Abstract
Clinically efficacious medication in anticancer therapy has been successfully designed with liposome-based nanomedicine. The liposomal formulation in cancer drug delivery can be facilitated with a functionalized peptide that mediates the specific drug delivery opportunities with increased drug penetrability, specific accumulation in the targeted site, and enhanced therapeutic efficacy. This review aims to focus on recent advances in peptide-functionalized liposomal formulation techniques in cancer diagnosis and treatment regarding recently published literature. It also will highlight different aspects of novel liposomal formulation techniques that incorporate surface functionalization with peptides for better anticancer effect and current challenges in peptide-functionalized liposomal drug formulation.
Keywords: Peptide, liposome, cancer, nanotechnology, bio-nanoparticle, active targeting, peptide-functionalized liposome, diagnostic liposomes
1. Introduction
Globally, cancer is a major public health concern; in the United States, it is the second-leading cause of death [1]. Cancer treatment with chemotherapeutic agents usually causes serious, undesired side-effects due to the harmful effects of chemotherapeutics on multiplying cells from normal, noncancerous tissues. Inefficient delivery is the leading cause of decreased drug effectiveness as well as serious adverse effects on non-targeted organs [2]. Therefore, researchers are now focusing on developing novel advanced drug delivery systems to improve the therapeutic index of chemotherapeutic agents by enhancing drug delivery and enabling the drug to reach a specific site of action with the total loaded amount [3, 4]. Progress in nanomedicine has enabled the design of nanoparticles consisting of different organic or synthetic materials for the diagnosis and treatment of cancer [5].
Liposomes are nanoparticles that are capable of introducing both lipid-soluble and water-soluble drugs [6]. This encapsulation protects the drug from rapid degradation and reduces drug toxicity by making it unavailable to the systemic circulation. Liposomes can also improve the therapeutic index of a new or established drug by changing the drug’s pharmacokinetic properties such as absorption and metabolism, increasing its biological half-life, or reducing its elimination. It has a hydrophilic exterior/interior and a hydrophobic layer that can enable it to incorporate various drugs and facilitate delivery to the desired site. To utilize this specialized vehicle, several anticancer drugs (including marketed) have been formulated to have special targeted delivery options.
Liposome surfaces can be functionalized using various methods to provide different beneficial properties for target-mediated cancer therapy, including increasing the stability of the entrapped compound while increasing the selectivity towards cancer cells. Liposomes can have specialized properties by virtue of their outer layer properties. These can achieve specific selectivity toward targeted cancer cell types, including specialized stimuli characteristics. Currently, various liposomal formulations are being developed, comprising various therapeutics compounds together with surface functionalization with active peptides to have specific delivery characteristics while maintaining their stealth property. Peptides as targeted agents enable the liposomal formulation technology to produce selective delivery characteristics in both treatment and diagnostic regimes while maintaining the biological properties of the peptide.
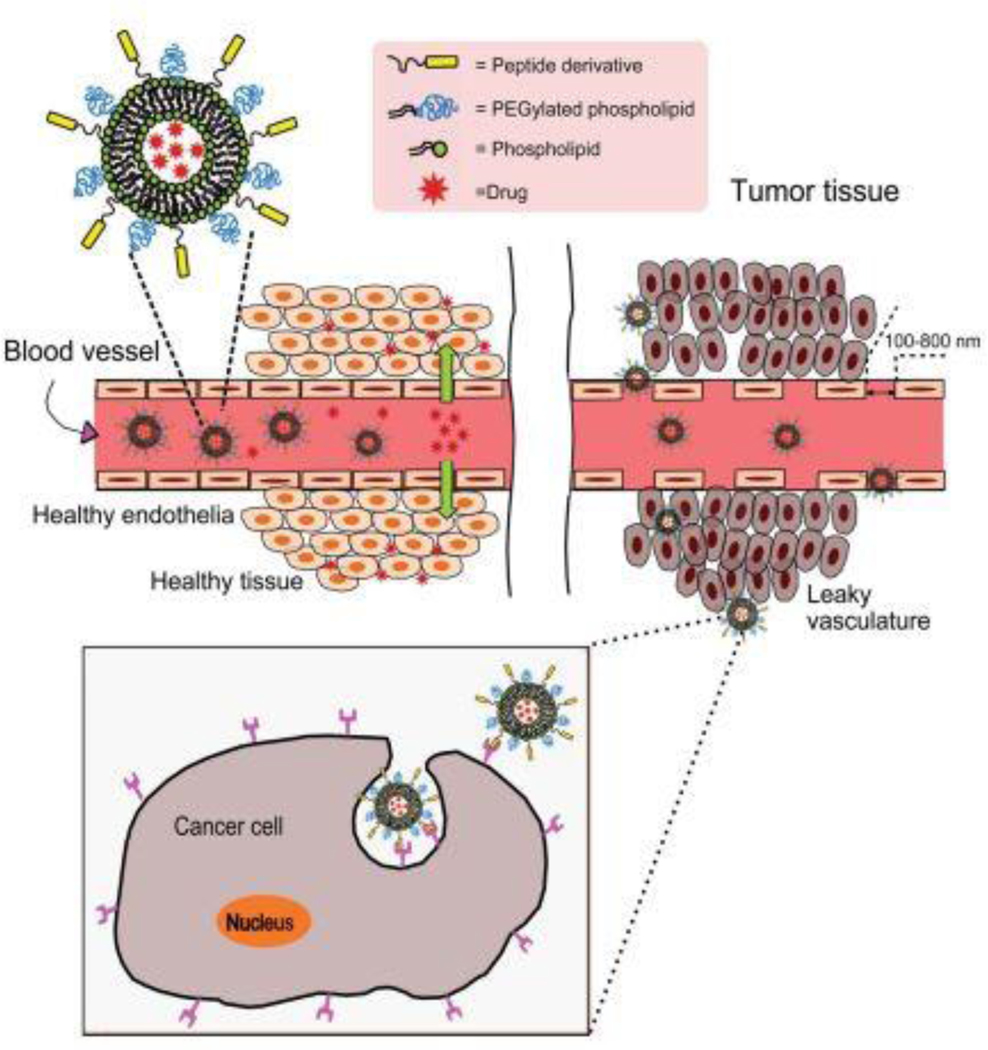
At present, liposomes have become one of the most popular delivery vehicles as an experimental model and commercially as a drug delivery system as they have low toxicity, biodegradability, biocompatibility, entrapment capacity of both hydrophilic and lipophilic drugs [7], and ease of target-specific drug delivery to malignant tissues [8]. Targeting particular cells and reduction of drug toxicity in the liposomal formulation has encouraged researchers to perform numerous studies focusing on liposome-based drug delivery [9–11]. The solubility of poorly water-soluble antitumor drugs can be increased through liposomes to improve existing cancer treatment regimens. Additionally, long-circulating liposomes can decrease the uptake by mononuclear phagocyte systems (MPS), which eventually increases a passive accumulation into the tumor area. Attaching targeted ligands (e.g., peptides) to the liposomal surface can also help to achieve active-tumoral targeting [12, 13]. The size of the nanoparticles has an effect on passive accumulation and effective retention on the targeted site of action (Fig. 1). These methods lessen drug deterioration and metabolic inactivation of a drug after treatment. Also, the amount of drug delivered and drug’s bioavailability within the affected area increases, thus improving efficacy and/or reducing drug toxicity [14].
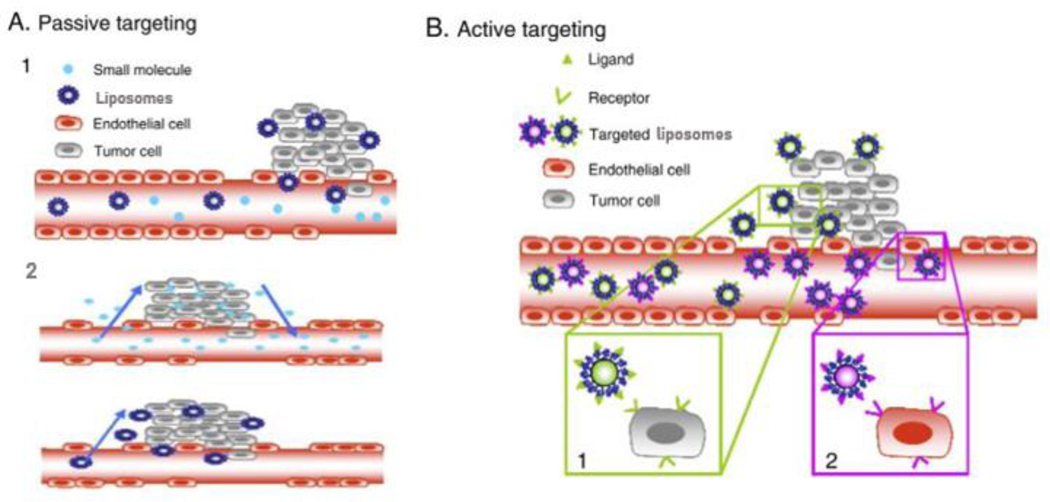
Fig 1.
A. Passive targeting of liposomes. (1) Selective accumulation of liposomes through leaky vasculature surrounding the tumors. (2) Size-dependent retention of liposomes in the tumor tissue. Drugs alone can diffuse back to the bloodstream, reducing drug concentration in the targeted site, while drug-loaded liposomes stay concentrated in the targeted site due to its particle size. B. Active targeting strategies. Targeting peptides grafted at the surface of liposomes bind to receptors (over)expressed by (1) cancer cells or (2) angiogenic endothelial cells (adapted with permission from [15]).
Peptide-functionalized liposomes that are tagged with imaging agents can be used to selectively and efficiently deliver diagnostic agents to the targeted site. For diagnostic purposes, peptide liposomes targeting particular receptors [16–18], irradiation-mediated diagnostic imaging with peptide-targeting liposomes [19], and peptide-conjugated theranostic liposomes possessing both therapeutic and diagnostic effects [20] can be effective methods for the detection of cancers at different stages. Some of these can be used for the development of personalized medicines [21, 22].
This review focuses on the advances in the last decade in liposomal formulation systems functionalized by active peptides for targeting effect containing therapeutic and diagnostic agents in the cancer treatment regime. The active peptides are mainly used in the reported liposomal formulations, either as a targeting agent (with or without biological actions) or cellular penetration facilitator, carrying chemotherapeutic and diagnostic agents into the targeted sites. In different segments we also cover some perspectives regarding peptide as a liposomal targeting agent, different properties of liposomal drug delivery system and specialized peptide-factionalized liposomal drug delivery systems for anticancer therapy. Surprisingly, very few attempts have been made to review peptide-functionalized liposomal systems covering different novel techniques and treatment approaches in anticancer therapy. Some of the previous reviews discussed particular targeting sites or particular treatment regimes with peptide-functionalized liposomal systems [23–25]. The current review comprehensively addresses different aspects of peptide-functionalized liposomal systems in anticancer therapy with recent updates, which we believe will present an overall scenario of novel anticancer approaches with targeted peptide-functionalized liposomal formulation systems and provide formulation scientists with an update on progress in this approach.
1.1. Bio-nanoparticles for targeted therapy
Microparticles and nanoparticles can also be used as an effective strategy for increasing oral bioavailability of peptides and proteins by protecting them from pH variations in different sites and enzymatic degradation from different proteolytic and other enzymes [26]. Macromolecules such as peptides or proteins can be encapsulated inside the polymeric carrier, which will protect them from pH and enzymatic degradation and also facilitate their absorption and control their release into the desired site [27, 28]. Nanoparticles can have sizes ranging from 1 to 1000 nm. However, a particle size around 200 nm is greatly desired for different drug delivery approaches [29, 30]. Nanoparticles can be generally classified as either nanospheres, a matrix system where a drug is evenly spread physically, or nanocapsules, in which the drug is encapsulated in vesicles of a polymeric membrane. The drug release and physicochemical properties of nanoparticles depend on the method of preparation [30, 31]. These can be pursued for targeted delivery of drugs and controlled drug release by changing the polymeric properties and surface chemistry [26]. Since peptides have the ability to bind to receptor proteins in the cells or on the surface of cells, peptides can be incorporated into liposomes and used for targeting or guiding the liposomes to particular target receptors or cells. A review of the literature suggests that peptides are used in liposomal delivery along with other drugs to enhance the effectiveness of the drug for treatment. Hence, a majority of the examples covered in this review are related to peptides that are used for enhancing the delivery of chemotherapeutic drugs and consequently increasing the therapeutic efficacy of drugs using peptide-conjugated liposomal formulations.
2. Formulation strategies for liposome-based drug delivery: peptide mediated receptor targeting for anticancer drug delivery
Liposomes are artificially prepared micro spherical vesicles composed of naturally derived phospholipids [10]. One of the major concerns regarding peptide drug delivery is in vivo stability. Peptides are enzymatically degraded by various protease enzymes along with other enzymes, reducing their bioavailability [26]. Methods are being investigated to improve the plasma stability of peptides to increase their biological effect. Protection for peptides and proteins in the GI tract can be achieved to some extent through a lipid-based bilayer drug delivery system [40]. Encapsulation in the liposomal bilayer of proteins and peptides can prevent their degradation. In peptide-functionalized liposomes, PEGylation of the liposomal surface helps to protect the attached targeting peptide from degradation. Liposomes can also reduce the toxicity of the encapsulated drug by delivering the drug to the specific target site. This feature can be achieved through various processes, and peptide or peptidomimetic incorporation for the target-specific release is becoming a popular method for targeted drug delivery.
The phospholipid bilayer is the main structural feature of conventional liposomes. Currently, liposomal formulations are modified through a different mechanism, such as surface functionalization of the lipid layer. This bilayer has an amphiphilic nature with a hydrophilic polar head and lipophilic backbone. These liposomal nanoparticles have electrostatic potential, which also plays a role in stability as segregated particles can be negatively or positively charged. The overall charge of the liposome depends on the nature of the electrostatic potential of the polar head region. Two of the main lipid components used for liposomal formulation are natural or synthetic double-chain lipids (composed of a glycerol backbone and polar phosphate group); another component is sterols, e.g., cholesterol.
The lipids that are commonly used are either zwitterionic, such as phosphatidylcholine and phosphatidylethanolamine, or negatively charged, e.g., phosphatidylglycerol. Some positively charged lipids (e.g., 1,2-dioleoyl-3-trimethylammoniopropane (DOTAP) and N-[1-(2,3-dioleyloxy)propyl]-N,N,N-triethylammonium (DOTMA)) are commonly used for the delivery of genes due to their interactive property with the negatively charged deoxyribonucleic acid (DNA) [32] and negatively charged cell membrane phospholipid layer.
Degradation pathways for phospholipids in aqueous liposomal dispersions can be divided into two distinct categories–oxidative and hydrolytic degradation. Liposomal oxidative degradation can be prevented by the incorporation of completely synthetic and saturated phospholipids such as 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dipalmitoylsn-glycero-3-phosphocholine (DPPC), and, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC). The level of oxidation of lipids can also be minimized by following some precautions during the preparation and storage of liposomes, which can affect the performance as well as create adverse effects on the phospholipid bilayer. Such initiatives include using freshly purified lipids and distilled solvents, taking care to avoid oxygen in the manufacturing processes, minimizing the production of high heat during various procedures, using nitrogen to deoxygenate the aqueous solutions, incorporation of antioxidant, e.g., α-tocopherol, as a component of the lipid layer, and storing the liposome suspensions in an inert environment [33].
One of the important components of liposomes is cholesterol, which has a modulatory effect on the characteristics of the lipid bilayer of the liposomes. It can maintain the stoutness of the liposomal structure [34] and enhance the compactness of the bilayer by increasing the rigidity between the lipid bilayer structure containing a phospholipid molecule [35]. This structural integrity provides orderly arrangement of the bilayer in the hydrophobic region of the bilayer of the liposome while reducing micro polarity [36], providing lipid bilayer rigidity by reducing the flexibility of the encompassing molecules (particularly water-soluble molecules), and enhancing the micro viscosity of the lipid bilayers through compaction [37]. Cholesterol also plays a role in the morphological stability of liposomal formulation from gastrointestinal environmental stress through its rigidifying property [37]. Liposomal size can also be modulated through the incorporation of cholesterol into the liposome (liposomal size, including shape transition, increases with an increased amount of cholesterol); this incorporates fluidity and permeability, which eventually controls the release of water-soluble compounds from liposomes [38].
Another strategic factor in providing various advantageous properties and reducing some limitations of the liposome is enabling surface functionalization through various active components. To increase the stability of liposomes in the blood with increasing circulation time, polyethylene glycol (PEG) has been used to provide stealth properties in various formulations, including marketed drugs [39]. Other surface-active agents such as peptides, proteins, antibodies, aptamers, ligands, small molecules, or carbohydrates can be attached to the liposome outer layer for specificity and selectivity. These selective components can also be attached for targeted delivery of imaging agents (e.g,, Gd-DOTA-DSPE for MRI) for diagnostic purposes [40].
Peptide conjugation to the liposomal system has become one of the major parts of the nano-drug delivery technique in cancer therapy. This procedure helps to develop a targeting liposomal system with the feature that the peptide targeting functionality is conserved due to its attachment on the liposomal outer layer (Fig. 2). Most of the peptides have the structural and biological functionality to confer a targeting effect toward the tumor site. Peptide-functionalized liposomal formulations reported in the literature are targeted toward an extensive number of selective receptors that usually are overexpressed on the cancer cell, tissue, or cancer vasculature. These peptide targets can be differentiated into three distinct categories such as G-protein-coupled receptors (GPCRs), growth factor receptors (GFRs), and integrin receptors (αvβ3 and αvβ5) [23]. Neuroendocrine peptides are used as a target for GPCRs, which are membrane receptors overexpressed in tumor tissues such as gastrin-releasing peptide (GRP) receptors, gastrin/cholecystokinin (CCKA and CCKB), and somatostatin (SSTR2 or SSTR5), and neurotensin type 1 receptors (NTS1). A popular peptide target in liposomal formulation is the growth factor receptor family such as epidermal growth factor receptor (EGFR/ErbB/HER); among the four types of the tyrosine kinase receptors, some subtypes are overexpressed in different subclasses of cancer. One of the ideal cancer vasculature targets is integrin receptors that are overexpressed on tumor vasculature and angiogenic endothelium [24].
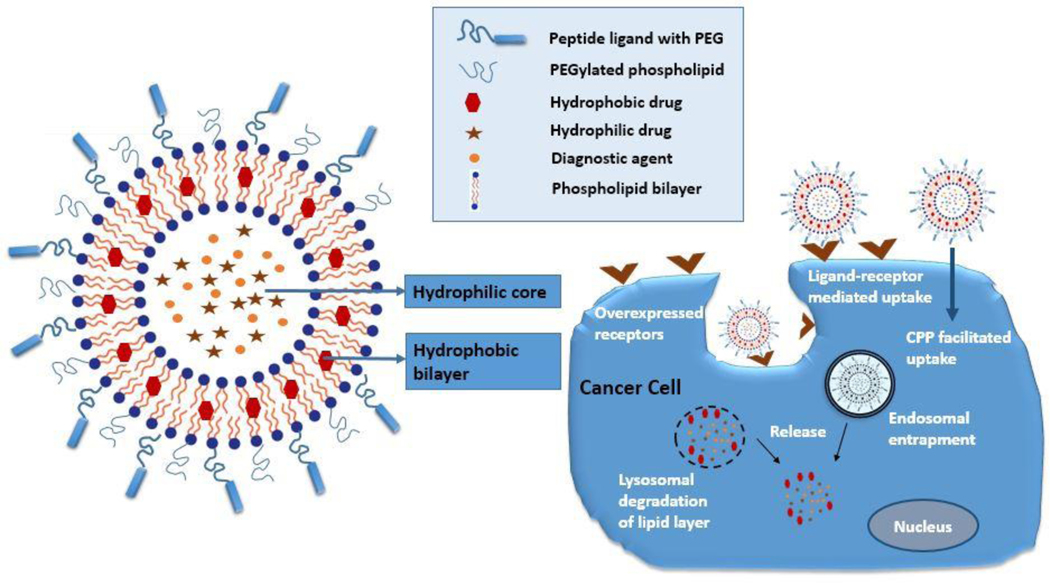
Fig. 2.
Schematic presentation of functionalized peptide-targeted liposomes drug delivery mechanism through an active targeting effect on targeted receptor overexpressed cancer cells. Active peptide-functionalized targeted liposomes either bind to the overexpressed receptors on the cancer cells selectively and go inside through receptor-mediated endocytosis or penetrate inside the cancer cells through cell-penetrating peptide (CPP)-mediated uptake. Drugs and diagnostic agents then can be released through endosomal or lysosomal degradation of the lipid layers.
A somatostatin hormone analog octreotide (OCT), a cyclic octapeptide, was reported the first time in 2008 in the liposomal formulation to target somatostatin receptor type 2 (SSTR2), a receptor subtype overexpressed vastly in small-cell lung cancer and breast cancer. The study reported a liposome formulation coupled with OCT on the liposomal surface and containing a natural anticancer agent cantharidin. In vitro and in vivo results indicated increased antitumor efficacy of the formulation with reduced drug toxicity [41]. A study reported an OCT-conjugated liposome containing two anticancer agents, doxorubicin (DOX) and platinum complexes. The results indicated that prepared liposomes represent a new target selective cargo system for delivery of platinum based drug (e.g. cisplatin) and cytotoxic drug DOX on cells overexpressing the SSTR2 and SSTR5 somatostatin receptors [42]. Another combinational stealth liposomal system comprising OCT loaded with DOX and combretastatin A-4 (CA-4) efficiently entrapped the drugs and provided a synergistic anticancer effect with targeted therapy [43]. Bombesin (BN) peptide, which targets GRP receptors overexpressed in various human cancers, e.g., pancreatic, ovarian, and breast cancers, had been used in the lipid layer of theranostic liposomes in two different formulations [44, 45]. Another peptide, CCK8, was utilized in two liposomal formulations containing DOX where the CCK8 peptide was targeted to selectively bind to cholecystokinin subtype receptors A (CCK1-R) and B (CCK-2R) [46] where CCK1-R has been observed to be highly overexpressed in certain neuroblastomas, gastroenteropancreatic neuroendocrine tumors, and meningiomas, while CCK-2R was found to be overexpressed in small-cell lung cancers, astrocytomas, thyroid cancers, and stromal ovarian cancers [47].
The EGFR receptor family (EGFR, HER-2, and HER-3) has been found to be overexpressed in various forms of cancers such as non-small cell lung cancer (NSCLC) [48] and breast cancer [49]. Two separate liposomal formulations were prepared to entrap doxorubicin and vincristine distinctively with surface modification by αHER-2 Fab’ fragments with targeted Asn-Gly-Arg (NGR) peptides to deliver the drug selectively into endothelial cells in tumor vasculature and tumor cells against HER2-positive breast cancer [50]. EGFR-targeted-peptide ligand D4 was detected through virtual peptide library screening and used with a liposomal formulation in which the EGRF-targeted peptide was conjugated with polyethylene glycol (PEG) moiety to insert into the liposomal layer [51]. These liposomes can also be applicable for the treatment of other solid tumors. Recently, HER-2 targeted peptide P6.1 (KCCYSL) in three multimeric forms conjugated with liposomes was investigated for selective delivery into the HER-2-overexpressed cell lines BT474 and MDA-MB-231. The P6.1 peptide, derivatized with different metal chelators (DOTA, NOTA, CB-TE2A, DAP) and labeled with radionuclide metal ions (111In, 64Cu, 99mTc), has been successfully used for in vivo imaging of HER-2 overexpressing tumor models [52–54]. Magnetic MRI experiments also revealed the potential of the liposomal formulation to function as a diagnostic tool [16].
Different integrin receptors such as α4β1 [55], α5β3 [56–59], and α5β1 [60], have been targeted with peptide-functionalized liposomes loaded with different anticancer drugs such as doxorubicin (DOX) [61–63], paclitaxel (PTX) [59], cisplatin (CDDP) [64], docetaxel (DTX) [65], and 5-fluorouracil (5-FU) [66]. Peptide-functionalized liposomal formulations carrying anticancer agents have been used to target α5β3 integrin which is overexpressed in different types of cancer, including lung cancer [57], glioma [58], and metastatic breast cancers [56].
3. Features of liposomes making them excellent targeting agents in tumors
Anticancer drugs can be delivered at the tumor site by passive targeting effect. Larger molecules and nanoparticles, such as liposomes, are accumulated in tumor tissues due to a characteristic feature of a tumor known as enhanced permeation and retention (EPR) effect [67]. The passive targeting of tumors by these nanoparticles is based upon the EPR effect, which is due to the nature of leaky vasculature resulting from rapid and defective angiogenesis in tumors shown in Fig. 3 [68, 69]. Other passive targeting is achieved using tumor microenvironment such as low pH at tumor site or using different stimuli that responds to the tumor microenvironment. Liposomes can become an effective drug delivery system by taking advantage of this effect.
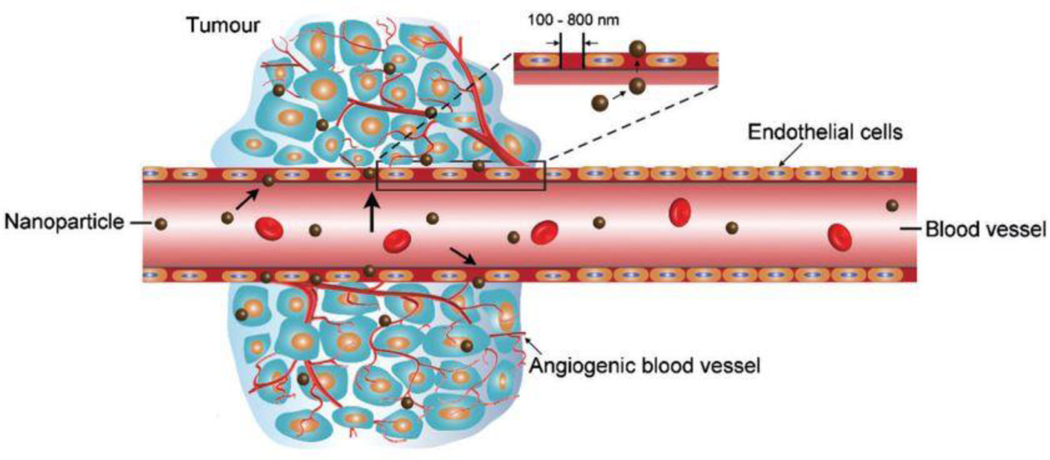
Fig. 3.
Schematic representation of the enhanced permeability and retention (EPR) effect. Large vascular fenestrae formation due to abnormality in the tumor site angiogenic vessels. (Reproduced with permission from [76]).
3.1. EPR Effect:
Cancer cells interact with the immune system cells in such a way that it supports the growth of malignant cells instead of suppressing the cancer cell. Such an interaction between cancer cells and various immune cells forms a tumor microenvironment (TME) [70]. Most of the non-malignant cells in TME include the immune cells, tumor vasculature, lymphatics cells, fibroblast, and adipocytes, which secrete proteins and cytokines that play a vital function in advance and progress of cancer [71]. The vasculature system in tumor tissues has significant differences compared to the control. The blood vessels in the tumor tissues are heterogeneous and their functions are significantly impaired. The vasculature system is flawed with the deficiency of the basement membrane, and the blood vessels are varied in diameter, irregular forms, and bulges [72, 73]. The neovascularization in tumor tissues is the development of new blood vessels also called angiogenesis. This process ensures that the tumor receives adequate nutrition and oxygen from the blood supply [74]. The newly formed vasculature in tumor tissues, which is due to the defective vasculature supportive tissues, leads to the formation of leaky vessels and pores through endothelial gaps varying in diameter from 100 nm to 2 μM. Also, there is a compromised lymphatic system, which leads to poor waste removal from the tumor tissues, resulting in high retention and accumulation. This phenomenon of high permeability due to leaky vasculature and retention in tumor tissues is called an EPR effect. This EPR effect of the cancer cells can be used for passive targeting of therapeutic agents, nanocarriers for both anticancer effect and diagnostic purposes [75].
Knowledge of the EPR effect has enabled several efforts in the development of targeted delivery of the drug to the tumor site. Nanoparticles such as liposomes can be used as targeting agents as they are highly accumulated and retained in tumor tissues compared to normal tissues due to the EPR effect resulting from leaky and unusual vasculature. Moreover, the liposomal drug delivery system enables the escape of drug molecules from high renal clearance, increasing their half-life in vivo significantly and thereby increasing the chances of targeting effects on tumor tissues through the EPR effect. Mononuclear phagocyte system (MPS) in the liver and spleen can reduce the EPR effect of the nanoparticles, leading to poor delivery of drug molecules. The protein binding and nanoparticle aggregation may have a detrimental effect on their delivery by the EPR effect. The effect of MPS on the liposomal drug delivery system can be resolved by employing the surface modification method on liposomes [77]. This might also help in solving the aggregation and protein binding problem and keeping the liposomes in blood circulation for a longer time. Coating the liposomes with polymers such as polyethylene glycol (PEG) has produced longevity in blood circulation. Rapid removal of liposomes from the circulation is prevented by the coating of PEG as it protects the liposomes from the MPS. As a result of its protection, liposomes remain in blood circulation for a longer period of time, which is required for efficient accumulation in the tumor [78]. A very detailed study on the EPR effect, its factors, and the mechanisms by which it is controlled is required for further knowledge on its use for the delivery approach of the liposomes and other nano-delivery approaches. Delivery of small molecules as a therapeutic and diagnostic approach for tumor-specific targeting is full of challenges owing to its rapid renal clearance, accumulation in normal tissues, and toxicity. Hence, nanoparticles systems such as liposomes can be designed as both passive and active targeting delivery systems to target cancer cells specifically through the EPR effect. The EPR effect will significantly limit the renal clearance and toxicity that makes liposomes superior delivery agents [79]. Targeting the tumor cells using the EPR effect must consider several factors, including particle size and shape, tumor perfusion, vascular permeability, vascular penetrations, retention and drainage from tumor tissues, and, cancer types. [80].
3.2. Liposomes making use of local stimuli for release of the drug:
Liposomes, because they are one of the most widely studied nanocarriers for targeted drug delivery, seem to have promising applications in stabilizing therapeutically active compounds, increasing the cellular and tissue uptake, and providing better biodistribution of therapeutic agents to target sites in vivo [81–83]. Tumor cells have a very peculiar nature in terms of their microenvironment and receptor expression. Such features of the tumor cell can be utilized for the formulation of better therapeutically efficacious liposomes. The tumor microenvironment has some unique features, including low pH, higher temperature, and unique enzymatic activity, that can serve as an endogenous stimulus. External stimuli like heat and light-triggered approaches have also been used for drug delivery approaches [84].
3.2.1. pH-triggered drug release by liposomes:
The tumor microenvironment has lower pH (acidic conditions) compared to normal tissue environment; this is the result of excessive metabolites, mainly lactic acid and CO2. This lower pH has been exploited for targeted drug delivery approaches using pH-responsive liposomes and nanoparticles. These pH changes can trigger the drug delivery from liposomes by protonation or deprotonation of lipid membranes, causing the destabilization of the lipid bilayer [85]. pH-sensitive lipids such as DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine) have polymorphic phase behavior that facilitates drug delivery by adopting the hexagonal state at lower pH, promoting the destabilizing of the lipid bilayer [86]. Dual pH-targeting liposomes have been used to significant effect to target the tumor cells specifically. Different types of lipid composition are formulated for pH sensitive liposomes [87, 88]. Apart from lipids, the use of pH-dependent peptides enables the release of liposomal contents at a lower pH [89].
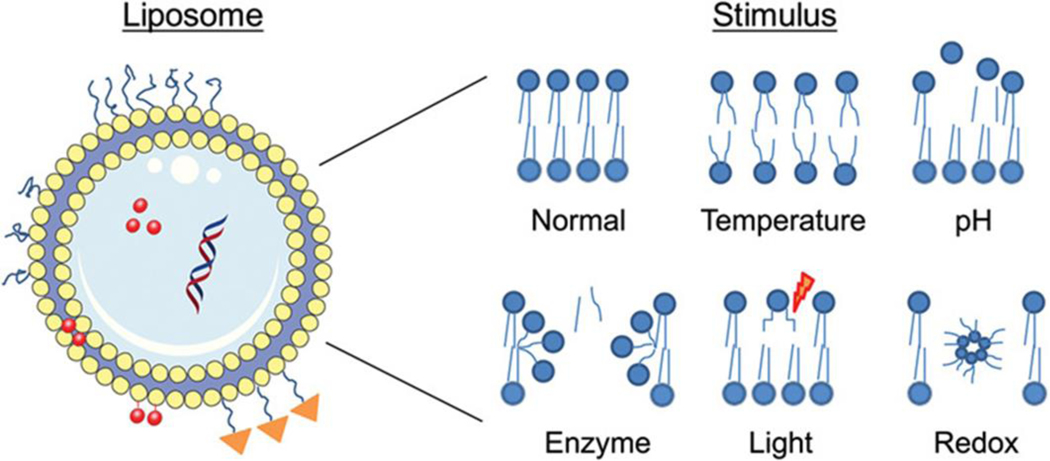
Conventional liposomes exhibit slow release of the constituents after the internalization in the cell because of the PEG barriers. To prevent this problem, stimuli-responsive liposomal preparations can be introduced, which can degrade and release its constituents only upon exposure to certain conditions (Fig.4). pH-sensitive liposomes (pHSLip) have garnered success in enhancing liposome-mediated drug delivery, which is designed to release the active constituents based on the pH conditions of the microenvironment.
Fig. 4.
Controlled drug delivery system using stimuli-responsive liposomes. (Adapted with permission from [90])
It is known that the average pH of the extracellular cancer microenvironment is between 6.0 and 7.0, while in normal tissues, it is 7.4 [91, 92]. This low extracellular tumor pH comes from the high glycolysis rate in cancer cells, which are more hypoxic than normal cells [93]. Hence, by utilizing pH-sensitive polymers and lipids, liposomes that can release the encapsulated active drugs in the presence of a low pH extracellular tumor microenvironment can be prepared. Hence, these pH-sensitive liposomal formulations overcome the limitations of the lack of selectivity of anticancer agents. Further, with the EPR effect of the liposome, they increase the efficacy of therapeutic entities such as siRNA, drugs, or radioisotopes.
Zhao et al. [87] reported that a tumor-specific, pH-responsive liposome could be prepared and targeted to the acidic environment of gliomas, where the pH-responsive liposome responds to the environment and triggers release of the drug (DOX). DOX-PSL-H7K(R2)2 liposomal formulations were designed using H7K(R2)2-modified pH-sensitive liposomes and evaluated for antitumor activity in mice with glioma tumor cells. It was observed that the liposomal formulation was able to deliver the active constituents of DOX in the acidic condition efficiently in in vitro and in vivo experiments.
Other important strategies reported that could be employed to target the acidic tumor extracellular environment include the use of cell-penetrating peptides (CPPs) and antimicrobial peptides (AMPs) based on liposomal formulations. Ding et al. formulated a liposomal formulation using PEG200-Hz-stearate on its surface along with CPPs. When it was compared with the PEGylated conventional liposomes, it was observed that the DOX accumulation in tumors increased up to 1.9-fold over the conventional liposomes and resulted in more cell apoptosis [94]. A similar strategy was also reported by Zhang et al., where a pH-responsive antimicrobial peptide was incorporated in the liposomal formulation; it was found that below pH 6.3, the liposome showed significantly improved efficiency in comparison to other liposomal formulations. Incorporation of paclitaxel (PTX) into the modified liposome further enhances the cellular toxicity [95]. Mozhi et al. described a liposomal formulation in which amphiphilic copolymer poly (β-amino esters)-poly (ethylene glycol) is conjugated with an antimicrobial peptide while DTX is encapsulated in the formulation. They found that once the liposome reaches the inside of the acidic endosomal compartment, the stimuli-responsive micellar carriers disassemble and release both peptides as well as DTX, which produces the antitumor effect [96].
3.2.2. Temperature-triggered release by liposomes:
Hyperthermia is observed at the site of inflammation in tumor tissues compared to normal tissues [15] [15, 97]. Hence, this strategy can be utilized for the preparation of liposomal formulations in cancer therapy to increase drug tumor permeability and enhanced drug uptake. The hyperthermia has a profound effect on tumor permeability and drug uptake, and this property has been utilized in the formulation of temperature-sensitive liposomes. Various studies have shown that local heating of tumor sites increases vascular permeability, blood flow, and pore size that results in enhanced extravasation of liposomes in tumor sites. Temperature-sensitive liposomes have been prepared from thermosensitive lipids and polymers that exhibit low critical solution temperature. The temperature at the tumor site is usually higher than the critical solution temperature of polymers, leading to precipitation of polymers and destabilization of lipid bilayers and releasing the liposomal contents [98].
In this technique, heat is applied to the tumor site, which causes an increase in microvascular pore size and blood flow and results in improved extravasation of drug-loaded nanocarriers. These types of temperature-sensitive liposomes can be prepared from thermoresponsive polymers or lipids with a low critical solution temperature (LCST) [99]. Some examples of temperature-sensitive polymer are poly (N-isopropylacrylamide (PNIPAM) and dipalmitoyl phosphatidylcholine. The polymer becomes water-soluble below its low critical solution temperature due to the formation of hydrogen bonds between the polymeric chain and the water molecules. Once the temperature is above the low critical solution temperature (generally at a tumor site), the polymer becomes insoluble and precipitates, disrupting the liposomes to release the drug [100]. Table 1 depicts some of the recent examples of thermosensitive liposomal formulation strategies used in cancer treatment.
Table 1.
Stimuli-responsive peptide-functionalized liposomal formulations entrapping anticancer agents for targeted therapy of cancer
| Surface attached peptide | Anticancer agent | Liposome formulation | Targeted cancer type | Peptide Target site/ Action | Size (nm) | Reference |
|---|---|---|---|---|---|---|
| CPP: H7K(R2)2 | DOX | DSPE-PEG-NHS | Brain (in vitro and in vivo) | Cell penetrating and pH responsive peptide targeting glioma tumor cells | 92.19 | [87] |
| CPP: GGRRRRRRRRR-amide | DOX | SPC-STR-EDCl-NHS-mPEG2000-hydrazone-stearate | Breast cancer (in vitro and in vivo) | CPP-facilitated delivery of anticancer agent in breast cancer. | 121.25 | [94] |
| Print3G | Calcein | DOPE:CHEMS:CHO L:PEG750-DSPE (43:21:30:6) |
Breast cancer (in vitro) | Antagonist of an oncoprotein involved in breast cancer growth and invasion | 162.6 163.8 | [120] |
| CPP: [D]-H6L9 | PTX | DSPE-PEG2000 | Breast cancer | Integrin αvβ3 targeting by peptide and drug release by local hyperthermia | 130–135 | [95] |
| D[KLAKLAK]2 (KLA) | PTX | SPC:CHOL:PTX = 10:1:1 SPC:DKD:CHOL:PTX = 8:2:1:1 | Lung cancer | Initiates apoptotic cell death | 132 | [121] |
| ELAAWCRWGFLL ALLPPGIAGGGC | Vaccine | DMPC:DMPG:Chol: DOPE | Breast cancer | Activation of cytotoxic T lymphocytes (CTLs) | 126–142 | [122] |
| CGKRKD(KLAKLAK)2 | DTX | PBAE-PEG | Breast cancer (in vitro) | Angiogenic blood vessels in tumors | 117 | [96] |
| TDSILRSYDWTY TDSILRSYDGGG | DOX | NHS-PEG-DSPE | Lung metastasis | Non-small cell lung cancer (NSCLC) cells | 65–75 | [123] |
| H peptide RF peptide K peptide | miR-200 Irinotecan | DSPE:PEG | Colorectal cancer | Tumor neovasculature undergoing angiogenesis, and one mitochondria-targeting peptide | 147–174 | [124] |
| KSSPHSRN(SG)5RG DSP | Calcein | DOPE:CHEMS:DSPE-PEG2000 | Colon cancer | Cell-adhesion domain of fibronectin, specifically integrin α5β1 receptor | 98.36 | [125] |
| NGR | DOX | DPPC:MSPC:DSPE-PEG2000-NGR:DiO:: 85.2:9.7:5:0.1 | Breast cancer and metastasis | CD13/ aminopeptidase N | 107.8 | [126] |
| CPP: CRGDRGPDC [iRGD] | DOX | DSPE-PEG2000:MPPC:DPPC | Liver cancer | αvβ3 integrins and neuropilin-1 | 84 | [127] |
| CPP: CKRRMKWKK | siRNA | DPPC:MSPC:DSPE-PEG2000:: 87:3:10 | Breast cancer (in vitro and in vivo) | CPP-facilitated delivery of anticancer agent | 90 | [128] |
| Cyclic RGD | DOX | DSPC/DPPC:DSPE-PEG (or DSPE-PEG-cRGD):cholesterol:E LP = 55:2:10:0.55 | Breast cancer (in vitro and in vivo distribution) | αvβ3 integrin | 181 | [129] |
| CPP: CKRRMKWKK | DOX | DPPC: MSPC: (DSPE-PEG2000-NGR or DSPE-PEG2000) :: 87:3:10 | Fibrosarcoma (in vitro and in vivo study) | CPP-facilitated delivery of anticancer agent | 82–89 | [130] |
| CREKA | DOX | DPPC, MSPC, DSPE-PEG, and DSPE-PEG-CREKA (86:10:2:2 molar ratio) | Breast cancer | Targeted clotted plasma proteins in tumor vessels and temperature triggered release of DOX | 83.8 | [131] |
| CCRGDKGPDC | DOX | DSPE-PEG2000-maleimide | Breast tumor model | αvβ3 integrin | 94.2 | [132] |
| CPP: CGRRMKWKK | Campothecin | DSPE-PEG2000, DSPC and DPPC (molar ratio of 10:10:90) | Cervical cancer (in vitro and in vivo | CPP-facilitated and ultrasound triggered delivery of anticancer agent | 189–190 | [103] |
| CPP: CKRRMKWKK NGR: CYGGRGNG | DOX | DSPE-PEG2000-NGR | Breast cancer (in vitro and in vivo) | CPP-facilitated delivery of anticancer agent | 195–202 | [104] |
| AG73: CGGRKRLQVQLSIRT | DOX | DSPC and DSPE-PEG2000-OMe | Colon cancer cells in-vitro study | Syndecan-2 targeting combined with ultrasound trigger release | 130–170 | [133] |
| Elastin like polypeptide | DOX | DPPC:DSPE-PEG-2000:cholesterol:SA-ELP3-NH2 = 55:2:15:0.4125 | Squamous cell carcinoma (in vitro and in vivo) | Facilitated stimuli-responsive release of anticancer agents | 161.8 | [134] |
| CPP: CKRRMKWKK | siRNA | DSPC and DPPC | In vivo distribution and cellular uptake in human breast adenocarcino ma cells | CPP-facilitated delivery of anticancer agent | 201 | [135] |
| CPP: CKRRMKWKK (derived from Penetratin) | DOX | DPPC:MSPC:DSPE-PEG2000 (87:3:10) Fe3O4 | Breast cancer (in vitro and in vivo study) | CPP facilitated magnetic hyperthermia-triggered release of DOX | 90–100 | [136] |
| CPP: R8 | PTX | Cholesterol:SPC: DSPE-PEG2K-R8(35:65:0.8) | Breast cancer (in vitro and in vivo study | CPP facilitated and pH sensitive release of PTX | 120 | [137] |
| PEGylated cleavable lipopeptide (PCL) H-G-Trp(Boc)-I-P-V-Ser(tBu)-L-Arg-(Pbf)-Ser(tBu)-G-Glu(tBu)-Glu(tBu)-Glu(tBu)-Glu(tBu)PEG2000 | DOX | POPC:Cholestrol:PCL (60:35:5) | Prostate adenocarcino ma (in vitro and in vivo) | MMP enzyme facilitated cleavage and ultrasound triggered release of DOX | 127 | [138] |
Abbreviations: CPP, cell penetrating peptide; Chol, cholesterol; CHEMS, cholesteryl hemisuccinate; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[poly(ethylene glycol) 2000]; DOX, doxorubicin; DTX, docetaxel; DPPG-Na, dipalmitoyl phosphatidylglycerol; DPPC, dipalmitoyl phosphatidylcholine; DOPE, Dioleoylphosphatidylethanolamine; DSPC, distearoylphosphatidylcholine; DSPE-PEG2000-OMe, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] EDCI, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; mPEG2000-hydrazone-stearate (mPEG2000-Hz-stearate); HSPC, hydrogenated soy phosphatidylcholine; MSPC, 1-myristoyl-2-stearoyl-sn-glycero-3-phosphocholine; MPPC, 1-myristoyl-2-palmitoyl-sn-glycero-3-phosphocholine; DSPE-PEG-NHS, 3-(N-succinimidyloxyglutaryl)aminopropyl; PBAE-PEG, poly(beta-amino ester) poly(ethylene glycol); PTX, paclitaxel; STR, stearate; SPC, soy phospholipids.
3.2.3. Ultrasound-responsive peptide liposomes:
In this approach, the tumor regions are exposed to an ultrasound wave, leading to localized and complete release of the active drug from the liposomal formulation. By utilizing low-frequency ultrasound (LFUS), the drug can be released from stabilized liposome formulations without disturbing its active physicochemical properties. In recent years, by using high intensity focused ultrasound (HIFU), local heating was induced, which causes a phase transition of the polymers and releases the drug from the liposomes [101]. The high-frequency sound waves can damage the liposomes and are able to produce chemical reactions that ultimately cleave chemical bonds between the peptides and the polymers [102]. Xie et al. [103] reported an ultrasound-responsive liposomal formulation using a combination of cell-permeable peptides and ultrasound strategy. Here, CPP-CPT conjugation was encapsulated in the liposome. Once the liposomes accumulate near the tumor region due to the EPR effects, ultrasound irradiation releases the CPP-CPT peptide-drug combination into the cells [103]. In another example in the literature, the cell-penetrating peptide-doxorubicin conjugate (CPP-DOX) was incorporated in peptide-modified nanobubbles (CPP-DOX/NGR-NB) and the penetration of CPP-DOX was temporally masked. It was found that the CPP-DOX/NGR-NB with ultrasound treatment? exhibited greater cytotoxic activity than that without ultrasound [104].
3.2.4. Magnetic field-responsive peptide liposomes:
Magnetic liposomes use the magnetic force for the site-specific delivery of chemotherapeutic agents and maintain them at the site until the drug is completely released [105]. In this case, liposomes are magnetized by incorporating magnetites, such as Fe3O4 or γ-Fe2O3, that are less than 10 nm in size [97]. Applications of these liposomes include magnetic hyperthermia, transfection, and manipulation of cells and proteins. Because of their magnetic properties as well as their nanoscale size, they are also called superparamagnetic iron oxide nanoparticles (SPIONs) [15]. Once an external magnetic field is exposed to the liposomes, the liposomes loaded with a drug with a ferromagnetic material can be guided to a particular target site where they release the drug [106].
3.2.5. Enzyme responsive liposomes:
Elevated expressions and altered compositions of several enzymes such as matrix metalloproteinases (MMPs) are distinctive features of the tumor microenvironment that is hugely involved in the pathogenesis, invasion, metastasis, and progression of the tumor. In particular, MMP-2 and MMP-9 enzymes are found in the extracellular matrix sites of different types of cancers such as breast, colorectal, pancreatic, and lung [147]. These altered enzymes can be biomarkers for diagnosis of the types and stages of tumors and provide the platform for targeted specific delivery of nanoparticles such as liposomes by enzyme-triggered mechanism [107, 108]. Extensive design and use of MMPs responsive peptides, proteins, and polymers for delivery of drugs and imaging agents to the tumor have been studied [109]. In one of the studies of a multifunctional liposomal carrier composed of MMP-2-responsive components, it was shown that it significantly enhanced the tumor-targeting effect and internalization of the liposomes in the tumor [110]. Studies of cathepsin B- and D-responsive liposomes, glucose oxidase-triggered liposomes, and other enzyme-responsive liposomes are promising [111–113].
Site-specific enzymatical activation of prodrugs is another liposomal-targeted therapy to specifically target the tumor cells. In this case, an unfunctionalized prodrug, which can be converted to a cytotoxic drug by certain enzymes present on the extracellular site of tumor cells, is administered to the tumor site [114]. MMP-sensitive peptides are incorporated into liposome formulations, where the peptides act as linkers between the polymer and the lipid. The polymer hinders the uptake of liposomes due to a physicochemical barrier effect. Once the liposomes are in contact with MMPs at the tumor site, the peptide is cleaved, which leads to the cleavage of the polymer and is followed by uptake of the liposomes. Zhu et al. [110] reported a liposomal formulation containing two different types of lipopolymers: mAb 2C5-PEG(3400)-MMP2-cleavable peptide-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and TATp-PEG(2000)-1,2-dioctadecanoyl-sn-glycero-3-phosphoethanolamine (DSPE). The DOPE forms a protective shield over the DSPE lipopolymer containing the TAT peptide. Once the peptide is cleaved at the tumor site, the long-chain PEG will emerge and remove its shielding effect. The hydrolysis of the liposome leads to the contact of the TAT peptide and increased uptake of liposomes [110].
Some other stimuli (external and internal)-responsive liposomal formulation strategies such as redox/thiol responsive liposomes [115, 116] and light-responsive liposomes [117–119] have been reported in the literature.
4. Peptide-functionalized liposome-mediated delivery approaches in cancer therapy
Functionalized peptides are being attached to the surface of liposomal formulation for both treatment and targeting purposes, which eventually increases the therapeutic efficacy of cancer treatment. These procedures also maintain the stability of the peptide drug, as well as in the case of cancer therapy, facilitate the targeted delivery of liposomal components into the tumor site. Different drug molecules are encapsulated or attached along with the peptide-functionalized liposomes for the combinational effect or synergistic effect (in addition to targeting) in different treatment purposes (Fig. 5) e.g., multidrug-resistant (Table 2).
Fig. 5.
Schematic representation of peptide-functionalized PEGylated liposomes containing an active drug. Small drug molecules can efflux through the healthy blood vessels endothelial cells while the liposomal formulation remains contained in the vessel (left). In contrast, in the tumor tissue vessels (right) form large vascular fenestrae due to the rapid vascular growth which facilitate liposomal passage through the vessel and impaired lymphatic drainage helps extravasation also of large liposomal drugs. Upon accumulation, the bioactive peptide on the liposome outer layer binds to the overexpressed targeted receptor on the cancer cells, which promotes intracellular uptake through receptor-mediated endocytosis and anticancer drug accumulation in vessels or in the nucleus (Reproduced with permission from [23]).
Table 2.
Peptide-functionalized liposomal formulations entrapping anticancer drug and diagnostic agents for targeted therapy of cancer
| Surface attached peptide | Anticancer agent | Liposome formulation | Targeted cancer type | Peptide target site or activity | Size (nm) | Reference |
|---|---|---|---|---|---|---|
| Arginine unit (R8) | DOX | HSPC:Chol:mPEG-DSPE:RhPE (molar ratio 59:38.21:2:1) | Ovarian carcinoma (in vitro and in vivo) | CPP-facilitated delivery of anticancer agent | 214.5 | [139] |
| Peptide S1 (LIDHEWKENYFPLSF) | DOX | SPC:Chol: S1-PEG2000-DSPE and DOX ( weight ratio 8:1:1:1 w/w/w/w) | Tumor-associated angiogenesis (in vitro and in vivo) | VEGFR2 receptor | 143 | [17] |
| P1 peptide (TVRTSAD) | DOX | DPPC: DPPG-Na: DPPE-PDP:Chol: Chol-PEG (15:15:30; 4:36 molar ratio) | Gastric cancer (in vivo) | CLDN7 marker protein | 160 | [19] |
| Fatty acid-conjugated, elastin-like peptide (FELP) | DOX | DPPC: DSPE-PEG: Cholesterol: FELP at a 55:2:15; 0.4125 molar ratio | Non-thermal acoustic cancer treatment (in vitro and in vivo) | Tumor site-specific drug delivery | 134.97 | [140] |
| pH responsive CPP H7K(R2)2 | DOX | DOPE: CHEMS: DSPE-PEG and DSPE-PEGH7K(R2)2 | Glioma tumor (in vitro and in vivo) | CPP-facilitated delivery of anticancer agents in gliomas | 92.19 | [87] |
| Integrin α5β1 antagonist (Ac-PHSCN-NH2) | DOX | HSPC/DPPE /Chol/OHCPEG-CHO (15:5:10:10 mole ratio) | Melanoma B16F10 cells (In vitro and in vivo) | Targeted-drug therapy toward integrin α5β1 receptor | 96.0 | [141] |
| Cell penetrating peptide (CPP) | DOX | SPC/Chol/DSPE-PEG2000 (100:50:8 mol/mol/mol) | Human breast cancer tumor (in vitro and in vivo) | CPP-facilitated delivery of anticancer agents in breast cancer. | 121.25 | [94] |
| TAT cell-penetrating peptide | DOX | 1.5% dsPe-PeG2000, 1% dsPe-PeG2000-tat, 2.5% dsPe-PeG2000-ang, 59% SPC, 36% CHO | Glioma cells (in vitro) | Low-density lipoprotein receptor related protein-1 (LRP1) | 93.7 | [142] |
| DCDX and c(RGDyK) peptides | DOX | HSPC/cholesterol/mPEG2000-DSPE/DCDX-PEG3400-DSPE/ c(RGDyK)-PEG3400-DSPE (52/43/2/2/1, by mole) | Glioma (in vitro and in vivo) | Nicotine acetylcholine receptors (nAChRs) on the BBB and integrin highly expressed on the BBTB and glioma cells | 93.9 | [143] |
| APRPG | miRNA and DOX | DOPE, cholesterol, DPPC, and DCP-TEPA (4:4:3:1 as a molar ratio) | Colon (in vitro and in vivo) | VEGFR-1 | 163 | [144] |
| RIV (DFDSDMDEDGDIDHDQ DGDQDHDPDKDIDRDM DIDQDMDTDI) | DOX and CA-4 | Hydrogenated soybean phosphatidylcholine/cholest erol, 55/45, mol/mol | Skin (in vitro and in vivo) | VEGFR-2 | 70–110 | [145] |
| RIPL peptide (IPLVVPLRRRRRRRRC) | DTX | PC, TW80, and DP2KM (8.8:1:0.2 molar ratio) | Prostate and ovarian tumor (in vitro and in vivo) | Targeted delivery to hepsin-expressing cancer cells | 162.4 | [146] |
| LDV and YSA peptides | DTX | DOPC, Cholesterol, C16-LDV, C16-YSA, DX (16:4:8:8:1 ratio) | Melanoma cell (in vitro) | Integrin (α4β1) and ephrin (EphA2) receptors | - | [55] |
| Histidine tagged EphA2 receptor specific peptide (YSA) | DTX | DTX:DOGS-NTA-Ni:DOPC:Cholesterol:DSP E-PEG 2000 (0.5:5:20:5:2.5 weight ratio) | Lung cancer (in vitro and in vivo) | EphA2 receptor | 189.3 | [147] |
| Angiopep-2 and tLyP-1 peptides | DTX & VEGF siRNA | DOTAP:SPC:Chol:DSPE-PEG2000 (25:40:30:4, mol/mol) | Brain tumor glioma cell (in vitro and in vivo) | Protein receptor (angiopep-2) and neuropilin-1 receptor (tLyP-1) | 110 – 150 | [148] |
| ELP and tethered GRP | DTX | 1.5× ELP-GRP/C/ACD-1/DTX | Prostate cancer cells (in vitro) | Gastrin-releasing peptide receptor (GRPR) | 23.5 – 154.8 | [149] |
| CPP Penetratin | 5-FU and Tf | DOTAP/DOPE/ CHEMS /Pen-PEG(2000)-DSPE (43.5:43.5:5:4 mole %) | Brain tumor glioblastoma cell (in vitro) | Transferrin (Tf) receptors in brain tumors | 178.12 | [150] |
| PR_b | 5-FU | (65-xy):35:x:y mol% of DPPC:CHOL:PEG:peptide-amphiphile, where x is the indicated mol% of PEG and y is the mol% of peptide-amphiphile | Colon cancer cells (in vitro) | Integrin α5β1 | 80 – 150 | [60, 66] |
| YIGSR peptide | 5-FU | DSPC/CH/DSPE-PEG(2000)-MPB (6:4:0.5) | Angiogenic endothelial cells (in vitro and in vivo) | Laminin receptors | 103 | [151] |
| Cyclic RGD peptide | 5-FU | DSPC/cholesterol/DSPE – PEG – RGD (56:39:5) | Angiogenic endothelial cells (in vitro and in vivo) | avb3 integrins | 105 | [152] |
| Peptide AA13 | DNR | S100PC/CHOL/mPEG2000-DSPE (4:1:0.2, molar ratio) | Acute myeloid leukemia (AML) cells (in vitro and in vivo) | Low density lipoprotein receptor (LDLR) | 95 | [153] |
| Arginine8-Glycine-Aspartic acid (R8GD) peptide | DNR and emodin | EPC, Chol, DSPE-PEG2000, emodin and DSPE-PEG2000-R8GD at a mass ratio of 100:25:8:6:40 | Cancer therapy (in vitro and in vivo) | Vasculogenic mimicry (VM) channels | 100 | [154] |
| PFV peptide | DNR and dioscin | EPC, Chol, DSPE-PEG2000, DSPE-PEG2000-PFV and dioscin (100:30:3:2:7, molar ratio) | Non-small-cell lung cancer (NSCLC) (in vitro and in vivo) | Vasculogenic mimicry (VM) channels and tumor metastasis | 121.13 | [155] |
| R8-dGR peptide | PTX | SPC, cholesterol and DSPE-PEG2000-OMe (molar ratio = 62:33:5) | Malignant melanoma (in vitro and in vivo) | Neuropilin-1 receptors and integrin αvβ3 receptors | 100 | [156] |
| Glu6-RGD peptide | PTX | SPC/cholesterol/Ligand Glu6-RGD-Chol (molar ratio = 62:33:3) | Bone metastatic breast cancer (in vitro and in vivo) | αvβ3 integrin | 121.9 | [56] |
| RGD peptide | PTX and CUR | PTX (5 mg), CUR (3 mg), CHOL (15 mg), DSPE-PEG 2000 (12.5 mg) and SPC (120 mg) | Lung cancer (in vitro and in vivo) | α5β3 integrin | 120.6 | [57] |
| TR peptide | PTX | SPC, Cho, DSPE-PEG2000OMe, DSPE-PEG2000-peptide (59:33:2:6, molar ratio) | Glioma (in vitro and in vivo) | α5β3 integrin | 131.8 | [58] |
| Peptide R8-RGD | PTX | SPC/cholesterol/DSPE-PEG2000/DSPE-PEG2000- R8-RGD (molar ratio ¼ 62: 33:4.2:0.8) | Glioma (in vitro and in vivo) | α5β3 integrin | 105.9 | [59] |
| CAP peptide | PTX and albumin nanoparticles | 2.5 mg of DPPC, 0.1 mg of CAP, 0.51 mg of DSPE-PEG2KOMe, and 0.2 mg of IR-780 | Pancreatic ductal adenocarcinoma (PDAC) (in vitro and in vivo) | Membrane biomarker FAP-α | 123.9 | [157] |
| TH peptide | PTX and αGC immunoadjuvant | Cholesterol/SPC/DSPE-PEG2000/DSPE-PEG2000-TH (molar ratio = 33:59:2:6) | Melanoma (in vitro and in vivo) | pH-responsive delivery of anticancer agent in melanoma | 118.3 | [158] |
| Cell-penetrating peptide TAT | PTX and DOX | SPC, CHO,DSPE-PEG2000, DSPE-PEG1000-TAT, DSPE-PEG3500-Tf in 60, 33, 3, 2, 2 % respectively. | Melanoma (in vitro and in vivo) | CPP-facilitated delivery of anticancer agent | 124.5 | [159] |
| CPP PFVYLI | PTX | 15.9 mg of EPC, 4.1 mg of Chol, 4.7 mg of PEG2000DSPE and 1.5 mg of PFVPEG2000-DSPE | Breast cancer (in vitro and in vivo) | CPP-facilitated delivery of anticancer agent | 120 | [160] |
| TH peptide | PTX and LST | SPC, Cholesterol, DSPE-PEG2000-OMe and DSPE-PEG2000-TH (molar ratio = 59:33:2:6) | Breast cancer (in vitro and in vivo) | pH-responsive delivery of anticancer agent in breast cancer | 109.3 | [161] |
| Peptide H7K(R2)2 | PTX and SPIO NPs | PTX, EPC, cholesterol, DSPE-PEG, and DSPE-PEG-H7K(R2)2 | Breast cancer (in vitro and in vivo) | pH-responsive delivery of anticancer agent in breast cancer | 168.30 | [20] |
| Gonadorelin peptide | MXT | HSPC, cholesterol, and mPEG 2000-DSPE in a mole ratio of 90:10:0.4 | Breast cancer (in vitro and in vivo) | Luteinizing hormonere-leasing hormone targeted delivery of anticancer agent | 118.7 | [162] |
| RGD and ATWLPPR peptide | Gd-DTPA | Egg PC/ cholesterol/ mPEG2000-DSPE at a molar ratio of 1.85/1/ 0.15 | Tumor tissue - (in vitro and in vivo) | Angiogenesis targeting molecular imaging of tumor | 103.50 | [163] |
| cRGD peptide | Iron oxide (Fe3O4) nanoparticles | DMPC: DMPG (9:1) in TES buffer were mixed at a lipid/ Fe3O4 weight ratio of 1:5 | Glioma and ovarian cancer (in vitro and in vivo) | α5β3 integrin | 57.8 | [164] |
| Anti-HER2 peptide | Methylene blue (MB) attached NaYF4:Yb,Er upconversion nanoparticles (UCNPs) | 500 μL soy lecithin (10 mM) and 47% cholesterol | Breast cancer (in vitro and in vivo) | HER2-positive breast cancer | 90.0 | [165] |
| L-peptide (RLLDTNRPLLPY) | Rhenium-188 (188Re) radioisotope | Peptide-PEGylated-liposomes (1 ml) were added to a solution of 188Re-BMEDA (50–250 MBq), and incubated at 60 ºC for 30 min | Nasopharyngeal carcinoma (in vitro, in vivo and, in silico) | GRP78, a specific cancer cell-surface marker | - | [166] |
| Bombesin peptide | Technetium-99m (99mTc) isotope | DOPE, CHEMS, and DSPE-PEG2000 (lipid concentration 40 mM; molar ratio5.7:3.8:0.5, respectively | Breast cancer (in vitro and in vivo) | Different tumors including lung, prostate, breast, pancreas, and colon tumors, express receptors for these peptides [167] | 124.1 | [168] |
Abbreviations: CPP, cell-penetrating peptide; Chol, cholesterol; CUR, Curcumin; CHEMS, cholesteryl hemisuccinate; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[poly(ethylene glycol) 2000]; DOX, doxorubicin; DTX, docetaxel; DNR, daunorubicin; DPPC, DPPG-Na, dipalmitoyl phosphatidylglycerol dipalmitoyl phosphatidylcholine; DPPE-PDP, (N-[3-(2-pyridinyldithio)-1-oxopropyl]-L-a-dipalmitoyl phosphatidylcholine; DOPE, dioleoylphosphatidylethanolamine; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane chloride; DSPC, distearoylphosphatidylcholine; DMPG, 1,2-Dimyristoyl-sn-glycero-3-phosphorylglycerol sodium salt; DMPC, 1,2-Dimyristoyl-sn-glycero-3-phosphocholine; ELP, elastin-like polypeptide; EPC, egg yolk phosphatidylcholine; GRP, gastrin-releasing peptide; Gd-DTPA, gadolinium-diethylenetriamine pentaacetic acid; HSPC, hydrogenated soy phosphatidylcholine; LST, losartan; PTX, paclitaxel; RhPE, rhodamine-PE; SPC, soy phospholipids; SPIO NPs, superparamagnetic iron oxide nanoparticle; Tf, transferrin; TES, 2-[(2-hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]ethanesulfonic acid; VEGF, vascular endothelial growth factor; 5-FU, 5-fluorouracil, MXT, mitoxantrone.
4.1. Doxorubicin-loaded peptide liposome:
Peptide-functionalized liposomal formulations have been prepared as a cargo to deliver various chemotherapeutic agents to cancer cells. Anthracyclines such as doxorubicin (DOX), daunorubicin (DNR), and their derivatives are widely used in various hematological and solid tumors; they are considered to be a first-line therapy for breast cancer, but can be associated with both cumulative and irreversible cardiotoxicity [64]. Also, cardiotoxicity can increase approximately fourfold if these are administered with other chemotherapeutic drugs [65]. For these reasons, the development of peptide-functionalized liposomal formulations is the focus in clinical and preclinical studies aimed at reducing the acute and cumulative cardiotoxicity, as well as mitigating other drug-related side effects (e.g., nausea, bone marrow depression, and alopecia) [66] while increasing drug efficacy. Doxorubicin (DOX) has been encapsulated in liposomal formulation alone or with other active components, facilitating targeting or synergistic effects. A targeted liposome was used to prepare arginine-rich cell-penetrating peptides (AR-CPP), which is a general approach for active tumor targeting. In a study by Deshpande et al., eight arginine units (R8) were used to attain optimal chain length for efficient translocation. In this study, dual factionalized liposome was prepared with surface modification through R8 and transferrin (Tf) to target A2780 ovarian cancer cells with R8-mediated intracellular delivery of DOX. In a different in vitro study, the researchers had found that dual DOX liposomes showed enhanced cytotoxicity. In comparison to other treatments, this dual DOX liposome was more effective in mitigating the tumor growth in vivo in an A2780 ovarian xenograft model [139].
Targeting liposomes to the vascular epithelial growth factor receptor 2 (VEGFR2) was done using a novel affinity peptide S1, which could specifically recognize and bind to the VEGFR2 receptor. The liposomal formulation was an effective nanoscale drug delivery system reported in vitro and in vivo by Han et al. Peptide S1 has 9 amino acids in the sequence, and the S1-functionalized liposomes (S1-LS) containing DOX showed promising VEGFR2-targeting drug delivery, which could be an effective method for cancer therapy and diagnosis [17]. In another study, peptide-conjugated DOX liposome has been prepared to recognize and bind effectively to the Claudin 7 (CLDN7) marker protein. In this study, the gastric tumor model was established with a tumor xenograft mouse model and an initial ionizing radiation dose was given, followed by a phage-displayed peptide library injection. Tightly bound peptides were recovered as well as a counterpart protein that was consequently recognized. The prepared peptide-conjugated liposome showed substantial improvement in therapeutic efficacy with the possibility of irradiation-mediated diagnostic imaging [19].
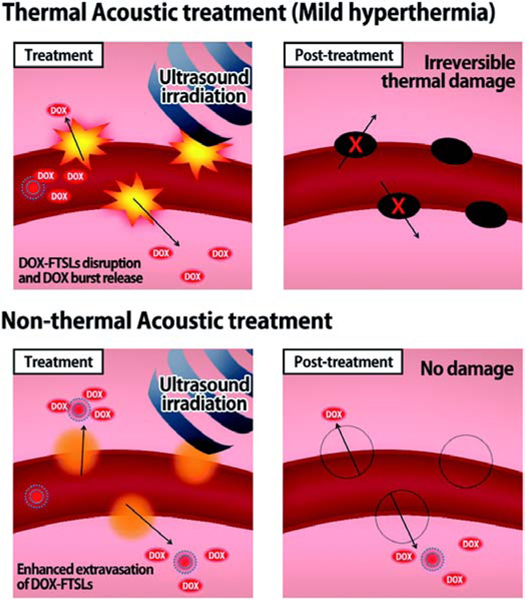
A promising approach for efficient drug delivery for cancer treatment was achieved through a spatiotemporally controlled heat-triggered ultrasound-mediated mild hyperthermia and thermosensitive liposome (Fig. 6).
Fig. 6.
Schematic illustration representing the drug delivery mechanisms of thermal and non-thermal acoustic treatment using DOX-FTSLs. (Reproduced with permission from [140]).
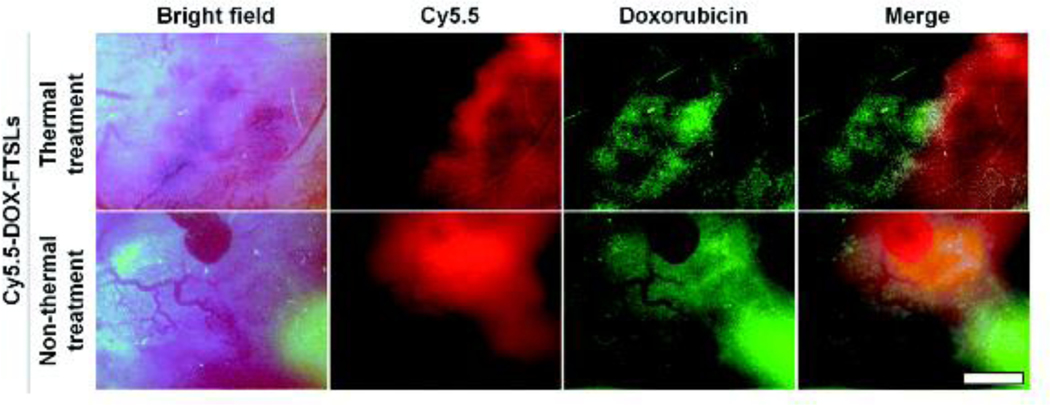
Um et al. proposed an alternative mild hyperthermia approach for site-specific drug delivery because the conventional thermal approach can cause vascular damage, which decreases the extravasation of the drug into the tumor tissue and affects the therapeutic effectiveness in follow-up treatments. In this study, fatty acid-conjugated elastin-like polypeptide (FELP) had been used to prepare thermosensitive liposomes (FTSLs) that have a long blood circulation time with selective collapse at or above 40°C and have a synergistic effect together with mild hyperthermia for enhanced anticancer efficacy. In this system, unlike thermal-mediated drug delivery, which causes irreversible damage to the tumor vasculature; the use of acoustic treatment increased the permeability of tumor vessels for a short time through the acoustic cavitation effect along with the release of DOX in the tumor tissue. Thermal and non-thermal treatments on extravasation of the DOX-FTSLs and DOX were compared; in vivo near-infrared fluorescent (NIRF) dye live imaging of tumor tissue was conducted at 12 h post-injection of Cy5.5-DOX-FTSLs (Fig. 7) The researchers revealed that non-thermal acoustic treatment may be a safe alternative to thermosensitive liposome-involved hyperthermia for liposomal chemotherapy [140].
Fig. 7.
(a) In-vivo NIRF images of tumor vessels and tissues at 12 h after intravenous injection of Cy5.5-labeled DOX-FTSLs combined with thermal or non-thermal acoustic treatment (red: Cy5.5, green: DOX). Scale bar represents 150 μm. (Reproduced with permission from [140]).
A pH-responsive liposomal formulation, which contained peptide H7K(R2)2 as a targeting agent and also possessed a cell-penetrating peptide (CPP) characteristic in acidic conditions, was investigated. This peptide H7K(R2)2-transformed pH-responsive liposome containing doxorubicin (DOX-PSLH7K(R2)2) was investigated for targeted activity toward glioma tumor cells in vitro and antitumor properties in vivo. A selective targeting effect initiated by an acidic pH environment was found in these in vitro experiments in U87-MG and C6 glioma cells. pH-sensitive release of DOX from pH-responsive liposome was also confirmed by in vitro drug release in the study carried out in different pH conditions. Antitumor properties of DOX-PSLH7K(R2)2 were found in the U87-MG orthotopic tumor-containing mice and C6 tumor-bearing mice examined in in vivo experiments [87]. Dai et al. reported liposome containing an integrin α5β1 antagonist N-acetyl-proline-histidine-serine-cysteine-asparagine-amide (Ac-PHSCN-NH2) as a novel targeting peptide, which is in clinical trials for cancer therapy. The liposomal formulation (PHSCNK-PL-DOX) also contains doxorubicin as a chemotherapeutic agent. PHSCNK-PL-DOX showed improved intracellular incorporation as well as enhanced cytotoxicity against melanoma B16F10 cells compared to control DOX-only liposome PL-DOX. This targeted formulation showed promising results in terms of better tumor inhibition and increased survival time compared to a control in an in vivo study, at the same time demonstrating lowered cardiovascular toxicity in tissue analysis. The pharmacokinetics and biodistribution results indicated that the integrin α5β1 antagonist-incorporated liposomal formulation could be used as a potential therapeutic agent for targeted cancer therapy [141].
In an in vivo study by Ding et al., novel pH-sensitive liposomes CPPL(DOX) with doxorubicin-loaded cell-penetrating peptide (CPP) were studied in BALB/c nude mice containing tumors of human breast cancer. This study revealed some detailed information regarding the distribution of liposome CPPL(DOX) in vivo with histological examination on tumors as well as organ tissues including other bioaccumulation studies. The results showed that enhanced DOX buildup in tumors reaches up to 1.9-fold (p<0.01), causing a relatively lower tumor growth ratio as well as more tumoral cell apoptosis through DNA disruption. Histological evidence showed promising signs indicating the absence of any inflammation or necrosis on normal tissues. In contrast, large cellular damage with dissolving areas was found in tumor tissue on animals treated with CPPL(DOX) [94].
To overcome the problem of crossing the blood-brain barrier (BBB) when delivering the drug to the brain, glioma-targeted, dual-modified liposomes (DOX-TATAng-LIP) were formulated. Angiopep-2, a targeted ligand for low-density lipoprotein receptor-related protein-1 (LRP1) overexpressed in both glioma and BBB cell membranes was employed. TAT peptide was used to facilitate the penetrability of the liposomal formulation. In vitro results indicated that the drug DOX-TATAng-LIP not only facilitates permeability through transcytosis across BBB but increases glioma necrosis upon selective accumulation into the glioma cells [142].
The efficient delivery of the drug into the glioma is a challenging mission, and liposomal formulations have been successfully studied for this purpose. Glioma-targeted liposomal formulation have been prepared to facilitate the delivery of drug load into the tumor crossing the BBB and the blood-brain tumor barrier (BBTB). Proteolytically stable peptides DCDX and c(RGDyK) have been used; DCDX is a nicotine acetylcholine receptor (nAChRs) D-peptide ligand on the BBB and c(RGDyK) is an integrin ligand overexpressed on the BBTB and glioma cells. DOX-loaded double peptide-conjugated liposomes produced better survival rates with inhibition of glioma in glioma-bearing mice. The result suggested that the proposed liposomal formulation with multiple peptide ligands can achieve glioma-targeted delivery of DOX, crossing multiple barriers and accomplishing improved therapeutic efficacy of DOX treatment for glioma [143].
4.2. Daunorubicin-loaded peptide liposome:
Daunorubicin is an anthracycline antibiotic that is used as a chemotherapeutic agent for different types of leukemia. A daunorubicin (DNR)-loaded therapeutic liposome was also evaluated in various cancer treatment approaches. Low-density lipoprotein receptor (LDLR) overexpression in acute myeloid leukemia (AML) cells was targeted by a novel peptide AA13 (14 amino acids) that selectively binds to LDLR. As a general approach of linking the peptide to the liposome outer layer for targeted effect, AA13 was attached to the DSPE-PEG2000-maleimide by the distal end connection. The AA13-conjugated liposomes showed LDLR selectivity, including enhanced DNR cytotoxicity in AML cells. An in vivo study in BALB/c nude bearing the human acute promyelocytic leukemia cell line, NB4 xenografts revealed enhanced drug accumulation with DNR-loaded targeted liposomes in tumors compared to untargeted liposome, increased inhibition of tumor volume, and elongation in survival times [153].
DNR and emodin separately loaded dual-targeted liposomal formulations were evaluated in breast cancer therapy; they were modified with Arg8-Gly-Asp (R8GD) peptide for selective accumulation and destruction of vasculogenic mimicry (VM) channels expressing in most proliferating cancer cells. The combination therapy effectively inhibited MDA-MB-435S cancer cells as well as VM channel formation, decreasing cancer cell metastasis [154]. Dioscin is a natural steroid saponin and is known to induce apoptosis in cancer cells [169]. Combinational liposomal delivery of DNR and dioscin with surface treatment with a neutral cell penetrating peptide PFVYLI (PFV) was also applied for the improvement of targeted tumor therapy with inhibition of tumor cell metastasis. The incorporation of dioscin mediates the augmentation of the repressing effect of DNR on lung cancer cell A549 and vasculogenic mimicry (VM) channels as well as tumor metastasis [155].
4.3. Docetaxel-loaded peptide liposome:
Docetaxel (DTX) is a cytotoxic chemotherapeutic agent used in the treatment of various cancers. Peptide molecules have been attached to liposomes for selectivity or other functional effects along with DTX as an anti-neoplastic agent. Yoon et al. reported the use of RIPL (IPLVVPLRRRRRRRRC) peptide for targeted delivery of RIPL peptide-conjugated liposomes (RIPL-L) into hepsin-expressing cancer cells. RIPL-L facilitated the accumulation of the liposome into the cancer tissue examined by fluorescent-conjugated probe. Compared to free DTX, liposome formulation DTX-RIPL-L showed significantly increased activity in tumor growth inhibition as well as lengthening the survival time of the experimental BALB/c nude mice bearing SK-OV-3 cell tumors [146].
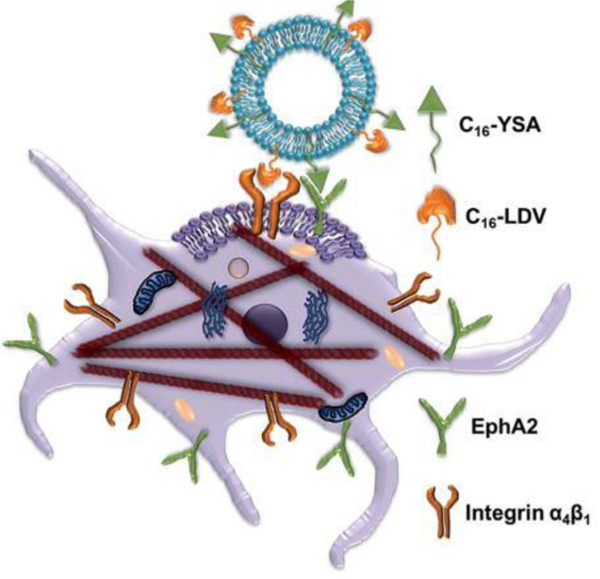
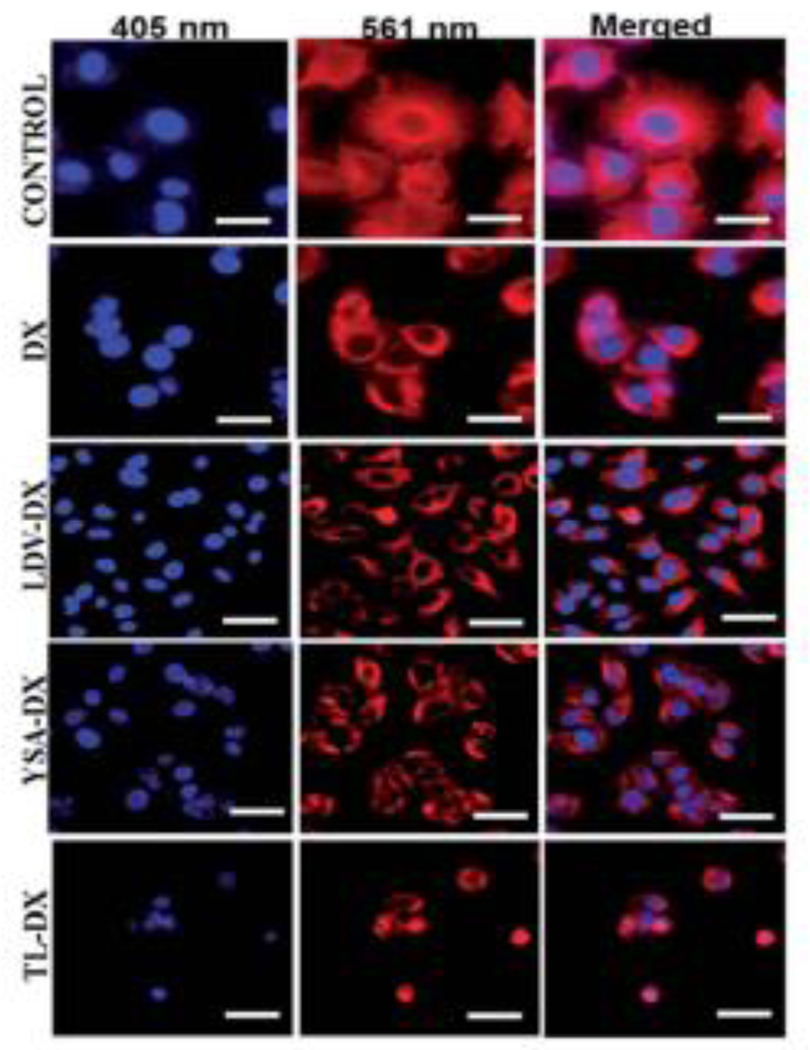
Bi-functional liposomes prepared with two lipopeptides carrying docetaxel for targeting integrin (α4β1) and ephrin (EphA2) receptors was also reported with improved activity of docetaxel in melanoma cells. Both the reported peptide-conjugated lipid molecules C16-LDV and C16-YSA (LDV and YSA are peptide molecules) were entrapped in three modified liposomal formulations LDV-DX (only LDV conjugated), YSA-DX (only YSA conjugated), and Lip. C16-LDV-YSA-DX or TL-DX (both LDV and YSA conjugated) containing docetaxel as an anticancer agent (Fig 8).
Fig. 8.
Schematic representation of dual functionalized liposome targets both integrin (α4β1) and ephrin (EphA2) receptors of the melanoma cell. (Reproduced with permission from [55]).
The therapeutic efficacy of docetaxel was significantly increased by the use of targeted liposomal formulations. Compared to DTX alone, TL-DX showed significant apoptotic death of melanoma cells, microtubule bundle formation, cell cycle arrest in G2/M + S, and inhibition of multicellular 3D melanoma spheroid growth. In fluorescence microscopic images, upon treatment of A375 cells with DX, LDV-DX, YSA-DX, and TL-DX liposome solutions and solution of DTX, the researchers found that TL-DX significantly increased intracellular microtubules bundle-like structure formation compared to only DX or with other liposomes, indicating that TL-DX significantly increased the efficiency of DTX into the melanoma cells (Fig. 9) [55].
Fig. 9.
Fluorescent microscopic images of DX, LDV-DX, YSA-DX, and TL-DX on the microtubule network of A375 cell. TL-DX treatment caused higher microtubule formation and disruption. Scale bars correspond to 20 mm. (Reproduced with permission from [55]).
Liposomal therapeutic efficacy was enhanced by incorporating active targeting agents as reported by Patel et al. An oral antifibrotic agent (telmisartan) was used to study its effect on tumor uptake and antitumor activity of a liposomes-targeting EphA2 receptor. Histidine-tagged EphA2 receptor-specific peptide (YSA) was used as a targeting agent to entrap docetaxel-loaded PEGylated liposomes (DPL) functionalized with a nickel-chelated phospholipid. The study revealed that pretreatment with telmisartan successfully increased tumoral uptake of liposomes. This approach had a promising effect that can be utilized with different types of solid tumors to give potent antitumor effects by compromising tumor barriers [147].
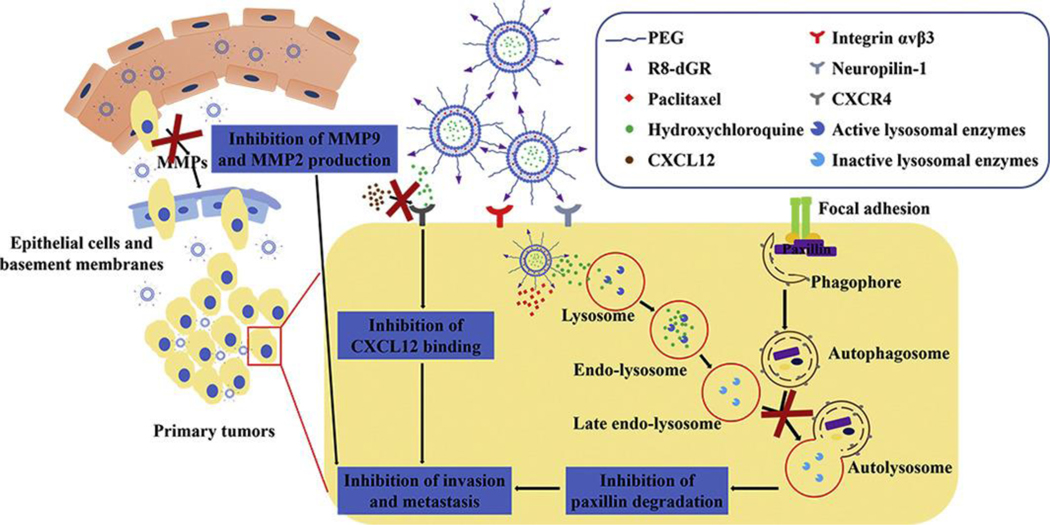
Combinations of drugs were also used in liposome formulations with dual-targeted peptides to deliver an effective anticancer effect. A study was reported by Yang et al. on dual peptide-modified liposomes in which docetaxel and siRNA were used in combination with two receptor-specific peptides—specifically, neuropilin-1 receptor (tLyP-1) and low-density lipoprotein receptor-related protein receptor (Angiopep-2)—to target brain tumor and accumulation in the tumor (Fig. 10). This study revealed the co-delivery of two different drugs of different solubility characteristics, one a large hydrophilic molecule and the other a small lipophilic molecule. The first one involves endocytosis and subsequent release from endosome or lysosome, whereas the latter enters through passive diffusion of lipophilic small drugs upon release. This combinational peptide-modified liposome binds effectively to glioma cells, which facilitates internalization through specific receptor-mediated endocytosis and was found to be more effective than non-modified or single modified liposomes due to dual functionality of loading VEGF siRNA and DTX [148].
Fig. 10.
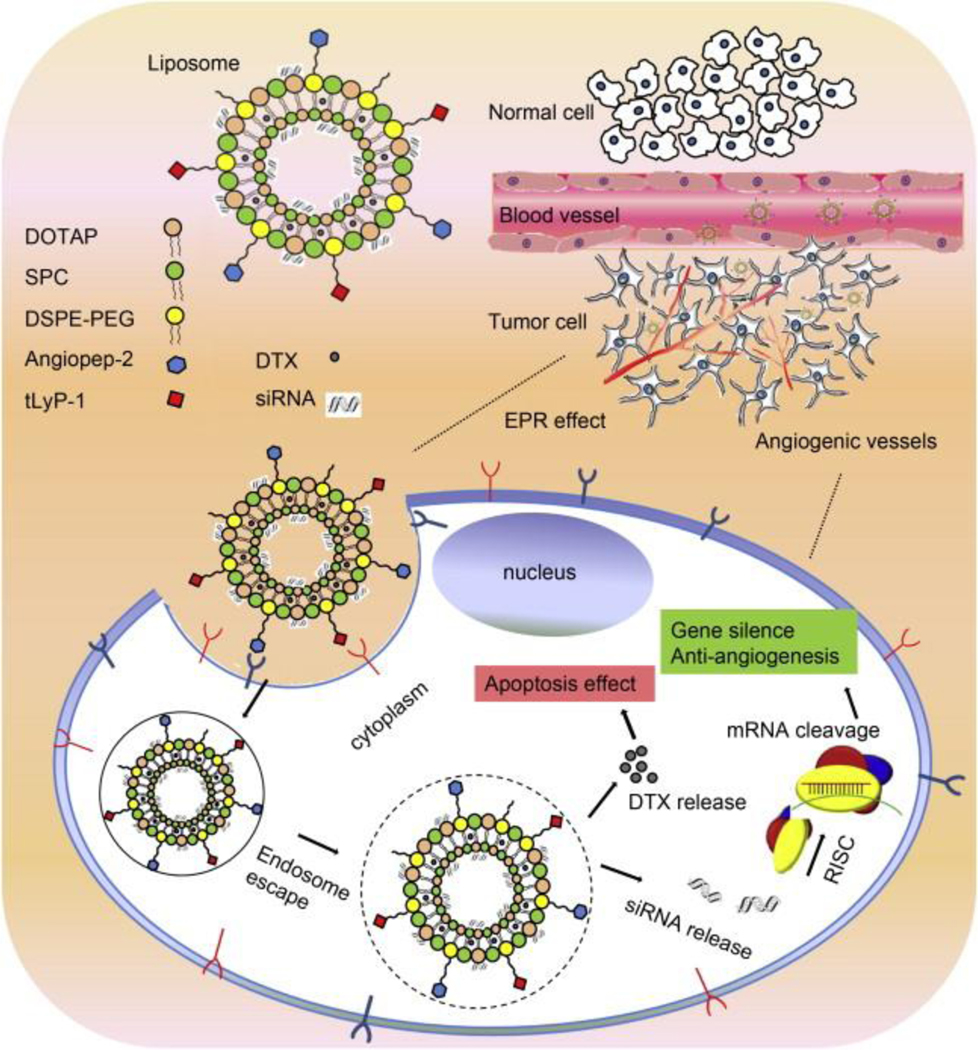
Schematic representation of two receptor-specific peptides, Angiopep-2 and tLyP-1, mediated liposomes and their tumor-targeting delivery mechanism. Tumor penetrations and delivery of the dual peptides-modified liposomes were achieved by targeting effect of peptides as well as via the EPR effect. Anti-angiogenesis gene (VEGF siRNA) and apoptosis-inducing chemotherapy (DTX) were used as a combinational therapeutic strategy. (Reproduced with permission from [148]).
Liposomes also acted to facilitate drug retention properties of elastin-like polypeptide (ELP)-based self-assembling micelles and hybrid ELP/liposome nanoparticles, which have the characteristic of self-assembly in response to changes in temperature with a high loading of DTX and slow release have been reported by Zhang et al. They contained a gastrin-releasing peptide (GRP) on the outer surface working as a targeting ligand to target gastrin-releasing peptide receptor (GRPR), which is frequently overexpressed in prostate cancer cells. The researchers developed nanoparticles that showed rapid self-assembly in PBS at physiological temperature, and DTX was successfully entrapped into the nanoparticles at high concentrations. The reported nanoparticles had the potential for tumor retention by GRP activity, which was demonstrated in vitro using PC-3 cells in flow cytometry experiments. It also significantly reduced the cell viability of PC-3 cells [149].
4.4. 5-Fluorouracil-loaded peptide liposome:
5-fluorouracil (5-FU) is an antimetabilite nucleoside analog used to treat various cancers. Different strategies have been used to facilitate the delivery of 5-FU to the target site as an effective therapeutic approach. In a recent study by S. Lakkadwala and J. Singh, a dual-functionalized liposomal delivery system was assessed to face the major hurdle of transporting the drug to the brain and crossing the BBB. For the enhancement of transport of the anticancer chemotherapeutic drug, 5-FU, across the BBB into the tumor cells, conjugation of the cell-penetrating peptide penetratin to transferrin-liposomes (Tf-Pen-conjugated liposomes) was evaluated. An in vitro cellular uptake study showed that the dual-functionalized liposomes are capable of higher cellular uptake in glioblastoma (U87) and brain endothelial (bEnd.3) cells monolayers. Similarly, higher apoptosis was found in U87 cells due to the action of dual-functionalized liposomes. This dual-functionalized liposome was also tested across a co-cultured endothelial barrier into a scaffold housing 3-d glioblastoma tumors and showed enhanced delivery of this therapeutic agent compared to the control [150].
A fibronectin-mimetic peptide was also used as a targeting agent in a PEGylated stealth liposome to target colon cancer cells. A novel peptide PR_b (peptide-amphiphile sequence) was designed to imitate the cell-attaching domain of fibronectin to target integrin α5β1, which is expressed in various cancer cells including colon cancer. 5-FU was used as a cytotoxic agent in the liposomal formulations as well as a positive control in various studies, which revealed that the formulation was effective in targeted delivery and in delivering the therapeutic load directly to the colon cancer cells [66].
Angiogenic endothelial cells were targeted through YIGSR peptide overexpressing laminin receptors attached in liposomes (YIGSR-SL). Liposome formulation YIGSR-SL showed significant effectiveness against lung angiogenesis and metastasis [151]. A similar study was done with cyclic RGD peptide targeting angiogenic endothelial cells which have avb3 integrins overexpression and deliver the 5-FU to the targeted site [152].
4.5. Paclitaxel-loaded peptide liposome:
Paclitaxel (PTX) is another important drug in cancer chemotherapy. It is an alkaloid that prevents endothelial cell motility, proliferation, and mitotic spindle assembly, and also stabilizes microtubules [67]. The prototype of PTX was from a natural source [68] and has been used for decades in various types of cancer including ovarian, head and neck, breast, and non-small cell lung carcinoma [69]. As of now, numerous peptide-functionalized liposomal formulations containing PTX have been investigated as effective anticancer therapies. A recent study reported by Yin et al. showed that autophagy inhibition could help to reduce tumor growth and increase chemotherapeutic efficacy. The researchers prepared liposomes (PTX/HCQ-R8-dGR-Lip) modified with R8-dGR peptide co-loaded with PTX and hydroxychloroquine (HCQ) for targeted delivery by selective recognition of neuropilin-1 receptors and integrin αvβ3 receptors on B16F10 melanoma cells (Fig. 11). In vitro and in vivo analyses revealed that (PTX/HCQ-R8-dGR-Lip) showed more significant antitumor effect synergistically along with anti-metastatic effect. The results signified that a combination of chemotherapeutic drugs PTX and HCQ with R8-dGR peptide-modified liposomal formulation can have both autophagy-dependent and independent effects that may be useful as a promising treatment for primarily as well as metastatic melanoma [156].
Fig. 11.
Schematic illustration of PTX/HCQ-R8-dGR-Lip delivered into tumor cells. (Reproduced with permission from [156]).
Recently, a dual-active targeting liposomal formulation (Glu6-RGD-Lip) containing PTX focused on bone metastatic breast cancer was studied. For an efficient distribution of PTX into the desired site of action, a unique bone-directed glutamic oligopeptides-RGD peptide (Glu6-RGD) was designed and used as a liposomal ligand. It was found in in vitro and in vivo studies that Glu6-RGD-Lip formulation showed activity superior to that of PTX alone or only single-modified liposomes [56]. Another study with RGD peptide containing PTX co-loaded with curcumin was carried out for the treatment of lung cancer. It was found that a PTX and curcumin co-loaded RGD-modified liposomal formulation showed an improved antitumor effect in vivo compared to non-modified liposomes [57].
To overcome the multiple hurdles faced to deliver the drug into the glioma, one of the aggressive forms of malignancy, a PTX-loaded and TR peptide-modified liposome formulation (PTX-TR-Lip) was investigated. TR peptide is an integrin αvβ3-specific vector with a pH-responsive cell penetration attribute that facilitates permeation across the BBB. The in vitro results showed increased accumulation of TR peptide-conjugated liposome (TR-Lip) in the targeted site through transport across BBB, destruction of VN channels, and destruction of brain cancer stem cell (CSC) and glioma cells. An in vivo study revealed that TR-Lip could substantially increase glioma targeting and elimination of CSC as well as VM channels in tumor tissues. PTX-TR-Lip administration increased mean survival time significantly compared to free PTX or other controls, which signifies its effectiveness as a therapeutic approach to brain glioma [170]. In another study, glioma targeting was also challenged through incorporating cyclic RGD peptide as targeting agent conjugated with a cell-penetrating peptide R8. Cyclic RGD peptide was conjugated to R8 through amide bond to obtain a tandem peptide R8-RGD [RRRRRRRR-c(RGDfK) The study showed that R8-RGD peptide-conjugated liposome (R8-RGD-lipo) had increased cellular uptake (~30-fold) compared to controls. In vivo study showed higher PTX delivery capacity of R8-RGD-lipo in C6 glioma-bearing mice and selective accumulation in glioma foci. The multifunctional peptide R8-RGD showed a unique approach to tackling difficult BBB drug transportation and targeting, while liposomal formulation provided the added advantage of selectivity demonstrated by in vitro and in vivo studies [59].
Pancreatic cancer treatment was evaluated with a dual functional lipid-albumin liposome formulation targeting pancreatic ductile adenocarcinoma (PDAC). PDAC has a glut of cancer-associated fibroblasts (CAFs) producing tumor stroma, which hinder drug delivery into the pancreatic tumor tissue. The small nanoparticle of PTX with albumin (HSA-PTX) that has substantial tumor penetration capacity was encapsulated in a FAP-α-responsive peptide CAP-conjugated thermosensitive liposome (CAP-TSL). Additionally, a photothermal compound IR-780 was incorporated into the liposomal formulation (CAP-ITSL) to give thermosensitive properties. The formulated HSA-PTX-loaded CAP-ITSL formulation produced efficient combinational photothermal chemotherapy, which could facilitate complicated PDAC treatment [157].
Enhanced chemo-immunotherapy was assessed against melanoma through the mechanism of cholesterol esterification inhibition in CD8+ T cells. T-cell receptor development occurs with the help of cholesterol on CD8+ T lymphocytes (CTLs), which is cytotoxic to tumor cells initiating necrosis and apoptosis. pH-sensitive TH peptide-modified PTX and αGC immunoadjuvant co-entrapped liposomes (PTX/αGC-TH-Lip) were prepared, in which an acetyl-CoA acetyltransferase-1 (ACAT-1) inhibitor avasimibe was co-administered as chemo-immunotherapy [158]. The results indicated that the avasimibe and PTX/αGC-TH-Lip combinational therapy is a promising approach to upregulate the antitumor effects of chemo-immunotherapy by decreasing the suppression of CD8+ T cells [158]. PTX and DOX combinations were studied for the targeted treatment of melanoma facilitated by transferrin and TAT-co-functionalized liposomes (Tf/TAT-PTX/DOXLP). In vivo study revealed the highest distribution into the targeted site of Tf/TAT-PTX/DOX-LP compared to other controls. Furthermore, dual PTX and DOX therapy showed a synergistic effect with enhanced therapeutic efficacy in melanoma treatment [159].
A lipophilic cell-penetrating peptide (CPP) with lower toxicity was incorporated-resulting in liposomal formulation PFV-Lip-PTX containing PTX was investigated in breast cancer treatment. In vitro and in vivo studies demonstrated that PFV-Lip-PTX produced reduced systemic toxicity and acted as a potential therapeutic candidate for tumor-targeted therapy in breast cancer treatment [160]. A combined liposomal strategy was reported by Xia et al., where PTX loaded, peptide-functionalized pH-sensitive liposome (PTX-TH-Lip) and losartan-loaded liposomal formulation (LST-Lip) were co-administered. Tumors consisted of a dense collagen circuit, which hinders the transport of drugs into tumors. Losartan can inhibit this collagen network, but it could cause hypotension; consequently, it requires targeted administration that is mediated through liposomal formulation in this study. The results indicated that the combinational strategy could potentially exert enhanced therapeutic efficacy in breast cancer therapy [161].
4.6. Mitoxantrone loaded peptide liposome:
The peptide-functionalized liposomal formulation prepared by using mitoxantrone (MXT) [162], an anthracenedione antineoplastic drug, is commonly used in the treatment of different types of cancer, for example breast cancer, lymphomas, leukemias, and prostate cancer [171]. Studies indicated that MXT showed cardiotoxic potential, although less severe than free DOX due to its diverse mechanism of action [172]. In a recent study, luteinizing hormone-releasing hormone (LHRH) receptor-specific peptide conjugated liposomes containing MXT (LHRH-MTO-LIPs) revealed enhanced anticancer properties with inhibition of breast cancer cell growth in vivo compared with non-targeted MTO-loaded liposomes (MTO-LIPs) and free MXT [162].
5. Diagnostic agent-loaded peptide liposomes
Targeted delivery of diagnostic agents to tumors represents a significant advance in cancer diagnosis. Several studies have reported peptide-functionalized liposomes loaded with diagnostic agents–sometimes along with drug molecules–for cancer diagnosis and therapy. Song et al. reported dual-targeted paramagnetic liposomes functionalized with two angiogenesis-targeting peptide ligands, the αVβ3 integrin-specific RGD (Arg-Gly-Asp) and the neuropilin-1 (NRP-1) receptor-specific ATWLPPR (Ala-Thr-Trp-Leu-Pro-Pro-Arg) (A7R). Effective encapsulation of MRI contrast agent Gd-DTPA (gadolinium-diethylenetriamine pentaacetic acid) was achieved using prepared dual peptide-functionalized liposomes. The results showed that the highest SER (signal enhancement rate) was achieved through using dual targeting liposomes in MR imaging of mice bearing A549 cells in vivo. It was also found that dual-targeted liposomes were likely to exert a synergistic effect on the specificity of delivering Gd-DTPA to the tumor site and might be a potent system for molecular imaging of tumors [163].
RGD peptide-functionalized magnetoliposomes (cRGD-MLs) have shown great potential as magnetic resonance imaging contrast agents and as delivery vehicles for cancer therapy as evaluated by Ribeiro et al. It was found that cRGD-MLs can be visualized by both MRI and fluorescence imaging (FLI), although FLI was less sensitive due to lower depth penetration. Magnetoliposomes (MLs) has potential as a theranostic agent for the follow-up of antivascular therapies as they target tumor neovasculature [173]; however, T2-weighted fast spin echo MRI revealed some limitations due to potential misguiding changes in background signal (T2 values) caused by physiological changes (edema, hemorrhages, etc.) [164].
In a recent study, Panikar et al. reported novel peptide-conjugated ligand-targeted nanoliposomes (LTLs) for chemo-photodynamic therapy against HER2-positive breast cancer. The LTLs carried in their hydrophilic core methylene blue (MB)-attached NaYF4:Yb,Er upconversion nanoparticles (UCNPs) for NIR-activated bioimaging and leveraging visible emission for photoexciting MB for enhanced photodynamic therapy (PDT). In addition, for chemotherapy, it contained DOX in the core as well. The in vitro and in vivo data suggested greater efficacy of LTLs as theranostic agents of breast cancer management [165].
Wang et al. developed a structure-based approach with various computational and analytical techniques to optimize cancer-targeting peptides for molecular imaging and therapy. A targeted L-peptide (12 amino acids) was identified to specifically target GRP78, a specific cancer cell-surface marker. Then the researchers conducted in vitro binding studies with cell lines and clinical cancer specimens, and in vivo tumor imaging and targeted chemotherapeutic studies. MicroSPECT/CT imaging was done to observe the uptake of peptide attached rhenium-188 (188Re) radioisotope conjugated liposomes (188Re-liposomes) and found greater uptake efficiency compared to control group. It was reported that the structure-based optimization strategy for peptides can be an effective tool for developing peptide drugs for cancer imaging and therapy [166]
In another study, a tetradecapeptide bombesin (BBN), which specifically binds to gastrin-releasing peptide receptors in humans, was used to prepare long-circulating and pH-sensitive liposomes (SpHL) containing technetium-99m (99mTc) isotope to produce 99mTc-HYNIC-βAla-Bombesin(7–14) as a radiotracer for breast tumor detection. In vitro and in vivo data suggested that pHLG-99mTc-BBN(7–14) presented promising characteristics suitable for a diagnostic component and could be a potential candidate for tumor identification [168].
A new approach of theranostic (possessing both therapeutic and diagnostic effects) liposomal formulation PTX/SPIO-SSL- H7K(R2)2 was reported containing PTX and iron oxide superparamagnetic nanoparticles (SPIO NPs) with pH-responsive peptide H7K(R2)2 modification where peptide H7K(R2)2 was used as a targeting ligand. SPIO NPs enabled the diagnostic imaging of the tumor site as a magnetic resonance imaging (MRI) agent and PTX worked to render an antitumor effect. PTX/SPIO-SSL-H7K(R2)2 liposomes showed effective antitumor activity with an MRI imaging effect producing dual functionality with a single therapeutic dosage form [20].
6. Limitations and challenges of peptide functionalized liposomes
6.1. Industry-scale production of functionalized liposomes:
Most of the liposomes that are clinically approved and have been manufactured on a large scale are very well characterized, which was possible due to their simple composition and structure. The scenario might not be the same in the large-scale production of these targeted liposomes. The functionalized liposomes have been studied mostly in a small laboratory setting where the product is a small amount. Shifting from the small scale to large scale comes with a massive challenge in the case of functionalized liposomes. The fact that it has been tremendously difficult to quantify the ligand conjugation with the liposomes accurately will undoubtedly lead to batch-to-batch variations in liposomes. This may result in variations in physicochemical properties of liposomes that affect their stability, biodistribution, and pharmacodynamics aspects of the functionalized liposomes [174]. Major challenges in the development of peptide-factionalized liposomes are shown in Fig. 12.
Fig. 12.
Challenges that need to be addressed and overcome in the development of peptide-functionalized targeting liposomal drugs.
6.2. Characterization of functionalized liposomes:
Several methods have been outlined for the characterization of liposomes. The characteristics that have been measured include liposome size and polydispersity by dynamic and static light scattering, surface charge by measuring zeta potential, drug encapsulation efficiency by spectrophotometry techniques, and morphology as well as physical state by cryo-transmission electron microscopy and atomic force microscopy [175]. Proper methods to characterize functionalized ligand-directed liposomes are lacking, making it difficult to control batch-to-batch variability. Moreover, quantifying the exact amount of ligand incorporated is another challenge that may significantly affect the regulatory approval of such liposomes as it is challenging to control or correct the amount of ligand attached to the surface [176]. There is no proper development of biochemical and biophysical assays that can be employed to quantify the peptide or protein incorporated on to the surface [177]. Flow-cytometric approaches have been used to explore the ability to measure peptides and proteins functionalized on liposomes, but this technique has been semi-quantitative at best [178]. Hence, a uniform and well-documented method to characterize these liposomes is very much essential to developing a clinically approved candidate.
6.3. Strict storage conditions:
Liposomes as a formulation need stringent storage conditions. Liposome formulations must be stored in the refrigerator as their integrity is challenged at higher temperatures. Moreover, liposomes cannot be stored in the freezer as the ice crystals formed disintegrate their lipid bilayer [179].
6.4. Aggregation due to high peptide ligand density:
Functionalization strategy is very useful in producing the directly targeting liposomes, but it comes with great challenges. The surface ligand plays a vital role in increased uptake in the desired target site, and the ligand density on the surface of the liposomes must be optimum to get the desired effect. It has been observed that the high ligand density may be responsible for the aggregation of liposomes, which has a detrimental effect on drug delivery. Proper study and optimization of ligand density, type of cancer cells, and shape and size of liposomes are required to get the enhanced effect of functionalized liposomes [180].
6.5. Masking of peptide ligands by polymers:
Coating of the liposomal surface by PEG has dramatically solved the problem of aggregation and also aids in enhancing the blood circulation time of liposomes [78]. However, the use of PEG for surface modification also has several challenges and requires extensive optimization. Different liposomal formulations use a varied range of PEG length and density in the formulation; hence, a proper establishment for the use of optimum PEG in the various liposomal formulation is required. Longer PEGs, due to their tendency to attend globular structure, have a significant masking effect of the ligand interaction with the receptor due to steric hindrance. This problem must be addressed if one elects to use PEG, and it can be done by choosing the proper PEG. The consideration of binding affinity of the ligand to the receptor is also of prime importance [181].
6.6. Serum-protein binding:
The serum proteins are known to bind nonspecifically to the surface of the liposomes, altering their properties significantly. In the case of functionalized liposomes, such a shield of proteins around liposomes and plasma protein binding of ligand will block the binding of the ligand to the intended receptor, thereby reducing the targeting ability of these liposomes. This serum-protein binding will lead to altering pharmacokinetics, biodistribution, pharmacodynamics, and toxicity of liposomes in vivo [182, 183]. These factors must be taken into consideration during the formulation of the functionalized liposomes.
6.7. Lack of accurate tumor models:
Functionalized liposome formulation depends on the type of tumor and its receptor expression, microenvironment, and vasculature system. Most of the targeted liposomes are studied in vitro on the 2D cancer cell model, which is not the perfect replica of the cancer disease model. The information obtained from using such a non-resembling model may ultimately lead to the failure of the liposomes in vivo. 3D tumor culture systems provide multicellular structure to study the liposomes. However, depending on the type of 3D tumor culture system the microenvironment may not be different compared to the 2D cell culture models. 3D models are known to bridge the gap between in vitro and in vivo models for drug screening [184]. The 3D model of cancer cell allows the cell to grow in clusters and layers and forms spheroids that resemble the cancer in vivo to a greater extent. Hence, the functionalized liposomes must be studied more on the 3D disease model to obtain reliable in vitro data. Moreover, the resistant tumor cells and models also need to be considered to obtain physiologically relevant information about the liposomes’ effect on cancer. There are significant interactions between cancer cells and immune system cells to form a tumor microenvironment. Hence, one must select the animal model wisely and choose one that resembles the disease state very closely [185].
7. Conclusion and future directions
Peptide-functionalized liposomal formulations containing chemotherapeutic agents provide successful therapeutic approaches to overcoming difficult drug delivery problems, particularly in drug-resistant cancers. This is also very promising for producing lower toxicity in chemotherapy due to targeting effects and other attributes related to nanomedicines. Recent reported advancements in peptide-conjugated liposomal formulations showed clear evidence that combinational therapies with peptide-functionalized liposomes containing anticancer agents can indeed help to inhibit cancer progression through antitumoral effects compared to drugs, drug-containing liposomes, or peptides alone. Various formulations have been prepared with the peptide-functionalized liposomal system entrapping different anticancer drugs. However, these liposomal formulations are required to face further challenges for industrial production, considering the limitations of liposomal formulations, and must be proven for safety issues. An ideal liposomal formulation needs to protect the entrapped drugs from drug loss and prevent enzymatic degradation or serum binding for the effective treatment of cancer. The in vivo antitumor model needs to be carefully designed so it can depict the effectiveness of the prepared liposomal formulation. Efficient formulation technologies need to be established with large-scale production capability for the peptide-functionalized liposomal system.
With increasing research on peptide-functionalized liposomal systems to treat and diagnose cancer, it can be anticipated that the knowledge of innovative peptide-functionalized liposomal delivery strategies will be enhanced in the future. It is undeniable that the development of peptide-functionalized liposomal systems represents a significant advance in cancer therapy and diagnosis. Future efforts are required to design precisely targeting peptide-functionalized liposomal systems to mitigate adverse outcomes of chemotherapy. Additionally, industrial development in peptide-functionalized liposomal formulations is needed to produce more efficient therapeutic or diagnostic agents to fight against cancer.
Highlights.
Liposome surfaces can be functionalized for target-mediated cancer therapy.
Surface-functionalized liposomes with peptides enhance therapeutic efficacy.
Peptide-functionalized liposomes can be tagged with imaging agents for cancer diagnostic.
Large scale production and storage of liposomes is still challenging
Acknowledgments
Funding
Funding for this project was supported by the National Institute of General Medical Sciences of the National Institute of Health under grant number P20GM10324-18.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA: A Cancer Journal for Clinicians, 66 (2016) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Ferrari M, Frontiers in cancer nanomedicine: directing mass transport through biological barriers, Trends Biotechnol, 28 (2010) 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guarnieri D, Falanga A, Muscetti O, Tarallo R, Fusco S, Galdiero M, Galdiero S, Netti PA, Shuttle-mediated nanoparticle delivery to the blood-brain barrier, Small, 9 (2013) 853–862. [DOI] [PubMed] [Google Scholar]
- [4].Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y, Engineered nanoparticles for drug delivery in cancer therapy, Angew Chem Int Ed Engl, 53 (2014) 12320–12364. [DOI] [PubMed] [Google Scholar]
- [5].Lim E-K, Kim T, Paik S, Haam S, Huh Y-M, Lee K, Nanomaterials for Theranostics: Recent Advances and Future Challenges, Chemical Reviews, 115 (2015) 327–394. [DOI] [PubMed] [Google Scholar]
- [6].Torchilin VP, Recent advances with liposomes as pharmaceutical carriers, Nature Reviews Drug Discovery, 4 (2005) 145–145. [DOI] [PubMed] [Google Scholar]
- [7].Johnston MJW, Semple SC, Klimuk SK, Ansell S, Maurer N, Cullis PR, Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin, Biochimica et Biophysica Acta (BBA) - Biomembranes, 1768 (2007) 1121–1127. [DOI] [PubMed] [Google Scholar]
- [8].Hofheinz R-D, Gnad-Vogt SU, Beyer U, Hochhaus A, Liposomal encapsulated anti-cancer drugs, Anti-Cancer Drugs, 16 (2005). [DOI] [PubMed] [Google Scholar]
- [9].Omri A, Suntres ZE, Shek PN, Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection, Biochemical Pharmacology, 64 (2002) 1407–1413. [DOI] [PubMed] [Google Scholar]
- [10].Schiffelers RM, Storm G, Bakker-Woudenberg IAJM, Host Factors Influencing the Preferential Localization of Sterically Stabilized Liposomes in Klebsiella Pneumoniae-Infected Rat Lung Tissue, Pharmaceutical Research, 18 (2001) 780–787. [DOI] [PubMed] [Google Scholar]
- [11].Stano P, Bufali S, Pisano C, Bucci F, Barbarino M, Santaniello M, Carminati P, Luisi PL, Novel Camptothecin Analogue (Gimatecan)‐Containing Liposomes Prepared by the Ethanol Injection Method, Journal of Liposome Research, 14 (2004) 87–109. [DOI] [PubMed] [Google Scholar]
- [12].Torchilin V, Multifunctional and stimuli-sensitive pharmaceutical nanocarriers, European Journal of Pharmaceutics and Biopharmaceutics, 71 (2009) 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tran MA, Watts RJ, Robertson GP, Use of liposomes as drug delivery vehicles for treatment of melanoma, Pigment cell & melanoma research, 22 (2009) 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lopes S.C.d.A. , Liposomes as Carriers of Anticancer Drugs, in: Giuberti C.d.S (Ed.), IntechOpen, Rijeka, 2013, pp. Ch. 4-Ch. 4. [Google Scholar]
- [15].Danhier F, Feron O, Preat V, To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery, J Control Release, 148 (2010) 135–146. [DOI] [PubMed] [Google Scholar]
- [16].Ringhieri P, Mannucci S, Conti G, Nicolato E, Fracasso G, Marzola P, Morelli G, Accardo A, Liposomes derivatized with multimeric copies of KCCYSL peptide as targeting agents for HER-2-overexpressing tumor cells, Int J Nanomedicine, 12 (2017) 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Han Q, Jia X, Qian Y, Wang Z, Yang S, Jia Y, Wang W, Hu Z, Peptide functionalized targeting liposomes: for nanoscale drug delivery towards angiogenesis, Journal of Materials Chemistry B, 4 (2016) 7087–7091. [DOI] [PubMed] [Google Scholar]
- [18].Molavipordanjani S, Hosseinimehr SJ, Strategies for Conjugation of Biomolecules to Nanoparticles as Tumor Targeting Agents, Curr Pharm Des, 25 (2019) 3917–3926. [DOI] [PubMed] [Google Scholar]
- [19].Lee KJ, Shin SH, Lee JH, Ju EJ, Park YY, Hwang JJ, Suh YA, Hong SM, Jang SJ, Lee JS, Song SY, Jeong SY, Choi EK, A strategy for actualization of active targeting nanomedicine practically functioning in a living body, Biomaterials, 141 (2017) 136–148. [DOI] [PubMed] [Google Scholar]
- [20].Zheng XC, Ren W, Zhang S, Zhong T, Duan XC, Yin YF, Xu MQ, Hao YL, Li ZT, Li H, Liu M, Li ZY, Zhang X, The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles, Int J Nanomedicine, 13 (2018) 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Perche F, Torchilin VP, Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting, J Drug Deliv, 2013 (2013) 705265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Al-Jamal WT, Kostarelos K, Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine, Acc Chem Res, 44 (2011) 1094–1104. [DOI] [PubMed] [Google Scholar]
- [23].Accardo A, Morelli G, Review peptide-targeted liposomes for selective drug delivery: Advantages and problematic issues, Biopolymers, 104 (2015) 462–479. [DOI] [PubMed] [Google Scholar]
- [24].Wu HC, Chang DK, Peptide-mediated liposomal drug delivery system targeting tumor blood vessels in anticancer therapy, J Oncol, 2010 (2010) 723798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rezler EM, Khan DR, Tu R, Tirrell M, Fields GB, Peptide-mediated targeting of liposomes to tumor cells, Methods Mol Biol, 386 (2007) 269–298. [DOI] [PubMed] [Google Scholar]
- [26].Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK, Approaches for enhancing oral bioavailability of peptides and proteins, International Journal of Pharmaceutics, 447 (2013) 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].O’Hagan DT, McGee JP, Holmgren J, Mowat AM, Donachie AM, Mills KHG, Gaisford W, Rahman D, Challacombe SJ, Biodegradable microparticles for oral immunization, Vaccine, 11 (1993) 149–154. [DOI] [PubMed] [Google Scholar]
- [28].O’Hagan DT, Rahman D, McGee JP, Jeffery H, Davies MC, Williams P, Davis SS, Challacombe SJ, Biodegradable microparticles as controlled release antigen delivery systems, Immunology, 73 (1991) 239–242. [PMC free article] [PubMed] [Google Scholar]
- [29].Kreuter J, Colloidal drug delivery systems, CRC Press, 2014. [Google Scholar]
- [30].Yun Y, Cho YW, Park K, Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery, Advanced drug delivery reviews, 65 (2013) 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Singh R, Lillard JW Jr, Nanoparticle-based targeted drug delivery, Experimental and molecular pathology, 86 (2009) 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Munye MM, Ravi J, Tagalakis AD, McCarthy D, Ryadnov MG, Hart SL, Role of liposome and peptide in the synergistic enhancement of transfection with a lipopolyplex vector, Sci Rep, 5 (2015) 9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Olusanya TOB, Haj Ahmad RR, Ibegbu DM, Smith JR, Elkordy AA, Liposomal Drug Delivery Systems and Anticancer Drugs, Molecules (Basel, Switzerland), 23 (2018) 907–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Briuglia ML, Rotella C, McFarlane A, Lamprou DA , Influence of cholesterol on liposome stability and on in vitro drug release, Drug Deliv Transl Res, 5 (2015) 231–242. [DOI] [PubMed] [Google Scholar]
- [35].Demel RA, De Kruyff B, The function of sterols in membranes, Biochim Biophys Acta, 457 (1976) 109–132. [DOI] [PubMed] [Google Scholar]
- [36].Liu W, Wei F, Ye A, Tian M, Han J, Kinetic stability and membrane structure of liposomes during in vitro infant intestinal digestion: Effect of cholesterol and lactoferrin, Food Chem, 230 (2017) 6–13. [DOI] [PubMed] [Google Scholar]
- [37].Cogan U, Shinitzky M, Weber G, Nishida T, Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes, Biochemistry, 12 (1973) 521–528. [DOI] [PubMed] [Google Scholar]
- [38].Kaddah S, Khreich N, Kaddah F, Charcosset C, Greige-Gerges H, Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule, Food Chem Toxicol, 113 (2018) 40–48. [DOI] [PubMed] [Google Scholar]
- [39].Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, Szebeni J, Ishida T, PEGylated liposomes: immunological responses, Sci Technol Adv Mater, 20 (2019) 710–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Riaz KM, Riaz AM, Zhang X, Lin C, Wong HK, Chen X, Zhang G, Lu A, Yang Z, Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review, International Journal of Molecular Sciences, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chang CC, Liu DZ, Lin SY, Liang HJ, Hou WC, Huang WJ, Chang CH, Ho FM, Liang YC, Liposome encapsulation reduces cantharidin toxicity, Food Chem Toxicol, 46 (2008) 3116–3121. [DOI] [PubMed] [Google Scholar]
- [42].Accardo A, Mangiapia G, Paduano L, Morelli G, Tesauro D, Octreotide labeled aggregates containing platinum complexes as nanovectors for drug delivery, J Pept Sci, 19 (2013) 190–197. [DOI] [PubMed] [Google Scholar]
- [43].Dai W, Jin W, Zhang J, Wang X, Wang J, Zhang X, Wan Y, Zhang Q, Spatiotemporally controlled co-delivery of anti-vasculature agent and cytotoxic drug by octreotide-modified stealth liposomes, Pharm Res, 29 (2012) 2902–2911. [DOI] [PubMed] [Google Scholar]
- [44].Accardo A, Mansi R, Salzano G, Morisco A, Aurilio M, Parisi A, Maione F, Cicala C, Ziaco B, Tesauro D, Aloj L, De Rosa G, Morelli G, Bombesin peptide antagonist for target-selective delivery of liposomal doxorubicin on cancer cells, Journal of Drug Targeting, 21 (2013) 240–249. [DOI] [PubMed] [Google Scholar]
- [45].Accardo A, Salsano G, Morisco A, Aurilio M, Parisi A, Maione F, Cicala C, Tesauro D, Aloj L, De Rosa G, Morelli G, Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: a potential theranostic agent, Int J Nanomedicine, 7 (2012) 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morisco A, Accardo A, Tesauro D, Palumbo R, Benedetti E, Morelli G, Peptide-labeled supramolecular aggregates as selective doxorubicin carriers for delivery to tumor cells, Biopolymers, 96 (2011) 88–96. [DOI] [PubMed] [Google Scholar]
- [47].Reubi JC, Peptide receptors as molecular targets for cancer diagnosis and therapy, Endocr Rev, 24 (2003) 389–427. [DOI] [PubMed] [Google Scholar]
- [48].Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A, Liposomal encapsulated anti-cancer drugs, Anticancer Drugs, 16 (2005) 691–707. [DOI] [PubMed] [Google Scholar]
- [49].Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM, A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy, Cancer Res, 68 (2008) 5878–5887. [DOI] [PubMed] [Google Scholar]
- [50].Hare JI, Moase EH, Allen TM, Targeting combinations of liposomal drugs to both tumor vasculature cells and tumor cells for the treatment of HER2-positive breast cancer, J Drug Target, 21 (2013) 87–96. [DOI] [PubMed] [Google Scholar]
- [51].Song S, Liu D, Peng J, Deng H, Guo Y, Xu LX, Miller AD, Xu Y, Novel peptide ligand directs liposomes toward EGF-R high-expressing cancer cells in vitro and in vivo, FASEB J, 23 (2009) 1396–1404. [DOI] [PubMed] [Google Scholar]
- [52].Kumar SR, Gallazzi FA, Ferdani R, Anderson CJ, Quinn TP, Deutscher SL, In vitro and in vivo evaluation of (6)(4)Cu-radiolabeled KCCYSL peptides for targeting epidermal growth factor receptor-2 in breast carcinomas, Cancer Biother Radiopharm, 25 (2010) 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kumar SR, Quinn TP, Deutscher SL, Evaluation of an 111In-radiolabeled peptide as a targeting and imaging agent for ErbB-2 receptor expressing breast carcinomas, Clin Cancer Res, 13 (2007) 6070–6079. [DOI] [PubMed] [Google Scholar]
- [54].Deutscher SL, Figueroa SD, Kumar SR, In-labeled KCCYSL peptide as an imaging probe for ErbB-2-expressing ovarian carcinomas, J Labelled Comp Radiopharm, 52 (2009) 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bhunia D, Saha A, Adak A, Das G, Ghosh S, A dual functional liposome specifically targets melanoma cells through integrin and ephrin receptors, RSC Advances, 6 (2016) 113487–113491. [Google Scholar]
- [56].Zhao Z, Zhao Y, Xie C, Chen C, Lin D, Wang S, Lin D, Cui X, Guo Z, Zhou J, Dual-active targeting liposomes drug delivery system for bone metastatic breast cancer: Synthesis and biological evaluation, Chem Phys Lipids, 223 (2019) 104785. [DOI] [PubMed] [Google Scholar]
- [57].Jiang K, Shen M, Xu W, Arginine, glycine, aspartic acid peptide-modified paclitaxel and curcumin co-loaded liposome for the treatment of lung cancer: in vitro/vivo evaluation, Int J Nanomedicine, 13 (2018) 2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shi K, Long Y, Xu C, Wang Y, Qiu Y, Yu Q, Liu Y, Zhang Q, Gao H, Zhang Z, He Q, Liposomes Combined an Integrin αvβ3-Specific Vector with pH-Responsible Cell-Penetrating Property for Highly Effective Antiglioma Therapy through the Blood–Brain Barrier, ACS Applied Materials & Interfaces, 7 (2015) 21442–21454. [DOI] [PubMed] [Google Scholar]
- [59].Liu Y, Ran R, Chen J, Kuang Q, Tang J, Mei L, Zhang Q, Gao H, Zhang Z, He Q, Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting, Biomaterials, 35 (2014) 4835–4847. [DOI] [PubMed] [Google Scholar]
- [60].Zhang C, Cai R, Lazerson A, Delcroix G, Wangpaichitr M, Mirsaeidi M, Griswold AJ, Schally AV, Jackson RM, Growth Hormone-Releasing Hormone Receptor Antagonist Modulates Lung Inflammation and Fibrosis due to Bleomycin, Lung, 197 (2019) 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Amin M, Badiee A, Jaafari MR, Improvement of pharmacokinetic and antitumor activity of PEGylated liposomal doxorubicin by targeting with N-methylated cyclic RGD peptide in mice bearing C-26 colon carcinomas, Int J Pharm, 458 (2013) 324–333. [DOI] [PubMed] [Google Scholar]
- [62].Battistini L, Burreddu P, Sartori A, Arosio D, Manzoni L, Paduano L, D’Errico G, Sala R, Reia L, Bonomini S, Rassu G, Zanardi F, Enhancement of the uptake and cytotoxic activity of doxorubicin in cancer cells by novel cRGD-semipeptide-anchoring liposomes, Mol Pharm, 11 (2014) 2280–2293. [DOI] [PubMed] [Google Scholar]
- [63].Zong T, Mei L, Gao H, Cai W, Zhu P, Shi K, Chen J, Wang Y, Gao F, He Q, Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals, Mol Pharm, 11 (2014) 2346–2357. [DOI] [PubMed] [Google Scholar]
- [64].Wang F, Chen L, Zhang R, Chen Z, Zhu L, RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer, Journal of Controlled Release, 196 (2014) 222–233. [DOI] [PubMed] [Google Scholar]
- [65].Li Y, Zheng X, Sun Y, Ren Z, Li X, Cui G, RGD-fatty alcohol-modified docetaxel liposomes improve tumor selectivity in vivo, Int J Pharm, 468 (2014) 133–141. [DOI] [PubMed] [Google Scholar]
- [66].Garg A, Tisdale AW, Haidari E, Kokkoli E, Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide, Int J Pharm, 366 (2009) 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Torchilin V, Tumor delivery of macromolecular drugs based on the EPR effect, Adv Drug Deliv Rev, 63 (2011) 131–135. [DOI] [PubMed] [Google Scholar]
- [68].Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK, Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment, Proc Natl Acad Sci U S A, 95 (1998) 4607–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jain RK, Transport of molecules, particles, and cells in solid tumors, Annu Rev Biomed Eng, 1 (1999) 241–263. [DOI] [PubMed] [Google Scholar]
- [70].Balkwill FR, Capasso M, Hagemann T, The tumor microenvironment at a glance, J Cell Sci, 125 (2012) 5591–5596. [DOI] [PubMed] [Google Scholar]
- [71].Mbeunkui F, Johann DJ Jr., Cancer and the tumor microenvironment: a review of an essential relationship, Cancer Chemother Pharmacol, 63 (2009) 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ruoslahti E, Specialization of tumour vasculature, Nat Rev Cancer, 2 (2002) 83–90. [DOI] [PubMed] [Google Scholar]
- [73].Mura M, Swain RK, Zhuang X, Vorschmitt H, Reynolds G, Durant S, Beesley JFJ, Herbert JMJ, Sheldon H, Andre M, Sanderson S, Glen K, Luu NT, McGettrick HM, Antczak P, Falciani F, Nash GB, Nagy ZS, Bicknell R, Identification and angiogenic role of the novel tumor endothelial marker CLEC14A, Oncogene, 31 (2012) 293–305. [DOI] [PubMed] [Google Scholar]
- [74].Byrne JD, Betancourt T, Brannon-Peppas L, Active targeting schemes for nanoparticle systems in cancer therapeutics, Adv Drug Deliv Rev, 60 (2008) 1615–1626. [DOI] [PubMed] [Google Scholar]
- [75].Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK, Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer, Mater Sci Eng C Mater Biol Appl, 98 (2019) 1252–1276. [DOI] [PubMed] [Google Scholar]
- [76].Dai Y, Xu C, Sun X, Chen X, Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment, Chem Soc Rev, 46 (2017) 3830–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat Biotechnol, 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gabizon AA, Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet, Clin Cancer Res, 7 (2001) 223–225. [PubMed] [Google Scholar]
- [79].Steichen SD, Caldorera-Moore M, Peppas NA, A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics, Eur J Pharm Sci, 48 (2013) 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kobayashi H, Watanabe R, Choyke PL, Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target?, Theranostics, 4 (2014) 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR, Advanced drug delivery systems that target the vascular endothelium, Mol Interv, 6 (2006) 98–112. [DOI] [PubMed] [Google Scholar]
- [82].Koning GA, Storm G, Targeted drug delivery systems for the intracellular delivery of macromolecular drugs, Drug Discov Today, 8 (2003) 482–483. [DOI] [PubMed] [Google Scholar]
- [83].Metselaar JM, Storm G, Liposomes in the treatment of inflammatory disorders, Expert Opin Drug Deliv, 2 (2005) 465–476. [DOI] [PubMed] [Google Scholar]
- [84].Lee Y, Thompson DH, Stimuli-responsive liposomes for drug delivery, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Felber AE, Dufresne MH, Leroux JC, pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates, Adv Drug Deliv Rev, 64 (2012) 979–992. [DOI] [PubMed] [Google Scholar]
- [86].Soares DC, de Oliveira MC, dos Santos RG, Andrade MS, Vilela JM, Cardoso VN, Ramaldes GA, Liposomes radiolabeled with 159Gd-DTPA-BMA: preparation, physicochemical characterization, release profile and in vitro cytotoxic evaluation, Eur J Pharm Sci, 42 (2011) 462–469. [DOI] [PubMed] [Google Scholar]
- [87].Zhao Y, Ren W, Zhong T, Zhang S, Huang D, Guo Y, Yao X, Wang C, Zhang WQ, Zhang X, Zhang Q, Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity, J Control Release, 222 (2016) 56–66. [DOI] [PubMed] [Google Scholar]
- [88].Almurshedi AS, Radwan M, Omar S, Alaiya AA, Badran MM, Elsaghire H, Saleem IY, Hutcheon GA, A novel pH-sensitive liposome to trigger delivery of afatinib to cancer cells: Impact on lung cancer therapy, Journal of Molecular Liquids, 259 (2018) 154–166. [Google Scholar]
- [89].Turk MJ, Reddy JA, Chmielewski JA, Low PS, Characterization of a novel pH-sensitive peptide that enhances drug release from folate-targeted liposomes at endosomal pHs, Biochimica et Biophysica Acta (BBA)-Biomembranes, 1559 (2002) 56–68. [DOI] [PubMed] [Google Scholar]
- [90].Lee Y, Thompson DH, Stimuli-responsive liposomes for drug delivery, WIREs Nanomedicine and Nanobiotechnology, 9 (2017) e1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ, In vivo imaging of extracellular pH using 1H MRSI, Magn Reson Med, 41 (1999) 743–750. [DOI] [PubMed] [Google Scholar]
- [92].Cardone RA, Casavola V, Reshkin SJ, The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis, Nat Rev Cancer, 5 (2005) 786–795. [DOI] [PubMed] [Google Scholar]
- [93].Feron O, Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells, Radiother Oncol, 92 (2009) 329–333. [DOI] [PubMed] [Google Scholar]
- [94].Ding Y, Cui W, Sun D, Wang GL, Hei Y, Meng S, Chen JH, Xie Y, Wang ZQ, In vivo study of doxorubicin-loaded cell-penetrating peptide-modified pH-sensitive liposomes: biocompatibility, bio-distribution, and pharmacodynamics in BALB/c nude mice bearing human breast tumors, Drug Des Devel Ther, 11 (2017) 3105–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang Q, Lu L, Zhang L, Shi K, Cun X, Yang Y, Liu Y, Gao H, He Q, Dual-functionalized liposomal delivery system for solid tumors based on RGD and a pH-responsive antimicrobial peptide, Sci Rep, 6 (2016) 19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mozhi A, Ahmad I, Okeke CI, Li C, Liang X-J, pH-sensitive polymeric micelles for the Co-delivery of proapoptotic peptide and anticancer drug for synergistic cancer therapy, RSC Advances, 7 (2017) 12886–12896. [Google Scholar]
- [97].Deshpande PP, Biswas S, Torchilin VP, Current trends in the use of liposomes for tumor targeting, Nanomedicine (Lond), 8 (2013) 1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhu L, Torchilin VP, Stimulus-responsive nanopreparations for tumor targeting, Integr Biol (Camb), 5 (2013) 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chountoulesi M, Kyrili A, Pippa N, Meristoudi A, Pispas S, Demetzos C, The modulation of physicochemical characterization of innovative liposomal platforms: the role of the grafted thermoresponsive polymers, Pharm Dev Technol, 22 (2017) 330–335. [DOI] [PubMed] [Google Scholar]
- [100].Yingchoncharoen P, Kalinowski DS, Richardson DR, Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come, Pharmacol Rev, 68 (2016) 701–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Schroeder A, Kost J, Barenholz Y, Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes, Chem Phys Lipids, 162 (2009) 1–16. [DOI] [PubMed] [Google Scholar]
- [102].Paris JL, Cabanas MV, Manzano M, Vallet-Regi M, Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers, ACS Nano, 9 (2015) 11023–11033. [DOI] [PubMed] [Google Scholar]
- [103].Xie X, Lin W, Liu H, Deng J, Chen Y, Liu H, Fu X, Yang Y, Ultrasound-responsive nanobubbles contained with peptide-camptothecin conjugates for targeted drug delivery, Drug Deliv, 23 (2016) 2756–2764. [DOI] [PubMed] [Google Scholar]
- [104].Lin W, Xie X, Deng J, Liu H, Chen Y, Fu X, Liu H, Yang Y, Cell-penetrating peptide-doxorubicin conjugate loaded NGR-modified nanobubbles for ultrasound triggered drug delivery, J Drug Target, 24 (2016) 134–146. [DOI] [PubMed] [Google Scholar]
- [105].Arias JL, Drug targeting strategies in cancer treatment: an overview, Mini Rev Med Chem, 11 (2011) 1–17. [DOI] [PubMed] [Google Scholar]
- [106].Mok H, Zhang M, Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics, Expert Opin Drug Deliv, 10 (2013) 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Meers P, Enzyme-activated targeting of liposomes, Adv Drug Deliv Rev, 53 (2001) 265–272. [DOI] [PubMed] [Google Scholar]
- [108].Gialeli C, Theocharis AD, Karamanos NK, Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting, FEBS J, 278 (2011) 16–27. [DOI] [PubMed] [Google Scholar]
- [109].Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D, Multistage nanoparticle delivery system for deep penetration into tumor tissue, Proc Natl Acad Sci U S A, 108 (2011) 2426–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhu L, Kate P, Torchilin VP, Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting, ACS Nano, 6 (2012) 3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Song SJ, Lee S, Lee Y, Choi JS, Enzyme-responsive destabilization of stabilized plasmid-lipid nanoparticles as an efficient gene delivery, Eur J Pharm Sci, 91 (2016) 20–30. [DOI] [PubMed] [Google Scholar]
- [112].Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, Guo D, Fu L, Cui Y, Liu X, Arledge KC, Chen YH, Zhang L, Wang X, Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4, Cell Res, 23 (2013) 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jo SM, Lee HY, Kim JC, Glucose-sensitivity of liposomes incorporating conjugates of glucose oxidase and poly(N-isopropylacrylamide-co-methacrylic acid-co-octadecylacrylate), Int J Biol Macromol, 45 (2009) 421–426. [DOI] [PubMed] [Google Scholar]
- [114].Guillen KP, Kurkjian C, Harrison RG, Targeted enzyme prodrug therapy for metastatic prostate cancer - a comparative study of L-methioninase, purine nucleoside phosphorylase, and cytosine deaminase, J Biomed Sci, 21 (2014) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ding C, Tong L, Feng J, Fu J, Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment, Molecules, 21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Goldenbogen B, Brodersen N, Gramatica A, Loew M, Liebscher J, Herrmann A, Egger H, Budde B, Arbuzova A, Reduction-sensitive liposomes from a multifunctional lipid conjugate and natural phospholipids: reduction and release kinetics and cellular uptake, Langmuir, 27 (2011) 10820–10829. [DOI] [PubMed] [Google Scholar]
- [117].Aznar E, Oroval M, Pascual L, Murguia JR, Martinez-Manez R, Sancenon F, Gated Materials for On-Command Release of Guest Molecules, Chem Rev, 116 (2016) 561–718. [DOI] [PubMed] [Google Scholar]
- [118].Bisby RH, Mead C, Morgan CG, Active uptake of drugs into photosensitive liposomes and rapid release on UV photolysis, Photochem Photobiol, 72 (2000) 57–61. [DOI] [PubMed] [Google Scholar]
- [119].Mei X, Yang S, Chen D, Li N, Li H, Xu Q, Ge J, Lu J, Light-triggered reversible assemblies of azobenzene-containing amphiphilic copolymer with beta-cyclodextrin-modified hollow mesoporous silica nanoparticles for controlled drug release, Chem Commun (Camb), 48 (2012) 10010–10012. [DOI] [PubMed] [Google Scholar]
- [120].Ducat E, Deprez J, Gillet A, Noel A, Evrard B, Peulen O, Piel G, Nuclear delivery of a therapeutic peptide by long circulating pH-sensitive liposomes: benefits over classical vesicles, Int J Pharm, 420 (2011) 319–332. [DOI] [PubMed] [Google Scholar]
- [121].Riaz MK, Riaz MA, Zhang X, Lin C, Wong KH, Chen X, Zhang G, Lu A, Yang Z, Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review, Int J Mol Sci, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shariat S, Badiee A, Jalali SA, Mansourian M, Yazdani M, Mortazavi SA, Jaafari MR, P5 HER2/neu-derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer, Cancer Lett, 355 (2014) 54–60. [DOI] [PubMed] [Google Scholar]
- [123].Chang DK, Li PC, Lu RM, Jane WN, Wu HC, Peptide-mediated liposomal Doxorubicin enhances drug delivery efficiency and therapeutic efficacy in animal models, PLoS One, 8 (2013) e83239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Juang V, Chang CH, Wang CS, Wang HE, Lo YL, pH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and microRNA to Enhance Tumor-Specific Therapy, Small, 15 (2019) e1903296. [DOI] [PubMed] [Google Scholar]
- [125].Garg A, Kokkoli E, pH-Sensitive PEGylated liposomes functionalized with a fibronectin-mimetic peptide show enhanced intracellular delivery to colon cancer cell, Curr Pharm Biotechnol, 12 (2011) 1135–1143. [DOI] [PubMed] [Google Scholar]
- [126].Negussie AH, Miller JL, Reddy G, Drake SK, Wood BJ, Dreher MR, Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome, J Control Release, 143 (2010) 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yan F, Wang S, Yang W, Goldberg SN, Wu H, Duan WL, Deng ZT, Han HB, Zheng HR, Tumor-penetrating Peptide-integrated Thermally Sensitive Liposomal Doxorubicin Enhances Efficacy of Radiofrequency Ablation in Liver Tumors, Radiology, 285 (2017) 462–471. [DOI] [PubMed] [Google Scholar]
- [128].Yang Y, Xie X, Xu X, Xia X, Wang H, Li L, Dong W, Ma P, Yang Y, Liu Y, Mei X, Thermal and magnetic dual-responsive liposomes with a cell-penetrating peptide-siRNA conjugate for enhanced and targeted cancer therapy, Colloids Surf B Biointerfaces, 146 (2016) 607–615. [DOI] [PubMed] [Google Scholar]
- [129].Kim MS, Lee DW, Park K, Park SJ, Choi EJ, Park ES, Kim HR, Temperature-triggered tumor-specific delivery of anticancer agents by cRGD-conjugated thermosensitive liposomes, Colloids Surf B Biointerfaces, 116 (2014) 17–25. [DOI] [PubMed] [Google Scholar]
- [130].Yang Y, Yang Y, Xie X, Cai X, Zhang H, Gong W, Wang Z, Mei X, PEGylated liposomes with NGR ligand and heat-activable cell-penetrating peptide-doxorubicin conjugate for tumor-specific therapy, Biomaterials, 35 (2014) 4368–4381. [DOI] [PubMed] [Google Scholar]
- [131].Wang C, Wang X, Zhong T, Zhao Y, Zhang WQ, Ren W, Huang D, Zhang S, Guo Y, Yao X, Tang YQ, Zhang X, Zhang Q, The antitumor activity of tumor-homing peptide-modified thermosensitive liposomes containing doxorubicin on MCF-7/ADR: in vitro and in vivo, Int J Nanomedicine, 10 (2015) 2229–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Deng Z, Xiao Y, Pan M, Li F, Duan W, Meng L, Liu X, Yan F, Zheng H, Hyperthermia-triggered drug delivery from iRGD-modified temperature-sensitive liposomes enhances the anti-tumor efficacy using high intensity focused ultrasound, J Control Release, 243 (2016) 333–341. [DOI] [PubMed] [Google Scholar]
- [133].Hamano N, Negishi Y, Omata D, Takahashi Y, Manandhar M, Suzuki R, Maruyama K, Nomizu M, Aramaki Y, Bubble liposomes and ultrasound enhance the antitumor effects of AG73 liposomes encapsulating antitumor agents, Mol Pharm, 10 (2013) 774–779. [DOI] [PubMed] [Google Scholar]
- [134].Park SM, Kim MS, Park SJ, Park ES, Choi KS, Kim YS, Kim HR, Novel temperature-triggered liposome with high stability: formulation, in vitro evaluation, and in vivo study combined with high-intensity focused ultrasound (HIFU), J Control Release, 170 (2013) 373–379. [DOI] [PubMed] [Google Scholar]
- [135].Xie X, Lin W, Li M, Yang Y, Deng J, Liu H, Chen Y, Fu X, Liu H, Yang Y, Efficient siRNA Delivery Using Novel Cell-Penetrating Peptide-siRNA Conjugate-Loaded Nanobubbles and Ultrasound, Ultrasound Med Biol, 42 (2016) 1362–1374. [DOI] [PubMed] [Google Scholar]
- [136].Lin W, Xie X, Yang Y, Fu X, Liu H, Yang Y, Deng J, Thermosensitive magnetic liposomes with doxorubicin cell-penetrating peptides conjugate for enhanced and targeted cancer therapy, Drug Deliv, 23 (2016) 3436–3443. [DOI] [PubMed] [Google Scholar]
- [137].Zhang L, Wang Y, Yang Y, Liu Y, Ruan S, Zhang Q, Tai X, Chen J, Xia T, Qiu Y, Gao H, He Q, High Tumor Penetration of Paclitaxel Loaded pH Sensitive Cleavable Liposomes by Depletion of Tumor Collagen I in Breast Cancer, ACS Appl Mater Interfaces, 7 (2015) 9691–9701. [DOI] [PubMed] [Google Scholar]
- [138].Olsman M, Sereti V, Andreassen K, Snipstad S, van Wamel A, Eliasen R, Berg S, Urquhart AJ, Andresen TL, Davies CL, Ultrasound-mediated delivery enhances therapeutic efficacy of MMP sensitive liposomes, J Control Release, 325 (2020) 121–134. [DOI] [PubMed] [Google Scholar]
- [139].Deshpande P, Jhaveri A, Pattni B, Biswas S, Torchilin V, Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer, Drug Deliv, 25 (2018) 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Um W, Kwon S, You DG, Cha JM, Kim HR, Park JH, Non-thermal acoustic treatment as a safe alternative to thermosensitive liposome-involved hyperthermia for cancer therapy, RSC Advances, 7 (2017) 29618–29625. [Google Scholar]
- [141].Dai W, Yang T, Wang Y, Wang X, Wang J, Zhang X, Zhang Q, Peptide PHSCNK as an integrin alpha5beta1 antagonist targets stealth liposomes to integrin-overexpressing melanoma, Nanomedicine, 8 (2012) 1152–1161. [DOI] [PubMed] [Google Scholar]
- [142].Han W, Yin G, Pu X, Chen X, Liao X, Huang Z, Glioma targeted delivery strategy of doxorubicin-loaded liposomes by dual-ligand modification, Journal of Biomaterials Science, Polymer Edition, 28 (2017) 1695–1712. [DOI] [PubMed] [Google Scholar]
- [143].Wei X, Gao J, Zhan C, Xie C, Chai Z, Ran D, Ying M, Zheng P, Lu W, Liposome-based glioma targeted drug delivery enabled by stable peptide ligands, J Control Release, 218 (2015) 13–21. [DOI] [PubMed] [Google Scholar]
- [144].Okamoto A, Asai T, Ryu S, Ando H, Maeda N, Dewa T, Oku N, Enhanced Efficacy of Doxorubicin by microRNA-499-Mediated Improvement of Tumor Blood Flow, J Clin Med, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Su T, Long Y, Deng C, Feng L, Zhang X, Chen Z, Li C, Construction of a two-in-one liposomal system (TWOLips) for tumor-targeted combination therapy, International Journal of Pharmaceutics, 476 (2014) 241–252. [DOI] [PubMed] [Google Scholar]
- [146].Yoon HY, Kwak SS, Jang MH, Kang MH, Sung SW, Kim CH, Kim SR, Yeom DW, Kang MJ, Choi YW, Docetaxel-loaded RIPL peptide (IPLVVPLRRRRRRRRC)-conjugated liposomes: Drug release, cytotoxicity, and antitumor efficacy, Int J Pharm, 523 (2017) 229–237. [DOI] [PubMed] [Google Scholar]
- [147].Patel K, Doddapaneni R, Sekar V, Chowdhury N, Singh M, Combination Approach of YSA Peptide Anchored Docetaxel Stealth Liposomes with Oral Antifibrotic Agent for the Treatment of Lung Cancer, Molecular Pharmaceutics, 13 (2016) 2049–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yang ZZ, Li JQ, Wang ZZ, Dong DW, Qi XR, Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas, Biomaterials, 35 (2014) 5226–5239. [DOI] [PubMed] [Google Scholar]
- [149].Zhang W, Song Y, Eldi P, Guo X, Hayball JD, Garg S, Albrecht H, Targeting prostate cancer cells with hybrid elastin-like polypeptide/liposome nanoparticles, Int J Nanomedicine, 13 (2018) 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lakkadwala S, Singh J, Dual Functionalized 5-Fluorouracil Liposomes as Highly Efficient Nanomedicine for Glioblastoma Treatment as Assessed in an In Vitro Brain Tumor Model, J Pharm Sci, 107 (2018) 2902–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Dubey PK, Singodia D, Vyas SP, Liposomes modified with YIGSR peptide for tumor targeting, Journal of Drug Targeting, 18 (2010) 373–380. [DOI] [PubMed] [Google Scholar]
- [152].Dubey PK, Mishra V, Jain S, Mahor S, Vyas SP, Liposomes Modified with Cyclic RGD Peptide for Tumor Targeting, Journal of Drug Targeting, 12 (2004) 257–264. [DOI] [PubMed] [Google Scholar]
- [153].Liu M, Li W, Larregieu CA, Cheng M, Yan B, Chu T, Li H, Mao SJ, Development of synthetic peptide-modified liposomes with LDL receptor targeting capacity and improved anticancer activity, Mol Pharm, 11 (2014) 2305–2312. [DOI] [PubMed] [Google Scholar]
- [154].Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao XM, Jing M, Cai FY, Li XT, Ju RJ, Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer, J Drug Target, 28 (2020) 245–258. [DOI] [PubMed] [Google Scholar]
- [155].Wang Y, Fu M, Liu J, Yang Y, Yu Y, Li J, Pan W, Fan L, Li G, Li X, Wang X, Inhibition of tumor metastasis by targeted daunorubicin and dioscin codelivery liposomes modified with PFV for the treatment of non-small-cell lung cancer, Int J Nanomedicine, 14 (2019) 4071–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Yin S, Xia C, Wang Y, Wan D, Rao J, Tang X, Wei J, Wang X, Li M, Zhang Z, Liu J, He Q, Dual receptor recognizing liposomes containing paclitaxel and hydroxychloroquine for primary and metastatic melanoma treatment via autophagy-dependent and independent pathways, J Control Release, 288 (2018) 148–160. [DOI] [PubMed] [Google Scholar]
- [157].Yu Q, Qiu Y, Li J, Tang X, Wang X, Cun X, Xu S, Liu Y, Li M, Zhang Z, He Q, Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy, J Control Release, 321 (2020) 564–575. [DOI] [PubMed] [Google Scholar]
- [158].Li M, Yang Y, Wei J, Cun X, Lu Z, Qiu Y, Zhang Z, He Q, Enhanced chemo-immunotherapy against melanoma by inhibition of cholesterol esterification in CD8(+) T cells, Nanomedicine, 14 (2018) 2541–2550. [DOI] [PubMed] [Google Scholar]
- [159].Yuan M, Qiu Y, Zhang L, Gao H, He Q, Targeted delivery of transferrin and TAT co-modified liposomes encapsulating both paclitaxel and doxorubicin for melanoma, Drug Deliv, 23 (2016) 1171–1183. [DOI] [PubMed] [Google Scholar]
- [160].Zhang Q, Wang J, Zhang H, Liu D, Ming L, Liu L, Dong Y, Jian B, Cai D, The anticancer efficacy of paclitaxel liposomes modified with low-toxicity hydrophobic cell-penetrating peptides in breast cancer: an in vitro and in vivo evaluation, RSC Advances, 8 (2018) 24084–24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Xia T, He Q, Shi K, Wang Y, Yu Q, Zhang L, Zhang Q, Gao H, Ma L, Liu J, Losartan loaded liposomes improve the antitumor efficacy of liposomal paclitaxel modified with pH sensitive peptides by inhibition of collagen in breast cancer, Pharm Dev Technol, 23 (2018) 13–21. [DOI] [PubMed] [Google Scholar]
- [162].Zhang L, Ren Y, Wang Y, He Y, Feng W, Song C, Pharmacokinetics, distribution and anti-tumor efficacy of liposomal mitoxantrone modified with a luteinizing hormone-releasing hormone receptor-specific peptide, Int J Nanomedicine, 13 (2018) 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Song Y, Li W, Meng S, Zhou W, Su B, Tang L, Zhao Y, Wu X, Yin D, Fan M, Zhou C, Dual integrin alphavbeta 3 and NRP-1-Targeting Paramagnetic Liposome for Tumor Early Detection in Magnetic Resonance Imaging, Nanoscale Res Lett, 13 (2018) 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Garcia Ribeiro RS, Belderbos S, Danhier P, Gallo J, Manshian BB, Gallez B, Banobre M, de Cuyper M, Soenen SJ, Gsell W, Himmelreich U, Targeting tumor cells and neovascularization using RGD-functionalized magnetoliposomes, Int J Nanomedicine, 14 (2019) 5911–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Panikar SS, Ramirez-Garcia G, Vallejo-Cardona AA, Banu N, Patron-Soberano OA, Cialla-May D, Camacho-Villegas TA, de la Rosa E, Novel anti-HER2 peptide-conjugated theranostic nanoliposomes combining NaYF4:Yb,Er nanoparticles for NIR-activated bioimaging and chemo-photodynamic therapy against breast cancer, Nanoscale, 11 (2019) 20598–20613. [DOI] [PubMed] [Google Scholar]
- [166].Wang SH, Lee AC, Chen IJ, Chang NC, Wu HC, Yu HM, Chang YJ, Lee TW, Yu JC, Yu AL, Yu J, Structure-based optimization of GRP78-binding peptides that enhances efficacy in cancer imaging and therapy, Biomaterials, 94 (2016) 31–44. [DOI] [PubMed] [Google Scholar]
- [167].Langer M, Beck-Sickinger AG, Peptides as carrier for tumor diagnosis and treatment, Curr Med Chem Anticancer Agents, 1 (2001) 71–93. [DOI] [PubMed] [Google Scholar]
- [168].De Barros AL, Mota LD, Coelho MM, Correa NC, De Goes AM, Oliveira MC, Cardoso VN, Bombesin Encapsulated in Long-Circulating pH-Sensitive Liposomes as a Radiotracer for Breast Tumor Identification, J Biomed Nanotechnol, 11 (2015) 342–350. [DOI] [PubMed] [Google Scholar]
- [169].Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y, Qi Y, Han X, Peng J, Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: a potential new drug for treatment of glioblastoma multiforme, Food Chem Toxicol, 59 (2013) 657–669. [DOI] [PubMed] [Google Scholar]
- [170].Shi K, Long Y, Xu C, Wang Y, Qiu Y, Yu Q, Liu Y, Zhang Q, Gao H, Zhang Z, He Q, Liposomes Combined an Integrin alphavbeta3-Specific Vector with pH-Responsible Cell-Penetrating Property for Highly Effective Antiglioma Therapy through the Blood-Brain Barrier, ACS Appl Mater Interfaces, 7 (2015) 21442–21454. [DOI] [PubMed] [Google Scholar]
- [171].Kucherov FA, Egorova KS, Posvyatenko AV, Eremin DB, Ananikov VP, Investigation of Cytotoxic Activity of Mitoxantrone at the Individual Cell Level by Using Ionic-Liquid-Tag-Enhanced Mass Spectrometry, Anal Chem, 89 (2017) 13374–13381. [DOI] [PubMed] [Google Scholar]
- [172].Alderton PM, Gross J, Green MD, Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model, Cancer Res, 52 (1992) 194–201. [PubMed] [Google Scholar]
- [173].Kazmierczak PM, Todica A, Gildehaus F-J, Hirner-Eppeneder H, Brendel M, Eschbach RS, Hellmann M, Nikolaou K, Reiser MF, Wester H-J, Kropf S, Rominger A, Cyran CC, 68Ga-TRAP-(RGD)3 Hybrid Imaging for the In Vivo Monitoring of αvß3-Integrin Expression as Biomarker of Anti-Angiogenic Therapy Effects in Experimental Breast Cancer, PLOS ONE, 11 (2016) e0168248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Honary S, Zahir F, Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2), Tropical Journal of Pharmaceutical Research, 12 (2013) 265–273. [Google Scholar]
- [175].Kang MH, Yoo HJ, Kwon YH, Yoon HY, Lee SG, Kim SR, Yeom DW, Kang MJ, Choi YW, Design of multifunctional liposomal nanocarriers for folate receptor-specific intracellular drug delivery, Molecular pharmaceutics, 12 (2015) 4200–4213. [DOI] [PubMed] [Google Scholar]
- [176].Saul JM, Annapragada AV, Bellamkonda RV, A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers, J Control Release, 114 (2006) 277–287. [DOI] [PubMed] [Google Scholar]
- [177].Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL, Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities, Journal of controlled release, 277 (2018) 1–13. [DOI] [PubMed] [Google Scholar]
- [178].Mack K, Rüger R, Fellermeier S, Seifert O, Kontermann RE, Dual targeting of tumor cells with bispecific single-chain Fv-immunoliposomes, Antibodies, 1 (2012) 199–214. [Google Scholar]
- [179].Franze S, Selmin F, Samaritani E, Minghetti P, Cilurzo F, Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging, Pharmaceutics, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Verma A, Stellacci F, Effect of surface properties on nanoparticle-cell interactions, Small, 6 (2010) 12–21. [DOI] [PubMed] [Google Scholar]
- [181].Barenholz Y, Liposome application: problems and prospects, Current opinion in colloid & interface science, 6 (2001) 66–77. [Google Scholar]
- [182].Shi J, Kantoff PW, Wooster R, Farokhzad OC, Cancer nanomedicine: progress, challenges and opportunities, Nature Reviews Cancer, 17 (2017) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Walkey CD, Chan WC, Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment, Chemical Society Reviews, 41 (2012) 2780–2799. [DOI] [PubMed] [Google Scholar]
- [184].Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC, In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform, Frontiers in bioengineering and biotechnology, 4 (2016) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Kitamura T, Qian B-Z, Pollard JW, Immune cell promotion of metastasis, Nature Reviews Immunology, 15 (2015) 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]