Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of the joints leading to tissue damage. Despite the availability of potent drugs including the biologics, many patients fail to respond to them, whereas others suffer adverse effects following long-term use of these drugs. Accordingly, the use of natural herbal products by RA patients has been increasing over the years. However, limited information about the mechanism of action of these natural products is a major shortcoming that prevents the widespread acceptance of herbal therapy by professionals and patients alike. In this study, we demonstrated the anti-arthritic activity of Tinospora cordifolia extract (TCE) using the rat adjuvant-induced arthritis model of human RA and elaborated the immune mechanisms underlying this effect. TCE treatment suppressed arthritic inflammation and bone and cartilage damage. The anti-inflammatory effect of TCE was mediated via reduction of the pro-inflammatory cytokines such as: IL-1β, TNF-α, IL-6, and IL-17; the frequency of IL-17-producing T cells; and the production of chemokines such as RANTES. Furthermore, TCE treatment limited bone damage by shifting the balance of mediators of bone remodeling (e.g., receptor activator of nuclear factor-kB ligand [RANKL] and MMP-9) in favor of anti-osteoclastic activity. Our results suggest that TCE and its bioactive components should be evaluated for their utility as therapeutic adjuncts to conventional drugs against RA.
Keywords: adjuvant arthritis, autoimmunity, bone remodeling, chemokines, cytokines, natural products, rheumatoid arthritis, Tinospora cordifolia
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects about 1% of the population and its prevalence is higher in women than in men.1,2 The major pharmacological agents currently being used for the treatment of RA are the nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin; the disease-modifying anti-rheumatic drugs (DMARDs) like methotrexate; the immunosuppressive agents such as prednisone; and the anti-cytokine agents like anti-TNFα receptor antibody.3–6 These drugs are quite effective, but their prolonged use may be associated with significant adverse effects such as gastrointestinal toxicity, kidney damage, or infections.6–9 Accordingly, increasing numbers of RA patients are resorting to the use of natural herbal products.10 Such products have been used in the Indian traditional system of medicine (Ayurveda) and traditional Chinese medicine (TCM) for a long period of time and generally display minimal side effects. However, there is limited information about the mechanisms of action of many of these natural products.
Tinospora cordifolia (TC) is a climbing shrub that belongs to the family Menispermaceae. It is native to India, but is also found in neighboring countries.11,12 The Tinospora cordifolia extract (TCE) has been used in many herbal formulations to treat various inflammatory conditions.13,14 TCE and the compounds isolated from T. cordifolia have been shown to possess immunomodulatory, anti-proliferative, and anti-angiogenic effects in various in vitro models.15 The anti-bacterial activity of T. cordifolia has also been reported.16 It has previously been shown in the rat collagen-induced arthritis (CIA) model that T. cordifolia reduced the severity of arthritis and protected against joint destruction.17 In this study, we validated the arthritis-suppressive effect of TCE using the rat adjuvant-induced arthritis (AA) model of RA and elaborated the immunological and biochemical mechanisms involved therein. Our results show that TCE inhibits key pro-inflammatory cytokines and chemokines along with mediators of bone remodeling and matrix degradation.
Materials and methods
Animals
Male Lewis rats (LEW/Hsd, RT.1l), aged 5–6 weeks, were purchased from Harlan Sprague-Dawley (Indianapolis, IN, USA) and housed in the vivarium of University of Maryland School of Medicine, Baltimore, MD (UMB). UMB Institutional Animal Care and Use Committee (IACUC) specifically approved this study via IACUC protocol #0514012. All experimental procedures were conducted in accordance with IACUC guidelines.
Tinospora cordifolia extract: Source and characteristics
The methanol extract of aerial part of T. cordifolia extract was a kind gift from Sami Labs Limited, Bangalore, India/ Sabinsa Corporation, NJ, USA (Product code: 2020, Batch No. C111811). The percentage of yield of the extract as specified by the commercial source was 10%, and well-defined standardization procedures were performed for purity and identity of the extract. The level of heavy metals such as lead, arsenic, cadmium, and mercury was not more than 3 ppm, 1 ppm, 1 ppm, and 0.1 ppm, respectively, and the level of total heavy metals was not more than 20 ppm. Chemical standardization, nature of bioactive components, and toxicity of TCE are described previously by other investigators.14–17 Several bioactive phytochemical constituents belonging to the sub-categories of alkaloids (e.g., berberine, tinosporin), glycosides (e.g., cordiofolioside A, palmatosides C), sesquiterpenoids (e.g., tinocordifolin), diterpenoid lactones (e.g., tinosporon, columbin), aliphatic compounds (e.g., octacosanol), and steroids (e.g., makisterone A, giloinsterone) have been identified in T. cordifolia.14–17
Induction and evaluation of adjuvant arthritis (AA)
Lewis rats were immunized subcutaneously at the base of the tail with 1.5 mg of grinded heat-killed M. tuberculosis H37Ra (Mtb) (Difco, Detroit, MI, USA) suspended in 200 μL of mineral oil for each rat. The rats were observed regularly for erythema, swelling, and induration in each paw after Mtb injection and the severity of arthritis in each paw was graded on a scale of 0 to 4.18,19 A typical disease course consisted of incubation, onset, peak, and recovery phase of AA.
Treatment of arthritic Lewis rats with T. cordifolia extract (TCE)
TCE was suspended in water and fed to Lewis rats (1 g/kg in a 2 mL volume) daily using a gavage needle (FNC-16–3, Kent Scientific Corp., Torrington, CT, USA). TCE was fed from the onset of AA (day 9) and then continued uninterrupted for the entire duration of the observation period. The control rats received vehicle (water). All rats were graded regularly for the severity of arthritis in this period.
Histological examination of hind paws of TCE-/vehicle-treated rats
The hind paws were harvested from the Lewis rats treated with vehicle/TCE on day 19 and immersed in Cal-Ex decalcifying solution CS510–1D (Fisher) for 9 days. Thereafter, the paws were immersed in 70% ethanol for 5 days followed by embedding in paraffin as described elsewhere.20 The embedded tissue was then sectioned serially using the microtome and mounted on microscope slides. The microtome sections were then stained with hematoxylin and eosin (H&E) (Histology Core, University of Maryland School of Medicine, Baltimore, MD, USA)21 or safranin-O22 and examined under a microscope for arthritis-related changes such as synovial hyperplasia, pannus formation, and cartilage and bone damage, and digital images were obtained.21
Preparation and restimulation of lymph node cells (LNC)
Arthritic Lewis rats treated with TCE/vehicle were euthanized on day 19 after disease induction and their draining lymph nodes (para-aortic, inguinal, and popliteal) were harvested. A single cell suspension of LNC was prepared. These LNC were washed three times with Hanks’ balanced salt solution (Invitrogen) and cultured (5 × 105 cells/well) in a flat-bottomed 96-well plate in HL-1 serum-free medium (Ventrex Laboratories, Portland, ME, USA) supplemented with 2 mM L-glutamine, 100 units/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate in an atmosphere of 95% air and 5% CO2.19 The cells were re-stimulated with antigen (Mtb sonicate, 10 ug/mL) for 24–48 h and then their RNA or culture supernate was used for cytokine/chemokine testing.19, 23
Preparation and restimulation of spleen adherent cells (SAC)
Spleens were harvested from rats treated as described above under LNC and a single cell suspension was prepared.24 The spleen cells (2.0 × 106 cells/well) were placed in a six-well plate (Corning, Corning, NY, USA) and incubated at 37°C for 90 min in RPMI 1640 medium supplemented with 5% FBS, 2 mM L-glutamine, 100 units/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate. The non-adherent cells were removed. The remaining cells consisted of SAC. These cells were restimulated with Mtb sonicate (10 μg/mL) for 6–24 h and then their RNA or culture supernate was used for cytokine/chemokine testing.
Preparation and restimulation of synovial-infiltrating cells (SIC)
Hind paws of the TCE-fed and water-fed rats were collected on day 19 after Mtb immunization and SIC were harvested by opening the ankle joint using a sterile blade as described elsewhere.19 These SIC were then washed three times with HBSS and cultured (5 × 105 cells/well) in a flat-bottomed 96-well plate in HL-1 serum-free medium (Ventrex Laboratories, Portland, ME, USA) supplemented with 2 mM L-glutamine, 100 units/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate in an atmosphere of 95% air and 5% CO2. The cells were re-stimulated with Mtb sonicate for 24–48 h and their RNA or culture supernate was used for cytokine/chemokine testing.
Methods of testing of cytokines, chemokines, and mediators of bone remodeling
Measurements of cytokines / chemokines / mediators of bone remodeling were performed using LNC, SAC, SIC, and sera of TCE- and vehicle-treated arthritic Lewis rats. Specific mediators of bone remodeling tested included receptor activator of nuclear factor-kB (RANK), receptor activator of NF-kB ligand (RANKL), osteoprotegerin (OPG), osteopontin (OPN), osteocalcin (OSC, also known as OCN), and macrophage colony-stimulating factor (M-CSF). The testing was done employing mRNA expression by quantitative real-time PCR (qRT-PCR) and/or protein levels by Multiplex assay (Cytokine Core facility, UMB). The preparation and restimulation of various cell types are described above. These cells were used for RNA isolation. RNA was isolated by Trizol reagent (Invitrogen) and cDNA prepared from it using an iScript cDNA synthesis kit (Bio-Rad).19 The cDNA was amplified by quantitative RT-PCR using SYBR Green PCR Master Mix (Applied Biosystems) and appropriate primers for different cytokines in an ABI PRISM 7900HT cycler (Applied Biosystems, Foster City, CA, USA). The expression of hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene served as a reference for normalization of the test values.19 The relative gene expression levels were expressed as “fold change”.
Flow cytometric analysis
Spleen and hind paws were harvested from water-fed arthritic control rats and TCE-fed rats at peak phase of adjuvant arthritis. Single-cell suspensions from the spleens and synovial-infiltrating cells (SIC) from the joints were prepared and stimulated under in vitro conditions for 6 h with 5 μg/mL phorbol 12-myristate 13-acetate (PMA) (Promega) and 100 μM ionomycin (Sigma-Aldrich) in the presence of 100 μg/mL Brefeldin A (Life Technologies) for the final 4 h of culture.25 Cells were surface-stained with anti-CD3 APC and anti-CD4 FITC (all from eBioscience). Thereafter, cells were fixed and permeabilized using a BD Fixation/Permeabilization Kit (BD Bioscience) and stained for intracellular cytokines using anti-IL-17A eFluor 450 (eBioscience) and anti-IFN-γ PE (Biolegend), and then analyzed on an LSRII flow cytometer (BD Biosciences). The data were analyzed with FlowJo Software (TreeStar).
Matrix metalloproteinase (MMP) activity
The SIC were collected from vehicle-/TCE-treated rats, washed, and cultured in a 6-well plate following the method described elsewhere.19 After 24 h, the supernates were collected and their MMP activity was analyzed by a zymogram assay.26,27 Briefly, the supernatant (10 uL) was separated on a gelatin-coated, precast SDS-polyacrylamide gel under non-reducing conditions at constant voltage. The SDS from the gel was removed by incubating it with Triton X-100 (2.5%) at room temperature for 1 h. After thorough washing, the gel was incubated overnight at 37°C in a developing buffer (Tris-HCl, pH 7.4) containing 5 mM CaCl2, 0.2 M NaCl, and 0.02% Brij 35 followed by staining with Coomassie Brilliant Blue R-250. The MMP activity was evident in the form of translucent bands after de-staining. The gel was then scanned and the intensity of bands was quantitated by densitometry using Image J software.
Statistical analysis
The data were expressed as mean ± SEM. Student’s t test was used to assess the significance of the difference between vehicle and TCE group. Two-way analysis of variance with the Bonferroni post-test was carried out for in vivo clinical experiments.
Results
TCE reduces the severity of AA in Lewis rats
To assess the efficacy of TCE in reducing the severity of arthritis, we tested this natural product extract in the rat AA model. Arthritic Lewis rats were fed TCE (test group) or water (vehicle) (control group) orally by gavage after the onset of disease (beginning on day 9 and then continuing throughout the course of AA), and thereafter these rats were observed daily for the signs of disease. The TCE-fed group consistently had lower arthritic scores when compared with the water-fed group (Figure 1a). For example, at the peak phase of disease, day 18 post-Mtb immunization, the swelling and redness of the hind paws were markedly reduced in the TCE-treated rats compared to control rats (Figure 1b). To further validate the effectiveness of TCE in suppressing arthritis severity, we examined the hind paws of the two groups of rats for histological changes in the joints using H&E and safranin-O staining. The sections from TCE-treated rat had decreased pannus formation, reduced cellular infiltrates, and reduced bone and cartilage damage compared with that of the control rat (Figure 1c, d). Taken together, these results show that TCE is effective in reducing both clinical and histological features of arthritis severity.
Figure 1.
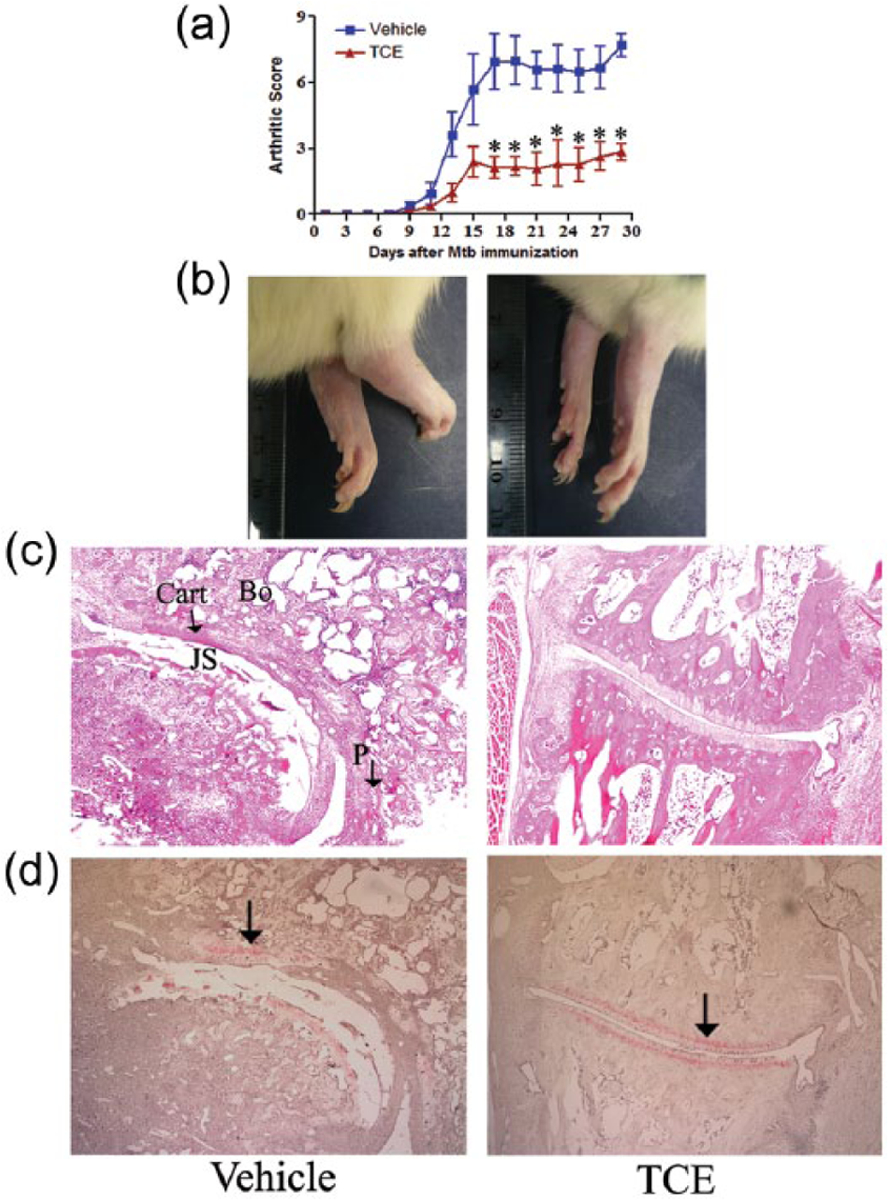
Suppression of AA in Lewis rats by TCE treatment. Groups (n = 4 per group) of Mtb-immunized Lewis rats were fed with either TCE or vehicle (water) beginning at the onset of the disease. The arthritic scores of rats (a), hind paw photographs (b), H&E stained sections (c), and Safranin-O-Stained sections (d) of hind paws of the two groups of rats on day 19 after Mtb injection are shown. The histology sections show the joint space (JS), the pannus (P) containing the mononuclear cell infiltrate, bone (Bo), and cartilage (Cart) (c) and the extent of cartilage damage (shown by arrow) (d) (*, P <0.05, statistically significant difference between TCE-treated and control groups from day 17 to day 29). The results are representative of two independent experiments. In (c) and (d), each section is at 10X.
TCE treatment inhibits key pro-inflammatory cytokines and chemokines involved in arthritis pathogenesis
The RNA prepared from SAC and LNC of the TCE- and water-fed rats was tested by qRT-PCR for various cytokines and chemokines. In the case of SAC (Figure 2a), there was a marked decrease (P <0.05) in the expression of key pro-inflammatory cytokines interleukin-1β (IL-1β), IL-6, IL-23, and tumor necrosis factor-α (TNF-α) as well as the chemokine “regulated on activation, normal T cell expressed and secreted” (RANTES) in the TCE-treated rats when compared with the control rats. In the case of LNC, the cytokine IL-17 was reduced significantly (P <0.05) without much change in IFN-γ in the TCE-treated rats compared to the control rats (Figure 2b). In addition, IL-23 and TNF-α showed no significant change, while RANTES was decreased (data not shown).
Figure 2.
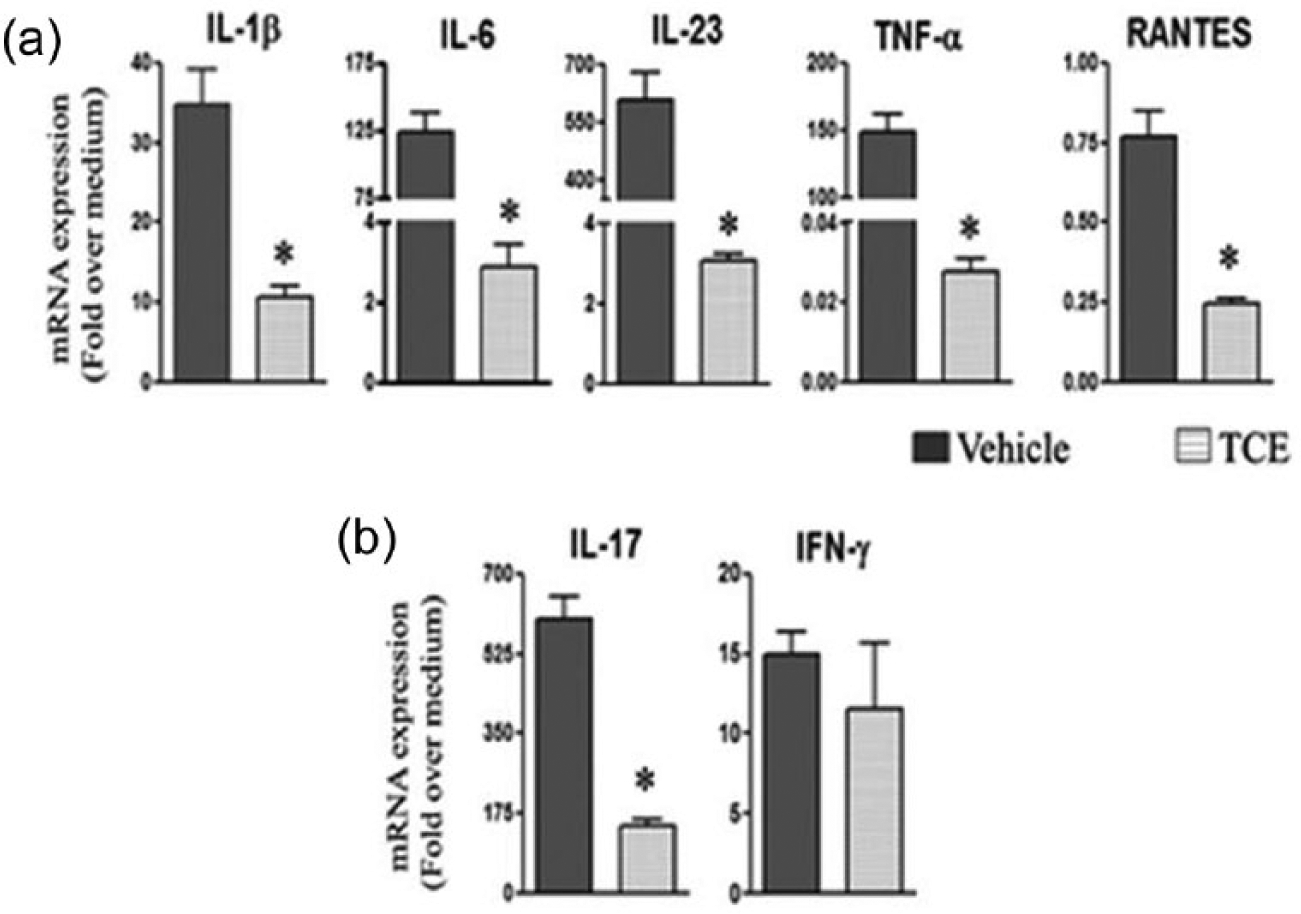
The influence of TCE treatment on cytokine and chemokine expression in SAC and LNC. Spleen adherent cells (SAC) (a) and lymph node cells (LNC) (b) were harvested from arthritic Lewis rats fed either with TCE or with water, on day 19 after Mtb challenge. These cells were restimulated for 6 and 24 h, respectively with Mtb sonicate (10 ug/mL) (n = 3 each). The cytokine/chemokine mRNA expression in these cells was quantified by qRT-PCR and the results were expressed as “fold over medium” after normalization to HPRT (*, P <0.05, when comparing TCE and control samples).
In another set of experiments, we tested various cytokines and chemokines in the culture supernates of SIC and in sera of TCE-treated and control rats. In the case of SIC (Figure 3a), the levels of the cytokines IL-1β, IL-17, IL-6, and TNF-α were decreased significantly (P <0.05) in TCE-fed rats compared to control rats. The chemokines monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α) were also decreased, but the difference was statistically significant for MCP-1 but not MIP-1α (Figure 3a). In the case of serum (Figure 3b), IL-1β and IL-17 were decreased significantly (P <0.05), whereas IL-10 was not altered following TCE treatment. Furthermore, the chemokines RANTES and MCP-1 showed a reduction (P <0.05) in TCE-treated rats.
Figure 3.
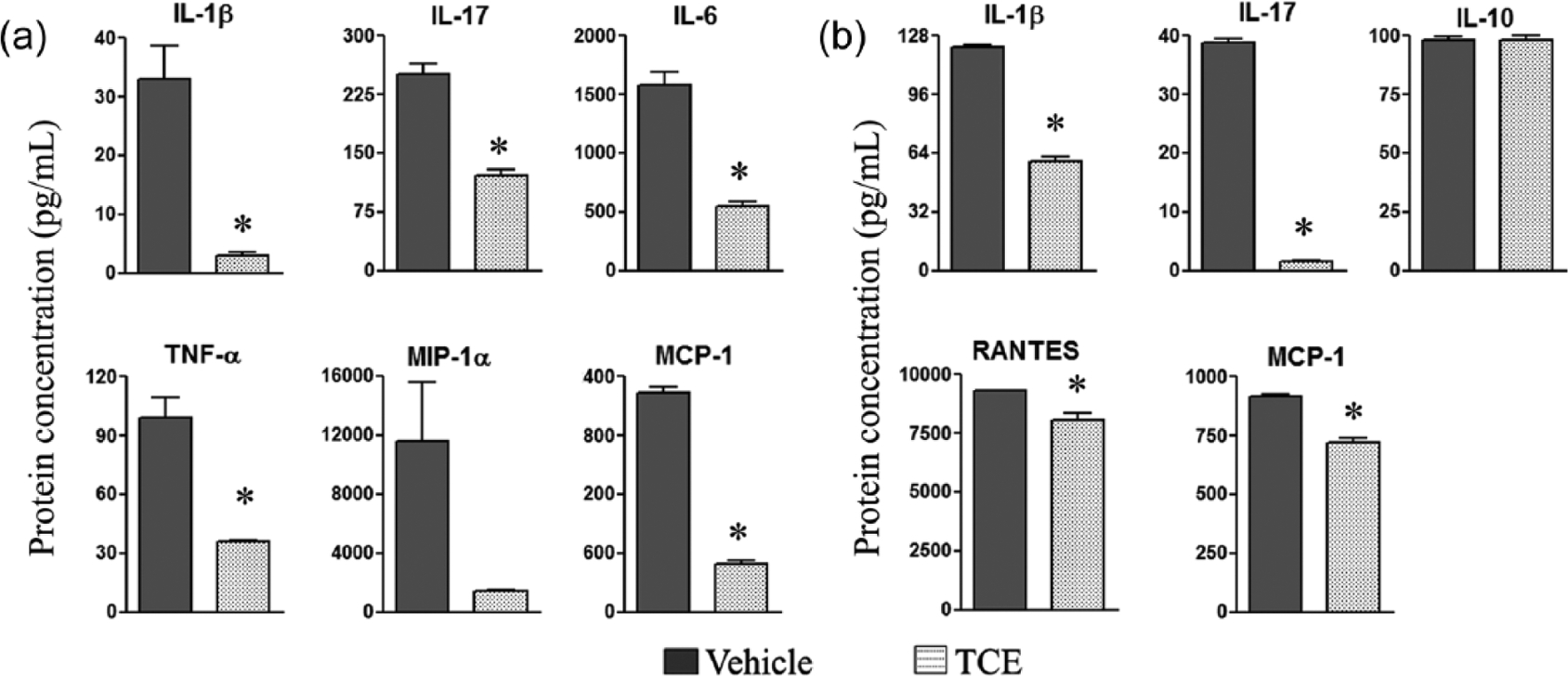
Alteration of cytokines and chemokines in SIC and sera following TCE treatment. Synovial-infiltrating cells (SIC) (a) and sera (b) were collected from arthritic rats fed either with TCE or with water (n = 3/each group), on day 19 after Mtb injection. SIC were restimulated for 24 h with Mtb sonicate (10 ug/mL) and culture supernates were collected. The levels of cytokines (top panels and TNF-α in lower panel) and chemokines (lower panel, except TNF-α) in the culture supernates of SIC and sera were measured by using a Multiplex assay. The results are expressed as pg/mL (*, P <0.05, when comparing TCE and control samples).
TCE treatment reduces the frequency of the IL-17/IFN-γ double-producing cells in the spleen
To gain more insight into the modulation of the pro-inflammatory cytokines by TCE, we determined the frequency of CD3+ and CD4+ T cells producing IL-17 and/or IFN-γ in splenic cells of TCE-fed arthritic rats compared with water-fed control rats using flow cytometry (Figure 4). IL-17+IFN-γ+ CD4+ T cells (double producers) were found to be significantly reduced from 2.2% in water-fed control rats to 0.6% in TCE-fed rats. Although, there was a slight decrease in the percentages of IL-17-producing only and IFN-γ-producing only (single producers) cells, the difference was not statistically significant (Figure 4a). The testing of SIC revealed a similar trend of reduced double producers, but the difference was not statistically significant (Figure 4b). Nevertheless, as both IL-17 and IFN-γ have pro-inflammatory properties, the reduction of double producers in the spleen along with the results of cytokine testing (Figures 2 and 3) reveal one of the mechanisms by which TCE suppresses arthritis.
Figure 4.
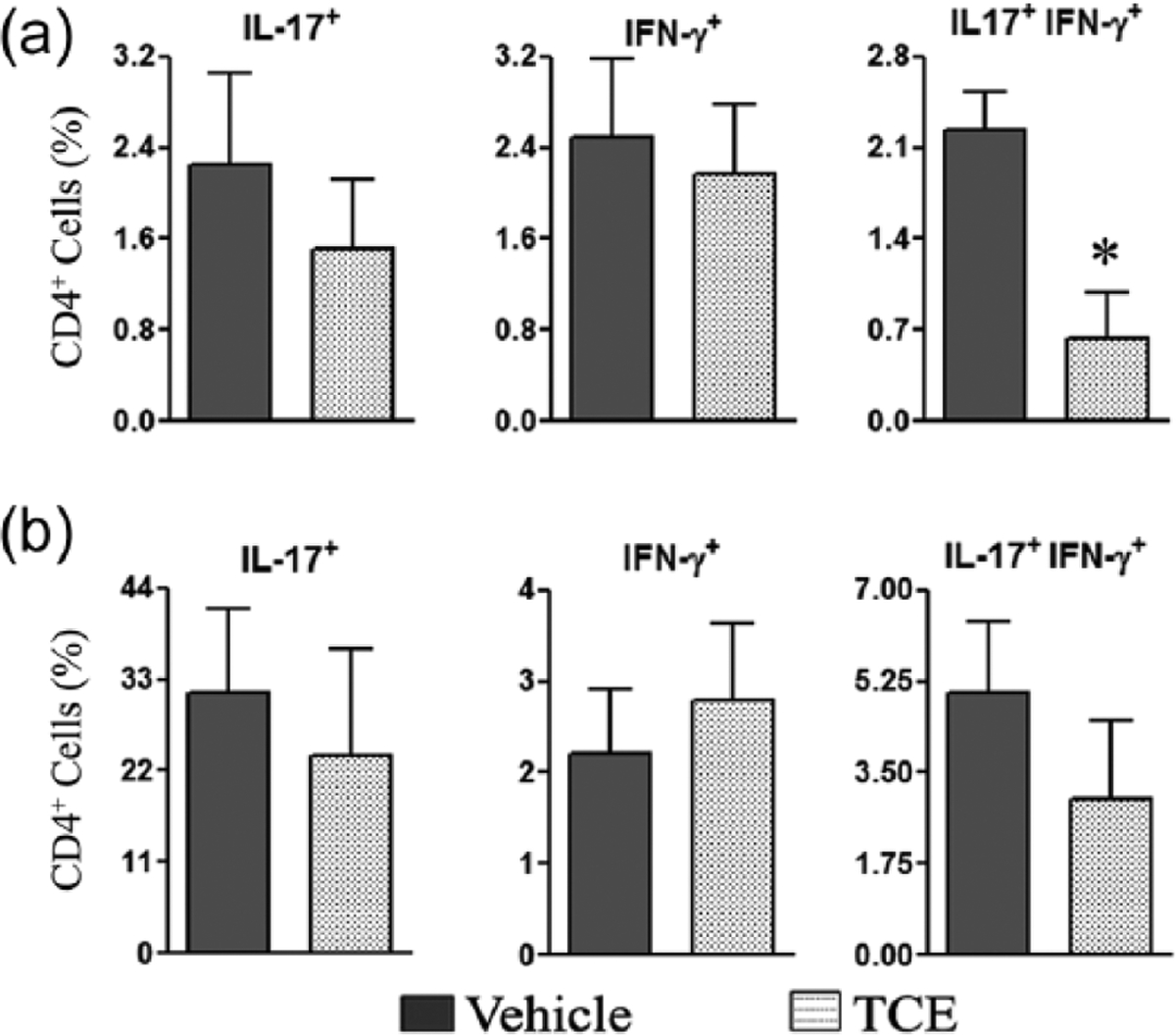
Treatment of arthritic rats with TCE reduces the frequency of IL-17 and IFN-γ double-producing cells. Spleen cells (a) and synovial-infiltrating cells (SIC) (b) were prepared from TCE-fed and control arthritic rats (n = 3 each), on day 19 after Mtb immunization. These cells were then stimulated with PMA and ionomycin for 6 h, followed by staining for CD3, CD4, IL-17, and IFN-γ. The cells were analyzed by flow cytometry for IL-17- and/or IFN-γ-expressing CD4+ T cells (*, P <0.05, when comparing TCE and control samples).
TCE treatment alters the balance of immune mediators of bone remodeling
To examine the mechanism underlying TCE-induced protection against bone damage in rats with AA, we determined the effect of TCE on the immune mediators of bone remodeling such as RANK, RANKL, OPG, OPN, OSC (also known as OCN), and M-CSF in TCE-fed and control rats. The expression levels of these mediators were measured in both SAC and SIC by qRT-PCR. SAC (Figure 5a) and SIC (Figure 5b) displayed a similar profile: OPN, M-CSF, and RANKL were decreased, whereas OSC and RANK were increased in TCE-fed rats compared with control rats, and these alterations were statistically significant. Although OPG was not altered much, the RANKL/OPG ratio was decreased significantly in both SAC and SIC in TCE group. This altered ratio favors anti-osteoclastic activity. Combined with an increase in OSC, a marker of osteoblastic activity, the reduced RANKL/OPG ratio offers insight into the bone damage-protective effect of TCE.
Figure 5.
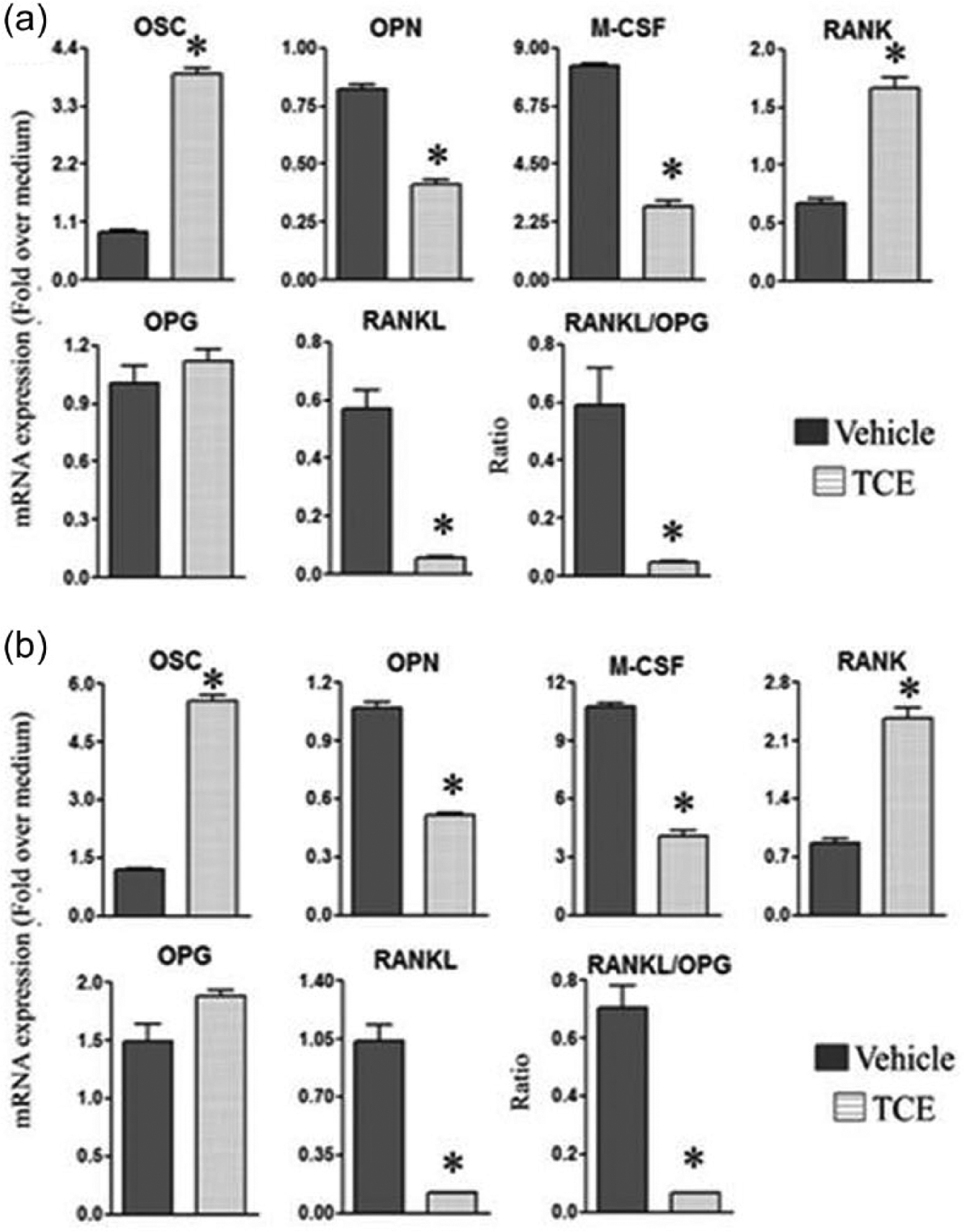
TCE alters the levels of mediators of bone remodeling. Spleen adherent cells (SAC) (a) and synovial-infiltrating cells (SIC) (b) were harvested from arthritic Lewis rats fed either with TCE or with water, on day 19 after Mtb challenge. The SIC and SAC were then restimulated for 24 h with Mtb sonicate (10 ug/mL) (n = 3 each). The mRNA expression of the bone remodeling-related mediators in these cells was quantified by qRT-PCR and the results were expressed as “fold over medium” after normalization to HPRT (*, P <0.05, when comparing TCE and control samples).
TCE suppresses matrix metalloproteinase-9 (MMP-9) activity
We also evaluated the effect of TCE on the MMPs, which mediate damage to the cartilage and bone in arthritic joints, in culture supernate of SIC obtained from the joints of TCE-treated and control rats. Treatment with TCE reduced MMP-9 activity, but without any effect on MMP-2 activity (Figure 6).
Figure 6.
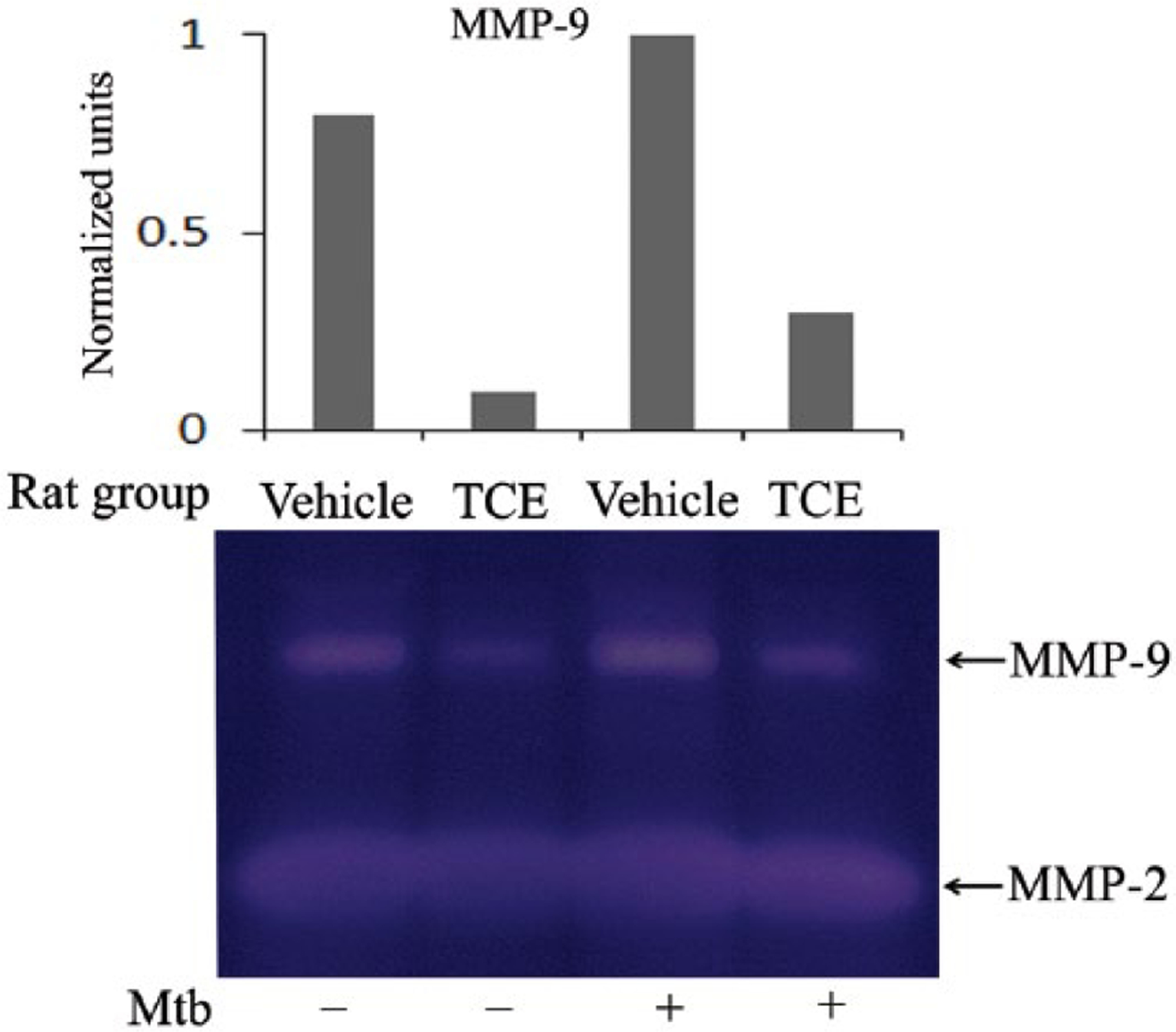
TCE reduces the level of MMP-9. Synovial-infiltrating cells (SIC) from the joints of TCE-fed and water-fed rats were collected on day 19 after Mtb injection. The SIC were then restimulated for 24 h in vitro with or without Mtb sonicate (10 ug/mL) and the activity of MMP-9 and MMP-2 was measured in the culture supernatant by using a gelatin zymogram assay. A representative pattern of MMP activity is shown. The graph above the gel shows the normalized units obtained by densitometric analysis.
Discussion
We describe in this study that T. cordifolia extract (TCE) can effectively inhibit the severity of autoimmune arthritis in the rat AA model. We also unraveled the immunological and molecular mechanisms underlying the anti-inflammatory and anti-bone resorptive effects of TCE. It has previously been reported that TCE can inhibit arthritis in the CIA model17 and modulate the mediators of immune response in other diseases.14,15 However, there is limited information on the arthritis-related immune mechanisms influenced by TCE. The results of our study discussed below have filled this gap.
RA is a chronic inflammatory disease leading to joint destruction mediated by the migration of CD4+ T cells and macrophages infiltrating the synovial tissue.1,2 Pro-inflammatory cytokines such as TNF-α, IL-17, IL-6, and IL-1β; chemokines such as RANTES and MCP-1; as well as biochemical mediators such as RANKL and MMPs initiate and propagate joint inflammation and tissue damage.28–30 Among the pro-inflammatory cytokines, IL-17 has been shown to play a vital role in arthritis pathogenesis.31,32 This cytokine is produced by Th17 cells and it can facilitate the production of other pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6.
Our results showed that the pro-inflammatory cytokines were significantly reduced in SAC (e.g., IL-1β, IL-6, IL-23, and TNFα) and draining LNC (e.g., IL-17) of arthritic rats treated with TCE. These results were reinforced by those showing that the pro-inflammatory cytokines were also reduced in SIC culture supernatants (e.g., IL-1β, IL-17, IL-6, and TNFα) and sera (e.g., IL-1β and IL-17) of TCE-treated rats. However, there was not much change in the levels of anti-inflammatory IL-10 (Figure 3b) and other cytokines (data not shown). Thus, TCE altered the balance of pro-versus anti-inflammatory cytokines primarily by downregulating the pro-inflammatory cytokines, particularly IL-17 and IL-1β.
Activated synovial fibroblasts and monocytes/macrophages in the inflamed arthritic joint are the source of chemokines, such as RANTES, MCP-1, and MIP-1α.35,36 Our study revealed TCE-induced reduction in RANTES in SAC, the draining LNC, and serum, as well as decrease in MCP-1 in both serum and SIC. These results suggest that TCE-induced suppression of arthritis might be mediated in part by altering the chemotactic cellular migration of immune cells into the joints.
Th17 cells produce IL-17, which plays a critical role in arthritis pathogenesis. IL-17 upregulates other pro-inflammatory cytokines, chemokines, and additional mediators of bone damage. IL-17 facilitates neutrophil influx into the inflamed synovial joints and increases synoviocyte survival, angiogenesis, osteoclast differentiation, and matrix-degrading enzymes.32,33,37 Other investigators have reported that Th17 cells were significantly increased at all stages of the disease in RA patients.38,39 Similarly, increased frequency of Th17 in mice/rats with arthritis has been reported by others and us.40,41 Studies based on the testing of natural products in animal models of RA have revealed that Th17 frequency was reduced in animals treated with defined natural products. For example, the treatment of arthritic animals by grape seed proanthocyanidin extract or pristimerin reduces the Th17 frequency in splenocytes and SIC of mice/rats with CIA and AA, respectively.25,40 In our present study based on TCE, a statistically significant decrease in IL-17-producing T cells was observed, but that difference was primarily in IL-17+IFN-γ+ (double producer) cells of spleen but not in IL-17+ (single producer) cells in the periphery (spleen) or the target organ (the joints). Nevertheless, the reduced frequency of IL-17+ IFN-γ+ (double producer) cells correlates with reduced production of IL-17 as measured by qPCR and Multiplex assay.
We described above the anti-inflammatory activity of TCE as evident from attenuation of the severity of clinical arthritis. We further investigated whether TCE also limits bone damage in arthritic rats, and also examined the mechanisms involved. Bone homeostasis is maintained by a balance between bone resorption and bone formation. The RANKL/RANK/OPG system regulates bone resorption by osteoclasts.30 Osteoclastogenesis and bone resorption can be inhibited by decreased production of RANKL and consequently decreased activation of RANK. The reduction in RANKL may or may not be accompanied by an increase in OPG, resulting in a decrease in RANKL/OPG ratio.20,30 In our study, we observed that TCE-treated rats showed reduction in the expression of RANKL as well as the RANKL/OPG ratio in both SAC and SIC compared with control rats. Thus, TCE restored the RANKL/OPG balance in AA. In addition, TCE treatment resulted in a marked reduction in the osteoclastic mediators, namely M-CSF and OPN, coupled with an increase in OSC, which is required for osteoblastic activity. Taken together, TCE shifted the balance of mediators of bone remodeling in favor of anti-osteoclastic activity. A previous report by other investigators has shown the anti-osteoporotic effects of TCE in an animal model of osteoporosis.42 Furthermore, a recent study has shown that TCE promotes the proliferation of osteoblast cells and mineralization of bone-like matrix on human osteoblast-like cells MG-63 and rat primary culture of osteoblasts.43
Among the MMPs, MMP-9 plays an important role in cartilage and bone destruction in arthritic joints.44 TCE-treated rats showed marked reduction in MMP-9 activity, but without much effect on MMP-2 activity. TCE might inhibit MMP-9 either by a direct effect on the enzyme or by an indirect effect via inhibiting the key inducers of MMP-9. In regard to the latter, TCE reduced three of the positive inducers of MMP-9, namely RANKL, IL-6, and IL-17. Similar to TCE, other herbal products have previously been shown to protect against osteoclastogenesis and bone loss. For example, Celastrus extract and its bioactive component Celastrol have been shown to protect against bone damage in AA by inhibiting pro-inflammatory cytokines, RANKL production, and MMP-9 activity, but increasing RANKL/OPG ratio.20 Similarly, green tea protects against inflammation-induced bone loss in rats by reducing TNF-α production as well as inflammation.45
In summary, the TCE treatment of arthritic rats inhibited two inter-related features of arthritis, namely inflammation and bone damage compared with the control rats. These effects were the integrated outcomes of TCE-induced changes in defined cytokines, chemokines, and mediators of bone remodeling, which play critical roles in arthritis pathogenesis. On the basis of our results, we suggest that TCE should be further examined as a potential anti-arthritic therapy in conjunction with conventional medications in patients with RA.
Acknowledgements
The authors thank Steven Dudics, Bodhraj Acharya, Chithrashree Chamegowda, and Rajesh Rajaiah for discussions and/or help in experimental work. The authors also thank Dr. SN Vogel for providing the real-time PCR facility, Lisa Hester for Multiplex assay, and Sabinsa Corporation, New Jersey for the kind gift of Tinospora cordifolia extract.
Funding
This work was supported by grants from the Department of Biotechnology, Government of India, New Delhi, India (to KMS) and the National Institutes of Health, Bethesda, MD (to KDM).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Goronzy J and Weyand CM (2001) Rheumatoid arthritis: Epidemiology, pathology, and pathogenesis. Atlanta, GA: The Arthritis Foundation. [Google Scholar]
- 2.Kvien TK (2004) Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 22: 1–12. [DOI] [PubMed] [Google Scholar]
- 3.No authors listed (1996) Guidelines for the management of rheumatoid arthritis. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis and Rheumatism 39: 713–722. [PubMed] [Google Scholar]
- 4.Abramson SB and Amin A (2002) Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 41: 972–980. [DOI] [PubMed] [Google Scholar]
- 5.Kremers HM, Nicola P, Crowson CS, et al. (2004) Therapeutic strategies in rheumatoid arthritis over a 40-year period. Journal of Rheumatology 31: 2366–2373. [PubMed] [Google Scholar]
- 6.Simon LS and Yocum D (2000) New and future drug therapies for rheumatoid arthritis. Rheumatology (Oxford) 39 (Suppl. 1): 36–42. [DOI] [PubMed] [Google Scholar]
- 7.Couzin J (2004) Drug safety. Withdrawal of Vioxx casts a shadow over COX-2 inhibitors. Science 306: 384–385. [DOI] [PubMed] [Google Scholar]
- 8.Scheiman JM (2001) The impact of nonsteroidal anti-inflammatory drug-induced gastropathy. American Journal of Managed Care 7: S10–14. [PubMed] [Google Scholar]
- 9.Weir M (2002) Renal effects of nonselective NSAIDs and coxibs. Cleveland Clinic Journal of Medicine 69: SI53–58. [DOI] [PubMed] [Google Scholar]
- 10.Tindle HA, Davis RB, Phillips RS and Eisenberg DM (2005) Trends in use of complementary and alternative medicine by US adults. Alternative Therapies in Health and Medicine 11: 42–49. [PubMed] [Google Scholar]
- 11.Sharma A, Gupta A, Singh S and Batra A (2010) Tinospora cordifolia (Willd.) Hook. F. & Thomson - A plant with immense economic potential. Journal of Chemical and Pharmaceutical Research 2: 327–333. [Google Scholar]
- 12.Sinha K, Mishra NP, Singh J and Khanuja SPS (2004) Tinospora cordifolia (Guduchi), a reservoir plant for therapeutic application: A review. Indian J Traditional Knowledge 3: 257–270. [Google Scholar]
- 13.Saha S and Ghosh S (2012) Tinospora cordifolia: One plant, many roles. Ancient Science of Life 31: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma U, Bala M, Kumar N, et al. (2012) Immunomodulatory active compounds from Tinospora cordifolia. Journal of Ethnopharmacology 141: 918–926. [DOI] [PubMed] [Google Scholar]
- 15.Mishra R and Kaur G (2013) Aqueous ethanolic extract of Tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas. PLoS One 8: e78764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra P, Jamdar P, Desai S, et al. (2014) Phytochemical analysis and assessment of in vitro antibacterial activity of Tinospora cordifolia. International Journal of Current Microbiology and Applied Sciences 3: 224–234. [Google Scholar]
- 17.Paval J, Kaitheri SK, Kumar A, Govindan S, et al. (2011) Anti-arthritic activity of the plant Tinospora cordifolia willd. Journal of Herbal Medicine and Toxicology 5: 11–16. [Google Scholar]
- 18.Moudgil KD, Chang TT, Eradat H, et al. (1997) Diversification of T cell responses to carboxy-terminal determinants within the 65-kD heat-shock protein is involved in regulation of autoimmune arthritis. Journal of Experimental Medicine 185: 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajaiah R, Puttabyatappa M, Polumuri SK, et al. (2011) Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. Journal of Biological Chemistry 286: 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanjundaiah SM, Venkatesha SH, Yu H, et al. (2012) Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. Journal of Biological Chemistry 287: 22216–22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YH, Rajaiah R, Lee DY, et al. (2011) Suppression of ongoing experimental arthritis by a chinese herbal formula (huo-luo-xiao-ling dan) involves changes in antigen-induced immunological and biochemical mediators of inflammation. Evidence-based Complementary and Alternative Medicine 2011: 642027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellers RS, Peluso D and Morris EA (1997) The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. Journal of Bone and Joint Surgery: American Volume 79: 1452–1463. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesha SH, Yu H, Rajaiah R, et al. (2011) Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. Journal of Biological Chemistry 286: 15138–15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durai M, Gupta RS and Moudgil KD (2004) The T cells specific for the carboxyl-terminal determinants of self (rat) heat-shock protein 65 escape tolerance induction and are involved in regulation of autoimmune arthritis. Journal of Immunology 172: 2795–2802. [DOI] [PubMed] [Google Scholar]
- 25.Tong L, Nanjundaiah SM, Venkatesha SH, et al. (2014) Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clinical Immunology 155: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hembry RM, Atkinson SJ and Murphy G (2007) Assessment of gelatinase expression and activity in articular cartilage. Methods in Molecular Medicine 135: 227–238. [DOI] [PubMed] [Google Scholar]
- 27.Mahadeswaraswamy YH, Devaraja S, Kumar MS, et al. (2009) Inhibition of local effects of Indian Daboia/Vipera russelli venom by the methanolic extract of grape (Vitis vinifera L.) seeds. Indian Journal of Biochemistry and Biophysics 46: 154–160. [PubMed] [Google Scholar]
- 28.Brennan FM and McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. Journal of Clinical Investigation 118: 3537–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorman CL and Cope AP. Immune-mediated pathways in chronic inflammatory arthritis. Best Practice & Research - Clinical Rheumatology 22: 221–238. [DOI] [PubMed] [Google Scholar]
- 30.Nanjundaiah SM, Astry B and Moudgil KD (2013) Mediators of inflammation-induced bone damage in arthritis and their control by herbal products. Evidence-based Complementary and Alternative Medicine 2013: 518094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaffen SL (2009) The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Current Rheumatology Reports 11: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Berg WB and Miosssec P (2009) IL-17 as a future therapeutic target for rheumatoid arthritis. Nature Reviews - Rheumatology 5: 549–553. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Oukka M and Kuchroo VK (2007) T(H)-17 cells in the circle of immunity and autoimmunity. Nature Immunology 8: 345–350. [DOI] [PubMed] [Google Scholar]
- 34.Manel N, Unutmaz D and Littman DR (2008) The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nature Immunology 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwamoto T, Okamoto H, Toyama Y, et al. (2008) Molecular aspects of rheumatoid arthritis: Chemokines in the joints of patients. FEBS Journal 275: 4448–4455. [DOI] [PubMed] [Google Scholar]
- 36.Koch AE (2005) Chemokines and their receptors in rheumatoid arthritis: Future targets? Arthritis and Rheumatism 52: 710–721. [DOI] [PubMed] [Google Scholar]
- 37.Astry B, Harberts E and Moudgil KD (2011) A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. Journal of Interferon & Cytokine Research 31: 927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leipe J, Grunke M, Dechant C, et al. (2010) Role of Th17 cells in human autoimmune arthritis. Arthritis and Rheumatism 62: 2876–2885. [DOI] [PubMed] [Google Scholar]
- 39.Shen H, Goodall JC and Hill Gaston JS (2009) Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis and Rheumatism 60: 1647–1656. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad SF, Zoheir KM, Abdel-Hamied HE, et al. (2013) Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. International Immunopharmacology 17: 79–87. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar S, Cooney LA and Fox DA (2010) The role of T helper type 17 cells in inflammatory arthritis. Clinical and Experimental Immunology 159: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapur P, Jarry H, Wuttke W, et al. (2008) Evaluation of the antiosteoporotic potential of Tinospora cordifolia in female rats. Maturitas 59: 329–338. [DOI] [PubMed] [Google Scholar]
- 43.Abiramasundari G, Sumalatha KR and Sreepriya M (2012) Effects of Tinospora cordifolia (Menispermaceae) on the proliferation, osteogenic differentiation and mineralization of osteoblast model systems in vitro. Journal of Ethnopharmacology 141: 474–480. [DOI] [PubMed] [Google Scholar]
- 44.Ohashi K, Kawai R, Hara M, et al. (1996) Increased matrix metalloproteinases as possible cause of osseo-articular tissue destruction in long-term haemodialysis and beta 2-microglobulin amyloidosis. Virchows Archiv 428: 37–46. [DOI] [PubMed] [Google Scholar]
- 45.Shen CL YJ, Samathanam C, Cao JJ, et al. (2011) Green tea polyphenols attenuate deterioration of bone microarchitecture in female rats with systemic chronic inflammation. Osteoporosis International 22: 327–337. [DOI] [PubMed] [Google Scholar]


