Abstract
Passive leg movement (PLM) evokes a robust and predominantly nitric oxide (NO)-mediated increase in blood flow that declines with age and disease. Consequently, PLM is becoming increasingly accepted as a sensitive assessment of endothelium-mediated vascular function. However, a substantial PLM-induced hyperemic response is still evoked despite nitric oxide synthase (NOS) inhibition. Therefore, in nine young healthy men (25 ± 4 yr), this investigation aimed to determine whether the combination of two potent endothelium-dependent vasodilators, specifically prostaglandin (PG) and endothelium-derived hyperpolarizing factor (EDHF), account for the remaining hyperemic response to the two variants of PLM, PLM (60 movements) and single PLM (sPLM, 1 movement), when NOS is inhibited. The leg blood flow (LBF, Doppler ultrasound) response to PLM and sPLM following the intra-arterial infusion of NG-monomethyl-l-arginine (l-NMMA), to inhibit NOS, was compared to the combined inhibition of NOS, cyclooxygenase (COX), and cytochrome P-450 (CYP450) by l-NMMA, ketorolac tromethamine (KET), and fluconazole (FLUC), respectively. NOS inhibition attenuated the overall LBF [area under the curve (LBFAUC)] response to both PLM (control: 456 ± 194, l-NMMA: 168 ± 127 mL, P < 0.01) and sPLM (control: 185 ± 171, l-NMMA: 62 ± 31 mL, P = 0.03). The combined inhibition of NOS, COX, and CYP450 (i.e., l-NMMA+KET+FLUC) did not further attenuate the hyperemic responses to PLM (LBFAUC: 271 ± 97 mL, P > 0.05) or sPLM (LBFAUC: 72 ± 45 mL, P > 0.05). Therefore, PG and EDHF do not collectively contribute to the non-NOS-derived NO-mediated, endothelium-dependent hyperemic response to either PLM or sPLM in healthy young men. These findings add to the mounting evidence and understanding of the vasodilatory pathways assessed by the PLM and sPLM vascular function tests.
NEW & NOTEWORTHY Passive leg movement (PLM) evokes a highly nitric oxide (NO)-mediated hyperemic response and may provide a novel evaluation of vascular function. The contributions of endothelium-dependent vasodilatory pathways, beyond NO and including prostaglandins and endothelium-derived hyperpolarizing factor, to the PLM-induced hyperemic response to PLM have not been evaluated. With intra-arterial drug infusion, the combined inhibition of nitric oxide synthase (NOS), cyclooxygenase, and cytochrome P-450 (CYP450) pathways did not further diminish the hyperemic response to PLM compared with NOS inhibition alone.
Keywords: nitric oxide, vascular function, vasodilation
INTRODUCTION
Passive leg movement (PLM) induces a rapid and robust hyperemic response, evoked by mechanical deformation of the vasculature and an increase in shear stress that sets in motion a cascade of vasodilatory signals. As dysfunction of the vascular system is an early, modifiable step in the progression of many cardiovascular diseases, there is demand for methods to monitor the health of the vascular system, noninvasively, in both clinical and research settings. Consequently, PLM is becoming increasingly accepted as a sensitive assessment of vascular function (1–7). Indeed, the PLM response is attenuated with age (2–4, 8, 9) and in a number of diseases (heart failure, peripheral artery disease, chronic obstructive pulmonary disease) that are characterized by reduced vascular function (7, 10–13). However, the exact vasodilatory signals responsible for the PLM-induced hyperemia have not been completely elucidated.
To date, the interrogation of the vasodilatory pathways that contribute to PLM-induced hyperemia has been limited to nitric oxide (NO) (1, 3–5, 8), with the PLM-induced hyperemic response being 60–80% NO dependent in young healthy men (1, 3, 5, 8). However, despite the substantial reduction in the leg blood flow (LBF) response to PLM with nitric oxide synthase (NOS) inhibition, a considerable hyperemic response is still evident. The mechanism responsible for this non-NOS-derived NO-mediated PLM-induced hyperemia remains unexplained. Identifying the underlying factors contributing to this persistent hyperemic response will not only advance our understanding of PLM as a novel approach to assess vascular function but will also add to our understanding of blood flow regulation and, potentially, age- and disease-induced compensatory vasodilation.
Although NO is thought to be the predominant endothelium-derived vasodilatory molecule, both prostaglandins (PGs) and endothelium-derived hyperpolarizing factors (EDHFs), specifically epoxyeicosatrienoic acids (EETs), have been implicated in the regulation of vascular tone at rest and blood flow during reactive hyperemia and exercise (14–25). PG and EDHF are both metabolites of arachidonic acid, formed enzymatically in the endothelium by the metabolism of arachidonic acid by cyclooxygenase (COX) and cytochrome P-450 (CYP450), respectively (26, 27). Both PG and EDHF exert potent vasoactive effects by acting on various K+ channels on the vascular smooth muscle. However, it remains to be determined whether inhibiting the endothelium-derived formation of PG and EDHF, in conjunction with NO inhibition, further attenuates the hyperemic response to PLM.
In addition to identifying the predominant vasodilatory pathway involved in PLM-induced hyperemia, significant effort has been expended to refine the PLM test, with the goal of improving usability, interpretability, physiological specificity, and, ultimately, clinical relevance (1, 2, 28). Indeed, these efforts have resulted in a variant of the PLM test, single PLM (sPLM), in which only a single passive movement is performed (1, 2, 29). sPLM results in a hyperemic response that qualitatively resembles that observed with PLM, albeit with an attenuated magnitude. Also, similar to PLM, sPLM-induced hyperemia is highly NO dependent and capable of clearly identifying age- and disease-related vascular dysfunction (1, 2, 12, 13). Finally, as with PLM, a substantial hyperemic response to sPLM persists after NOS inhibition, and the contribution of PG and EDHF to this residual response is currently unknown.
Therefore, the purpose of this study was to determine whether the endothelium-derived vasodilatory factors PG and EDHF contribute to the non-NOS-derived NO-mediated hyperemic response to PLM and sPLM in young healthy men. Specifically, we tested the hypothesis that the combined pharmacological inhibition of COX and CYP450 would result in an additional reduction in the PLM and sPLM-induced hyperemic response, beyond that of inhibiting NOS alone. Testing this hypothesis will strengthen the clinical utility of PLM and sPLM and advance our understanding of the vasodilatory pathways assessed by these novel vascular function tests.
METHODS
Subjects
Nine healthy, recreationally active men volunteered to participate in this research study (subject characteristics are presented in Table 1). Subjects were not taking any medications and were free from overt cardiovascular disease. Written informed consent was obtained, and protocols were approved by the University of Utah and the Salt Lake City Veterans Affairs Medical Center (VAMC) Institutional Review Boards, in accordance with the principles outlined in the Declaration of Helsinki. All data collection took place at the Salt Lake City VAMC Geriatric Research, Education, and Clinical Center in the Utah Vascular Research Laboratory.
Table 1.
Subject characteristics
| Age, yr | 25 ± 4 |
| Height, cm | 180 ± 6 |
| Weight, kg | 83 ± 11 |
| Body mass index, kg m−2 | 25± 3 |
| Leg volume, dL | 76 ± 17 |
| Glucose, mg·dL−1 | 91 ± 7 |
| Cholesterol, mg·dL−1 | 171 ± 12 |
| Triglycerides, mg·dL−1 | 100 ± 33 |
| HDL, mg·dL−1 | 45 ± 10 |
| LDL, mg·dL−1 | 105 ± 20 |
| Hct, % | 44.8 ± 2.4 |
| Hgb, g·dL−1 | 15.5 ± 1.1 |
Values are means ± SD; n = 9 subjects. Hgb, hemoglobin.
Experimental Protocol
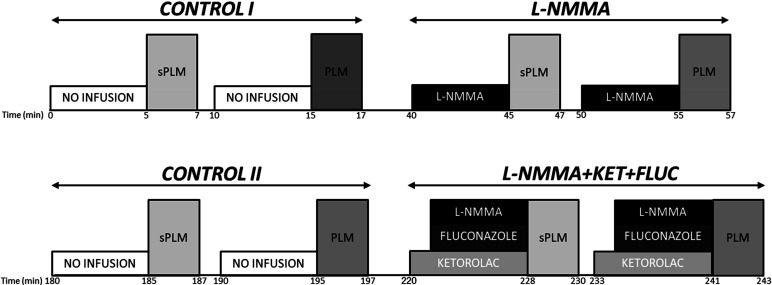
Subjects arrived at the laboratory after an overnight fast and having abstained from vigorous activity for at least 24 h. Body mass and stature were recorded, and the femoral artery of the experimental leg was catheterized (18-gauge central arterial catheter; Arrow International, Reading, PA) under sterile conditions with the Seldinger technique. Specifically, the catheter was inserted into the superficial femoral artery and then advanced proximally, past the bifurcation, allowing infusates to be administered at the level of the common femoral artery. After catheterization, subjects were given at least 30 min to rest before further instrumentation and commencement of the experiments. All experimental protocols were performed in an upright-seated position and involved passive knee flexion-extension through a 90° range of motion at 1 Hz (metronome guided) over 60 s for PLM or 1 s for sPLM. Both PLM and sPLM were initiated with the knee fully extended at 180°. Both PLM and sPLM were performed by a member of the research team moving the leg through the desired range of motion. In an effort to localize the drug infusions to the upper portion of the leg and emphasize the specificity of the thigh vasculature to PLM and sPLM, an occlusion cuff (Hokanson, Bellevue, WA) was placed immediately distal to the knee and inflated to 250 mmHg (30). To minimize any hemodynamic alterations evoked by the cuff, baseline hemodynamics measurements were performed 5 min after cuff inflation (30). To avoid a startle reflex and minimize active resistance during either PLM or sPLM, subjects were informed that the movement would take place in ∼1 min, but they were not informed of the exact timing of the movement. The order and timing of the PLM protocols are presented in Fig. 1. The order of testing for PLM and sPLM was randomized. In the majority of subjects (n = 7), as illustrated in Fig. 1, there were two control trials of PLM and sPLM without drug infusion. The first was the initial trial (CONTROL I), and the second (CONTROL II) was repeated 120 min after the NG-monomethyl-l-arginine (l-NMMA) infusion trial. In the other two subjects, who were actually studied first, there was no CONTROL II preceding the combined infusion of l-NMMA, ketorolac tromethamine (KET), and fluconazole (FLUC), the l-NMMA+KET+FLUC trial, following the trial with only the infusion of l-NMMA. This protocol change, the addition of CONTROL II (n = 7), was instigated when it was recognized that, because of the length of time between trials, it would be more rigorous to include an additional PLM measurement before the final drug infusions. As a CONTROL II was not performed in these two subjects, the CONTROL I data were used as both CONTROL I and II data in the analyses, such that a complete data set (n = 9 subjects) was available for the CONTROL I, l-NMMA, CONTROL II, and l-NMMA+KET+FLUC trials. This approach was justified, as a statistical comparison of the results from CONTROL I and II, in the seven subjects who performed both, revealed no difference between the data collected at these time points. Of importance, based on previously published research, the window of effect of l-NMMA in the human vasculature is <60 min (31), indicating that our 120-min period between trials was more than adequate to wash out any vasoactive impact of the previously administered l-NMMA before CONTROL II. Finally, KET and FLUC were not infused independently from l-NMMA, or one another, as the purpose of this investigation was to identify whether PG and EDHF contribute to the remaining hyperemic response once NOS-derived production of NO has been inhibited.
Figure 1.
Experimental protocol. The order of testing for passive leg movement (PLM) and single passive leg movement (sPLM) was randomized. Two of the nine subjects did not perform CONTROL II. FLUC, fluconazole; KET, ketorolac tromethamine; l-NMMA, NG-monomethyl-l-arginine.
Thigh volume measurement and drug delivery.
Anthropometric measurements were used to determine thigh volume (32). All drugs were infused intra-arterially and dosed according to thigh volume.
l-NMMA infusion.
To inhibit NOS, l-NMMA (Bachem, Bubendorf, Switzerland) was diluted in normal saline to a concentration of 5 mg/mL from 250 mg of lyophilized powder and infused at a loading dose of 0.24 mg·dL−1·min−1 for 5 min. This dosing was based on previous dose-response curves from our group that demonstrated a plateau in the reduction in resting arm blood flow at this dose (33). Furthermore, additional prior work from our group using this dose of l-NMMA documented a significant attenuation in the hyperemic response to PLM and sPLM in healthy young men (1, 5). A maintenance dose of 0.12 mg·dL−1·min−1 was infused for the second PLM test in the counterbalanced design (Fig. 1).
KET infusion.
To inhibit PG synthesis by COX, KET (Hospira, Lake Forrest, IL) was diluted in normal saline to a concentration of 2 mg/mL from 30 mg/mL and was infused at a dose of 0.03 mg·dL−1·min−1 for 5 min, according to previous reports (22, 34).
FLUC infusion.
To inhibit the cytochrome P-450 production of EETs from arachidonic acid, FLUC (Sagent, Schaumburg, IL), premixed in normal saline at a concentration of 2 mg/mL, was infused at a dose of 0.049 mg·dL−1·min−1 for 8 min, according to previous reports (14, 15, 23).
Measurements
Femoral blood flow.
Measurements of common femoral arterial blood velocity and vessel diameter were performed in the experimental leg, distal to the inguinal ligament and proximal to the deep and superficial femoral bifurcation, with a Doppler ultrasound system (Logic e9; General Electric Medical Systems, Milwaukee, WI) in accordance with recently published guidelines (35). The ultrasound system was equipped with a linear transducer operating at an imaging frequency of 9 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was measured with the same transducer with a pulse wave frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was centered within the vessel and then maximized according to vessel size. Femoral artery diameter was measured by the investigators, whereas mean blood velocity (Vmean; angle-corrected and intensity-weighted area under the curve) was automatically calculated by the Doppler ultrasound system (Logic e9). With the use of femoral arterial diameter and Vmean, LBF was calculated as LBF = Vmean·π (vessel diameter/2)2·60, where LBF is in milliliters per minute.
Central hemodynamic variables.
Heart rate (HR), stroke volume (SV), and cardiac output (CO) were determined with a finometer (Finapres Medical Systems, Amsterdam, The Netherlands). SV was calculated from beat-by-beat pressure waveforms assessed by photoplethysmography using the Modelflow method (Beatscope version 1.1; Finapres Medical Systems), which, in combination with HR, has been documented to accurately estimate CO during a variety of experimental protocols (5, 16, 36–38). Intravascular systolic and diastolic arterial pressures were measured with an in-line pressure transducer (Baxter, Deerfield, IL) placed at the level of the catheter. Mean arterial pressure (MAP) was calculated as the mean of the femoral arterial catheter pressure.
Data acquisition.
Throughout each protocol, HR, SV, CO, and femoral arterial pressure underwent analog-to-digital conversion and were simultaneously acquired (200 Hz) with a data acquisition system (AcqKnowledge; Biopac Systems, Goleta, CA).
Data and Statistical Analysis
HR, SV, CO, and femoral MAP were averaged to second by second by the data acquisition software (AcqKnowledge; Biopac Systems). The second-by-second blood velocities acquired by Doppler ultrasound (Logic e9) were analyzed for 60 s of movement. All analyses were performed using a 3-s moving average. Baseline was analyzed using the 60 s before initiation of either PLM or sPLM. A one-way repeated-measures ANOVA was used to determine significant differences in baseline, absolute change from baseline to peak for HR, SV, CO, and MAP, as well as area under the curve (AUC) for LBF within each PLM condition (sPLM and PLM). Comparisons between PLM and sPLM were not performed. LBFAUC was calculated as the summed second-by-second response above baseline over 60 s for PLM and 45 s for sPLM. Post hoc comparisons were made with the Holm–Sidak method, when appropriate. Statistical power analysis based on 1) previous results from our group reporting a 60–70% reduction in PLM-induced hyperemic response after l-NMMA (1, 5) and 2) the hypothesis that the combined inhibition of l-NMMA+KET+FLUC would nearly abolish (85–95% reduction) the PLM-induced hyperemic response was performed (GPower, version 3.1). This analysis indicated that 8 subjects would be required to determine statistical differences, assuming a 65 ± 25% and 90 ± 25% reduction in the PLM-induced hyperemic response as measured by AUC for the l-NMMA and l-NMMA+KET+FLUC infusions, respectively. An expected correlation between groups of 0.8 was utilized, and an effect size of 1.58 was determined. Statistical significance was accepted at P < 0.05. Values are presented as means ± SD, with the exception of some figures where SE is used for clarity.
RESULTS
Central and Peripheral Hemodynamic Response to PLM
Baseline central hemodynamics before PLM with and without the intra-arterial infusion of l-NMMA and the combined infusion of l-NMMA, KET, and FLUC are presented in the top portion of Table 2. Central hemodynamics (HR, SV, CO, and MAP) remained stable during baseline, before PLM, and there were no differences in these variables at baseline between the l-NMMA and l-NMMA+KET+FLUC infusions (Table 2, top portion). As documented in the top portion of Table 3, the PLM-induced peak changes in HR, SV, CO, and MAP were significantly different from baseline, but there were no differences in these responses between the l-NMMA and l-NMMA+KET+FLUC infusions.
Table 2.
Baseline central hemodynamics with and without intra-arterial infusion of l-NMMA and combined infusion of l-NMMA, KET, and FLUC
| CONTROL I | l-NMMA | CONTROL II | l-NMMA+KET+FLUC | P Value (main effect) | |
|---|---|---|---|---|---|
| PLM | |||||
| MAP, mmHg | 103 ± 12 | 105 ± 13 | 106 ± 15 | 107 ± 13 | 0.24 |
| HR, beats/min | 67 ± 12 | 64 ± 11 | 65 ± 14 | 65 ± 11 | 0.22 |
| SV, mL/beat | 104 ± 32 | 102 ± 23 | 101 ± 23 | 99 ± 18 | 0.71 |
| CO, L/min | 6.5 ± 1.4 | 6.3 ± 1.3 | 6.2 ± 1.4 | 6.4 ± 0.7 | 0.37 |
| sPLM | |||||
| MAP, mmHg | 102 ± 12 | 104 ± 10 | 105 ± 14 | 108 ± 12 | 0.04 |
| HR, beats/min | 65 ± 12 | 65 ± 11 | 64 ± 11 | 64 ± 12 | 0.99 |
| SV, mL/beat | 103 ± 34 | 101 ± 22 | 98 ± 21 | 99 ± 18 | 0.76 |
| CO, L/min | 6.3 ± 0.8 | 6.2 ± 1.2 | 6.1 ± 1.4 | 6.0 ± 1.1 | 0.74 |
Means ± SD; n = 9 subjects for all data except CONTROL II (n = 7 subjects). CO, cardiac output; FLUC, fluconazole; HR, heart rate; KET, ketorolac; l-NMMA, NG-monomethyl-l-arginine; MAP, mean arterial pressure; PLM, passive leg movement; sPLM, single passive leg movement; SV, stroke volume. There were no statistically significant differences between conditions.
Table 3.
Peak central hemodynamic changes during PLM and sPLM with and without intra-arterial infusion of l-NMMA and combined infusion of l-NMMA, KET, and FLUC
| CONTROL I | l-NMMA | CONTROL II | l-NMMA+KET+FLUC | P Value (main effect) | |
|---|---|---|---|---|---|
| PLM | |||||
| MAP, Δ mmHg | −6 ± 5 | −7 ± 5 | −8 ± 3 | −10 ± 5 | 0.12 |
| HR, Δ beats/min | 11 ± 10 | 12 ± 3 | 12 ± 9 | 11 ± 8 | 0.95 |
| SV, Δ mL/beat | 16 ± 6 | 14 ± 7 | 12 ± 6 | 17 ± 6 | 0.44 |
| CO, Δ L/min | 2.1 ± 1.5 | 1.6 ± 0.7 | 1.9 ± 0.9 | 1.8 ± 0.8 | 0.67 |
| sPLM | |||||
| MAP, Δ mmHg | −9 ± 7 | −6 ± 3 | −9 ± 4 | −9 ± 5 | 0.17 |
| HR, Δ beats/min | 9 ± 4 | 9 ± 5 | 11 ± 4 | 11 ± 8 | 0.82 |
| SV, Δ mL/beat | 11 ± 5 | 10 ± 5 | 13 ± 6 | 11 ± 5 | 0.42 |
| CO, Δ L/min | 1.4 ± 1.3 | 0.9 ± 0.4 | 1.3 ± 0.5 | 0.9 ± 0.5 | 0.30 |
Means ± SD; n = 9 subjects for all data except CONTROL II (n = 7 subjects). CO, cardiac output; FLUC, fluconazole; HR, heart rate; KET, ketorolac; l-NMMA, NG-monomethyl-l-arginine; MAP, mean arterial pressure; PLM, passive leg movement; sPLM, single passive leg movement; SV, stroke volume. There were no statistically significant differences between conditions.
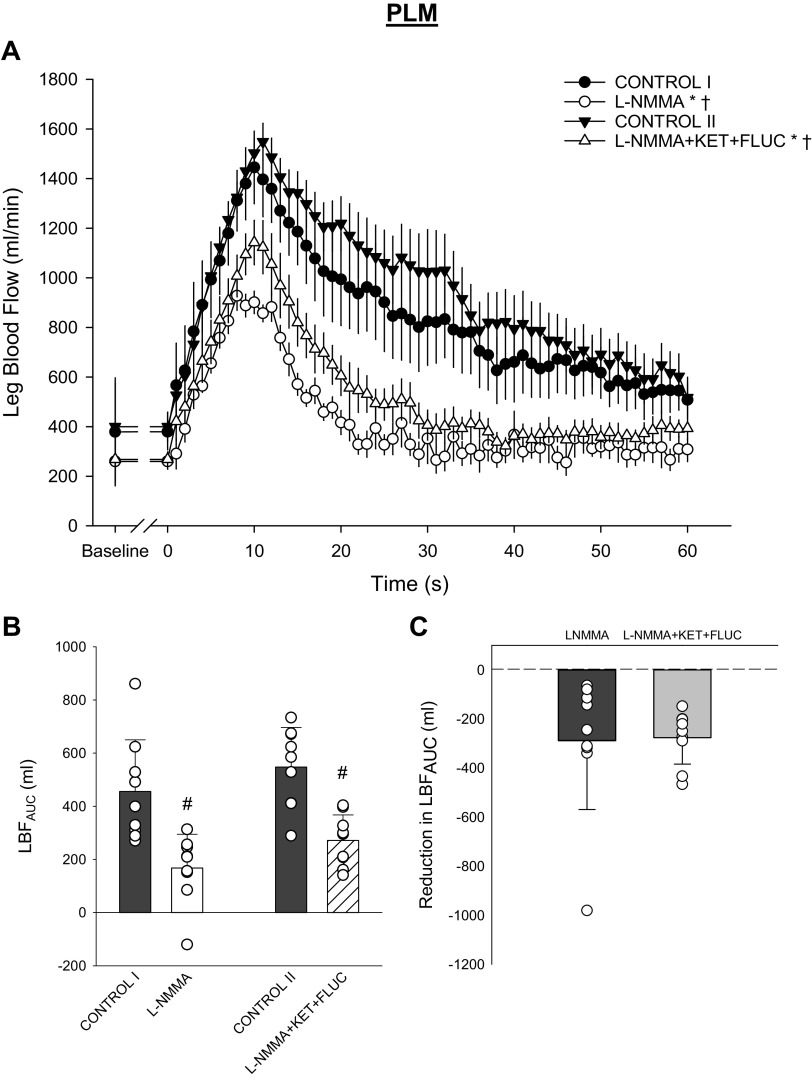
Baseline and PLM-induced peripheral hemodynamics are presented in Fig. 2. Before PLM, l-NMMA significantly lowered baseline LBF (CONTROL I: 379 ± 218 vs. l-NMMA: 260 ± 95 mL/min, P = 0.021; Fig. 2A). A similar reduction in baseline LBF was evident after the combined administration of l-NMMA+KET+FLUC (CONTROL II: 400 ± 177 vs. l-NMMA+KET+FLUC: 268 ± 90 mL/min, P = 0.012; Fig. 2A). Indeed, there was no difference between the l-NMMA- and l-NMMA+KET+FLUC-induced reductions in baseline LBF (P = 0.69; Fig. 2A). l-NMMA and l-NMMA+KET+FLUC significantly attenuated the PLM-induced LBFAUC (P < 0.01; Fig. 2, A and B). However, there was no difference between the l-NMMA- and l-NMMA+KET+FLUC-induced reduction in the PLM-induced LBFAUC (P = 0.89; Fig. 2C).
Fig. 2.
Leg blood flow response to passive leg movement (PLM) with and without intra-arterial infusion of NG-monomethyl-l-arginine (l-NMMA) and combined infusion of l-NMMA, ketorolac tromethamine (KET), and fluconazole (FLUC). A: average second-by-second leg blood flow (LBF) response to PLM (CONTROL I vs. l-NMMA: main effect: P = 0.02, interaction effect: P < 0.01; CONTROL II vs. l-NMMA+KET+FLUC: main effect: P < 0.01, interaction effect: P < 0.01). B: overall LBF response assessed by LBF area under the curve (LBFAUC) (main effect: P < 0.01). C: the drug-induced reduction in LBFAUC (P = 0.89). A: data presented as means ± SE. *Significant main effect, †significant interaction from respective control trial. B and C: data presented as individual (circles), mean (bar), and SD. #Significant difference from respective control trial, P < 0.05. n = 9 subjects for all data except CONTROL II (n = 7 subjects).
Central and Peripheral Hemodynamic Response to sPLM
Baseline central hemodynamics before sPLM with and without the intra-arterial infusion of l-NMMA and the combined infusion of l-NMMA, KET, and FLUC are presented in the bottom portion of Table 2. Central hemodynamics (HR, SV, CO, and MAP) remained stable during baseline, before sPLM, and there were no differences in these variables at baseline between the l-NMMA and l-NMMA+KET+FLUC infusions (Table 2, bottom portion). As documented in the bottom portion of Table 3, the sPLM-induced peak changes in HR, SV, CO, and MAP were significantly different from baseline, but there were no differences in these responses between the l-NMMA and l-NMMA+KET+FLUC infusions.
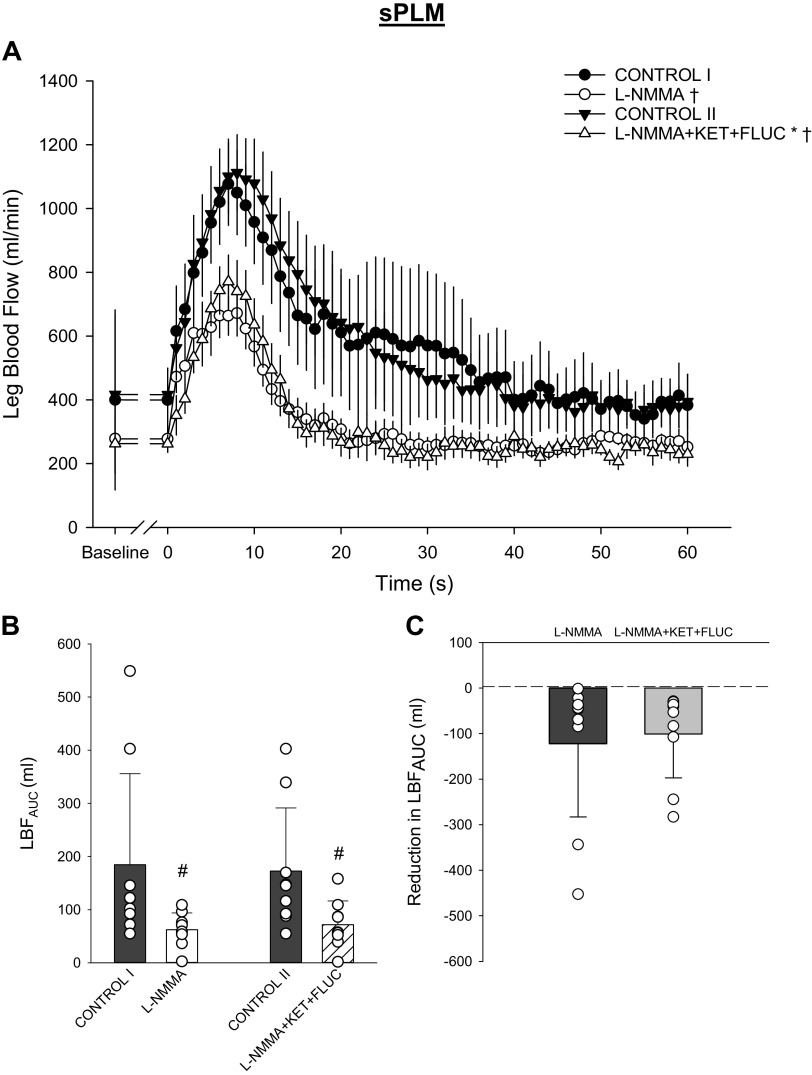
Baseline and sPLM-induced peripheral hemodynamic responses are presented in Fig. 3. There was a significant main effect of the drug interventions on baseline LBF; however, likely because of greater variance in baseline LBF before sPLM, the post hoc analysis was unable to identify pairwise differences in baseline LBF (P > 0.05; Fig. 3A). It should be noted that the magnitudes of the LBF reductions due to l-NMMA and the combined administration of l-NMMA+KET+FLUC were similar to each other and to that exhibited before PLM. l-NMMA and l-NMMA+KET+FLUC significantly attenuated the PLM-induced LBFAUC (P = 0.026 and P = 0.047, respectively; Fig. 3, A and B). There was no difference between the l-NMMA- and l-NMMA+KET+FLUC-induced reduction in the PLM-induced LBFAUC (P = 0.43; Fig. 3C).
Fig. 3.
Leg blood flow response to single passive leg movement (sPLM) with and without intra-arterial infusion of NG-monomethyl-l-arginine (l-NMMA) and combined infusion of l-NMMA, ketorolac tromethamine (KET), and fluconazole (FLUC). A: average second-by-second leg blood flow (LBF) response to sPLM (CONTROL I vs. l-NMMA: main effect: P = 0.09, interaction effect: P < 0.01; CONTROL II vs. l-NMMA+KET+FLUC: main effect: P = 0.02, interaction effect: P < 0.01). B: overall LBF response assessed by LBF area under the curve (LBFAUC) (main effect: P < 0.01). C: the drug-induced reduction in LBFAUC (P = 0.54). A: data presented as means ± SE. *Significant main effect, †significant interaction from respective control trial. B and C: data presented as individual (circles), mean (bar), and SD. #Significant difference from respective control trial, P < 0.05. n = 9 subjects for all data except CONTROL II (n = 7 subjects).
DISCUSSION
Passive leg movement (PLM) and single PLM (sPLM) are becoming increasingly accepted as sensitive approaches to assess endothelium-mediated vascular function, as the majority of the hyperemic response in young healthy adults is attributed to nitric oxide (NO) bioavailability. However, a robust and transient PLM- and sPLM-induced hyperemia persists in the presence of nitric oxide synthase (NOS) inhibition, indicating that factors in addition to NO are likely contributing to the remaining hyperemia. This study sought to determine whether other endothelium-dependent pathways, specifically prostaglandin (PG) and endothelium-derived hyperpolarizing factor (EDHF), contribute to the remaining hyperemic response to PLM and sPLM when NOS is inhibited. The main finding of this work, contrary to our hypothesis, was that the combined inhibition of nitric oxide synthase (NOS), cyclooxygenase (COX), and cytochrome P-450 (CYP450) to inhibit NO, PG, and EDHF formation, respectively, did not further attenuate the hyperemic response, to either PLM or sPLM, beyond that which was accomplished by NOS inhibition alone. Therefore, the combined inhibition of PG and EDHF, two potent endothelium-dependent vasodilators, does not contribute to the non-NOS-derived NO-mediated hyperemic response to either PLM or sPLM in healthy young men. The present findings confirm previous work from our group and others that the majority of the hyperemic response to PLM and sPLM is NO dependent (1, 3, 5) and that the remaining PLM-induced hyperemic response is likely endothelium independent. These findings add to the mounting, clinically relevant, evidence and understanding of the vasodilatory pathways assessed by the PLM and sPLM vascular function tests.
Contribution of NO to PLM- and sPLM-Induced Hyperemia
The pharmacological dissection of the vasodilatory pathways contributing to the PLM-induced hyperemic response has, to date, been limited to the NO pathway and accomplished by the intra-arterial administration of the NOS inhibitor l-NMMA (1, 3, 5, 8). This prior work and the present study have clearly documented that in young healthy subjects the hyperemic response to both PLM and sPLM is largely NOS-derived NO mediated (50–80%) (Figs. 2 and 3, A and B) (1, 3, 5, 8). Because of the recognized antiatherogenic properties of NO, this high level of NO dependence, in conjunction with several reports of age- and disease-related reductions in PLM and sPLM-induced hyperemia (2, 4, 7–9, 11–13), support the clinical relevance of these vascular function assessments. Intriguingly, despite the substantial reduction in hyperemia following NOS inhibition, there remains a persistent, and considerable, hyperemic response to PLM and sPLM, the cause of which remains unexplained. The magnitude of this persistent hyperemic response following NOS inhibition in young individuals, ranges between 50 and 200 mL as assessed by LBFAUC (1, 4, 5, 8). In the present study, l-NMMA significantly attenuated the hyperemic response (LBFAUC) to both PLM and sPLM by ∼55% and ∼50%, respectively, which, although on the lower end of the NOS-induced inhibition effect, aligns with previous findings (1, 4, 5, 8). In absolute terms, the remaining hyperemia equates to ∼290 mL for PLM and ∼120 mL for sPLM. The magnitude of the remaining response is not trivial and is, in fact, of a similar magnitude to that which is observed in older healthy individuals without NOS blockade (i.e., control conditions) (4, 8). With the magnitude of the remaining hyperemic response in context, the aim of the present study was to examine the contribution of endothelium-dependent vasodilators, beyond NO, to the hyperemic response to PLM and sPLM in healthy young men. Identifying the vasodilatory pathways responsible for both PLM and sPLM adds to the mounting, clinically relevant, evidence and understanding of the vasodilatory mechanisms assessed by these vascular function tests.
Contribution of PG and EDHF to PLM- and sPLM-Induced Hyperemia
PG and EDHF are potent endothelium-dependent vasodilators. Indeed, vasoactive roles for PG and EDHF have been identified in many studies, including those focused on healthy and diseased subject cohorts (14–16, 20–25). Moreover, the contributions of these molecules to blood flow regulation have been established in models examining resting blood flow, reactive hyperemia, exercise-induced hyperemia, but not PLM. Although it should be recognized that not all studies have reported a significant impact of inhibiting PG and/or EDHF on hyperemia (21, 22, 39), our hypothesis that the combined inhibition of NOS, COX, and CYP450 would further attenuate the reduction in PLM- and sPLM-induced hyperemia, beyond NOS inhibition alone, is reasonable based upon the preponderance of data.
In contrast to our hypothesis, compared with NOS inhibition alone there was no further attenuation in the PLM-induced hyperemic response when NOS, COX, and CYP450 were inhibited. Although the reason for the lack of an additive reduction in PLM- and sPLM-induced hyperemia with combined triple blockade is not entirely clear, there are several potential explanations. Specifically, although a clear vasoactive role for PG has been identified in many investigations (21, 22, 24, 25, 40, 41), often the magnitude of the PG contribution to the vasodilation was relatively small and/or transient. Furthermore, the majority of studies examining the vasoactive role of PG report that PG is not obligatory for resting blood flow, reactive hyperemia, or exercise hyperemia (21, 22, 39, 42). Interestingly, most investigations examining the role of EDHF are performed with the proviso that COX inhibition is accomplished by either oral indomethacin administration or intra-arterial KET infusion (14–16, 20, 23), which effectively minimizes a possible vasoactive role of PG. Therefore, in combination with both NO and PG blockade, the lack of an effect of CYP450 inhibition on both PLM- and sPLM-induced hyperemia was also rather surprising. This is especially true considering the important role that both pulsatile stretch and increased shear stress play in the stimulation of CYP450 metabolites in the vascular bed of human skeletal muscle (19).
Several reports indicate an important role of EDHFs in the regulation of baseline vasomotor tone and reactive hyperemia, which are both responses that are predominantly regulated in the microvasculature (14, 16, 20, 23). Current dogma, based largely on animal models and in vitro preparations, states that the contribution of EDHF to endothelium-dependent vasodilation increases as vessel diameter decreases (27, 38, 43). However, EDHF has been reported to play a major role in conduit arteries in coronary, renal, and peripheral vascular beds (14–16, 38, 44) Indeed, among the most impressive human-based findings supporting the localized vasoactive role of EDHF was the report of blunted radial artery dilation, without a concomitant reduction in forearm blood flow, during sustained hand heating with CYP450 inhibition (14). Both PLM and sPLM are primarily microvascular assessments, and therefore this previous report is well aligned with the present finding of no effect of EDHF on the movement-induced hyperemia. Another possible explanation for why our hypothesis was not supported may be related to differences in the regulation of vascular tone between limbs (45) and the high degree of heterogeneity in endothelium-dependent vasodilatory mechanisms across the length of the vascular bed (27). Although a growing body of literature supports the role of CYP450 metabolites as EDHFs, the exact identity of EDHF may vary among tissues and species (18, 26, 38, 46, 47). During active single-leg knee-extension exercise in humans, EDHF has been reported to contribute significantly, albeit minimally (16%), to exercise hyperemia (19), whereas others have reported no role for EDHF (42), leaving overall conclusions regarding the role of EDHF in the exercising human leg somewhat equivocal. However, the present findings, focused upon PLM and sPLM, support there being no role for EDHF in movement-induced hyperemia when combined with PG and NOS inhibition.
The novel finding of no further attenuation in the PLM- or sPLM-induced hyperemic response when NOS, COX, and CYP450 were inhibited, compared with NOS inhibition alone, has several important implications beyond the fact that these dilators do not explain the remaining leg blood flow response. First, as the combined triple blockade did not further attenuate the hyperemic response to either PLM or sPLM, it appears that PG and EDHF did not compensate for the reduced NO bioavailability when NOS was inhibited alone. It remains to be determined whether a similar lack of compensatory vasodilation by PG and EDHF is evident in conditions where NO bioavailability is chronically decreased, such as aging and cardiovascular disease. Second, additional endothelium-dependent pathways, not targeted in this investigation, may be contributing to PLM vasodilation, including K+ efflux from the endothelium (48), ATP acting through endothelial purinergic receptors (49–51), or hydrogen peroxide (H2O2) derived from endothelial cell mitochondria (36, 37, 52, 53). Furthermore, recent evidence indicates that nitrate can be stored in skeletal muscle of humans and utilized during exercise (54). The contribution of this skeletal muscle nitrate reservoir to exercise and movement-induced hyperemia remains unknown. In light of the present findings, and the recognition that we did not mechanistically evaluate all possible vasodilatory pathways, it is possible that the hyperemic response to PLM and sPLM is actually comprised of both endothelium-dependent and endothelium-independent mechanisms.
Potential Contribution of Endothelium-Independent Vasodilation to PLM- and sPLM-Induced Hyperemia
The finding that the addition of COX and CYP450 inhibition failed to further diminish the response to both PLM and sPLM adds credence to the potential contribution of endothelium-independent regulation of PLM- and sPLM-induced hyperemia. Indeed, somewhat related studies of mechanical compression, utilizing isolated artery preparations with and without endothelial denudation (55–58), indicate that both endothelium-dependent and endothelium-independent mechanisms regulate vasodilation. Additionally, with the use of an in vivo protocol to compare hyperemia during eccentric and concentric cycling, it was determined that mechanical tension accounts for ∼26% of the blood flow response to exercise (59). Clearly, mechanical deformation/compression of the vasculature contributes to the regulation of blood flow through an endothelium-independent mechanism(s). Although not assessed in the present study, a growing body of literature based on elegant mechanistic investigations performed in the human arm has identified a critical role for K+ and several K+ channels (inwardly rectifying KIR channels, Ca2+ voltage-gated K+ channels, Na+-K+-ATPase) in the vascular smooth muscle that govern hyperpolarization and vasodilation in response to mechanical compression, postcirculatory occlusion, single-muscle contraction, and exercise (34, 40, 58, 60–64). Unfortunately, examining the vasoactive role of these K+ channels in the leg with the present pharmacological approach may not be possible, as the dose of the drugs (i.e., barium chloride, ouabain) required to adequately inhibit these channels may evoke unwanted, and potentially dangerous, systemic effects.
Experimental Considerations
The overall goal of this study was to determine whether PG and EDHF, two potent endothelium-dependent vasodilators, contribute to the non-NOS-derived NO-mediated hyperemic response to either PLM or sPLM in healthy young men. Therefore, the effects of PG and EDHF independent of NOS inhibition were not examined, and the contribution of each of these vasodilators to the PLM-induced hyperemic response during control conditions (i.e., without any inhibitory drugs on board) remains unknown. This is not an uncommon approach, as most investigations examining the role of EDHF are performed with COX inhibition (14–16, 20, 23). Furthermore, the vasoactive role of CYP450-derived EDHF is only evident when combined with NOS inhibition (14, 19), indicating an interesting interaction between NO and EDHF. Indeed, studies that have interrogated the independent role of EDHF in the regulation of blood flow often report an increase in blood flow when the contribution of EDHF is inhibited by CYP450-blocking drugs or by inhibiting channels (14, 15). Given this increase in blood flow with CYP450 inhibitors, as used here, it is not possible to perform a dose response for this drug or independently assess the contribution of EDHF to PLM or sPLM. Previous studies, examining the vasoactive roles of EDHF, have used tetraethylammonium chloride (TEA) to inhibit channels (14, 15, 20, 42), the CYP450 inhibitor fluconazole (or other azole-type drugs), or a combination of these drugs. This study focused upon the upstream endothelial production of EDHF, by inhibiting CYP450 with fluconazole, because channel inhibition is likely not endothelium specific, as these channels are found on the vascular smooth muscle. Additionally, although the lack of an effect of PG and EDHF inhibition raises interest in endothelium-independent vasodilatory mechanisms, as discussed above, other endothelium-dependent pathways, not targeted in this investigation, may be contributing to PLM- and sPLM-induced hyperemia. These include K+ efflux from the endothelium (48), ATP acting through endothelial purinergic receptors (49–51), as well as H2O2 derived from endothelial cell mitochondria (36, 37, 52). These pathways remain potential targets for future investigations that aim to completely characterize the vasodilatory mechanisms responsible for PLM- and sPLM-induced hyperemia.
An additional consideration regarding the present study is that only young healthy men were studied, which limits the generalizability of the findings. Further research is warranted to examine how PG and EDHF might contribute to PLM-induced hyperemia in women, older adults, and individuals with existing cardiovascular disease. Of note, we previously reported that age-related reductions in PLM-induced hyperemia observed in men are recapitulated in women (65), suggesting similar regulation of blood flow during PLM; however, mechanistic evaluation of the vasodilatory pathways to PLM has not been directly performed in women. With aging and disease (heart failure, chronic obstructive pulmonary disease, and peripheral artery disease) the hyperemic response to PLM is markedly diminished (4, 11, 13, 66), yet clearly present. Given the much lower hyperemic responses to PLM in older and diseased individuals, a compensatory response that preserves vasodilatory capacity, which has been reported in resistance vessels obtained from older adults and patients with coronary artery disease (53, 67) or after increased systemic or intraluminal pressure (36, 52), does not appear to be occurring with PLM. However, it remains to be determined whether PG and EDHF alone or other endothelium-derived vasodilators (e.g., H2O2) contribute to the significantly reduced hyperemic response.
Conclusions
In healthy young men, PG and EDHF, produced enzymatically from COX and CYP450, respectively, do not contribute to the remaining PLM- and sPLM-induced hyperemic response when NO bioavailability is diminished by NOS inhibition. These findings advance our understanding of the vasodilatory pathways that contribute to the hyperemic response during PLM and sPLM and indicate that endothelium-independent mechanisms responsive to mechanical compression and increased shear stress likely contribute to the remaining hyperemic response. Overall, these findings add to the mounting, clinically relevant, evidence and understanding of the vasodilatory pathways assessed by the PLM and sPLM vascular function tests.
GRANTS
This study was supported by Department of Veterans Affairs (VA) Rehabilitation Research and Development Career Development (IK2RX001215), VA Merit Awards (I01 CX001999, E6910-R, E1697-R, E1572-P, and E3207-R), VA Senior Research Career Scientist (E9275-L) awards, the American Heart Association (14SDG18850039), National Heart, Lung, and Blood Institute Grants P01 HL-091830 and R01 HL-142603, and Ruth L. Kirschstein National Research Service Awards T32 HL-139451 and T32 HL-007576.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.T. and R.S.R. conceived and designed research; J.D.T., O.S.K., R.M.B., J.R.G., A.C.K., J.R.H., C.L.J., K.L.S., A.V.B., S.H.P., J.C.C., A.D.N., D.E.M., J.E.J., and A.D.B. performed experiments; J.D.T., R.M.B., J.R.G., A.C.K., J.R.H., C.L.J., K.L.S., A.V.B., S.H.P., and J.C.C. analyzed data; J.D.T., O.S.K., R.M.B., J.R.G., and R.S.R. interpreted results of experiments; J.D.T. prepared figures; J.D.T. drafted manuscript; J.D.T., O.S.K., R.M.B., J.R.G., A.C.K., J.R.H., C.L.J., K.L.S., A.V.B., S.H.P., J.C.C., A.D.N., D.E.M., J.E.J., A.D.B., and R.S.R. edited and revised manuscript; J.D.T., O.S.K., R.M.B., J.R.G., A.C.K., J.R.H., C.L.J., K.L.S., A.V.B., S.H.P., J.C.C., A.D.N., D.E.M., J.E.J., A.D.B., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD, Richardson RS. Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol (1985) 123: 1468–1476, 2017. doi: 10.1152/japplphysiol.00533.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hydren JR, Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Richardson RS. Delineating the age-related attenuation of vascular function: Evidence supporting the efficacy of the single passive leg movement as a screening tool. J Appl Physiol (1985) 126: 1525–1532, 2019. doi: 10.1152/japplphysiol.01084.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe A, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol 308: H672–H679, 2015. doi: 10.1152/ajpheart.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinity JD, Richardson RS. Physiological impact and clinical relevance of passive exercise/movement. Sports Med 49: 1365–1381, 2019. doi: 10.1007/s40279-019-01146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker MA, Hoier B, Walker PJ, Schulze K, Bangsbo J, Hellsten Y, Askew CD. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 246: 98–105, 2016. doi: 10.1016/j.atherosclerosis.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol 593: 3917–3928, 2015. doi: 10.1113/JP270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Walter Wray D, Bader F, Gilbert EM, Richardson RS. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol 299: H1653–H1659, 2010. doi: 10.1152/ajpheart.00580.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoier B, Walker M, Passos M, Walker PJ, Green A, Bangsbo J, Askew CD, Hellsten Y. Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol (1985) 115: 1777–1787, 2013. doi: 10.1152/japplphysiol.00979.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J, Richardson RS. The mechanoreflex and hemodynamic response to passive leg movement in heart failure. Med Sci Sports Exerc 48: 368–376, 2016. doi: 10.1249/MSS.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol 178: 232–238, 2015. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellien J, Iacob M, Gutierrez L, Isabelle M, Lahary A, Thuillez C, Joannides R. Crucial role of NO and endothelium-derived hyperpolarizing factor in human sustained conduit artery flow-mediated dilatation. Hypertension 48: 1088–1094, 2006. doi: 10.1161/01.HYP.0000246672.72188.bd. [DOI] [PubMed] [Google Scholar]
- 15.Bellien J, Joannides R, Iacob M, Arnaud P, Thuillez C. Evidence for a basal release of a cytochrome-related endothelium-derived hyperpolarizing factor in the radial artery in humans. Am J Physiol Heart Circ Physiol 290: H1347–H1352, 2006. doi: 10.1152/ajpheart.01079.2005. [DOI] [PubMed] [Google Scholar]
- 16.Bellien J, Thuillez C, Joannides R. Contribution of endothelium-derived hyperpolarizing factors to the regulation of vascular tone in humans. Fundam Clin Pharmacol 22: 363–377, 2008. doi: 10.1111/j.1472-8206.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 17.Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjær M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol (1985) 97: 393–403, 2004. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 19.Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol 546: 307–314, 2003. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation 123: 2244–2253, 2011. doi: 10.1161/CIRCULATIONAHA.110.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol (1985) 98: 1251–1257, 2005. doi: 10.1152/japplphysiol.00966.2004. [DOI] [PubMed] [Google Scholar]
- 22.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spilk S, Herr MD, Sinoway LI, Leuenberger UA. Endothelium-derived hyperpolarizing factor contributes to hypoxia-induced skeletal muscle vasodilation in humans. Am J Physiol Heart Circ Physiol 305: H1639–H1645, 2013. doi: 10.1152/ajpheart.00073.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson JR, Kapoor S. Contribution of endothelium-derived relaxing factor to exercise-induced vasodilation in humans. J Appl Physiol (1985) 75: 2740–2744, 1993. doi: 10.1152/jappl.1993.75.6.2740. [DOI] [PubMed] [Google Scholar]
- 25.Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol Heart Circ Physiol 265: H171–H175, 1993. doi: 10.1152/ajpheart.1993.265.1.H171. [DOI] [PubMed] [Google Scholar]
- 26.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 27.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce‐Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2011. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 28.Gifford GJ, Richardson RS. CORP: ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venturelli M, Layec G, Trinity J, Hart CR, Broxterman RM, Richardson RS. Single passive leg movement-induced hyperemia: a simple vascular function assessment without a chronotropic response. J Appl Physiol (1985) 122: 28–37, 2017. doi: 10.1152/japplphysiol.00806.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shields KL, Broxterman RM, Jarrett CL, Bisconti AV, Park SH, Richardson RS. The passive leg movement technique for assessing vascular function: defining the distribution of blood flow and the impact of occluding the lower leg. Exp Physiol 104: 1575–1584, 2019. doi: 10.1113/EP087845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kithas AC, Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Hydren JR, Nelson AD, Jessop JE, Bledsoe AD, Morgan DE, Richardson RS. Nitric oxide synthase inhibition with NG-monomethyl-L-arginine: determining the window of effect in the human vasculature. Nitric Oxide 104-105: 51–60, 2020. doi: 10.1016/j.niox.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Layec G, Venturelli M, Jeong EK, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum: from able-bodied adults to individuals with a spinal cord injury. J Appl Physiol (1985) 116: 1142–1147, 2014. doi: 10.1152/japplphysiol.01120.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res 113: 1023–1032, 2013. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ, Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Heart Circ Physiol 318: H301–H325, 2020. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durand MJ, Dharmashankar K, Bian JT, Das E, Vidovich M, Gutterman DD, Phillips SA. Acute exertion elicits a H2O2-dependent vasodilator mechanism in the microvasculature of exercise-trained but not sedentary adults. Hypertension 65: 140–145, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium‐dependent vasodilation in health and disease. Microcirculation 20: 239–247, 2013. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quilley J, Fulton D, McGiff JC. Hyperpolarizing factors. Biochem Pharmacol 54: 1059–1070, 1997. doi: 10.1016/S0006-2952(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 39.Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol (1985) 81: 1516–1521, 1996. doi: 10.1152/jappl.1996.81.4.1516. [DOI] [PubMed] [Google Scholar]
- 40.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffy SJ, New G, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol Heart Circ Physiol 276: H663–H670, 1999. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- 42.Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 45.Nishiyama SK, Wray DW, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol (1985) 103: 843–851, 2007. doi: 10.1152/japplphysiol.00273.2007. [DOI] [PubMed] [Google Scholar]
- 46.Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res 84: 484–488, 1999. doi: 10.1161/01.RES.84.4.484. [DOI] [PubMed] [Google Scholar]
- 47.Fisslthaler B, Fleming I, Busse R. EDHF: a cytochrome P450 metabolite in coronary arteries. Semin Perinatol 24: 15–19, 2000. doi: 10.1016/S0146-0005(00)80048-8. [DOI] [PubMed] [Google Scholar]
- 48.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 49.Dora KA. Conducted dilatation to ATP and K+ in rat skeletal muscle arterioles. Acta Physiol (Oxf) 219: 202–218, 2017. doi: 10.1111/apha.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, Ali F, Carrier MJ, Mitchell JA. Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol 72: 1132–1136, 2007. doi: 10.1124/mol.107.037325. [DOI] [PubMed] [Google Scholar]
- 51.Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol 582: 335–347, 2007. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am J Physiol Heart Circ Physiol 307: H1587–H1593, 2014. doi: 10.1152/ajpheart.00557.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol 112: 5, 2017. doi: 10.1007/s00395-016-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wylie LJ, Park JW, Vanhatalo A, Kadach S, Black MI, Stoyanov Z, Schechter AN, Jones AM, Piknova B. Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J Physiol 597: 5565–5576, 2019. doi: 10.1113/JP278076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawes M, Sieniawska C, Delves T, Dwivedi R, Chowienczyk PJ, Ritter JM. Barium reduces resting blood flow and inhibits potassium-induced vasodilation in the human forearm. Circulation 105: 1323–1328, 2002. doi: 10.1161/hc1102.105651. [DOI] [PubMed] [Google Scholar]
- 57.Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol 557: 1013–1020, 2004. doi: 10.1113/jphysiol.2004.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohrman DE, Sparks HV. Role of potassium ions in the vascular response to a brief tetanus. Circ Res 35: 384–390, 1974. doi: 10.1161/01.RES.35.3.384. [DOI] [PubMed] [Google Scholar]
- 59.Dufour SP, Doutreleau S, Lonsdorfer-Wolf E, Lampert E, Hirth C, Piquard F, Lonsdorfer J, Geny B, Mettauer B, Richard R. Deciphering the metabolic and mechanical contributions to the exercise-induced circulatory response: insights from eccentric cycling. Am J Physiol Regul Integr Comp Physiol 292: R1641–R1648, 2007. doi: 10.1152/ajpregu.00567.2006. [DOI] [PubMed] [Google Scholar]
- 60.Duling BR. Effects of potassium ion on the microcirculation of the hamster. Circ Res 37: 325–332, 1975. doi: 10.1161/01.RES.37.3.325. [DOI] [PubMed] [Google Scholar]
- 61.Hazeyama Y, Sparks HV. Exercise hyperemia in potassium-depleted dogs. Am J Physiol Heart Circ Physiol 236: H480–H486, 1979. doi: 10.1152/ajpheart.1979.236.3.H480. [DOI] [PubMed] [Google Scholar]
- 62.Hearon CM, Richards JC, Racine ML, Luckasen GJ, Larson DG, Dinenno FA. Amplification of endothelium-dependent vasodilatation in contracting human skeletal muscle: role of KIR channels. J Physiol 597: 1321–1335, 2019. doi: 10.1113/JP276998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiens B, Saltin B, Walløse L, Wesche J. Temporal relationship between blood flow changes and release of ions and metabolites from muscles upon single weak contractions. Acta Physiol Scand 136: 551–559, 1989. doi: 10.1111/j.1748-1716.1989.tb08701.x. [DOI] [PubMed] [Google Scholar]
- 64.Murray PA, Sparks HV. The mechanism of K+-induced vasodilation of the coronary vascular bed of the dog. Circ Res 42: 35–42, 1978. doi: 10.1161/01.RES.42.1.35. [DOI] [PubMed] [Google Scholar]
- 65.Groot HJ, Rossman MJ, Trinity JD, Layec G, Ives SJ, Richardson RS. Passive leg movement-induced vasodilation in women: the impact of age. Am J Physiol Heart Circ Physiol 309: H995–H1002, 2015. doi: 10.1152/ajpheart.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ives SJ, Layec G, Hart CR, Trinity JD, Gifford JR, Garten RS, Witman MA, Sorensen JR, Richardson RS. Passive leg movement in chronic obstructive pulmonary disease: evidence of locomotor muscle vascular dysfunction. J Appl Physiol (1985) 128: 1402–1411, 2020. doi: 10.1152/japplphysiol.00568.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003. doi: 10.1161/01.RES.0000054200.44505.AB. [DOI] [PubMed] [Google Scholar]