antibody-mediated killing in most meningococcal isolates. Front Microbiol 2019;10:2847. 10.3389/fmicb.2019.02847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol 2006;176:7566–75. 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- [42].Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun 2009;77:764–9. 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, et al. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect Immun 2009;77:292–9. 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog 2010;6:e1001027. 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. mBio 2013;4:e00339–13. 10.1128/mBio.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 2006;177:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin Vaccine Immunol 2011;18:1002–14. 10.1128/CVI.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lucidarme J, Lekshmi A, Parikh SR, Bray JE, Hill DM, Bratcher HB, et al. Frequent capsule switching in ‘ultra-virulent’ meningococci - Are we ready for a serogroup B ST-11 complex outbreak? J Infect 2017;75:95–103. 10.1016/j.jinf.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010;28:6086–93. 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- [50].Giuntini S, Pajon R, Ram S, Granoff DM. Binding of complement factor H to PorB3 and NspA enhances resistance of Neisseria meningitidis to anti-factor H binding protein bactericidal activity. Infect Immun 2015;83:1536–45. 10.1128/IAI.02984-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A 2006;103:10834–9. 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010;51:1127–37. 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- [53].Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J 2010;29:e71–9. 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- [54].Toneatto D, Ismaili S, Ypma E, Vienken K, Oster P, Dull P. The first use of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) in humans. Hum Vaccin 2011;7:646–53. 10.4161/hv.7.6.15482. [DOI] [PubMed] [Google Scholar]
- [55].Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis 2008;198:262–70. 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rajam G, Stella M, Kim E, Paulos S, Boccadifuoco G, Serino L, et al. Meningococcal antigen typing system (MATS)-based Neisseria meningitidis serogroup B coverage prediction for the MenB-4C vaccine in the United States. mSphere 2017;2:doi: 10.1128/mSphere.00261-17. 10.1128/mSphere.00261-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis 2013;13:416–25. 10.1016/S1473-3099(13)70006-9. [DOI] [PubMed] [Google Scholar]
- [58].Masignani V, Pizza M, Moxon ER. The development of a vaccine against meningococcus B using reverse vaccinology. Front Immunol 2019;10:751. 10.3389/fimmu.2019.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Giuliani MM, Biolchi A, Serruto D, Ferlicca F, Vienken K, Oster P, et al. Measuring antigen-specific bactericidal responses to a multicomponent vaccine against serogroup B meningococcus. Vaccine 2010;28:5023–30. 10.1016/j.vaccine.2010.05.014. [DOI] [PubMed] [Google Scholar]
- [60].Brunelli B, Del Tordello E, Palumbo E, Biolchi A, Bambini S, Comanducci M, et al. Influence of sequence variability on bactericidal activity sera induced by Factor H binding protein variant 1.1. Vaccine 2011;29:1072–81. 10.1016/j.vaccine.2010.11.064. [DOI] [PubMed] [Google Scholar]
- [61].Konar M, Granoff DM, Beernink PT. Importance of inhibition of binding of complement factor H for serum bactericidal antibody responses to meningococcal factor H-binding protein vaccines. J Infect Dis 2013;208:627–36. 10.1093/infdis/jit239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hong E, Giuliani MM, Deghmane AE, Comanducci M, Brunelli B, Dull P, et al. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine 2013;31:1113–6. 10.1016/j.vaccine.2012.12.022. [DOI] [PubMed] [Google Scholar]
- [63].Bambini S, De Chiara M, Muzzi A, Mora M, Lucidarme J, Brehony C, et al. Neisseria adhesin A variation and revised nomenclature scheme. Clin Vaccine Immunol 2014;21:966–71. 10.1128/CVI.00825-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med 2020;382:309–17. 10.1056/NEJMoa1901229. [DOI] [PubMed] [Google Scholar]
- [65].Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine 2006;24:5093–107. 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- [66].Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A 2010;107:19490–5. 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vu DM, Wong TT, Granoff DM. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and Neisserial heparin binding antigen. Vaccine 2011;29:1968–73. 10.1016/j.vaccine.2010.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Partridge E, Lujan E, Giuntini S, Vu DM, Granoff DM. The role of anti-NHba antibody in bactericidal activity elicited by the meningococcal serogroup B vaccine, MenB-4C. Vaccine 2017;35:4236–44. 10.1016/j.vaccine.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Frosi G, Biolchi A, Sapio ML, Rigat F, Gilchrist S, Lucidarme J, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine 2013;31:4968–74. 10.1016/j.vaccine.2013.08.006. [DOI] [PubMed] [Google Scholar]
- [70].Weynants VE, Feron CM, Goraj KK, Bos MP, Denoel PA, Verlant VG, et al. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect Immun 2007;75:5434–42. 10.1128/IAI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Luo Y, Friese OV, Runnels HA, Khandke L, Zlotnick G, Aulabaugh A, et al. The Dual Role of Lipids of the Lipoproteins in Trumenba, a Self-Adjuvanting Vaccine Against Meningococcal Meningitis B Disease. AAPS J 2016;18:1562–75. 10.1208/s12248-016-9979-x. [DOI] [PubMed] [Google Scholar]
- [72].Feavers IM, Fox AJ, Gray S, Jones DM, Maiden MC. Antigenic diversity of meningococcal outer membrane protein PorA has implications for epidemiological analysis and vaccine design. Clin Diagn Lab Immunol 1996;3:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].McNeil LK, Donald RGK, Gribenko A, French R, Lambert N, Harris SL, et al. Predicting the susceptibility of meningococcal serogroup B isolates to bactericidal antibodies elicited by bivalent rLP2086, a novel prophylactic vaccine. MBio 2018;9:e00036–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Harris SL, Tan C, Perez J, Radley D, Jansen KU, Anderson AS, et al. Selection of diverse strains to assess broad coverage of the bivalent FHbp meningococcal B vaccine. NPJ Vaccines 2020;5:8. 10.1038/s41541-019-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Perez JL, Absalon J, Beeslaar J, Balmer P, Jansen KU, Jones TR, et al. From research to licensure and beyond: Clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines 2018;17:461–77. 10.1080/14760584.2018.1483726. [DOI] [PubMed] [Google Scholar]
- [76].Ostergaard L, Vesikari T, Absalon J, Beeslaar J, Ward BJ, Senders S, et al. A Bivalent Meningococcal B Vaccine in Adolescents and Young Adults. N Engl J Med 2017;377:2349–62. 10.1056/NEJMoa1614474. [DOI] [PubMed] [Google Scholar]
- [77].Harris SL, Donald RGK, Hawkins JC, Tan C, O’Neill RE, McNeil LK, et al. Neisseria meningitidis serogroup B vaccine, bivalent rLP2086, induces broad serum bactericidal activity against diverse invasive disease strains including outbreak strains. Pediatr Infect Dis J 2017;36:216–23. 10.1097/INF.0000000000001399. [DOI] [PubMed] [Google Scholar]
- [78].Lujan E, Partridge E, Giuntini S, Ram S, Granoff DM. Breadth and duration of meningococcal serum bactericidal activity in healthcare workers and microbiologists immunized with the MenB-FHbp vaccine. Clin Vaccine Immunol 2017;24:doi: 10.1128/CVI.00121-17. 10.1128/CVI.00121-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Taha MK, Hawkins JC, Liberator P, Deghmane AE, Andrew L, Hao L, et al. Bactericidal activity of sera from adolescents vaccinated with bivalent rLP2086 against meningococcal serogroup B outbreak strains from France. Vaccine 2017;35:1530–7. 10.1016/j.vaccine.2017.01.066. [DOI] [PubMed] [Google Scholar]
- [80].Harris SL, Tan C, Andrew L, Hao L, Liberator PA, Absalon J, et al. The bivalent factor H binding protein meningococcal serogroup B vaccine elicits bactericidal antibodies against representative non-serogroup B meningococci. Vaccine 2018;36:6867–74. 10.1016/j.vaccine.2018.05.081. [DOI] [PubMed] [Google Scholar]
- [81].ClinicalTrials.gov. A study to describe the immunogenicity, safety, and tolerability of Neisseria meningitidis serogroup B bivalent recombinant lipoprotein 2086 vaccine (bivalent rLP2086) in healthy subjects aged ≥24 months to <10 years (NCT02531698), https://clinicaltrials.gov/ct2/show/NCT02531698; 2018. [accessed April 29, 2020].
- [82].ClinicalTrials.gov. Immunogenicity, safety and tolerability of a Neisseria meningitidis serogroup B bivalent recominant lipoprotein 2086 vaccine (bivalent rLP2086) in healthy toddlers (NCT02534935), https://clinicaltrials.gov/ct2/show/NCT02534935; 2018. [accessed June 25, 2020].
- [83].Beernink PT, Vianzon V, Lewis LA, Moe GR, Granoff DM. A meningococcal outer membrane vesicle vaccine with overexpressed mutant FHbp elicits higher protective antibody responses in infant rhesus macaques than a licensed serogroup B vaccine. MBio 2019;10:doi: 10.1128/mBio.01231-19. 10.1128/mBio.01231-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, et al. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 2011;29:4728–34. 10.1016/j.vaccine.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Granoff DM, Giuntini S, Gowans FA, Lujan E, Sharkey K, Beernink PT. Enhanced protective antibody to a mutant meningococcal factor H-binding protein with low-factor H binding. JCI Insight 2016;1:e88907. 10.1172/jci.insight.88907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hollingshead S, Jongerius I, Exley RM, Johnson S, Lea SM, Tang CM. Structure-based design of chimeric antigens for multivalent protein vaccines. Nat Commun 2018;9:1051. 10.1038/s41467-018-03146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martcheva M, Bolker BM, Holt RD. Vaccine-induced pathogen strain replacement: what are the mechanisms? J R Soc Interface 2008;5:3–13. 10.1098/rsif.2007.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013;10:e1001517. 10.1371/journal.pmed.1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Soeters HM, McNamara LA, Blain AE, Whaley M, MacNeil JR, Hariri S, et al. University-Based Outbreaks of Meningococcal Disease Caused by Serogroup B, United States, 2013–2018. Emerg Infect Dis 2019;25:434–40. 10.3201/eid2503.181574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi-Benissan C, et al. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis 2017;17:867–72. 10.1016/S1473-3099(17)30301-8. [DOI] [PubMed] [Google Scholar]
- [91].Public Health England. Invasive meningococcal disease in England: annual report for 2017 to 2018 supplementary data tables, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/752085/Laboratory_confirmed_cases_of_IMD_England_data_tables_2017to2018.pdf; 2018. [accessed August 16, 2019].
- [92].Bijlsma MW, Bekker V, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. Epidemiology of invasive meningococcal disease in the Netherlands, 1960–2012: an analysis of national surveillance data. Lancet Infect Dis 2014;14:805–12. 10.1016/S1473-3099(14)70806-0. [DOI] [PubMed] [Google Scholar]
- [93].Presa J, Findlow J, Vojicic J, Williams S, Serra L. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: A review. Infect Dis Ther 2019;8:307–33. 10.1007/s40121-019-0254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dorji D, Mooi F, Yantorno O, Deora R, Graham RM, Mukkur TK. Bordetella Pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance. Med Microbiol Immunol 2018;207:3–26. 10.1007/s00430-017-0524-z. [DOI] [PubMed] [Google Scholar]
- [95].Bambini S, Piet J, Muzzi A, Keijzers W, Comandi S, De Tora L, et al. An analysis of the sequence variability of meningococcal fHbp, NadA and NHBA over a 50-year period in the Netherlands. PLoS One 2013;8:e65043. 10.1371/journal.pone.0065043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hoiseth SK, Murphy E, Andrew L, Vogel U, Frosch M, Hellenbrand W, et al. A multi-country evaluation of Neisseria meningitidis serogroup B factor H-binding proteins and implications for vaccine coverage in different age groups. Pediatr Infect Dis J 2013;32:1096–101. 10.1097/INF.0b013e31829aa63b. [DOI] [PubMed] [Google Scholar]
- [97].Abad R, Garcia C, Navarro C, Vazquez JA. Genetic variability of the meningococcal serogroup B vaccine antigens: analysis of 2015–2016 invasive MenB strains in Spain. In: 15th European Meningococcal and Haemophilus Disease Society Congress; 2019 May 27–30; Lisbon, Portugal. [Google Scholar]
- [98].Cartwright KA, Stuart JM, Jones DM, Noah ND. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect 1987;99:591–601. 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Caugant DA, Maiden MC. Meningococcal carriage and disease--population biology and evolution. Vaccine 2009;27:B64–70. 10.1016/j.vaccine.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine 2009;27:B20–9. 10.1016/j.vaccine.2009.04.067. [DOI] [PubMed] [Google Scholar]
- [101].Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis 2008;197:737–43. 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Marshall HS, McMillan M, Koehler AP, Lawrence A, Sullivan TR, MacLennan JM, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med 2020;382:318–27. 10.1056/NEJMoa1900236. [DOI] [PubMed] [Google Scholar]
- [103].McMillan M, Walters L, Sullivan T, Leong LEX, Turra M, Lawrence A, et al. Impact of meningococcal B (4CMenB) vaccine on pharyngeal Neisseria meningitidis carriage density and persistence in adolescents. Clin Infect Dis 2020; 10.1093/cid/ciaa610. [DOI] [PubMed] [Google Scholar]
- [104].Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, MacNeil JR, Martin SW, et al. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a college-Rhode Island, 2015–2016. Clin Infect Dis 2017;64:1115–22. 10.1093/cid/cix091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].McNamara LA, Thomas JD, MacNeil J, Chang HY, Day M, Fisher E, et al. Meningococcal carriage following a vaccination campaign with MenB-4C and MenB-FHbp in response to a university serogroup B meningococcal disease outbreak-Oregon, 2015–2016. J Infect Dis 2017;216:1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Evaluating the effect of immunisation with group B meningococcal vaccines on meningococcal carriage (EudraCT number: 2017-004609-42), https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-004609-42/GB; [accessed July 17, 2020].
- [107].Mothibeli KM, du Plessis M, von Gottberg A, Murphy E, Hoiseth SK, Zlotnick G, et al. Distribution of factor H binding protein beyond serogroup B: variation among five serogroups of invasive Neisseria meningitidis in South Africa. Vaccine 2011;29:2187–92. 10.1016/j.vaccine.2010.11.072. [DOI] [PubMed] [Google Scholar]
- [108].Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res 2018;3:124. 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


