Abstract
Purpose of review:
Childhood obesity is escalating globally. Lifestyle and behavioral changes, which are the frequently used interventions in clinical practice lead to only modest improvements in children with established obesity. Bariatric surgery is currently the most effective obesity treatment but has very limited utilization in pediatric obesity and is preferentially used for children with worsening comorbidities. There exists a massive treatment gap for children suffering with obesity especially after the failure of lifestyle modifications. Pharmacotherapy which is an established management tool in adults is very infrequently used in children. Only two medications, Phentermine and Orlistat are approved by the Food and Drug Administration (FDA)for use in adolescent obesity. Herein, we discuss the current landscape and available literature on the use of anti-obesity pharmacotherapy in children.
Recent findings:
There are emerging pediatric data about the efficacy of the many weight loss medications which are FDA approved in adults. Moreover, more clinical trials are underway on the rarer, intractable forms of obesity such as monogenic, syndromic and hypothalamic obesity.
Summary:
Weight loss medications in children, like adults, have variable efficacy and similar side effect profile. Rigorous research and improved education of providers about weight loss medications may address the huge treatment gap in severe pediatric obesity.
Keywords: Pediatric obesity, Severe obesity, Pharmacotherapy, off label medications
Introduction
Pediatric obesity is an exponentially growing global public health concern. In the United States (US), the prevalence of obesity among children aged 2–19 years has more than tripled over the last four decades from 5% in 1978 to 18.5% in 20161. Almost 1 in 5 youth is afflicted with obesity2 and 9.5% of adolescents have severe obesity3. As the prevalence and severity of childhood obesity increases, associated acute and chronic comorbidities, previously considered to be adult diseases: type 2 diabetes mellitus (T2DM), nonalcoholic fatty liver disease, metabolic syndrome, cardiovascular disease and obstructive sleep apnea, are more prevalent among children and commonly encountered in routine clinical practice. Children with obesity face significant social stigma predisposing them to serious negative consequences on their emotional and mental health and school performance4,5 . Overall, obesity in childhood is a robust predictor of obesity in adulthood, and consequences may persist even if the excess childhood weight is lost6,7 . Pediatric obesity has lifelong effects on patients, their families and health care systems and warrants appropriate treatment.
With these massive negative effects, it is essential to understand and utilize the treatment approaches available to treat the disease early in its course and to prevent worsening health. A stepwise approach is imperative to treat obesity in childhood. Lifestyle modifications including behavioral change, increased physical activity, and a balanced diet remain the first line and fundamental component of treatment throughout. Studies assessing these interventions have shown only modest persistent effects on BMI in pediatric patients. Separate Cochrane reviews found weak evidence that lifestyle modification dedicated to food, physical activity, and other behavior modifications reduce BMI in children and adolescents. Across 70 randomized controlled trials (RCT) in children with overweight and obesity aged 6 to 11 years, with duration of follow up ranging from six months to three years, BMI and weight decreased on average by 0.06 kg/m2 and 1.45 kg , respectively in the intervention groups compared with the control groups8 .Similarly, across 44 RCTs in participants aged 12–17 years followed for six to twenty four months, the mean change in BMI z score was 0.13 units with a weight loss of 3.67 kg in lifestyle intervention group as compared with the controls9.
There is a vast body of literature demonstrating the tremendous benefit of weight loss during early years10,11. Unfortunately, lifestyle modification alone is modestly effective in severe obesity. While bariatric surgery guidelines are available for pediatric patients with severe obesity, guidelines on the use of pharmacotherapy for patients who fail lifestyle modifications and are not candidates or are hesitant to get bariatric surgery are lacking12. Furthermore, the use of pharmacotherapy for pediatric obesity is currently limited and only two medications are Food and Drug Administration (FDA) approved. This leads to frequent off label use of medications which are approved in the adults only.
Henceforth, we provide a summary of literature of the FDA approved and non-approved medications (off label) used in pediatric obesity (Table 1). We also discuss the relative efficacy of some of the commonly used medications13–17(Fig 1).
Table 1:
Summary of medications used for the treatment of pediatric obesity.
| Medication | Mechanism | Side effects | Contraindications | Efficacy from Pediatric data (different measures of weight change reported) |
|---|---|---|---|---|
| Orlistat13,18,19,78 |
|
GI symptoms -abdominal pain, oily stools and spotting, fecal urgency & incontinence, flatus, fat soluble vitamin deficiency | Chronic malabsorption, cholestasis, pregnancy | −2.61-kg placebo-subtracted weight loss after 1 year of treatment. |
| Phentermine14,22,28 |
|
Irritability, insomnia, dry mouth, dizziness, tremor, headache, HR and BP elevation. GI symptoms- abdominal pain, diarrhea, constipation, nausea | Cardiovascular disease, hyperthyroidism, glaucoma, co-use of monoamine oxidase inhibitors | 4.1% reduction in BMI and 3.2 kg reduction in weight at 6-months with phentermine and lifestyle modification compared to lifestyle modification only |
| *Topiramate15,26,29,79 |
|
Cognitive dysfunction, paresthesia, nephrolithiasis, metabolic acidosis | Pregnancy (teratogen), acute myopia and secondary angleclosure glaucoma | 2 – 4.9% BMI reduction with 75 mg of topiramate for 6 months |
| *Bupropion/Naltrexone24,32,33,80 |
|
Headache, dizziness, vomiting, constipation, dry mouth. irritability, dizziness, insomnia, headaches, anxiety, fatigue and tremor | A black box warning of increased suicidal risk and ideation in young adults | Data is not available for < 18 years |
| *Metformin26,36,78 |
|
Mostly GI symptoms- bloating, flatus, diarrhea, usually well tolerated | Severe Hepatic/Renal Disease | BMI Z score reduction of 0.1 and BMI reduction of 0.86 compared to placebo |
| *Lis dexamphetamine40,41 |
|
Diarrhea, dizziness, dry mouth, irritability, insomnia, nausea, abdominal pain, vomiting, HR and BP elevation | Cardiovascular diseases Psychiatric adverse reactions Serotonin syndrome with use of serotonergic agent. | 0.24–0.51-point reduction in BMI z score (dose dependent) |
|
*GLP-1 agonists17,26,47–49,55,57–59,81,82 Exenatide Liraglutide |
|
GI symptoms- nausea, vomiting, diarrhea Hypoglycemia risk in those on insulinotropic medications A small risk of cholelithiasis and pancreatitis |
Pregnancy, personal or family history of medullary thyroid carcinoma or type 2 multiple endocrine neoplasia | Absolute BMI reduction of 3.42% with exenatide for 3–6 months BMI SDS reduction of 0.23 with 56 weeks of Liraglutide vs placebo |
GI, gastrointestinal; HR, heart rate; BP, blood pressure; BMI, body mass index; GABA, gamma-Aminobutyric acid; CNS, central nervous system; GLP-1, glucagon-like peptide 1; SDS, standard deviation score;
Non-FDA- approved medications for indication of weight loss (off label use)
Fig 1: BMI SDS/BMI Z score change by weight loss medication.
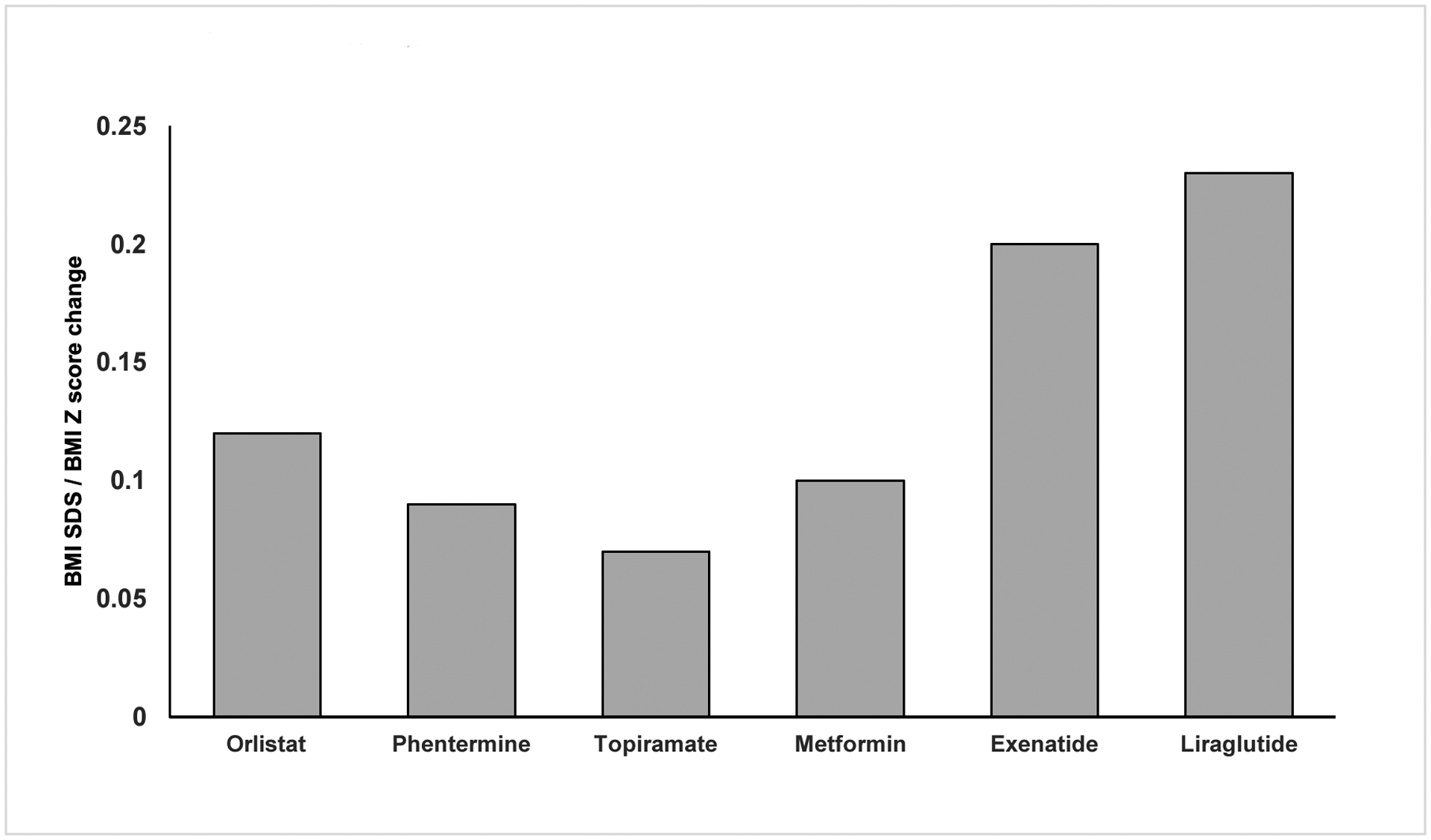
A BMI SDS change from representative controlled trials is reported for Orlistat, Metformin, Exenatide, Liraglutide. A BMI Z score change from representative studies is reported for Phentermine, Topiramate.10–14
Discussion
Discussion of pharmacotherapy with the patient and family needs complete disclosure of the dearth of long-term data and the need of chronic usage (per adult data). This should be done under close monitoring with continued focus on lifestyle interventions and finding the lowest effective dose of medications.
A. FDA-Approved Anti-Obesity Medications in Pediatrics (Table1)
Only two medications, Orlistat and Phentermine are currently approved for weight loss in adolescents with obesity.
1. Orlistat -
Orlistat is the lone medication for long-term management of obesity for adolescents ≥12 years that is FDA approved. It inhibits the pancreatic and gastric lipase and decreases lipid absorption. The usual prescribed dose is 120 mg three times a day with meals. Most of the time its use is limited by the associated gastrointestinal side effects such as oily stools,abdominal pain, fecal urgency and incontinence, flatus as well as deficiency of fat-soluble vitamins18,19. Contraindications include chronic malabsorption, cholestasis and pregnancy20.
2. Phentermine -
Phentermine is approved in the Unites States for adolescents>16 years for a short duration of up to 12 weeks. It reduces the reuptake of norepinephrine (NE) thereby stimulating the pro-opiomelanocortin (POMC) neurons in the hypothalamus21 and also affects serotonin and dopamine reuptake , which in the pre-frontal cortex improves inhibitory control of appetite22,23 (Fig 2) .The usual prescribed dosage is either 15 mg, 30 mg or 37.5 mg daily24 . The most common side effects are irritability, insomnia, mood alteration, dry mouth, dizziness, tremor, headache, heart rate and blood pressure elevation and gastrointestinal side effects14,25. Contraindications include history of past or uncontrolled cardiovascular disease, hyperthyroidism, glaucoma and current use of monoamine oxidase inhibitors22,26 (Table 1).
Figure 2: Sites and mechanism of action of various anti-obesity medications. (Original representation).
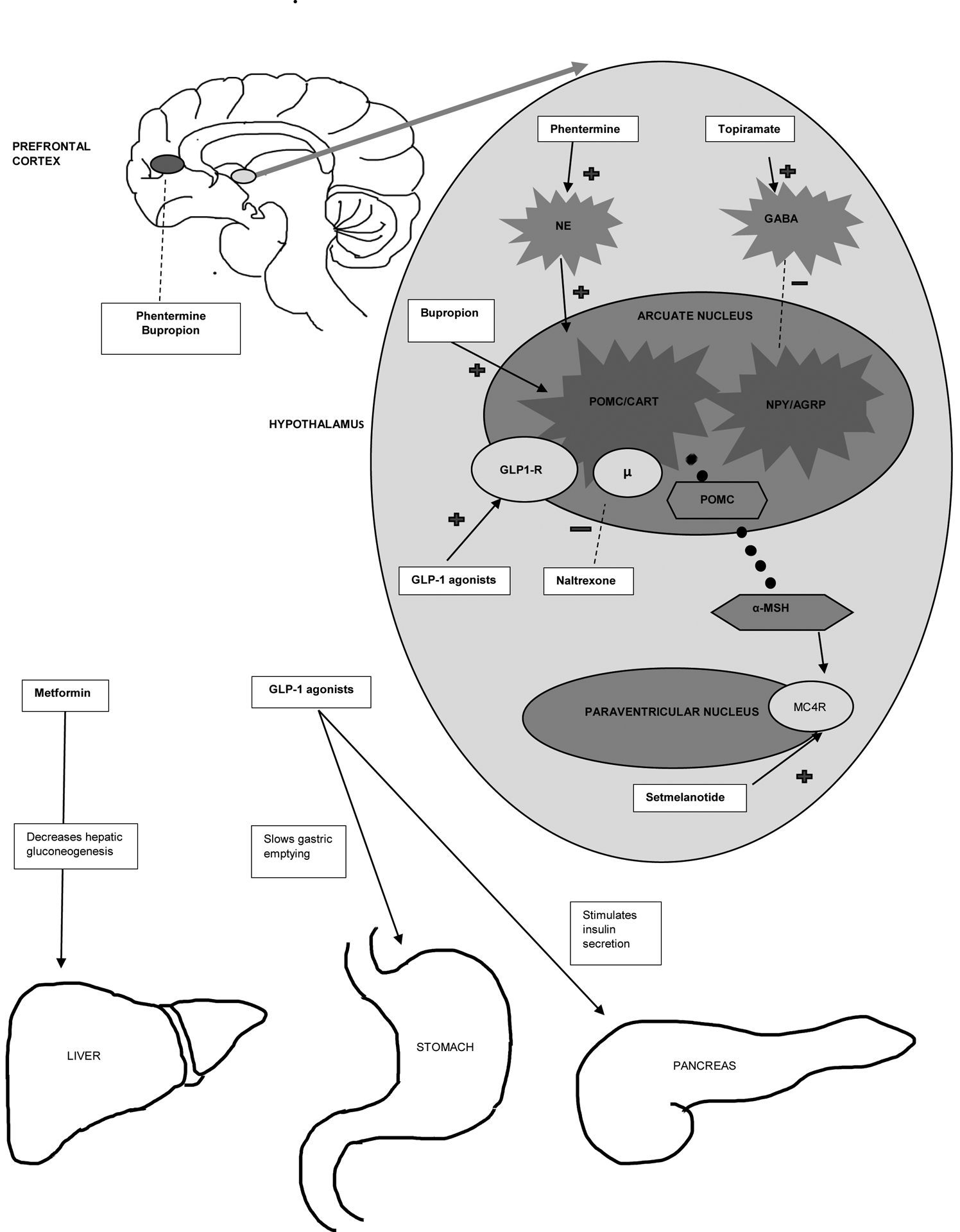
The hypothalamic region of the brain is the primary site of action of many anti-obesity medications. The arcuate nucleus of the hypothalamus harbors the proopiomelanocortin (POMC)/cocaine and amphetamine regulated transcript (CART) neurons as well as the agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons. Activation of POMC/CART results in decrease food intake and increased resting energy expenditure (REE)and activation of AgRP/NPY results in is orexigenic effects. The main product of POMC/CART neuron activation is POMC. POMC undergoes proteolytic cleavage to produce many active peptides. Alpha melanocyte stimulating hormone (α-MSH), a product of POMC acts as a ligand to Melanocortin 4 receptor (MC4R) located on the neurons of the paraventricular nucleus of the hypothalamus. Activation of the melanocortin pathway leads to increased REE and decrease in food intake. Various medications exert the weight loss effect by central and/or peripheral mechanisms as illustrated in the figure.
NE: Nor epinephrine; GABA, gamma-Aminobutyric acid; GLP-1, glucagon-like peptide
B. Non-FDA-Approved Anti-Obesity Medications in Pediatrics (Table1)
The lack of many FDA approved medications necessitates the off-label use of medications commonly used in adult obesity.
1. Topiramate
Topiramate is FDA approved for the treatment of epilepsy in ≥2 years of age and for migraine prophylaxis in ≥12 years of age and in combination with phentermine for obesity in ≥18 years of age. Topiramate blocks neuronal sodium channels, antagonizes glutamate receptors, inhibits carbonic anhydrase and is thought to suppress appetite via augmentation of the gamma-Aminobutyric acid (GABA)activity15 (Fig 2). Side effects of Topiramate include reversible cognitive dysfunction, metabolic acidosis, nephrocalcinosis and paresthesia15. The recommended doses range from 25 mg to 100 mg. Topiramate is a teratogen and can cause orofacial defects in the fetus. It might also decrease the efficacy of oral contraceptives (less likely at the commonly used dose of <200 mg). Adolescents should be counseled on using other methods of contraception and serial pregnancy testing is recommended22,26.
2. Phentermine/topiramate extended release (ER)
Phentermine/topiramate extended release (ER) combination is FDA approved for chronic weight management in adults. It was found to achieve greater weight loss than topiramate and phentermine monotherapy27. Side effects are similar to those seen when each compound is used alone and are dose dependent28,29.
For monitoring, heart rate, blood pressure, electrolytes and creatinine should be assessed in the beginning of the treatment and periodically while on treatment, especially during dose adjustment30.
3. Bupropion/ Naltrexone -
Bupropion is a selective reuptake inhibitor of dopamine and noradrenaline (Fig 2), used in depression and smoking cessation treatment while Naltrexone is an opioid receptor antagonist used in opioid use disorder in adults31. The combination is approved for obesity treatment in adults. Bupropion/ Naltrexone carries a black box warning regarding increased suicidal risk and ideation in young adults and hence requires careful monitoring. Bupropion monotherapy for adolescents with depression was associated with side effects including irritability, dizziness, insomnia, headaches, nausea/vomiting, decreased appetite, worsening anxiety, headaches, fatigue and tremor32. Usual side effects associated with Naltrexone are nausea, vomiting, headache, dizziness, insomnia33–35 (Table 1).
4. Metformin
Metformin is FDA approved for treatment of Type 2 Diabetes Mellitus > 10 years. Metformin inhibits hepatic gluconeogenesis (Fig 2) and enhances insulin-mediated glucose consumption in peripheral tissues (such as muscle and liver)36. Mechanism for its weight loss effects are largely unknown. Recommended dose ranges between 500 mg-2000 mg divided twice daily. Common adverse events are gastrointestinal in nature- bloating, flatus, diarrhea16. Metformin-associated lactic acidosis is rare but serious concern37.
5. Lis dexamphetamine
Lis dexamphetamine is a stimulant medication, FDA approved for children with ADHD≥ 6 years and for binge eating disorder in adults. It decreases dopamine and noradrenaline reuptake in the nucleus accumbens, thus decreasing the hedonic/reward-based eating behaviors38. Increase in blood pressure and heart rate and worsening of psychiatric disorders may occur with its use39–41 . In children and adolescents with cardiac abnormalities, sudden death has been reported42,43.
6. Glucagon-like peptide1 (GLP-1) analogues:
Exenatide/Dulaglutide/Liraglutide/Semaglutide:
GLP-1 receptor agonists are incretins which enhance insulin secretion and increase satiety by slowing gastric emptying as well as by effect on the arcuate nucleus of the hypothalamus, limbic/reward system in amygdala and the cortex44–46(Fig 2) . Adverse effects are primarily gastrointestinal-nausea, vomiting, and diarrhea47–49. Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or type 2 multiple endocrine neoplasia50.
The different GLP-1 agonists differ in their duration of action and efficacy to the receptors51. Liraglutide, due its slower degradation allows for once daily dosing and is FDA approved for obesity treatment in adults and T2DM in both adults and adolescents52. The initial recommended dose is 0.6 mg injected subcutaneously and gradually augmented in increments of 0.6 mg weekly to the maximum dose of 3.0 mg. This slow increase helps to minimize the associated gastrointestinal side effects52.
Semaglutide-
Semaglutide the newest GLP-1 agonist which demonstrated promising results in adults with obesity. It is a long acting GLP-1 receptor agonist with decreased degradation by dipeptidyl peptidase (DPP-4), allowing once weekly dosing53. There are ongoing clinical trials with weekly Semaglutide in adolescents with obesity (NCT04102189).
C. Pharmacotherapy for syndromic obesity
Syndromic obesity is characterized by a wide variety of features involving various organ systems such as intellectual disability, developmental delay, dysmorphic features, retinal changes and deafness. Obesity is typically early onset in nature and associated with hyperphagia. Over 25 syndromic forms of obesity have been identified.54,55. Prader-Willi syndrome (PWS) is the most common of these55. Others includes Bardet-Biedl syndrome (BBS), Alström syndrome, Albright hereditary osteodystrophy (pseudohypoparathyroidism type 1A) and more. Details of syndromic obesity are beyond the scope of this review.
Unfortunately, obesity management in these syndromes is challenging. Lifestyle based therapy are crucial in order to drop the risk of obesity comorbidities. Currently, there are no approved anti-obesity pharmacotherapy available for syndromic obesity, but studies are ongoing. GLP-1 agonists may have some beneficial role in PWS, but data are evolving. A small, single blinded crossover study in patients with PWS receiving a single 10-μg exenatide injection or placebo demonstrated increased satiety and lowered glucose level56 . One year of exenatide and liraglutide use in young females with PWS demonstrated marked reduction in BMI as well as food consumption and decreased ghrelin levels33,57,58. On the other hand, in a six-month, open-label, nonrandomized longitudinal study of patients with PWS receiving exenatide, weight and adiposity were unaffected even though hunger scores and hemoglobin A1c reduced after treatment.59
Oxytocin (OXT) is another compound under investigation. Oxytocin is produced by the hypothalamus and regulates food consumption via hedonic and homeostatic pathways and is administered intranasally. In adults, oxytocin decreases the total caloric and fat intake while increasing fat utilization and causing weight loss.60–62 In children, it has been mainly studied in PWS subjects has shown promising effects on food related behaviors and reduction in appetite drive63–65.
D. Pharmacotherapy for monogenic obesity
Monogenic obesity is caused by single-gene defects in which early onset obesity and hyperphagia are the key characteristics. Loss-of-function mutations in genes involved in the leptin-melanocortin pathway (pathway of homeostatic energy regulation) (Fig 2), are implicated in rare forms of monogenic obesity36. Metreleptin, the recombinant form of leptin produced extreme weight loss, significant improvement in hyperphagia , decrease in hunger scores and resolution of metabolic consequences when administered to patients with congenital leptin deficiency66,67.
Setmelanotide (RM-493), a synthetic melanocortin −4-receptor (MC4R) agonist (Fig 2), is being tested for the treatment of monogenic obesity. In a small open-label study in individuals with leptin receptor (LepR) deficiency and POMC deficiency, Setmelanotide led to reductions in body weight and decreased hunger scores68,69. Described adverse events included dry mouth, localized skin induration at injection sites and darkening of skin nevi68.
E. Pharmacotherapy for Hypothalamic Obesity (HO)
Hypothalamic Obesity is another severe form of obesity, mostly seen after craniopharyngioma resection or with other diseases affecting the medial hypothalamic region. It is characterized by exponential weight gain leading to severe obesity, voracious appetite and poor satiety, decreased resting energy expenditure and fatigue. Standardized and effective treatments are lacking for HO.
The efficacy of improvement of satiety by GLP-1 agonists is being evaluated in HO70. In a small prospective study of adults with HO, treated with exenatide for 52 weeks, subjects were noted to have decreased food intake and 75% of them had stable or decreasing trends in weight71. Several case reports and small studies also support the efficacy of Liraglutide72,73. A retrospective study of exenatide or liraglutide treatment in HO patients for up to 51 months, demonstrated a reduced BMI from 37.6 kg/m2 to 33.4 kg/m274 .
Dextroamphetamine, an adrenergic agonist may mitigate weight gain through central anorexigenic effects. A case series of 7 adolescent and young adult patients with HO treated with dextroamphetamine showed a reduction of 0.18 units in BMI z-score during the first year of treatment. However, the weight loss effects of treatment during the second year were heterogenous ,where some patients continued to lose weight and others showed significant increase in the BMI z score , making the long-term efficacy of this treatment suspicious.75
Diazoxide and Metformin in combination has also been evaluated in a small prospective study for 6 months in pediatric patients with HO. The decrease in the BMI standard deviation score (SDS) was significantly greater with treatment as compared with that of the pre-study period with lifestyle intervention alone (−0.04 ± 0.15 versus +0.11 ± 0.08)76. In another RCT of pediatric patients randomized to either diazoxide or placebo for 2 months, BMI was not significantly different between the groups77. Hence larger studies with longer follow up are required to establish the true efficacy of these medications.
Conclusion
Obesity, especially severe obesity, in the pediatric population is increasing. Large studies have demonstrated that lifestyle interventions alone only have modest benefits in reversing the trends of severe obesity. While lifestyle modification and physical activity remain the cornerstone for treating and managing obesity, pharmacologic interventions should be considered to slow the weight gain and decrease the risk of complications, particularly in children who fail to lose weight on lifestyle modifications and demonstrate new or worsening comorbidities. While there are many anti-obesity medications approved for adults, only one medication is approved for long-term use in the pediatric population and another one for short term use only. Although there are medications in the pipeline, further high-quality research in pediatric anti-obesity pharmacotherapy with subsequent FDA approval of more medications is critical to address this huge treatment gap.
KEY POINTS:
Severe obesity is escalating, and lifestyle-based therapies have modest effects in changing the trajectory of the disease.
Treatment of obesity needs additional and more efficacious treatment options which should be tailored based on the age of the patient, severity of disease and associated comorbidities.
Anti-obesity pharmacotherapy should be utilized in patients who have failed attempts to weight loss after lifestyle modifications.
Syndromic, monogenic and hypothalamic obesity are other severe forms of obesity which remain recalcitrant to standard treatment options.
Financial support and sponsorship:
None
Dr. Sonali Malhotra is on the speaker’s bureau for Rhythm pharmaceuticals.
Dr. Aluma Chovel Sella and Dr. Vibha Singhal report no disclosures.
Dr Aluma Chovel Sella received funding from the NIH- T32DK007028
Dr. Singhal received funding from the NIH - K23DK110419
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Anderson PM, Butcher KF, Schanzenbach DW. Understanding recent trends in childhood obesity in the United States. Econ Hum Biol. 2019;34:16–25. doi: 10.1016/j.ehb.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017;(288):1–8. [PubMed] [Google Scholar]
- 3.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141(3). doi: 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Haye K, Dijkstra JK, Lubbers MJ, van Rijsewijk L, Stolk R. The dual role of friendship and antipathy relations in the marginalization of overweight children in their peer networks: The TRAILS Study. PLoS ONE. 2017;12(6):e0178130. doi: 10.1371/journal.pone.0178130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rankin J, Matthews L, Cobley S, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolescent Health, Medicine and Therapeutics. 2016;7:125–146. doi: 10.2147/AHMT.S101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz WH. Childhood weight affects adult morbidity and mortality. J Nutr. 1998;128(2 Suppl):411S–414S. doi: 10.1093/jn/128.2.411S [DOI] [PubMed] [Google Scholar]
- 7.*.Ryder JR, Jacobs DR, Sinaiko AR, Kornblum AP, Steinberger J. Longitudinal Changes in Weight Status from Childhood and Adolescence to Adulthood. J Pediatr. 2019;214:187–192.e2. doi: 10.1016/j.jpeds.2019.07.035 [DOI] [PubMed] [Google Scholar]; This study shows the high risk for adult obesity in children and adolescents who have obesity This emphasizes the importance of early intervention.
- 8.Mead E, Brown T, Rees K, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6:CD012651. doi: 10.1002/14651858.CD012651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al‐Khudairy L, Loveman E, Colquitt JL, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst Rev. 2017;2017(6). doi: 10.1002/14651858.CD012691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EY, Yoon K-H. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med. 2018;12(6):658–666. doi: 10.1007/s11684-018-0640-1 [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt AB, Stein RI, Saelens BE, Theim KR, Epstein LH, Wilfley DE. Importance of early weight change in a pediatric weight management trial. Pediatrics. 2011;128(1):e33–39. doi: 10.1542/peds.2010-2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong SC, Bolling CF, Michalsky MP, Reichard KW, Section on Obesity S on S. Pediatric Metabolic and Bariatric Surgery: Evidence, Barriers, and Best Practices. Pediatrics. 2019;144(6). doi: 10.1542/peds.2019-3223 [DOI] [PubMed] [Google Scholar]
- 13.Safety Yanovski J. and efficacy of Xenical in children and adolescents with obesity-related diseases [NCT00001723]. ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT00001723. 2012. Accessed February 2, 2016. ClinicalTrials.gov website https://clinicaltrials.gov/ct2/show/NCT00001723. Published online February 2, 2016. [Google Scholar]
- 14.Ryder JR, Kaizer A, Rudser KD, Gross A, Kelly AS, Fox CK. Effect of phentermine on weight reduction in a pediatric weight management clinic. Int J Obes (Lond). 2017;41(1):90–93. doi: 10.1038/ijo.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CK, Marlatt KL, Rudser KD, Kelly AS. Topiramate for weight reduction in adolescents with severe obesity. Clin Pediatr (Phila). 2015;54(1):19–24. doi: 10.1177/0009922814542481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427–2444. doi: 10.1001/jama.2017.0332 [DOI] [PubMed] [Google Scholar]
- 17.*.Weghuber D, Forslund A, Ahlström H, et al. A 6-month randomized, double-blind, placebo-controlled trial of weekly exenatide in adolescents with obesity. Pediatr Obes. 2020;15(7):e12624. doi: 10.1111/ijpo.12624 [DOI] [PubMed] [Google Scholar]; First study to examine the efficacy, safety and tolerability of extended release exenatide in adolescents with obesity. study found a modest effect on BMI and other beneficial effects on glucose and cholesterol.
- 18.Maahs D, de Serna DG, Kolotkin RL, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12(1):18–28. doi: 10.4158/EP.12.1.18 [DOI] [PubMed] [Google Scholar]
- 19.Chanoine J-P, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873–2883. doi: 10.1001/jama.293.23.2873 [DOI] [PubMed] [Google Scholar]
- 20.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155 [DOI] [PubMed] [Google Scholar]
- 21.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. doi: 10.1210/er.2006-0034 [DOI] [PubMed] [Google Scholar]
- 22.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14(1):12–24. doi: 10.1038/nrendo.2017.122 [DOI] [PubMed] [Google Scholar]
- 23.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi: 10.1210/jc.2014-3415 [DOI] [PubMed] [Google Scholar]
- 24.Woodard K, Louque L, Hsia DS. Medications for the treatment of obesity in adolescents. Ther Adv Endocrinol Metab. 2020;11:2042018820918789. doi: 10.1177/2042018820918789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond). 2009;33(8):857–865. doi: 10.1038/ijo.2009.124 [DOI] [PubMed] [Google Scholar]
- 26.Srivastava G, Fox CK, Kelly AS, et al. Clinical Considerations Regarding the Use of Obesity Pharmacotherapy in Adolescents with Obesity. Obesity (Silver Spring). 2019;27(2):190–204. doi: 10.1002/oby.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21(11):2163–2171. doi: 10.1002/oby.20584 [DOI] [PubMed] [Google Scholar]
- 28.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330–342. doi: 10.1038/oby.2011.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5 [DOI] [PubMed] [Google Scholar]
- 30.Narayanaswami V, Dwoskin LP. Obesity: Current and potential pharmacotherapeutics and targets. Pharmacol Ther. 2017;170:116–147. doi: 10.1016/j.pharmthera.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greig SL, Keating GM. Naltrexone ER/Bupropion ER: A Review in Obesity Management. Drugs. 2015;75(11):1269–1280. doi: 10.1007/s40265-015-0427-5 [DOI] [PubMed] [Google Scholar]
- 32.Kweon K, Kim H-W. Effectiveness and Safety of Bupropion in Children and Adolescents with Depressive Disorders: A Retrospective Chart Review. Clin Psychopharmacol Neurosci. 2019;17(4):537–541. doi: 10.9758/cpn.2019.17.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khera R, Murad MH, Chandar AK, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events. JAMA. 2016;315(22):2424–2434. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4 [DOI] [PubMed] [Google Scholar]
- 35.Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of Combined Bupropion and Naltrexone Therapy for Obesity with Monotherapy and Placebo. J Clin Endocrinol Metab. 2009;94(12):4898–4906. doi: 10.1210/jc.2009-1350 [DOI] [PubMed] [Google Scholar]
- 36.Pilitsi E, Farr OM, Polyzos SA, et al. Pharmacotherapy of obesity: Available medications and drugs under investigation. Metab Clin Exp. 2019;92:170–192. doi: 10.1016/j.metabol.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 37.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in Patients With Type 2 Diabetes and Kidney Disease. JAMA. 2014;312(24):2668–2675. doi: 10.1001/jama.2014.15298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1397–1409. doi: 10.1016/j.biopsych.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 39.Frampton JE. Lisdexamfetamine Dimesylate: A Review in Paediatric ADHD. Drugs. 2018;78(10):1025–1036. doi: 10.1007/s40265-018-0936-0 [DOI] [PubMed] [Google Scholar]
- 40.Banaschewski T, Johnson M, Nagy P, et al. Growth and Puberty in a 2-Year Open-Label Study of Lisdexamfetamine Dimesylate in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. CNS Drugs. 2018;32(5):455–467. doi: 10.1007/s40263-018-0514-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichikawa H, Miyajima T, Yamashita Y, Fujiwara M, Fukushi A, Saito K. Long-term study of lisdexamfetamine dimesylate in Japanese children and adolescents with attention-deficit/hyperactivity disorder. Neuropsychopharmacol Rep. 2020;40(1):52–62. doi: 10.1002/npr2.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gould MS, Walsh BT, Munfakh JL, et al. Sudden death and use of stimulant medications in youths. Am J Psychiatry. 2009;166(9):992–1001. doi: 10.1176/appi.ajp.2009.09040472 [DOI] [PubMed] [Google Scholar]
- 43.Anders T, Sharfstein S. ADHD drugs and cardiovascular risk. N Engl J Med. 2006;354(21):2296–2298; author reply 2296–2298. doi: 10.1056/NEJMc061187 [DOI] [PubMed] [Google Scholar]
- 44.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(6):784–793. doi: 10.1038/ijo.2013.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farr OM, Tsoukas MA, Triantafyllou G, et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metab Clin Exp. 2016;65(7):945–953. doi: 10.1016/j.metabol.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlögl H, Kabisch S, Horstmann A, et al. Exenatide-induced reduction in energy intake is associated with increase in hypothalamic connectivity. Diabetes Care. 2013;36(7):1933–1940. doi: 10.2337/dc12-1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.**.Kelly AS, Auerbach P, Barrientos-Perez M, et al. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N Engl J Med. 2020;382(22):2117–2128. doi: 10.1056/NEJMoa1916038 [DOI] [PubMed] [Google Scholar]; This study showed liraglutide is superior to placebo in BMI reduction of adolescents with obesity over a long period of use and follow up.
- 48.Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167(4):355–360. doi: 10.1001/jamapediatrics.2013.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danne T, Biester T, Kapitzke K, et al. Liraglutide in an Adolescent Population with Obesity: A Randomized, Double-Blind, Placebo-Controlled 5-Week Trial to Assess Safety, Tolerability, and Pharmacokinetics of Liraglutide in Adolescents Aged 12–17 Years. J Pediatr. 2017;181:146–153.e3. doi: 10.1016/j.jpeds.2016.10.076 [DOI] [PubMed] [Google Scholar]
- 50.Patel DK, Stanford FC. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med. 2018;130(2):173–182. doi: 10.1080/00325481.2018.1435129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linnebjerg H, Kothare PA, Skrivanek Z, et al. Exenatide: effect of injection time on postprandial glucose in patients with Type 2 diabetes. Diabet Med. 2006;23(3):240–245. doi: 10.1111/j.1464-5491.2006.01800.x [DOI] [PubMed] [Google Scholar]
- 52.1, 2, 1, 3. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes/Metabolism Research & Reviews. 2019;35(1):N.PAG-N.PAG. doi: 10.1002/dmrr.3070 [DOI] [PubMed] [Google Scholar]
- 53.Marbury TC, Flint A, Jacobsen JB, Derving Karsbøl J, Lasseter K. Pharmacokinetics and Tolerability of a Single Dose of Semaglutide, a Human Glucagon-Like Peptide-1 Analog, in Subjects With and Without Renal Impairment. Clin Pharmacokinet. 2017;56(11):1381–1390. doi: 10.1007/s40262-017-0528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung WK. An Overview of Mongenic and Syndromic Obesities in Humans. Pediatr Blood Cancer. 2012;58(1):122–128. doi: 10.1002/pbc.23372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geets E, Meuwissen MEC, Hul WV. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clinical Genetics. 2019;95(1):23–40. doi: 10.1111/cge.13367 [DOI] [PubMed] [Google Scholar]
- 56.Sze L, Purtell L, Jenkins A, et al. Effects of a Single Dose of Exenatide on Appetite, Gut Hormones, and Glucose Homeostasis in Adults with Prader-Willi Syndrome. J Clin Endocrinol Metab. 2011;96(8):E1314–E1319. doi: 10.1210/jc.2011-0038 [DOI] [PubMed] [Google Scholar]
- 57.Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. 2012;59(10):889–894. doi: 10.1507/endocrj.ej12-0074 [DOI] [PubMed] [Google Scholar]
- 58.Kim Y-M, Lee YJ, Kim SY, Cheon CK, Lim HH. Successful rapid weight reduction and the use of liraglutide for morbid obesity in adolescent Prader-Willi syndrome. Ann Pediatr Endocrinol Metab. 2020;25(1):52–56. doi: 10.6065/apem.2020.25.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salehi P, Hsu I, Azen CG, Mittelman SD, Geffner ME, Jeandron D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr Obes. 2017;12(3):221–228. doi: 10.1111/ijpo.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017;13(12):700–709. doi: 10.1038/nrendo.2017.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H, Wu C, Chen Q, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE. 2013;8(5):e61477. doi: 10.1371/journal.pone.0061477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thienel M, Fritsche A, Heinrichs M, et al. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int J Obes (Lond). 2016;40(11):1707–1714. doi: 10.1038/ijo.2016.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller JL, Tamura R, Butler MG, et al. Oxytocin treatment in children with Prader–Willi syndrome: A double-blind, placebo-controlled, crossover study. Am J Med Genet A. 2017;173(5):1243–1250. doi: 10.1002/ajmg.a.38160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuppens RJ, Donze SH, Hokken-Koelega ACS. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin Endocrinol (Oxf). 2016;85(6):979–987. doi: 10.1111/cen.13169 [DOI] [PubMed] [Google Scholar]
- 65.Einfeld SL, Smith E, McGregor IS, et al. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet A. 2014;164A(9):2232–2239. doi: 10.1002/ajmg.a.36653 [DOI] [PubMed] [Google Scholar]
- 66.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–1103. doi: 10.1172/JCI15693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA. 2004;101(13):4531–4536. doi: 10.1073/pnas.0308767101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuhnen P, Clement K, Wiegand S, et al. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. New England Journal of Medicine. 2016;375(3):240–246. doi: 10.1056/NEJMoa1512693 [DOI] [PubMed] [Google Scholar]
- 69.Clément K, Biebermann H, Farooqi IS, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018;24(5):551–555. doi: 10.1038/s41591-018-0015-9 [DOI] [PubMed] [Google Scholar]
- 70.van Iersel L, Brokke KE, Adan RAH, Bulthuis LCM, van den Akker ELT, van Santen HM. Pathophysiology and Individualized Treatment of Hypothalamic Obesity Following Craniopharyngioma and Other Suprasellar Tumors: A Systematic Review. Endocr Rev. 2019;40(1):193–235. doi: 10.1210/er.2018-00017 [DOI] [PubMed] [Google Scholar]
- 71.Lomenick JP, Buchowski MS, Shoemaker AH. A 52-week pilot study of the effects of exenatide on body weight and energy balance in patients with hypothalamic obesity. Obesity (Silver Spring). 2016;24(6):1222–1225. doi: 10.1002/oby.21493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ando T, Haraguchi A, Matsunaga T, et al. Liraglutide as a Potentially Useful Agent for Regulating Appetite in Diabetic Patients with Hypothalamic Hyperphagia and Obesity. Intern Med. 2014;53(16):1791–1795. doi: 10.2169/internalmedicine.53.1646 [DOI] [PubMed] [Google Scholar]
- 73.Ashraf S, Nadkarni P, Bansal N, Stred SE. Liraglutide for the treatment of hypothalamic obesity. AACE Clinical Case Reports. 2018;4(4):e342–e345. doi: 10.4158/ACCR-2018-0009 [DOI] [Google Scholar]
- 74.Zoicas F, Droste M, Mayr B, Buchfelder M, Schöfl C. GLP-1 analogues as a new treatment option for hypothalamic obesity in adults: report of nine cases. Eur J Endocrinol. 2013;168(5):699–706. doi: 10.1530/EJE-12-0997 [DOI] [PubMed] [Google Scholar]
- 75.Denzer C, Denzer F, Lennerz BS, Vollbach H, Lustig RH, Wabitsch M. Treatment of Hypothalamic Obesity with Dextroamphetamine: A Case Series. Obes Facts. 2019;12(1):91–102. doi: 10.1159/000495851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamilton JK, Conwell LS, Syme C, Ahmet A, Jeffery A, Daneman D. Hypothalamic Obesity following Craniopharyngioma Surgery: Results of a Pilot Trial of Combined Diazoxide and Metformin Therapy. Int J Pediatr Endocrinol. 2011;2011:417949. doi: 10.1155/2011/417949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brauner R, Serreau R, Souberbielle J-C, et al. Diazoxide in Children With Obesity After Hypothalamic-Pituitary Lesions: A Randomized, Placebo-Controlled Trial. J Clin Endocrinol Metab. 2016;101(12):4825–4833. doi: 10.1210/jc.2016-2126 [DOI] [PubMed] [Google Scholar]
- 78.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427–2444. doi: 10.1001/jama.2017.0332 [DOI] [PubMed] [Google Scholar]
- 79.Fox CK, Kaizer AM, Rudser KD, et al. Meal-Replacements followed by Topiramate for the Treatment of Adolescent Severe Obesity: A Pilot Randomized Controlled Trial. Obesity (Silver Spring). 2016;24(12):2553–2561. doi: 10.1002/oby.21633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial. Obes Res. 2002;10(7):633–641. doi: 10.1038/oby.2002.86 [DOI] [PubMed] [Google Scholar]
- 81.Kelly AS, Metzig AM, Rudser KD, et al. Exenatide as a Weight-Loss Therapy in Extreme Pediatric Obesity A Randomized, Controlled Pilot Study. Obesity (Silver Spring). 2012;20(2):364–370. doi: 10.1038/oby.2011.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nathan BM, Rudser KD, Abuzzahab MJ, et al. Predictors of weight-loss response with glucagon-like peptide-1 receptor agonist treatment among adolescents with severe obesity. Clin Obes. 2016;6(1):73–78. doi: 10.1111/cob.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]


