Abstract
Stress stimulates colonic motor function and plays a role in functional bowel disorders, prevalently in women. We examined, in conscious female rats, the influence of water avoidance stress for 60min on colonic myenteric neuron activity using immunohistochemical detection of Fos as a marker of neuronal activity. In control rats, Fos immunoreactive nuclei were rare in proximal and distal colon and no defecation was observed. Water avoidance stimulated fecal pellet output, which was associated with Fos expression in myenteric ganglia of proximal and distal colon including in a population of peripheral choline acetyltransferase-immunoreactive neurons. Atropine blocked fecal pellet output but not Fos expression in myenteric ganglia. These results indicate that psychological stress stimulates the activity of colonic cholinergic myenteric neurons.
Keywords: colonic motor function, Fos, myenteric neurons, peripheral choline acetyltransferase, stress
Introduction
Knowledge of acute stress-related stimulation of colonic motor function is derived almost exclusively from experiments performed on male rats, except for a few studies on female rats, showing that wrap or cold-restraint stress reduces colonic transit time and induces defecation [1]. The predominant use of male rodents to assess stress-related alterations of gut motor functions contrasts with clinical evidence of higher prevalence of functional bowel disorders in women compared with men, particularly as it relates to irritable bowel syndrome characterized by abnormal bowel habits and pain exacerbated by stress [2]. Mechanisms underlying the colonic motor response to stress involve the activation of corticotropin-releasing factor (CRF) signalling pathways in the brain through the modulation of sacral parasympathetic outflow to the gut [3]. The existence of vagal and spinal efferent projections from hypothalamic and pontine nuclei to the colon [4] provides neuroanatomical support for autonomic pathways playing a role in stress-related changes in propulsive colonic motility [1].
The myenteric plexus is a ganglionated neuronal component of the enteric nervous system coordinating gastrointestinal motility and known to be modulated by the autonomic nervous system [5]. So far, however, no study has established the relationship between a stressful stimulus and the subsequent changes in the activity of colonic myenteric neurons and propulsive motility leading to fecal expulsion. This study was undertaken to determine whether water avoidance, a well-established model of psychological stress stimulating colonic motor function [6], induces changes in the activity of colonic myenteric neurons in female rats. The induction of the nuclear phosphoprotein, Fos, in ganglionic myenteric neurons was used as a marker of neuronal activity [7], whereas fecal pellet output was used as an index of propulsive colonic motility. The biochemical coding of activated myenteric neurons was also assessed by immunohistochemical double labelling of Fos with peripheral choline acetyltransferase (pChAT) [8].
Materials and methods
Animals
Female Sprague–Dawley rats (Harlan, Indianapolis, Indiana, USA) weighing 220–240g were maintained four per cage under controlled environmental conditions (12: 12-h light–dark cycle, 22±1ºC). They had free access to food (Purina Rat Chow) and tap water for at least 1 week before the experiments that were carried out between 08.00 h and 13.00 h under approved protocol no. 96–080-08 (West Los Angeles Veterans Affairs Institutional Animal Care and Use Committee).
Treatments
Rats were accustomed to single housing and handled daily for 2 days before the experiment. Water avoidance stress was conducted by placing a rat singly for a 60-min period on a rectangular platform (10 × 8 × 8 cm) anchored on the center of a standard plastic cage (45 cm length × 25 cm width × 25 cm height) filled with tap water up to 7 cm of the height of the platform as described elsewhere [9]. Control rats were kept individually in their standard housing cages without water or food during the same period (sham stress). To study whether activation of colonic motility per se induces Fos in the colon, groups of rats were injected subcutaneously with either saline (0.5 ml) or atropine sulfate (1 mg/kg dissolved in 0.5 ml saline; Sigma Chemical, St Louis, Missouri) and, 30 min later, rats were exposed to water avoidance or left undisturbed for 60 min. In naive or subcutaneous injected groups, fecal pellet output was monitored for the 60-min period. The animals were then euthanized with an overdose of sodium pentobarbital and the colon was harvested.
Immunohistochemical staining of Fos and peripheral choline acetyltransferase
Segments of both proximal and distal colon were opened longitudinally along the mesenteric border and placed in phosphate-buffered saline (PBS, pH 7.4) containing nifedipine (10−6 M, Sigma) to avoid muscle contractions. Segments were stretched and pinned flat on a Sylgard-coated Petri dish (Sylgard 184, DowCorning Corp, Midland, Michigan, USA) and fixed overnight in Zamboni’s fixative (pH 7.4) at 4°C. Tissues were washed (3 × 10 min) in PBS, and the longitudinal muscle/myenteric plexus whole mount preparations of approximately 0.25 cm2 each were dissected from the proximal and distal colon [10]. The immunohistochemical procedures used for Fos labelling were as described previously [10,11]. Briefly, longitudinal muscle/myenteric plexus whole mount preparations were rinsed (3 × 10 min) with PBS containing 0.1% Triton X-100 (PBS-T, pH 7.4; Sigma) and incubated for 60 min at room temperature with normal goat serum (3%) in PBS-T to block nonspecific binding. Tissues were then incubated at 4°C for 24 h for the immunoperoxidase procedure and 48 h for immunofluorescence with a polyclonal rabbit anti-Fos (1 : 10 000; fos Ab-5; Oncogene Research Products, Cambridge, Massachusetts, USA). After the first incubation with primary antibody, tissues were incubated for 60 min at room temperature with biotinylated goat anti-rabbit secondary antibody (1 : 500, Jackson ImmunoResearch, West Grove, Pennsylvania, USA) followed by the standard biotin–avidin–horseradish peroxidase methodology, or with tetramethylrhodamine ‘TRITC’ conjugated to goat anti-rabbit IgG (1 : 100; Jackson ImmunoResearch). Cholinergic neurons were assessed using double labelling of Fos and pChAT as described previously [11]. Fos immunoreactivity was examined using a Zeiss LSM 510 laser scanning microscope (Carl Zeiss, Thornwood, New York, USA). The number of Fos-immunoreactive (IR) nuclei was counted in 25 myenteric ganglia randomly selected in each examined piece (0.25 cm2) of longitudinal muscle/myenteric plexus whole mount preparation. The mean number of Fos-IR nuclei per myenteric ganglion per rat was used to generate a mean number. Neurons double-stained with Fos/pChAT are expressed as a percentage of the total number of pChAT-positive neurons.
Statistics
Data are expressed as means±SEM and analyzed by one-way analysis of variance followed by Dunn’s test to analyze differences between groups. The confidence limit for significance was set at P<0.05.
Results
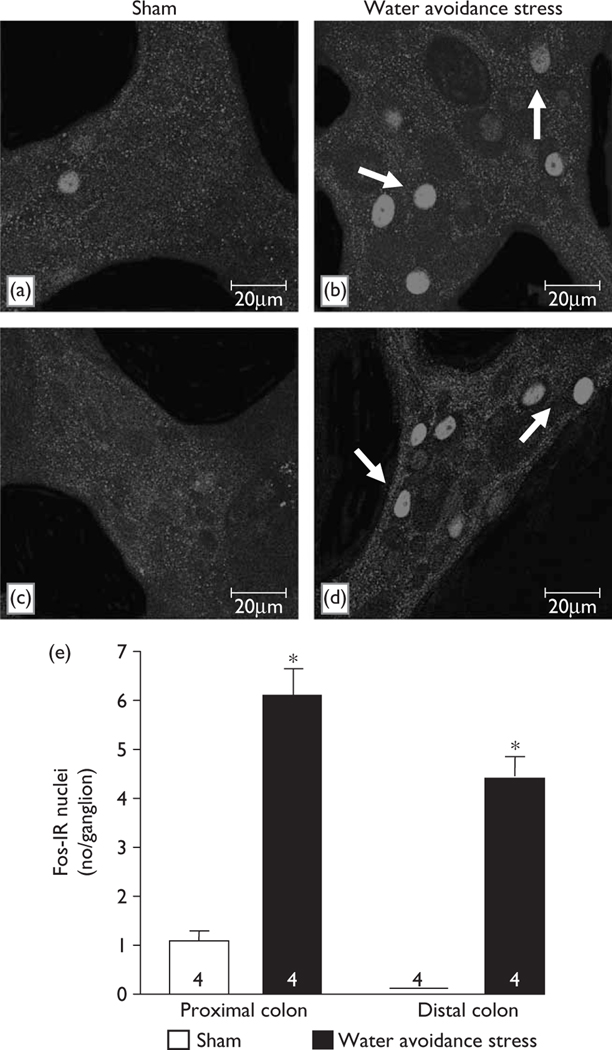
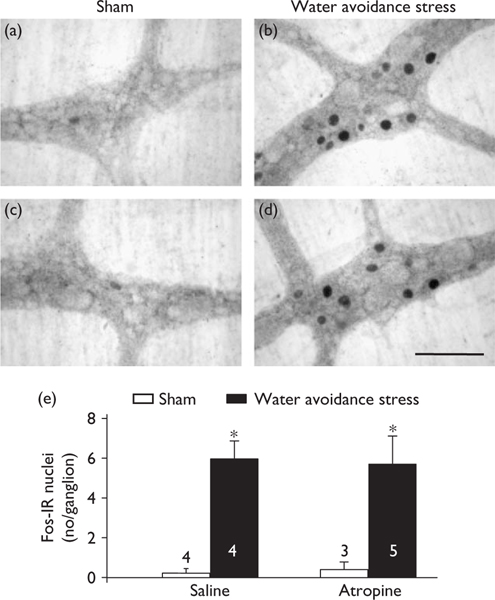
In nonpretreated (Fig. 1a, c, e) or saline-injected (Fig. 2a, c, e) rats, there was little or no Fos expression in the proximal and distal colonic myenteric ganglia. Water avoidance stress for 60 min significantly increased the number of Fos-IR nuclei/ganglion compared with sham stress nonpretreated rats in both the colon proximal (6.1±0.6 vs. 1.0±0.2; Fig. 1b, a, e) and distal (4.4±0.4 vs. 0.0±0.0; Fig. 1d, c, e). Similarly, in saline-injected rats, water avoidance increased the number of Fos-IR nuclei/ganglion in both the proximal (7.8±0.8 vs. 0.1±0.1, P<0.05, Fig. 2b, e) and distal colon compared with sham stress rats injected with saline (Fig. 2a, e). Double Fos/pChAT labelling revealed that 25.3±5.7 and 24.4±6.3% of total pChAT neurons in the proximal and distal colon, respectively, were Fos-positive after water avoidance compared with 0.0±0.0% in the sham stress group. Water avoidance for 60 min also induced fecal pellet output/h compared with naive (4.1±0.7 vs. 0.0±0.0, n=4/group) or saline-injected rats (8.0±0.8 vs. 0.0±0.0, n=4–5). In atropine-pretreated rats, avoidance increased also the number of Fos-positive nuclei/ganglia in the proximal (6.3±1.0, n=5, vs. saline 0.1±0.1, n=3, P<0.05) and distal (Fig. 2d, e) colon while the defecation response was blocked (pellet number/h: 0.0±0.0 vs. saline 8.0±0.8, n=4–5, P<0.05). Atropine alone had no effect on basal Fos and pellet output (Fig. 2e and data not shown).
Fig. 1.
Water avoidance stress induces Fos expression in the myenteric ganglia of proximal and distal colon in female rats. Confocal microscope images of representative myenteric ganglia show Fos-immunoreactive nuclei in longitudinal muscle/myenteric plexus whole mount preparations of proximal (a, b) and distal colon (c, d) of rats exposed for 60 min to water avoidance (b, d) or control rats maintained in their normal environment (a, c). Scale bar, 20 μm. (e) Average number (mean±SEM) of Fos-labelled nuclei/ganglion in number of rats indicated at the bottom of the columns. *P<0.05 vs. related shamrats. IR, immunoreactive.
Fig. 2.
Atropine did not influence Fos expression in myenteric ganglia of the distal colon in conscious female rats. (a–d)Photomicrographs of longitudinal muscle/myenteric plexus whole mount preparations in the distal colon of rats injected subcutaneously with saline (a, b) or atropine (1 mg/kg) (c, d) and exposed to water avoidance (b, d) or not (sham a, c). Scale bar, 100 μm. (e) Average number (mean±SEM) of Fos-labelled nuclei/ganglion from number of rats indicated at the bottom. *P<0.05 vs. corresponding sham groups.IR, immunoreactive.
Discussion
This study demonstrates that water avoidance stress for 60 min activates myenteric neurons in the colon, as shown by the induction of Fos-IR nuclei in the myenteric plexus from proximal and distal segments, and stimulates propulsive motility quantified by the defecation score in conscious female rats. These responses are specific to stress exposure as rats maintained undisturbed for 60 min in their normal environment had no Fos expression in the colonic myenteric ganglia and did not defecate. A similar increase was also reproduced in saline-injected rats exposed to water avoidance stress compared with saline-injected rats kept in their housing cages. This provides the first demonstration that psychological stress activates ganglionic myenteric neurons in the proximal and distal colon in association with functional colonic motor response. In previous studies, Fos induction in the myenteric neurons of the distal colon was observed in vivo under conditions of colonic mechanosensory or nociceptive stimulation (topical irritants placed into the colonic lumen or wall of the sigmoid colon) or in vitro after challenge with inflammatory mediators placed into segments of rat’s or guinea pig’s colon [7,12].
Mechanisms through which water avoidance stress activates colonic motor function have been established to involve brain autonomic rather than hormonal pathways. One of the hallmarks of acute stress, including water avoidance stress, is the activation of CRF-containing neurons located in the paraventricular nucleus of the hypothalamus [13]. These neurons release CRF into the hypophyseal portal blood which, in turn, activates CRF1 receptors located on the corticotroph cells in the anterior pituitary resulting in adrenocorticotropic hormone and β-endorphin secretion and adrenal corticosterone release [14]. The activation of the pituitary–adrenal axis induced by stress, however, does not contribute to colonic motor response as hypophysectomy, adrenalectomy, or naloxone did not block wrap restraint or conditioned fear-induced stimulation of colonic transit and motility in rats [15]. By contrast, ganglionic blockers, hexamethonium or chlorisondamine, inhibit the colonic motor responses (enhanced transit, motility and defecation) induced by water avoidance stress and wrap restraint in male rats, whereas sympathetic blockade has no effect [15,16]. The paraventricular nucleus of the hypothalamus and pontine locus coeruleus/Barrington’s nuclei are activated by water avoidance and are responsive sites of exogenous CRF to induce autonomic-mediated stimulation of colonic motor function in rats [17,18]. The paraventricular nucleus of the hypothalamus and locus coeruleus/Barrington’s nuclei complex have monosynaptic connections with the sacral parasympathetic centres [19]. In addition, defecation in rats has been shown to be regulated by these pontine-spinal pathways [20]. We have previously shown that water avoidance stress increases the activity of neurons in the intermediolateral column of the lumbosacral spinal cord segments in female rats [9]. This nucleus is the primary source of parasympathetic innervation of myenteric neurons in the rat distal colon through the pelvic nerves [4,21], whereas only 16% of distal colon myenteric neurons are vagally innervated [22]. Similar magnitude of Fos expression in the myenteric ganglia of both the proximal and distal colon after exposure to water avoidance stress supports the involvement of sacral parasympathetic activation in the distal colonic myenteric neurons. If the Fos induction had been driven solely by the vagal input to colonic myenteric neurons, this would have been reflected by a higher response in the proximal rather than the distal colon.
Although the complete biochemical coding of Fos-positive neurons in the colon myenteric ganglia was not characterized, double labelling revealed that water avoidance stress activates 24–25% of pChAT-positive neurons providing support for activation of cholinergic myenteric motoneurons in the colonic functional response. Indeed, 69% of myenteric neurons in the rat colon are immunoreactive to a specific pChAT antibody [8]. Muscarinic receptors are involved in the regulation of frequency of contractions [23] and are present in rat colonic smooth muscles [24]. Importantly, muscarinic receptor blockade with atropine inhibited water avoidance stress-induced defecation in female rats as observed previously in male rats [16], but did not alter Fos expression in proximal and distal colon. These data also indicate that Fos expression is not secondary to the activation of colonic motor function, and likely reflects a direct autonomic modulation of the enteric nervous system by stress.
Conclusion
In summary, our results show that a psychological stress induced by water avoidance activates a population of cholinergic myenteric neurons in the colon in association with propulsive motility in female rats. This provides a new means of gaining insight into stress-related interactions between the central and enteric nervous systems in non-anesthetized animals. It may also be useful as a model to evaluate irritable bowel syndrome for which stress plays an important role in the onset and/or magnitude of the colonic symptoms, which are more prevalent in women than men [2,25].
Acknowledgements
The authors thank Mrs Honghui Liang for her technical support and Miss Teresa Olivas for her help in the preparation of the manuscript. This work was supported by the NIH Grants, P50 AR 049550, R01 DK 33061, Center Grant DK 41301 (Animal Core), and Veterans Affairs Career Scientist Award.
References
- 1.Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol 2001; 280:G173–G177. [DOI] [PubMed] [Google Scholar]
- 2.Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs 2003; 5:56–65. [DOI] [PubMed] [Google Scholar]
- 3.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol 2004; 141:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vizzard MA, Brisson M, de Groat WC. Transneuronal labelling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res 2000; 299:9–26. [DOI] [PubMed] [Google Scholar]
- 5.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol 1999; 61:117–142. [DOI] [PubMed] [Google Scholar]
- 6.Bonaz B, Taché Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res 1994; 641:21–28. [DOI] [PubMed] [Google Scholar]
- 7.Miampamba M, Sharkey KA. c-Fos expression in the myenteric plexus, spinal cord and brainstem following injection of formalin in the rat colonic wall. J Auton Nerv Syst 1999; 77:140–151. [PubMed] [Google Scholar]
- 8.Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J Chem Neuroanat 2000; 18:31–40. [DOI] [PubMed] [Google Scholar]
- 9.Million M, Wang L, Martinez V, Taché Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res 2000; 877:345–353. [DOI] [PubMed] [Google Scholar]
- 10.Miampamba M, Maillot C, Million M, Taché Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF1 receptor in conscious rats. Am J Physiol Gastrointest Liver Physiol 2002; 282: G857–G865. [DOI] [PubMed] [Google Scholar]
- 11.Yuan PQ, Kimura H, Million M, Bellier JP, Wang L, Ohning GV, et al. Central vagal stimulation activates enteric cholinergic neurons in the stomach and VIP neurons in the duodenum in conscious rats. Peptides 2005; 26:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjwa ET, Bradley JM, Keenan CM, Kroese AB, Sharkey KA. Interleukin-1beta activates specific populations of enteric neurons and enteric glia in the guinea pig ileum and colon. Am J Physiol Gastrointest Liver Physiol 2003; 285:G1268–G1276. [DOI] [PubMed] [Google Scholar]
- 13.Kresse AE, Million M, Saperas E, Taché Y. Colitis induces CRF expression in hypothalamic magnocellular neurons and blunts CRF gene response to stress in rats. Am J Physiol Gastrointest Liver Physiol 2001; 281: G1203–G1213. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine response to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med 1997; 215:1–10. [DOI] [PubMed] [Google Scholar]
- 15.Lenz HJ. Neurohumoral pathways mediating stress-induced changes in rat gastrointestinal transit. Gastroenterology 1989; 97:216–218. [DOI] [PubMed] [Google Scholar]
- 16.Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotrophin-releasing factor. Gastroenterology 1993; 104:716–723. [DOI] [PubMed] [Google Scholar]
- 17.Mönnikes H, Schmidt BG, Tebbe J, Bauer C, Taché Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus stimulates colonic motor function in rats. Brain Res 1994; 644:101–108. [DOI] [PubMed] [Google Scholar]
- 18.Mönnikes H, Schmidt BG, Raybould HE, Taché Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol 1992; 262:G137–G143. [DOI] [PubMed] [Google Scholar]
- 19.Toth ZE, Gallatz K, Fodor M, Palkovits M. Decussations of the descending paraventricular pathways to the brainstem and spinal cord autonomic centers. J Comp Neurol 1999; 414:255–266. [PubMed] [Google Scholar]
- 20.Nagano M, Ishimizu Y, Saitoh S, Okada H, Fukuda H. The defecation reflex in rats: fundamental properties and the reflex center. Auton Neurosci 2004; 111:48–56. [DOI] [PubMed] [Google Scholar]
- 21.Luckensmeyer GB, Keast JR. Projections of pelvic autonomic neurons within the lower bowel of the male rat: an anterograde labelling study. Neuroscience 1998; 84:263–280. [DOI] [PubMed] [Google Scholar]
- 22.Berthoud H-R, Jedrzejewska A, Powley TL. Simultaneous labelling of vagal innervation of the gut and afferent projections from the visceral forebrain with Dil injected into the dorsal vagal complex in the rat. J Comp Neurol 1990; 301:65–79. [DOI] [PubMed] [Google Scholar]
- 23.Ono S, Mitsui R, Karaki S, Kuwahara A. Muscarinic and 5-HT4 receptors participate in the regulation of the frequency of spontaneous contractions of the longitudinal muscle in rat distal colon. Biomed Res 2005; 26:173–177. [DOI] [PubMed] [Google Scholar]
- 24.Gomez A, Martos F, Bellido I, Marquez E, Garcia AJ, Pavia J, et al. Muscarinic receptor subtypes in human and rat colon smooth muscle. Biochem Pharmacol 1992; 43:2413–2419. [DOI] [PubMed] [Google Scholar]
- 25.Mayer EA, Naliboff BD, Chang L, Coutinho SV. Stress and the gastrointestinal tract. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2001; 280:G519–G524. [DOI] [PubMed] [Google Scholar]