Abstract
Inflammatory bowel disease (IBD) management has changed dramatically over the past 20 years, after the introduction of targeted biological therapies. However, the impact of these new drugs in changing the natural history of disease is still under debate. Recent evidence seems to suggest that the extent of their efficacy might be, at least partially, dependent on the timing of their introduction and on the subsequent management strategy. In this complex landscape, the potential role for a more dynamic approach with treatments based on sequencing and combining targeted therapies has been explored only minimally so far. In this review, we aim to explore the potential biological rationale behind the use of sequential and combination therapies in IBD, to summarise the current knowledge on this topic and to propose a management algorithm that combines these notions.
Keywords: combination therapy, Crohn’s disease, dual targeted therapy, swap, switch, ulcerative colitis, ;, switch;, swap
Introduction
Inflammatory bowel diseases (IBD) are chronic conditions that require lifelong medical therapy. However, despite available treatments, a significant number of patients undergo abdominal surgery one or more times during their life.1 The advent of biologics has undoubtedly represented a significant advance in IBD management, but their usefulness in changing the natural history of the disease has not been clearly established.2 Several important limitations deserve attention with respect to biological therapy for IBD.
Are we proving unwise in delaying the introduction of biologics?
Data from national registries have disclosed that the majority of patients receive biologics in a late phase of their disease or do not receive them at all.3,4 Conversely, growing evidence has suggested that early treatment with these drugs might be associated with more favourable outcomes.5 It is conceivable that early introduction of biological therapies might grant deeper control over intestinal inflammation, possibly due to lower inflammatory burden or specific immunophenotypes that are more sensitive to therapies associated with early stages of disease. Furthermore, there are no reliable clinical tools that can currently predict patient response to a specific drug. Owing to the impossibility of a priori stratification and profiling, ineffective therapies are frequently started: this leads to protracted time with active disease and consequential damage progression, overshadowing the window of opportunity to introduce a therapy and maximise its efficacy.
Are we resting on the laurels of clinical remission and settling for insufficient outcomes?
Symptom control, although being the first goal of IBD treatment, is insufficient. The 2015 Selecting Therapeutic Targets in Inflammatory Bowels Disease (STRIDE) consensus and its 2020 update proposed a management algorithm for Crohn’s disease (CD) and ulcerative colitis (UC) aiming to optimise disease management and prevent long-term disability. A treat-to-target strategy involves identification of a pre-defined therapeutic target and, accordingly, strict monitoring and potentially an optimisation of therapy.6
Endoscopic healing has been proposed as the ultimate target to pursue, as it has proved to correlate with better long-term outcomes in both CD and UC. The benefits associated with histological healing have also been recognised in terms of reduction of clinical relapse7,8; however, due to the lack of standardised reporting methods and the uncertainties surrounding the advantage of optimising therapy to achieve it, it still not considered a clinical target.6,9 Furthermore, the ‘Effect of tight control management on Crohn’s disease’ (CALM) study has shown that tight monitoring – with therapy escalation based on either clinical and biomarkers [C-reactive protein (CRP) and faecal calprotectin] – is superior to symptom-based management to achieve better endoscopic and clinical outcomes in early CD.10 Accordingly, the STRIDE-II consensus included normalisation of CRP/erythrocyte sedimentation rate and faecal calprotectin as a clinical target to be pursued.
Are we playing our cards right to maintain long-term remission?
One of the main problems associated with biological therapies is the considerable rate of secondary loss of response,11 which prevents many patients from achieving stable, durable remission and, therefore, exposes them to damage progression associated with disease activity. Comparison studies evaluate drug efficacy in patients with active disease, but neglect to compare their capability of preventing relapses once the patient has achieved remission. Indeed, whether a drug that is more effective in inducing remission will also be more effective in maintaining it over the long term has not been demonstrated.
The overall notion suggested here is that IBD treatment needs to be improved not only by acquiring new drugs with different mechanisms of action, but also by developing new treatment strategies to optimise efficacy. IBD are characterised by significant disease-related burden that progresses over time in parallel with disease flares and damage accumulation. Aiming for timely and deeper control might be able to avert disease progression and damage accumulation; decisions to optimise or change therapy seeking for more ambitious therapeutic goals might help to prevent long-term disability associated with IBD. A more profound comprehension of the immunological landscape associated with IBD would help to identify the mechanism of action needed to achieve the best results in each patient.12 Multiple inflammatory pathways are contemporarily activated in the intestinal mucosa13; therefore, blocking only one of them – as we currently do with targeted monotherapies such as biologics – might not be sufficient to achieve adequate control over inflammation in each patient. A combination of targeted therapies [i.e. dual targeted therapy, (DTT)] might be able to partially overcome the intrinsic refractoriness that seems to be associated with some diseases. Finally, it has now been demonstrated clearly that the pathogenic mechanisms that sustain inflammation in IBD are not static, but rather evolve over time.14 Changing mechanism of action to comply with different ‘phases’ of the disease might prove beneficial to maintain strict control over inflammation over the long term and prevent damage accumulation.
In this review, we aim to summarise current knowledge on sequential and combined use of targeted therapies, and to provide a new viewpoint on its potential implementation in clinical practice.
What we have learnt so far. . .
Combining therapies: lessons learnt from anti-TNFα and thiopurines
The efficacy of the combination of infliximab and thiopurines has been proven in two double-blind randomised controlled trials (RCTs); conversely, combining immunosuppressors with other biologics is not supported by evidence.15–17 This might depend on the fact that thiopurine association is efficacious in improving infliximab pharmacokinetics by lowering its immunogenicity,18 which is higher compared with other biologics.19 The Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC) RCT showed that the association of infliximab and thiopurines was more effective at inducing steroid-free clinical remission (SFCR) and mucosal healing at week 26 compared with both infliximab and thiopurines monotherapy in CD patients naïve to both agents.20 Similarly, the UC-SUCCESS RCT proved that combining infliximab and azathioprine was superior to infliximab monotherapy in inducing SFCR after 16 weeks in bionaïve UC patients.21 There is also evidence suggesting that the addition of immunomodulators might be beneficial in some patients with secondary loss of response to anti-tumour necrosis factor alpha (TNFα) therapy.22 Specifically, in case of immunogenic loss of response with detectable anti-infliximab or anti-adalimumab antibodies, it has been shown that the introduction of an immunomodulator can make patients regain clinical response, in parallel with increasing drug concentrations and disappearance of anti-drug antibodies (ADAs).23
Sequencing therapies: maintenance and de-escalation – lessons learnt from traditional immunosuppressors
Therapeutic changes are usually reserved for IBD patients who experience primary or secondary failure to ongoing drugs. Conversely, clinicians are cautious when it comes to discontinuing effective treatments due to the considerable rates of short-term relapse observed in real-life studies after therapy withdrawal, even among patients in deep remission.24 Interestingly, there are two scenarios where remitting patients discontinue one of two therapies or transition from one therapy to another as a maintenance strategy.
Two randomised prospective studies demonstrated clearly that indefinite continuation of the combination of infliximab and thiopurines is not necessary once the therapeutic target has been achieved: in both studies, immunosuppressor discontinuation in stable patients (clinical remission >6 months) was not associated with any loss in terms of clinical benefit, despite a significant drop in median trough levels (TLs).25,26 Conversely, a 2012 retrospective study, including stable CD patients with infliximab and thiopurines, evaluated the strategy of biologic discontinuation; after 1 year of follow up, about 50% had relapsed, but no control group was included in the study.27 Finally, there is an ongoing open-label RCT aiming to compare the efficacy of three strategies in maintaining SFCR in stable CD patients: continuation of infliximab and immunosuppressors, continuation of infliximab alone and continuation of immunosuppressors alone.28
Another interesting case could be made for steroid-refractory acute severe ulcerative colitis (ASUC) treated with calcineurin inhibitors. Since it is recommended to discontinue them within 6–12 months, thiopurines are usually started in combination and then continued as maintenance monotherapy.29 Recently, vedolizumab has been proposed as an alternative to thiopurines after calcineurin inhibitor rescue therapy, with encouraging results.30–32 This represents an intriguing scenario, as different therapies are chosen based on the ‘clinical phase’ of disease: calcineurin inhibitors are used to achieve rapid response during the acute phase, while thiopurines or vedolizumab serve as maintenance regimens in responding patients.
Is there a rational for sequential and combination therapies? Evidence from translational studies
New insights into the role of cytokine-driven pathways in mucosal immunity have been described, based on several recent studies in animal models of acute intestinal injury, repair and chronic inflammation. Information derived from these studies reveals that intestinal homeostasis and inflammation are driven by cellular elements and soluble mediators that mediate both processes, with several cytokines exhibiting opposing roles, depending upon the specific phase of the inflammation. In this scenario, the same cytokine can possess both classic pro-inflammatory properties, as well as protective, anti-inflammatory functions, which are dependent primarily on the presence of receptor-bearing cells during the host disease state.33–44
As such, aside from the established pro-inflammatory properties of cytokines implicated in IBD pathogenesis [i.e. TNF-α, interleukin (IL)-12/23, IL-1α, IL-1β, IL-18 and IL-33], and their downstream signalling molecules, a growing body of evidence indicates that these mediators are necessary for the maintenance of mucosal homeostasis by effectively handling microbiota, as well as by protecting and restoring the integrity of the epithelial barrier.45–48 Epithelial disruption often occurs, facilitating translocation of luminal bacterial products and the recruitment of innate immune cells, primarily neutrophils and macrophages that are also a potent source of secreted (s)IL-1Ra, IL-1β, IL-18 and IL-33.49,50 Normally, early expression of these mediators dampens acute inflammation and promotes epithelial repair and restitution, with the ultimate goal of limiting gut mucosal damage and restoring intestinal homeostasis. Under conditions of either uncontrolled and/or persistent inflammation (e.g. as a result of innate immune dysfunction or host genetic predisposition), infiltration of adaptive immune cells occurs during the later phases of inflammation, making available an effector population able to respond to various cytokines.50 For instance, the presence of naïve CD4+ T cells expressing the IL-18R have the ability to respond to IL-18 and, in combination with IL-12, represent one of the most potent stimuli for interferon (IFN)γ production and Th1-polarised effector responses, thereby promoting chronic Th1-mediated inflammation. Similar effects can occur upon IL-33 stimulation of naïve CD4+ T cells but, in this case, a robust Th2 immune response results. Furthermore, several levels of regulation exist within each subfamily of inflammatory molecules, often including the presence of several agonist isoforms (both precursor and mature, cleaved forms) and receptor antagonists as well as soluble and cell-bound decoy receptors. In addition, the usual promiscuity of inflammatory ligands with both binding receptors as well as recruited accessory proteins, instils yet another level of regulation that should be considered when determining the overall biological effects of a specific biological drug. In fact, each cytokine cannot be considered in isolation, but with other related proteins that can influence their overall interactive effects. An imbalance in the equilibrium between these components, dependent on the prevalent isoform and receptor binding domain/accessory protein present on effector cells, may be responsible for either driving pathogenic events, including chronic intestinal inflammation and fibrosis, or for promoting protection by inducing epithelial repair, mucosal wound healing and restoration of gut homeostasis. Moreover, modifications in the composition of mucosal immune cells in response to therapeutic pressure are able to promote a molecular resistance to these drugs. Indeed, a critical action of evasion of apoptosis in response to anti-TNF drugs has been evidenced for IL-23. This leads to an expansion of apoptosis-resistant T cells and consequent drug resistance.51
Based on this new information, and the emerging concept that several inflammatory molecules can possess opposing roles in gut health and disease, novel pathogenic hypotheses can be formed with important translational implications in regard to the use of sequential and combination therapies in chronic intestinal inflammation.
Dual targeted therapy: real-life management of complicated patients
Recently, DTT has been proposed for the treatment of complex IBD patients in two archetypical scenarios: concomitant IBD and extraintestinal comorbidities (‘double indication’) or refractory IBD. Despite being a largely unexplored strategy with targeted immunomodulators, the rationale behind the use of DTT in patients with ‘double indication’ is easily intuitable: administration of drugs with diverse mechanisms of action to treat concomitant diseases that rely on different immunoinflammatory pathways. On the contrary, whether multiple inhibitions provide any benefit on a single immune-mediated disease remains elusive. There is strong evidence from other medical fields (i.e. oncology and haematology), that combination therapies might work in selected patients. Furthermore, it has been shown that IBD rely on multiple immunoinflammatory mechanisms that are activated concomitantly: therefore, it is conceivable that some patients might need multiple pathways to be targeted in order to achieve disease control.
In the 2018 work from Buer and colleagues, 10 IBD patients (4 CD and 6 UC) received the addition of vedolizumab after a median time of 13 months since anti-TNFα initiation (9 infliximab and 1 adalimumab). After a median follow up of 17 months (range 12–20), all patients were in clinical remission. At the end of follow up, normalisation of biomarkers was observed in all UC and in half of CD patients; all UC patients had endoscopic improvement (three achieved endoscopic remission, defined as endoscopic Mayo score ⩽ 1), while three of the four CD patients had endoscopic improvement (one achieved remission, defined as absence of ulcerations). Notably, therapeutic drug monitoring (TDM) was also performed in these patients: anti-TNFα drugs concentration was persistently above 3 mg/l, no patient developed anti-TNFα neutralising antibodies and vedolizumab TLs at the end of follow up ranged between 20 and 40 mg/l.52 In another dataset, outcomes of 15 IBD patients (14 CD and 1 UC) were reported: 8 patients received vedolizumab + anti-TNFα agent, 5 vedolizumab + ustekinumab and 2 ustekinumab + anti-TNFα. Clinical and endoscopic or radiologic improvement was reported by 11 (73%) and 4 (44%) patients, respectively, after a median follow up of 24 months (median time on DTT: 6 months); notably, 67% of patients was able to reduce corticosteroid use after DTT initiation.53 In a recent Italian report, 16 IBD patients (11 CD and 5 UC) receiving DTT were included: one group received DTT due to uncontrolled IBD (seven patients), while the other for uncontrolled extraintestinal manifestations (nine patients). In the former group, two patients received anti-TNFα + vedolizumab, two anti-TNFα + ustekinumab and two vedolizumab + ustekinumab, while the remaining patient had the addition of vedolizumab to secukinumab (which was initiated due to uncontrolled severe psoriatic disease and caused IBD relapse). After 2 months, all patients already had clinical improvement, 50% had CRP negativisation and six out of seven were not on concomitant steroid therapy.54 Another European report comprised 50 IBD patients (33 CD, 18 UC and 1 undetermined IBD) who were treated with DTT, 10% of whom also had extraintestinal manifestations. Vedolizumab was the most commonly used drug (25 times with ustekinumab, 7 times with anti-TNFα agents and 8 times with tofacitinib); notably, this cohort also included 21 patients receiving the combination of a small molecule (20 tofacitinib and 1 apremilast) with a biologic. Data on clinical efficacy was reported for 36 patients: the proportion of patients in clinical remission increased from 14% to 50% (p = 0.0018) after a median time of 4 months. Out of 32 patients with endoscopic evaluation, 34% of them were in endoscopic remission after a median follow up of 8 months, compared with 6% at baseline (p = 0.0039). A significant decrease in median CRP levels was observed after 3 months (5–2.35 mg/l, p = 0.002), and 65% of patients who were taking oral steroids at baseline were able to discontinue them by the time of last follow up.55 Finally, a study from a Canadian group including 22 CD patients has been published recently; 24 DTT were used: 13 anti-TNFα + vedolizumab, 3 anti-TNFα + ustekinumab and 8 vedolizumab + ustekinumab. DTT was associated with a significant reduction in median patient reported outcomes (PRO) 2 score (24.1–13.4, p = 0.002) and CRP levels (17.0–9.0, p = 0.02). Clinical response and remission occurred in 50% and 40% of patients, respectively, while endoscopic response and remission were recorded in 43% and 23%, respectively. The percentage of patients taking oral corticosteroids decreased from 33% to 17%. After 1 year of follow up, 38% of patients were still on DTT.56
The potentially harmful effect of combining two targeted immunomodulators unquestionably represents one of the main limitations to DTT further diffusion – alongside drug costs. These five studies represent the largest cohort of IBD patients receiving DTT reported so far, with a total of 113 patients included. Adverse events were reported in 13–30% of patients; as expected, infections were the most common, whereas no deaths occurred and only one malignancy (basal cell skin cancer) was diagnosed. Data from early experiences with DTT in rheumatological patients raised some concerns about its safety, as a trend towards an increased rate of serious adverse events was observed.57 However, based on current evidence, no specific safety issue in IBD has yet emerged, beside a potential increase in risk of infection. This might be attributable to the fact that vedolizumab and ustekinumab are probably associated with a more favourable safety profile. Nevertheless, it needs to be recognised that the exiguous number of patients and the short follow up do not allow us to draw firm conclusions.
Recently, DTT has also been tested in paediatric IBD patients. In one study, the outcomes of 13 patients (9 CD, 4 UC) were reported: 8 were receiving infliximab + vedolizumab due to uncontrolled IBD, while the remaining 5 received the addition of ustekinumab in response to the development of paradoxical psoriasis under infliximab. In particular, in the first group, four of eight patients achieved remission – three of whom were able to discontinue infliximab and were left with maintenance vedolizumab monotherapy – while the other four underwent colectomy.58 The other study comprised 16 IBD patients (7 CD, 9 UC, 1 undetermined IBD) treated with DTT due to persistently active intestinal disease; 9 received vedolizumab + tofacitinib, 4 ustekinumab + vedolizumab and 3 ustekinumab + tofacitinib. Among them, 75% achieved steroid-free clinical remission after 6 months, and a significant reduction in median disease activity scores and inflammatory markers was recorded. Treatment failure with need for therapy discontinuation occurred in three (19%) patients.59 Comprehensively, safety data from these two paediatric cohorts are in line with the experiences in adult patients. One child developed septic arthritis while on triple immunosuppression (vedolizumab, tofacitinib and prednisone 30 mg); later, he also developed deep vein thrombosis, which required anticoagulant therapy and tofacitinib dose de-escalation. Another patient developed aminotransferases elevation, which did not recede after infliximab discontinuation, and was being investigated for potential primary sclerosing cholangitis at the time of publication. Finally, two cutaneous eczematous reactions and one otitis were recorded.
Comprehensively, current evidence on DTT appears to suggest that it holds the potential to become a useful strategy to implement in IBD management. First, the encouraging data on its safety should prompt the idea to consider it in case of ‘double indication’, when the patient is already receiving one biologic that is able to control one disease but not the other, and out-of-class swap does not feel suitable. Furthermore, it has been shown that patients previously considered refractory to medical therapy can benefit from this approach. This might be particularly important for all those patients who lack valid alternatives, such as those who have already failed all licenced therapies, when surgery is not advisable and exclusion criteria prevents eligibility in clinical trials (i.e. patients with ileorectal anastomosis, pouchitis, stomas or short-bowel syndrome).
Sequential biologic therapy in failures: in-class ‘switch’ or out-of-class ‘swap’
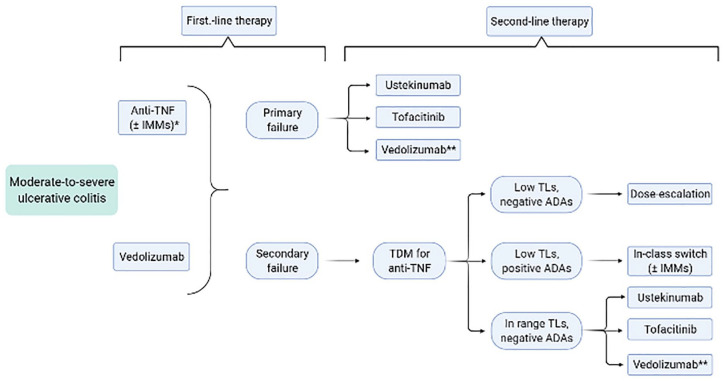
The choice of a specific biologic agent is often arbitrary in IBD – except for some cases of specific contraindications or comorbidities – and many society guidelines do not express specific recommendations.29,60–62 Figures 1 and 2 propose an algorithm for choosing therapies based on current evidence. Only one head-to-head trial comparing biological therapies has been published to date; in the VARSITY trial, vedolizumab proved superior to adalimumab to induce clinical remission in UC patients (31.3% versus 22.5%, p = 0.006), notably with a significant difference reported only in naïve patients.63 Of note, the 2020 AGA guidelines on the management of moderate-to-severe UC suggest the use of infliximab or vedolizumab over adalimumab for induction of remission in naïve patients.64 Indeed, in the recent network meta-analysis from Singh and colleagues, infliximab was ranked highest in biologic-naïve UC patients for inducing clinical remission, and infliximab and vedolizumab were ranked highest for inducing endoscopic remission.65 In a similar meta-analysis on CD, infliximab and adalimumab were ranked highest for inducing clinical remission in bionaïve patients, followed by ustekinumab and vedolizumab.66 Anti-TNFα biosimilars are often started in the majority of naïve patients who need to start a biological therapy, as they allow significant cost-saving compared with other biologics67; however, about one-third of patients fails to respond to these therapies,68 and 13–20% of them will experience yearly loss of response.69,70 Data on real-life effectiveness of second-line therapies, after failure of the first anti-TNFα agent, is accumulating. As a general consideration, subsequent lines are usually considered less effective compared with first-line therapies71; furthermore, a 2018 meta-analysis found that primary nonresponse was associated with an even lower likelihood of responding to second-line therapies, compared with both loss of response and intolerance.72 In case of primary failure, out-of-class swap should probably be preferred; however, a second-line anti-TNFα can be considered in patients who develop ADAs.73 After secondary failure, either in-class switch or out-of-class swap might be valid choices. In their 2015 work, Yanai et al. demonstrated that response to specific second-line therapies was correlated with the reason for failure of the first anti-TNFα agent.74 Patients with adequate TLs were more likely to regain and maintain response after the introduction of a non-TNFα antagonist second-line agent [compared with dose escalation and in-class switch, odds ratio (OR) 25, 95% confidence interval (CI) 3–220, p = 0.002], suggesting that mechanistic failure was responsible for treatment ineffectiveness; conversely, in patients with undetectable TLs and presence of anti-drug antibodies, second-line anti-TNFα was generally effective in inducing clinical remission.74 In a subsequent French work, Roblin et al. assessed the pharmacokinetic and clinical outcomes of IBD patients treated with second-line anti-TNFα agents according to the pharmacokinetic status at the time of first TNFα antagonist discontinuation75; patients with therapeutic TLs at withdrawal were the most likely to fail the second anti-TNFα despite adequate TLs, patients with positive anti-drug antibodies were likely to develop ADAs against the second agent and subtherapeutic or undetectable TLs were associated with higher rates of clinical response with the second anti-TNFα. In patients with immunogenic failure, it has been also shown that the addition of thiopurines to the second-line therapy can prevent clinical failure secondary to an unfavourable pharmacokinetic: in a randomised controlled prospective study, combination therapy with second-line anti-TNFα therapy was significantly associated with a higher likelihood of evolution without clinical failure compared with biologic monotherapy [hazard ratio (HR) 2.98, 95% CI 2.98–13.26, p < 0.001].76 In a large Spanish retrospective study including more than 1000 IBD patients, second- and third-line anti-TNFα therapy has been evaluated. At multivariate analysis, both primary and secondary nonresponse as reason for discontinuation were associated with a lower likelihood of achieving clinical remission with subsequent anti-TNFα therapy at 12 weeks, compared with treatment intolerance (OR 0.6, 95% CI 0.4–0.9, p = 0.007 for primary failure versus intolerance; OR 0.6, 95% CI 0.5–0.9, p = 0.005 for secondary failure versus intolerance); surprisingly, no difference between primary and secondary failure was observed. Furthermore, reason for therapy discontinuation did not correlate with remission at 52 weeks at multivariate analysis.77
Figure 1.
Proposed management algorithm for CD. Created with BioRender.com.
ADAs, anti-drug antibodies; CD, Crohn’s disease; IMMs, immunosuppressors; TDM, therapeutic drug monitoring; TLs, trough levels.
Figure 2.
Proposed management algorithm for UC. Created with BioRender.com.
*Infliximab preferred as first-line agent in bionaïve patients.65
**Tofacitinib or ustekinumab preferred over vedolizumab as second-line agents.65
ADAs, anti-drug antibodies; IMMs, immunosuppressors; TDM, therapeutic drug monitoring; TLs, trough levels; UC, ulcerative colitis.
In two retrospective studies on UC patients failing on first-line anti-TNFα, vedolizumab as second-line therapy has been shown to outperform both infliximab and adalimumab.78,79 In an Italian work, amongst patients with previous loss of response to infliximab, rates of clinical failure were significantly higher in the adalimumab-treated group compared with vedolizumab (48.0% versus 22.4%, p = 0.035)79; similarly, in a French study including UC patients who had experienced primary or secondary nonresponse to subcutaneous anti-TNFα therapy, persistence rates at 1 and 3 years were significantly higher in the vedolizumab group (80% and 50%) compared with the infliximab group (50% and 29%, p < 0.01 in both cases).78 A recent meta-analysis, based on the results of RCTs concluded that, after first anti-TNFα failure, tofacitinib and ustekinumab were ranked highest for inducing clinical remission in UC patients; notably, a clear superiority of adalimumab and vedolizumab over placebo as second-line agents has not been demonstrated in this analysis.65 In CD, three recent retrospective studies compared vedolizumab and ustekinumab in patients who had already failed at least one anti-TNFα agent, and all concluded that ustekinumab was superior to vedolizumab at 1 year. Two of these studies assessed persistence with therapy at 1 year; in both cases, ustekinumab was associated with higher rates of treatment persistence at 1 year (71.5% versus 49.7%, OR 2.54, 95% CI 1.40–4.62 in one study; 84.4% versus 61.5%, p = 0.007 in the other).80,81 Each study evaluated steroid-free clinical remission at 12 months. In two cases, a significant association between ustekinumab and SFCR was found compared with vedolizumab (OR 2.58, 95% CI 1.36–4.90, p = 0.004 in one study; OR 2.01, 95% CI 0.89–4.56, p = 0.095 in the other).81,82 Similarly, a 2018 meta-analysis found that ustekinumab and adalimumab were superior to vedolizumab in inducing clinical remission in CD patients who had already failed at least one anti-TNFα agent.66
Sequential biologic therapy in remitters: maintenance regimens and therapy de-escalation
Even though there is robust evidence from basic and translational works that the immunological landscape of the disease changes over time,14,83 the current therapeutic paradigm establishes that therapies are changed based only upon clinical features (symptom relapse and endoscopic or radiologic activity). Sequential therapy with biologics and/or small molecules for stable patients is not part of the standard of care for IBD management, as early experiences with elective switch have not provided positive results. In the 2011 SWITCH open-label RCT, elective switch from infliximab to adalimumab in patients with CD in stable remission was performed; the trial was discontinued prematurely due to the higher rate of relapses reported in the adalimumab arm. Specifically, a significantly higher proportion of patients in the adalimumab group needed to receive dose intensification or therapy discontinuation, compared with the infliximab arm (47% versus 16%, p = 0.003), and 10 (28%) adalimumab-treated patients discontinued therapy, compared with 1 (2%) infliximab-treated patient (p < 0.01).84 In a subsequent single-arm trial, 72% of stable CD patients switched from infliximab to adalimumab were still on adalimumab therapy at week 54 and 9.5% had to receive dose optimisation (three of whom discontinued adalimumab in the end).85 Comprehensively, these two studies raised enough concerns regarding elective switch from intravenous to subcutaneous anti-TNFα in IBD, so this strategy has not been incorporated into routine clinical practice, despite evidence of patients generally preferring subcutaneous administration.84
With the advent of new mechanisms of action, there is now the theoretical possibility of sequencing therapies with elective switch from anti-TNFα agents to other drugs; however, such a possibility has not been explored thus far. Two recent works focussed on the effect of starting second-line vedolizumab while still having circulating levels of previous anti-TNFα drug in IBD patients. While these studies did not show any significant effect on vedolizumab pharmacokinetics, effectiveness and safety, they focussed mainly on patients swapping therapy due to loss of response; thus, they only revealed that, when transitioning from anti-TNFα agents to vedolizumab, wash-out periods are probably not required.86,87 In a recent retrospective study, 41 patients (36 CD and 5 UC) who underwent elective switch from anti-TNFα therapy to vedolizumab while in stable clinical remission were included; in the majority of cases, the reason for discontinuing anti-TNFα treatment was adverse events. Clinical remission was maintained by 97.6%, 97.6% and 93.4% of patients at 12, 24 and 36 months, respectively; 4 (9.8%) patients had an immunomodulator added and 11 (26.8%) received therapy optimisation. After a median follow up of 30 months, only one (2.4%) patient had to discontinue vedolizumab due to disease relapse, and four more (9.8%) due to adverse events.88 Such figures support the idea that elective switch can be considered a safe and effective option in selected patients in stable remission. This study was not designed to compare the long-term outcomes of anti-TNFα and vedolizumab therapy; however, the cumulative probability of maintaining vedolizumab after 3 years was remarkably high in this study, possibly suggesting that elective switch to vedolizumab in remitting patients might deserve further investigation.
The concept of sequencing therapies might also be applicable to combinations, as already shown with infliximab and thiopurines. So far, combinations of targeted therapies have been employed mainly in add-on strategies. Furthermore, we do not know if a patient achieving remission with DTT should continue both agents or can withdraw one of them. Considering the two main indications for DTT in IBD (double indication or purely intestinal disease), it is likely that patients in the former group might require to continue both drugs, as each agent is required to control a specific disease. On the contrary, for patients in the latter group, the question remains controversial. An interesting line of evidence comes from the 2007 double-blind RCT, where CD patients not in remission while treated with scheduled infliximab were randomised to receive three infusions of natalizumab or placebo. The study was designed to evaluate the safety of the additional natalizumab infusions (of note, no significant differences observed), and was not powered enough to prove clinical benefit. However, efficacy analysis, despite not reaching statistical significance, showed a trend towards superiority of natalizumab over placebo: reduction in Crohn’s Disease Activity Index (CDAI) was numerically higher in the natalizumab group at week 6 (–37.7 points in the natalizumab group versus + 3.5 in the placebo group, p = 0.084).89 As this study was not meant to prove efficacy, and we cannot know whether three additional infusions of natalizumab are actually sufficient to provide any significant benefit, this study only suggests the potentiality of add-on and temporary DTT. In a more recent observational study, Buer and colleagues tested the efficacy the addition of vedolizumab in IBD patients receiving anti-TNFα treatment. Out of 10 patients receiving DTT, 8 were able to discontinue anti-TNFα therapy after a median time of 6 months; the remaining 2 had to resume it due to recurrence of intestinal symptoms in 1 patient and arthralgias in the other upon discontinuation. This study prompts the idea that temporary DTT is an effective strategy to achieve remission, and that subsequent monotherapy can be considered as maintenance regimen. However, it is impossible to know if the same results would have been obtained by directly swapping from anti-TNFα therapy to vedolizumab.52
Combination and sequential therapies for a dynamic management of IBD
The therapeutic paradigm of IBD treatment has evolved rapidly over the last decades. As both CD and UC are now recognised as progressive and disabling conditions, prevention of long-term disability has become one of the main treatment goals. With this shift, there are two main fields of research where advances are needed: development of new drugs with different mechanisms of actions and implementation of new strategies for diseases management (Figure 3). Together with treat-to-target management and personalised medicine, a ‘dynamic’ approach comprising combination and sequential therapies for IBD treatment – as we propose in the algorithm presented in Figure 4 – might be worthy of consideration in the near future.
Figure 3.
Implementations needed in IBD medical management. Created with BioRender.com.
IBD, inflammatory bowel disease; IL, interleukin; JAK, Janus kinases; RCTs, randomised controlled trials; S1P, sphingosine-1-phosphate; TNF, tumour necrosis factor.
Figure 4.
Proposed algorithm for IBD medical management, excluding cases where surgery is needed. Created with BioRender.com.
IBD, inflammatory bowel disease; DTT, dual targeted therapy; IMM, immunosuppressor; TNF, tumour necrosis factor.
In oncology and haematology, the association of multiple drugs represents the standard of care for many malignancies.90 Furthermore, it is not unusual in this field to start with combination therapies for the first cycles, than to continue with monotherapy in responding patients – as it happens, for instance, in some forms of advanced lung cancer or in multiple myeloma.91–93 There is also evidence that some therapies can be used effectively as maintenance regimens, after successful induction with different drugs, as has been proposed for some forms of BRCA-positive advanced ovarian cancers.94 Whether such an approach might be exported in immune-mediated diseases – and, in particular, IBD – represents an undoubtedly attractive possibility.
A recent meta-analysis, including more than 18,000 CD patients, has found that early (disease duration less than 2 years) biologic use was associated with higher rates of clinical remission and mucosal healing and with lower rates of relapse.95 The SONIC and UC-SUCCESS trials all concluded that early introduction of the combination of infliximab and thiopurines was more effective than monotherapy with each of the two agents in inducing clinical and endoscopic remission in IBD. Furthermore, the CALM study demonstrated that rapid therapeutic optimisation (adalimumab escalation followed by introduction of azathioprine), driven by clinical and objective markers, was more effective that conventional management in inducing mucosal healing in patients with early CD. Its long-term extension also showed that achieving mucosal healing or deep remission after the first year, regardless of the strategy used to obtain it, was associated with a lower risk of relapse in the subsequent follow up. These lines of evidence seem to suggest that, especially in the earliest phases of disease, the utmost effort should be invested in achieving the best results with induction, as early control seems to be associated with more favourable long-term outcomes. Different pathogenetic pathways are activated contemporarily in the inflamed intestinal mucosa of IBD patients,13,51 so it could be speculated that targeting multiple pathways at the same time might be needed to achieve deep remission in some patients. Accordingly, using DTT as a more effective induction strategy, possibly followed by maintenance monotherapy, represents an intriguing possibility. Real-life data on this issue is still missing, but it is worth mentioning two ongoing RCTs that are testing the efficacy of DTT in induction.96,97
So far, DTT has been used mainly as an add-on therapy in patients with partial response to one therapy or in patients who relapse while on maintenance therapy. In such cases, therapy optimisation is usually the first choice, as it has been shown that some patients might have accelerated drug clearance (even in absence of anti-drug antibodies) or might need higher drug concentrations to achieve disease control (i.e. patients with perianal disease).98 The management of therapy failures can benefit from TDM. The most convincing evidence is on infliximab and adalimumab for the management of secondary failures.73 Reactive TDM (performed upon symptoms relapse) can discriminate three different scenarios accountable for drug inefficacy: (1) low TLs with negative ADAs, a situation which could require dose-escalation, (2) low/undetectable TLs with positive ADAs, where therapy optimisation (via dose-escalation or immunosuppressants add on) or in-class switch are advisable, (3) normal/high TLs with negative ADAs, when out-of-class swap is advisable.74,99 In case of primary failure, evidence on the appropriateness of TDM is less convincing, and out-of-class swap is usually preferred,73 even though it has been observed that reactive TDM might be beneficial in some cases.100 Fewer data on reactive TDM with non-anti-TNFα agents exist, and it is not recommended in routine clinical practice; however, evidence from post hoc analysis of pivotal trials and observational studies have demonstrated that a dose-response correlation exists for these molecules101; therefore, it might be possible that reactive TDM could help discriminate patients who might benefit from dose-escalation from those who need out-of-class swap. Indeed, being able to identify patients with pharmacokinetic failure would be extremely useful; inadequate drug levels only account for certain cases of treatment inefficacy, and patients can also become ‘insensitive’ to the drug’s mechanism of action. For instance, it has been shown recently that CD4+ T cells can become insensitive to anti-TNFα-induced apoptosis due to the stimulation of IL23102; therefore, it is theoretically possible that IL23 inhibition might restore sensitivity to the mechanism of action of TNFα antagonists in such patients.
The correct management for IBD patients in remission has not been defined. Continuation of effective maintenance therapies is usually preferred, due to the higher risk of relapse after withdrawal and the absence of reliable predictive factors to identify patients who could harmlessly discontinue treatments. In a retrospective observational study including 1055 IBD patients in clinical remission, anti-TNFα discontinuation was associated with an incidence of relapse of 18% per patient-year.103 Fiorino et al. retrospectively compared the outcomes of UC patients who continued infliximab against those who discontinued it after at least 12 months of clinical remission and concluded that therapy discontinuation was associated with an increased risk of relapse.104 Notably, the same studies also demonstrated that restarting the previously effective treatment was generally successful and safe.103,104 Whether elective swap to a drug with a different mechanism of action could be proposed to IBD patients in deep remission is unknown; the appropriateness of elective swap has not been investigated appropriately so far and comparative data on treatment persistence are scarce. Two recent German studies reported that vedolizumab was associated with better treatment persistence, both as first and second line agent, compared with anti-TNFα agents.105,106 Such findings are in contrast with the results of the VICTORY consortium, where anti-TNF agents were reported to have better persistence compared with vedolizumab in CD patients.107 Considering that IBD are life-long conditions that usually arise in young adults,108 further investigations on the best strategies to maintain long-term remission are needed.
Separate considerations should be made for two specific situations that usually need therapy discontinuation, despite controlled IBD: (1) inefficacy on extraintestinal manifestations (EIMs), where out-of-class swap is usually preferred or DTT could be considered in selected cases; and (2) intolerance to current treatment, which could require either in-class switch or out-of-class swap, depending on the situation. An interesting case could be made for paradoxical psoriasis, which can be regarded either as a form of intolerance or an EIM. It has been estimated that it occurs with an incident rate of 5 per 100 person-years in IBD patients treated with anti-TNFα and is generally considered a class effect.109–111 For patients with concomitant psoriasis (including paradoxical), swap to ustekinumab has been reported to be safe and effective.112 Furthermore, ustekinumab has been reported to be superior compared with in-class switch in case of paradoxical psoriasis.111 A few cases of patients where ustekinumab was added to anti-TNFα have also been reported, with five out of eight patients reporting clinical benefit.58,113
Conclusions
A more dynamic approach to IBD treatment might represent, in the near future, a way to enhance its efficacy, together with the discovery of new mechanisms of action, the advances in personalised medicine and the implementation of new therapeutic targets and strategies. To prove if this dynamic approach, based on the use of combination and sequential therapies, has the potential to contribute to change the natural history of IBD, five main questions need to be answered in the following years:
Is DTT superior to monotherapy in accomplishing the new goals of IBD management (deep remission to prevent long-term disability)?
Should we invest more in the induction phase of early IBD, by using combination therapies, and then continue with monotherapy in patients who achieve remission?
If we were able to identify patients with more aggressive IBD, should DTT be considered the preferential option in such patients?
Can the addition of a second targeted therapy be implemented in the treat-to-target management algorithm?
Should we use different drugs in induction and maintenance, in order to optimise long-term treatment efficacy?
Acknowledgments
The authors wish to thank Alessia Leonetti for her support in proofreading the manuscript.
Footnotes
Author contributions: Giuseppe Privitera designed the project, performed literature research and drafted the manuscript.
Daniela Pugliese and Loris Riccardo Lopetuso also contributed to perform literature research and to writing the manuscript.
Franco Scaldaferri, Matteo Neri, Luisa Guidi, Antonio Gasbarrini and Alessandro Armuzzi contributed to literature research and revised the manuscript critically.
Alessandro Armuzzi oversaw the project and guarantees for the integrity of the work.
All authors reviewed and approved the final draft of the article before submission.
Conflict of interest statement: The authors declare the following conflicts of interest: Giuseppe Privitera received consultancy fees from Alphasigma. Daniela Pugliese received speaker fees from AbbVie, MSD, Takeda, Janssen and Pfizer. Franco Scaldaferri: advisory board for Abbvie, Janssen, MSD, Sanofi and Takeda. Luisa Guidi: consultancies and/or speaker fees from: AbbVie, Janssen, MSD, Mundipharma, Takeda, Vifor Pharma and Zambon. Antonio Gasbarrini reports personal fees for consultancy for Eisai S.r.l., 3PSolutions, Real Time Meeting, Fondazione Istituto Danone, Sinergie S.r.l. Board MRGE, and Sanofi S.p.A, personal fees for acting as a speaker for Takeda S.p.A, AbbVie, and Sandoz S.p.A, and personal fees for acting on advisory boards for VSL3 and Eisai. Alessandro Armuzzi: consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Ferring, Gilead, Janssen, Lilly, MSD, Mylan, Pfizer, Samsung Bioepis, Sandoz and Takeda; lecture and/or speaker bureau fees from AbbVie, Amgen, Biogen, Ferring, Giliead, Janssen, MSD, Mitsubishi-Tanabe, Nikkiso, Novartis, Pfizer, Sandoz, Samsung Bioepis and Takeda; and research grants from MSD, Pfizer and Takeda. All the other authors have no conflict of interest to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Luisa Guidi  https://orcid.org/0000-0003-3320-7094
https://orcid.org/0000-0003-3320-7094
Alessandro Armuzzi  https://orcid.org/0000-0003-1572-0118
https://orcid.org/0000-0003-1572-0118
Contributor Information
Giuseppe Privitera, Dipartimento Universitario di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Rome, Italy.
Daniela Pugliese, CEMAD – IBD UNIT – Unità Operativa Complessa di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Rome, Italy.
Loris Riccardo Lopetuso, CEMAD – IBD UNIT – Unità Operativa Complessa di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Rome, Italy; Department of Medicine and Ageing Sciences, ‘G. d’Annunzio’ University of Chieti-Pescara, Chieti, Italy; Center for Advanced Studies and Technology (CAST), ‘G. d’Annunzio’ University of Chieti-Pescara, Chieti, Italy.
Franco Scaldaferri, CEMAD – IBD UNIT – Unità Operativa Complessa di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Rome, Italy.
Matteo Neri, Department of Medicine and Ageing Sciences, ‘G. d’Annunzio’ University of Chieti-Pescara, Chieti, Italy; Center for Advanced Studies and Technology (CAST), ‘G. d’Annunzio’ University of Chieti-Pescara, Chieti, Italy.
Luisa Guidi, Dipartimento Universitario di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Rome, Italy; CEMAD – IBD UNIT – Unità Operativa Complessa di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Rome, Italy.
Antonio Gasbarrini, Dipartimento Universitario di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Rome, Italy; CEMAD – IBD UNIT – Unità Operativa Complessa di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Rome, Italy.
Alessandro Armuzzi, Dipartimento Universitario di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Rome, Italy; CEMAD – IBD UNIT – Unità Operativa Complessa di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Rome, Italy.
References
- 1. Verdon C, Reinglas J, Coulombe J, et al. No change in surgical and hospitalization trends despite higher exposure to anti-tumor necrosis factor in inflammatory bowel disease in the Québec Provincial Database from 1996 to 2015. Inflamm Bowel Dis. Epub ahead of print 17 July 2020. DOI: 10.1093/ibd/izaa166. [DOI] [PubMed] [Google Scholar]
- 2. Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut 2019; 68: 423–433. [DOI] [PubMed] [Google Scholar]
- 3. Targownik LE, Tennakoon A, Leung S, et al. Temporal trends in initiation of therapy with tumor necrosis factor antagonists for patients with inflammatory bowel disease: a population-based analysis. Clin Gastroenterol Hepatol 2017; 15: 1061–1070.e1. [DOI] [PubMed] [Google Scholar]
- 4. Siegel CA, Yang F, Eslava S, et al. Treatment pathways leading to biologic therapies for ulcerative colitis and Crohn’s disease in the United States. Clin Transl Gastroenterol 2020; 11: e00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berg DR, Colombel JF, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis 2019; 25: 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 7. Yoon H, Jangi S, Dulai PS, et al. Histologic remission is associated with lower risk of treatment failure in patients with Crohn disease in endoscopic remission. Inflamm Bowel Dis. Epub ahead of print 25 November 2020. DOI: 10.1093/ibd/izaa301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chateau T, Feakins R, Marchal-Bressenot A, et al. Histological remission in ulcerative colitis: under the microscope is the cure. Am J Gastroenterol 2020; 115: 179–189. [DOI] [PubMed] [Google Scholar]
- 9. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. Epub ahead of print 21 December 2020. DOI: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 10. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017; 390: 2779–2789. [DOI] [PubMed] [Google Scholar]
- 11. Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis 2019; 25: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 12. Kalla R, Borg-Bartolo SP, Boyapati RK, et al. Precision medicine in inflammatory bowel disease: concept, progress and challenges [version 1; peer review: 2 approved]. F1000Res 2020; 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verdier J, Begue B, Cerf-Bensussan N, et al. Compartmentalized expression of Th1 and Th17 cytokines in pediatric inflammatory bowel diseases. Inflamm Bowel Dis 2012; 18: 1260–1266. [DOI] [PubMed] [Google Scholar]
- 14. Zorzi F, Monteleone I, Sarra M, et al. Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One 2013; 8: e54562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dulai PS, Siegel CA, Colombel JF, et al. Systematic review: monotherapy with antitumour necrosis factor α agents versus combination therapy with an immunosuppressive for IBD. Gut 2014; 63: 1843–1853. [DOI] [PubMed] [Google Scholar]
- 16. Yzet C, Diouf M, Singh S, et al. No benefit of concomitant immunomodulator therapy on efficacy of biologics that are not tumor necrosis factor antagonists in patients with inflammatory bowel diseases: a meta-analysis. Clin Gastroenterol Hepatol. Epub ahead of print 3 July 2020. DOI: 10.1016/j.cgh.2020.06.071. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis 2016; 10: 1259–1266. [DOI] [PubMed] [Google Scholar]
- 18. Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007; 56: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vermeire S, Gils A, Accossato P, et al. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol 2018; 11: 1756283X17750355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 21. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014; 146: 392–400.e3. [DOI] [PubMed] [Google Scholar]
- 22. Macaluso FS, Sapienza C, Ventimiglia M, et al. The addition of an immunosuppressant after loss of response to anti-TNFα monotherapy in inflammatory bowel disease: a 2-year study. Inflamm Bowel Dis 2018; 24: 394–401. [DOI] [PubMed] [Google Scholar]
- 23. Strik AS, van den Brink GR, Ponsioen C, et al. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017; 45: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 24. Zhang B, Gulati A, Alipour O, et al. Relapse from deep remission after therapeutic de-escalation in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2020; 14: 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology 2008; 134: 1861–1868. [DOI] [PubMed] [Google Scholar]
- 26. Roblin X, Boschetti G, Williet N, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open-label, prospective and randomised clinical trial. Aliment Pharmacol Ther 2017; 46: 142–149. [DOI] [PubMed] [Google Scholar]
- 27. Louis E, Mary JY, Verniermassouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012; 142: 63–70.e5. [DOI] [PubMed] [Google Scholar]
- 28. ClinicalTrials.gov. A proSpective randomized controlled trial comParing infliximAb-antimetabolites combination therapy to anti-metabolites monotheRapy and infliximab monothErapy in Crohn’s disease patients in sustained steroid-free remission on combination therapy - full text view, https://clinicaltrials.gov/ct2/show/NCT02177071 (accessed 13 December 2020).
- 29. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 30. Ollech JE, Dwadasi S, Rai V, et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by vedolizumab maintenance in 71 patients with severe steroid-refractory ulcerative colitis. Aliment Pharmacol Ther 2020; 51: 637–643. [DOI] [PubMed] [Google Scholar]
- 31. Pellet G, Stefanescu C, Carbonnel F, et al. Efficacy and safety of induction therapy with calcineurin inhibitors in combination with vedolizumab in patients with refractory ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 494–501. [DOI] [PubMed] [Google Scholar]
- 32. Christensen B, Gibson PR, Micic D, et al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol 2019; 17: 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cominelli F, Nast CC, Clark BD, et al. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest 1990; 86: 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andus T, Daig R, Vogl D, et al. Imbalance of the interleukin 1 system in colonic mucosa - association with intestinal inflammation and interleukin 1 receptor agonist genotype 2. Gut 1997; 41: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishiyama T, Mitsuyama K, Toyonaga A, et al. Colonic mucosal interleukin 1 receptor antagonist in inflammatory bowel disease. Digestion 1994; 55: 368–373. [DOI] [PubMed] [Google Scholar]
- 36. Ferretti M, Casini-Raggi V, Pizarro TT, et al. Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest 1994; 94: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casini-Raggi V, Kam L, Chong YJ, et al. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol 1995; 154: 2434–2440. [PubMed] [Google Scholar]
- 38. Pizarro TT, Huybrechts M, Bentz M, et al. IL-18, a novel immunoregulatory cytokine, is upregulated in Crohn’s disease: expression and localization in intestinal mucosal cells. Gastroenterology 1998; 114: A1062. [PubMed] [Google Scholar]
- 39. Monteleone G, Trapasso F, Parrello T, et al. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol 1999; 163: 143–147. [PubMed] [Google Scholar]
- 40. McNamee EN, Masterson JC, Jedlicka P, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A 2011; 108: 16711–16716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A 2010; 107: 8017–8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seidelin JB, Bjerrum JT, Coskun M, et al. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett 2010; 128: 80–85. [DOI] [PubMed] [Google Scholar]
- 43. Bamias G, Corridoni D, Pizarro TT, et al. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine 2012; 59: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kobori A, Yagi Y, Imaeda H, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol 2010; 45: 999–1007. [DOI] [PubMed] [Google Scholar]
- 45. Kojouharoff G, Hans W, Obermeler F, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol 1997; 107: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol 2004; 34: 2347–2355. [DOI] [PubMed] [Google Scholar]
- 47. Tebbutt NC, Giraud AS, Inglese M, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med 2002; 8: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 48. Menghini P, Corridoni D, Buttó LF, et al. Neutralization of IL-1α ameliorates Crohn’s diseaselike ileitis by functional alterations of the gut microbiome. Proc Natl Acad Sci U S A 2019; 116: 26717–26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopetuso LR, De Salvo C, Pastorelli L, et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A 2018; 115: E9362–E9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bamias G, Cominelli F. Exploring the early phase of Crohn’s disease. Clin Gastroenterol Hepatol. Epub ahead of print 16 September 2020. DOI: 10.1016/j.cgh.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Atreya R, Neurath MF. Mechanisms of molecular resistance and predictors of response to biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2018; 3: 790–802. [DOI] [PubMed] [Google Scholar]
- 52. Buer LCT, Høivik ML, Warren DJ, et al. Combining anti-TNF-α and vedolizumab in the treatment of inflammatory bowel disease: a case series. Inflamm Bowel Dis 2018; 24: 997–1004. [DOI] [PubMed] [Google Scholar]
- 53. Kwapisz L, Raffals LE, Bruining DH, et al. Combination biologic therapy in inflammatory bowel disease: experience from a Tertiary Care Center. Clin Gastroenterol Hepatol. Epub ahead of print 14 February 2020. DOI: 10.1016/j.cgh.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 54. Privitera G, Onali S, Pugliese D, et al. Dual targeted therapy: a possible option for the management of refractory inflammatory bowel disease. J Crohns Colitis. Epub ahead of print 17 July 2020. DOI: 10.1093/ecco-jcc/jjaa149. [DOI] [PubMed] [Google Scholar]
- 55. Glassner K, Oglat A, Duran A, et al. The use of combination biologic or small molecule therapy in inflammatory bowel disease: a retrospective cohort study. J Dig Dis. Epub ahead of print 11 June 2020. DOI: 10.1111/1751-2980.12867. [DOI] [PubMed] [Google Scholar]
- 56. Yang E, Panaccione N, Whitmire N, et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment Pharmacol Ther 2020; 51: 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirten RP, Iacucci M, Shah S, et al. Combining biologics in inflammatory bowel disease and other immune mediated inflammatory disorders. Clin Gastroenterol Hepatol 2018; 16: 1374–1384. [DOI] [PubMed] [Google Scholar]
- 58. Olbjørn C, Rove JB, Jahnsen J. Combination of biological agents in moderate to severe pediatric inflammatory bowel disease: a case series and review of the literature. Pediatr Drugs 2020; 22: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dolinger MT, Spencer EA, Lai J, et al. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. Epub ahead of print 30 October 2020. DOI: 10.1093/ibd/izaa277. [DOI] [PubMed] [Google Scholar]
- 60. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 61. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113: 481–517. [DOI] [PubMed] [Google Scholar]
- 62. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline. Am J Gastroenterol 2019; 114: 384–413. [DOI] [PubMed] [Google Scholar]
- 63. Sands BE, Peyrin-Biroulet L, Loftus EV, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019; 381: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 64. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020; 158: 1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Singh S, Murad MH, Fumery M, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. Epub ahead of print 13 January 2020. DOI: 10.1016/j.cgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singh S, Fumery M, Sandborn WJ, et al. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther 2018; 48: 394–409. [DOI] [PubMed] [Google Scholar]
- 67. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q 2018; 7: 3. [PMC free article] [PubMed] [Google Scholar]
- 68. Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev 2014; 13: 24–30. [DOI] [PubMed] [Google Scholar]
- 69. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol 2011; 106: 674–684. [DOI] [PubMed] [Google Scholar]
- 70. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009; 104: 760–767. [DOI] [PubMed] [Google Scholar]
- 71. Gisbert JP, Marín AC, McNicholl AG, et al. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015; 41: 613–623. [DOI] [PubMed] [Google Scholar]
- 72. Singh S, George J, Boland BS, et al. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis 2018; 12: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017; 46: 1037–1053. [DOI] [PubMed] [Google Scholar]
- 74. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015; 13: 522–530.e2. [DOI] [PubMed] [Google Scholar]
- 75. Roblin X, Vérot C, Paul S, et al. Is the pharmacokinetic profile of a first anti-TNF predictive of the clinical outcome and pharmacokinetics of a second anti-TNF? Inflamm Bowel Dis 2018; 24: 2078–2085. [DOI] [PubMed] [Google Scholar]
- 76. Roblin X, Williet N, Boschetti G, et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut 2020; 69: 1206–1212. [DOI] [PubMed] [Google Scholar]
- 77. Casanova MJ, Chaparro M, Mínguez M, et al. Effectiveness and safety of the sequential use of a second and third anti-TNF agent in patients with inflammatory bowel disease: results from the Eneida Registry. Inflamm Bowel Dis 2020; 26: 606–616. [DOI] [PubMed] [Google Scholar]
- 78. Hupé M, Rivière P, Nancey S, et al. Comparative efficacy and safety of vedolizumab and infliximab in ulcerative colitis after failure of a first subcutaneous anti-TNF agent: a multicentre cohort study. Aliment Pharmacol Ther 2020; 51: 852–860. [DOI] [PubMed] [Google Scholar]
- 79. Favale A, Onali S, Caprioli F, et al. Comparative efficacy of vedolizumab and adalimumab in ulcerative colitis patients previously treated with infliximab. Inflamm Bowel Dis 2019; 25: 1805–1812. [DOI] [PubMed] [Google Scholar]
- 80. Alric H, Amiot A, Kirchgesner J, et al. The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther 2020; 51: 948–957. [DOI] [PubMed] [Google Scholar]
- 81. Townsend T, Razanskaite V, Dodd S, et al. Comparative effectiveness of ustekinumab or vedolizumab after one year in 130 patients with anti-TNF-refractory Crohn’s disease. Aliment Pharmacol Ther 2020; 52: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 82. Biemans VBC, van der Woude CJ, Dijkstra G, et al. Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther 2020; 52: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gui X, Li J, Ueno A, et al. Histopathological features of inflammatory bowel disease are associated with different CD4+ T cell subsets in colonic mucosal lamina propria. J Crohns Colitis 2018; 12: 1448–1458. [DOI] [PubMed] [Google Scholar]
- 84. Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61: 229–234. [DOI] [PubMed] [Google Scholar]
- 85. Hoentjen F, Haarhuis BJT, Drenth JPH, et al. Elective switching from infliximab to adalimumab in stable Crohn’s disease. Inflamm Bowel Dis 2013; 19: 761–766. [DOI] [PubMed] [Google Scholar]
- 86. Ben-Horin S, Ungar B, Kopylov U, et al. Safety, efficacy and pharmacokinetics of vedolizumab in patients with simultaneous exposure to an anti-tumour necrosis factor. Aliment Pharmacol Ther 2018; 47: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 87. Liefferinckx C, Verstockt B, Gils A, et al. Impact of first-line infliximab on the pharmacokinetics of second-line vedolizumab in inflammatory bowel diseases. United Eur Gastroenterol J 2019; 7: 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang Y, Wang J, Pekow J, et al. Outcome of elective switching to vedolizumab in inflammatory bowel disease patients under tumor necrosis factor antagonist-maintained clinical remission. J Gastroenterol Hepatol 2019; 34: 2090–2095. [DOI] [PubMed] [Google Scholar]
- 89. Sands BE, Kozarek R, Spainhour J, et al. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm Bowel Dis 2007; 13: 2–11. [DOI] [PubMed] [Google Scholar]
- 90. Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell 2017; 171: 1678–1691.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med 2018; 379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 92. Borghaei H, Langer CJ, Paz-Ares L, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non–small cell lung cancer without tumor PD-L1 expression: a pooled analysis of 3 randomized controlled trials. Cancer 2020; 126: 4867–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med 2017; 281: 365–382. [DOI] [PubMed] [Google Scholar]
- 94. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379: 2495–2505. [DOI] [PubMed] [Google Scholar]
- 95. Ungaro RC, Aggarwal S, Topaloglu O, et al. Systematic review and meta-analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment Pharmacol Ther 2020; 51: 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. ClinicalTrials.gov. Triple combination therapy in high risk Crohn’s Disease (CD) - full text view, https://clinicaltrials.gov/ct2/show/NCT02764762?term=triple+therapy&cond=Crohn+Disease&draw=2&rank=1 (accessed 6 September 2020).
- 97. ClinicalTrials.gov. A study of efficacy and safety of combination therapy with guselkumab and golimumab in participants with moderately to severely active ulcerative colitis - full text view, https://clinicaltrials.gov/ct2/show/NCT03662542?term=vega&cond=Inflammatory+Bowel+Diseases&draw=2&rank=1 (accessed 6 September 2020).
- 98. Argollo M, Kotze PG, Kakkadasam P, et al. Optimizing biologic therapy in IBD: how essential is therapeutic drug monitoring? Nat Rev Gastroenterol Hepatol 2020; 17: 702–710. [DOI] [PubMed] [Google Scholar]
- 99. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014; 63: 919–927. [DOI] [PubMed] [Google Scholar]
- 100. Sparrow MP, Papamichael K, Ward MG, et al. Therapeutic drug monitoring of biologics during induction to prevent primary non-response. J Crohns Colitis 2020; 14: 543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Alsoud D, Vermeire S, Verstockt B. Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: hype or hope? Curr Opin Pharmacol 2020; 55: 17–30. [DOI] [PubMed] [Google Scholar]
- 102. Schmitt H, Billmeier U, Dieterich W, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut. Epub ahead of print 30 May 2018. DOI: 10.1136/gutjnl-2017-315671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Casanova MJ, Chaparro M, García-Sánchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol 2017; 112: 120–131. [DOI] [PubMed] [Google Scholar]
- 104. Fiorino G, Cortes PN, Ellul P, et al. Discontinuation of infliximab in patients with ulcerative colitis is associated with increased risk of relapse: a multinational retrospective cohort study. Clin Gastroenterol Hepatol 2016; 14: 1426–1432.e1. [DOI] [PubMed] [Google Scholar]
- 105. Helwig U, Braegger F, Kostev K, et al. Comparative analysis of the 3-year persistence rate with second-line vedolizumab and tumor necrosis factor-α inhibitors in patients with inflammatory bowel disease followed in gastroenterology practices in Germany. Dig Dis 2020; 38: 466–473. [DOI] [PubMed] [Google Scholar]
- 106. Helwig U, Kostev K, Schmidt C. Comparative analysis of 3-year persistence with vedolizumab compared with antibodies against tumor necrosis factor-alpha in patients with inflammatory bowel disease in Germany: retrospective analysis of a large prescription database. J Clin Gastroenterol 2021; 55: e1–e7. [DOI] [PubMed] [Google Scholar]
- 107. Bohm M, Xu R, Zhang Y, et al. Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for Crohn’s disease. Aliment Pharmacol Ther 2020; 52: 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 109. Pugliese D, Guidi L, Ferraro PM, et al. Paradoxical psoriasis in a large cohort of patients with inflammatory bowel disease receiving treatment with anti-TNF alpha: 5-year follow-up study. Aliment Pharmacol Ther 2015; 42: 880–888. [DOI] [PubMed] [Google Scholar]
- 110. Cullen G, Kroshinsky D, Cheifetz AS, et al. Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: a new series and a review of 120 cases from the literature. Aliment Pharmacol Ther 2011; 34: 1318–1327. [DOI] [PubMed] [Google Scholar]
- 111. Alivernini S, Pugliese D, Tolusso B, et al. Paradoxical arthritis occurring during anti-TNF in patients with inflammatory bowel disease: histological and immunological features of a complex synovitis. RMD Open 2018; 4: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pugliese D, Daperno M, Fiorino G, et al. Real-life effectiveness of ustekinumab in inflammatory bowel disease patients with concomitant psoriasis or psoriatic arthritis: an IG-IBD study. Dig Liver Dis 2019; 51: 972–977. [DOI] [PubMed] [Google Scholar]
- 113. Yzet C, Dupas JL, Fumery M. Ustekinumab and anti-TNF combination therapy in patients with inflammatory bowel disease. Am J Gastroenterol 2016; 111: 748–749. [DOI] [PubMed] [Google Scholar]