Abstract
Background and Objective:
Mercury is one of the most harmful heavy metals and its toxicity causes severe multi-organ dysfunction. This study was designed to explore novel molecular pathways involved in the hepatoprotective effect of vitamin E (Vit-E) and Lactobacillius plantarum (Lac-B) against mercury toxicity.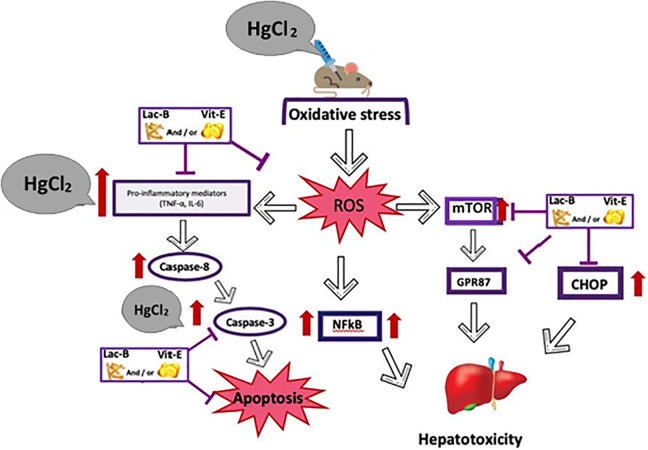
Method:
Acute hepatotoxicity was induced by administration of high dose of mercuric chloride (HgCl2) in male rats, Vit-E or/and Lac-B were given along with HgCl2 for 2 weeks. The effects of those antioxidants were studied focusing on their anti-apoptotic, anti-oxidative stress and anti-inflammatory eficacies. Histopathological examinations were also conducted.
Results:
The administration of HgCl2 induced liver injury which manifested by elevation in serum ALT and AST. Liver MDA, caspase-3 and TNF-α levels were markedly increased; whereas, GSH level and SOD activity were declined. HgCl2 significantly elevated the expressions of hepatic CHOP, GPR87, NF-κB and mTOR. Histopathological examination revealed massive hepatocyte degeneration following HgCl2 administration. Treatment with Vit-E or/and Lac-B restored the normal levels of the previously mentioned parameters, as well as improved hepatic architecture.
Conclusion:
Vit-E and Lac-B provided protective effect against HgCl2-induced hepatotoxicity via reduction of oxidative stress and inflammation, and downregulation of CHOP, GPR87, NF-κB and mTOR proteins’ expressions.
Keywords: mercury, hepatotoxicity, lactobacillius plantarum, vitamin E, CHOP, mTOR
Introduction
Mercury is extensively spread in the environment, which leads to a continuous human exposure to even low levels of this heavy metal.1 People can be exposed to mercury by consumption of specific types of fish, dental amalgam or even at work.2 The main source of mercury is the natural degassing of the earth’s crust, including oceans and rivers. It has been documented that low level of mercury exposure caused immune system alterations and reduced mice resistance to viral infections.3 Mercury toxicity is attributed to its capability to diminish free sulfhydryl groups of GSH and other antioxidants enzymes.
Numerous studies illustrated that oxidative stress evokes various intracellular events, such as proliferation, cell-cycle arrest, and apoptosis.4,5 In addition, exposure to inorganic mercury can exert a dose-dependent cytotoxicity by producing high levels of H2O2, which is normally quenched by pyruvate and catalase.6 Mercuric chloride (HgCl2) may damage the function of many organelles such as lysosomes that keep proton gradient through the membrane and decline renal glutathione peroxidase activity with upregulation of heme oxidase function. Several studies have found increased risk of pulmonary, renal, and CNS systems among dental workers.7 Mercury induces disruption of the cytochrome c oxidase system/ATP energy function,8,9 and inhibits enzymes needed to change porphyrins to ATP causing progressive porphyrinuria, leading to low energy and digestive injuries.10
Many studies indicated that oxidative stress represents a dangerous event correlated to the neurotoxic effects of HgCl2.11 The levels of various reactive species are dramatically increased upon HgCl2 exposure.12 Although it is broadly sulfhydryl reactive, yet signaling cascade implicated in mediating HgCl2-induced liver injury is not fully investigated.
This initiates our interest to study a new mechanistic role of HgCl2 hepatotoxicity at molecular level and to find a way to protect against this toxicity using a combination of vitamin E (Vit-E) and Lactobacillius plantarum (Lac-B). Vit-E is considered as a major lipid soluble element in the defense system inside the cell, and it can be obtained from diet. It has a powerful protection action against complications of various diseases due to its antioxidant role.13 Lac-B, a lactic acid bacterium, is used for dairy and meat fermentation, also it can be used as a probiotic with favorable actions on gut and metabolic illnesses.14
Materials and Methods
Chemicals
Vit-E and HgCl2 were obtained from Sigma Chemical Co. (Sigma, St. Louis, MO, USA). Lac-B was obtained from local pharmacy. The primary antibodies of CHOP, GPR87, mTOR and NF-κB were obtained from Santa Cruz (Santa Cruz Biotechnology, CA, USA).
Experimental Animals
Thirty Wistar adult male albino rats weighing 150-200 g were obtained from the Animal House, Faculty of Pharmacy, King Saud University. Animals were kept at temperature of 20-22°C; they were fed with standard rat pellet chow with free access to tap water ad libitum. The Experimental protocol was approved by the Research Ethics Committee, King Saud University (KSU-SE-19-38).
Experimental Design
After 1-week acclimation, rats were allocated into 5 groups each contains 6 rats; they were treated as follows: the first group was served as the normal control group and administered distilled water; in the second group, rats were intoxicated subcutaneously with 5 mg/kg HgCl2 15 once daily; the rats in the third group were treated with Vit-E at a dose of 100 mg/kg/day, orally16; the fourth group was orally treated with 6 × 1010 CFU of Lac-B 1.8701/kg in 1 mL normal saline17; and the fifth group was treated with the combination of Vit-E and Lac-B. All treatments were given daily along with HgCl2 for 2 weeks.
After completion of all treatments, rats were subjected to a gradual concentration of CO2, then sacrificed by decapitation. Blood samples were collected, and sera were separated by centrifugation at 3000 rpm for 20 min. The livers were also collected; parts of livers were homogenized in phosphate buffer to yield 20% homogenates. Then the homogenates were centrifuged for 20 min at 3000 rpm at 4°C, and the supernatants were kept at −80°C. Other parts of livers were rapidly frozen under liquid nitrogen and stored at −80°C for Western blotting. Parts of livers from each group were kept in 10% formalin for histopathological examination.
Biochemical Analysis
Examination of the liver enzymes
The activity of liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was measured in the serum using kits obtained from Randox (Crumlin, UK).
Determination of oxidative stress biomarkers
Malondialdehyde (MDA) was estimated in the liver tissue following the method of Mihara and Uchiyama.18 Reduced glutathione (GSH) was determined using the method of Ellman.19 Superoxide dismutase (SOD) activity was evaluated following the procedure of Marklund and Marklund.20
Determination of liver inflammatory and apoptotic biomarkers
Liver tumor necrosis factor alpha (TNF-α), cysteine–aspartic acid protease 3 (caspase-3) and interleukin 6 (IL-6) levels were measured using a highly sensitive ELISA kit (Immuno-Biological Laboratories Co., Ltd. Takasaki-Shi, Gunma, Japan).
Western blot analysis
Western blots were performed to determine the proteins expressions of CHOP, GPR87, mTOR, and NF-κB. Protein bands were visualized using the ECL-Plus detection system (Amersham Life Sciences, Little Chalfont, Buckinghamshire, UK) according to the manufacturer’s instructions. Positive immunoreactive bands were quantified densitometrically and compared with control.
Histological analysis
Liver samples were fixed in 10% formaldehyde, and thinly sliced sections were used for histopathological examination using hematoxylin and eosin (H&E) stain.
Statistical Analysis
Data were expressed as mean ± SEM for quantitative measures. The statistical comparisons were performed using 1-way analysis of variance (ANOVA), followed by Tukey-Kramer multiple comparisons test. The level of significance was set at P < 0.05, P < 0.01, and P < 0.001. Statistical tests were conducted using GraphPad Prism 5.00 (GraphPad Prism, San Diego, California, USA).
Results
Lac-B and/or Vit-E Retained Liver Functions in HgCl2-Induced Hepatotoxicity
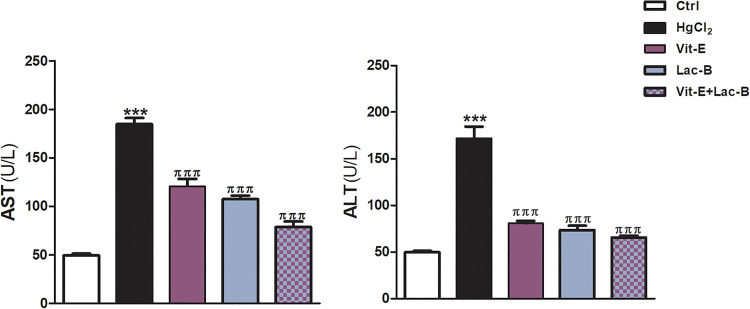
The current work showed marked elevation of the activity levels of serum ALT and AST enzymes in rat exposed to HgCl2 compared to control rats. While the administration Lac-B and Vit-E concurrently with HgCl2 significantly lowered the activity of liver enzymes (Figure 1).
Figure 1.
Vit-E, Lac-B and their combination prevents HgCl2-induced liver injury in rats. Vit-E, Lac-B reduced serum AST and ALT in HgCl2-intoxicated rats. The data are presented as the mean + SEM (n = 5). ***P ≤ .001 versus control, and πππ P ≤ .001 versus HgCl2-treated groups.
Lac-B and/or Vit-E Attenuated Oxidative Stress, Inflammation, and Apoptosis Induced by HgCl2
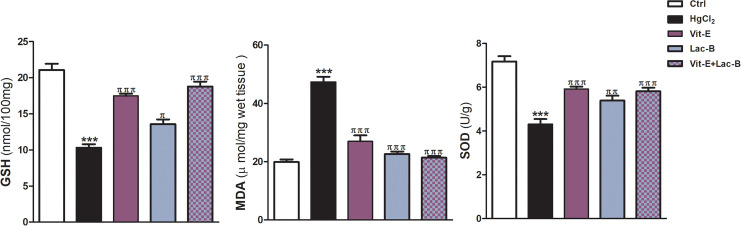
Further assessment of the hepatic protective effects of Lac-B and/or Vit-E was conducted by measuring the levels of oxidative, inflammatory, and apoptotic markers. Figure 2 shows the significant increase of MDA level and decrease of GSH and SOD following HgCl2 intoxication; accordingly, indicated high oxidative damage. In the same figure, the administration of Lac-B and/or Vit-E markedly ameliorated the toxic effects of HgCl2 on the previous parameters (Figure 2).
Figure 2.
Vit-E, Lac-B and their combination modulate hepatic GSH, MAD and SOD against HgCl2-induced liver injury in rats. The data are presented as the mean + SEM (n = 5). ***P ≤ .001 versus control, and πππ P ≤ .001 versus HgCl2-treated groups.
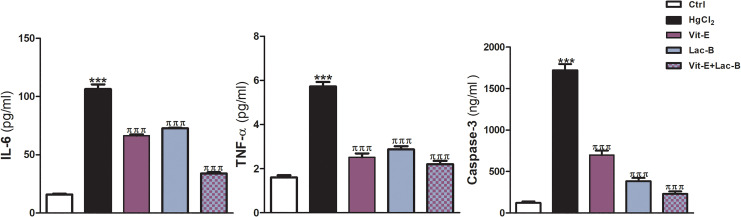
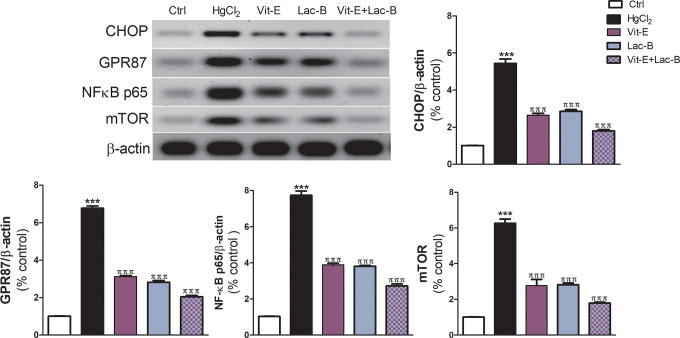
The expressions of the inflammatory biomarkers, IL-6 and TNF-α, were upregulated post HgCl2 administration; but they reduced in Lac-B and/or Vit-E treated groups (Figure 3). Moreover, HgCl2 intoxicated rats exhibited a significant elevation of the hepatic NF-κB (P ≤ 0.001) relative to control rats. On the other hand, rats received the antioxidants in question concurrently with HgCl2 showed a significant reduction in the expression of NF-κB (Figure 4).
Figure 3.
Vit-E, Lac-B and their combination downregulate hepatic inflammatory markers (IL-6 and TNF-α) and apoptotic marker (Caspase-3) in HgCl2-induced liver injury in rats. The data are presented as the mean + SEM (n = 5). ***P ≤ .001 versus control, and πππ P ≤ .001 versus HgCl2-treated groups.
Figure 4.
Representative blots of the expression of CHOP, GPR87, NF-кB and mTOR proteins, Vit-E, Lac-B, and their combination significantly downregulated their expressions in hepatic tissues. The data are presented as the mean + SEM (n = 5). ***P ≤ .001 versus control, and πππ P ≤ .001 versus HgCl2-treated groups.
In addition, HgCl2 toxicity provoked apoptosis by causing a significant increase in capsase-3 expression in the liver of intoxicated rats (P < 0.001). However, the use of Lac-B and/or Vit-E produced remarkable reduction in apoptosis by downregulating capsase-3 expression (Figure 3).
Lac-B and/or Vit-E Modulated the Expression of CHOP, GPR87, and mTOR
The CHOP, GPR87, and mTOR were significantly overexpressed following HgCl2 intoxication compared to control group (Figure 4). Interestingly, the administration of Lac-B or Vit-E either alone or in combination downregulated the expressions of those proteins in comparison to HgCl2 intoxicated group (Figure 4).
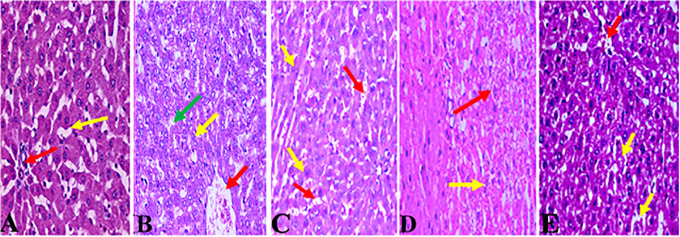
Lac-B and/or Vit-E Improved the Histopathological Changes Induced by HgCl2 Overdose
The hepatoprotective effects of Lac-B and/or Vit-E were confirmed by the histological examination of liver sections stained with H&E. The liver sections from the rats of the negative control group demonstrate normal hepatocytes and normal blood sinusoids (Figure 5A). The liver section from a rat administered HgCl2 shows moderate hepatocyte with ballooning and binucleated hepatocytes, central vein congestion (Figure 5B). The liver section from a rat administered Vit-E with HgCl2 shows a hepatic tissue with normal structure and architecture with dilated congested sinusoids, central vein congestion (Figure 5C). The liver section from a rat administered Lac-B concurrently with HgCl2 shows moderate healing of the necrosis and a slight decrease of the dilated sinusoids (Figure 5D). Lastly, the liver section from a rat administered a combined therapy of Vit-E and Lac-B with HgCl2 shows a marked improvement in hepatocyte degeneration with normal sinusoids (Figure 5E).
Figure 5.
Light photomicrographs of liver sections stained with H&E. (A) The liver section from a negative control-treated rat shows normal hepatocytes (red arrow) with normal blood sinusoids (yellow arrow). (B) The liver section from a rat administered HgCl2 shows moderate hepatocyte with ballooning (red arrow) and binucleated hepatocytes (yellow arrow), central vein congestion (green arrow). (C) The liver section from a rat administered Vit-E with HgCl2 shows a hepatic tissue with normal structure and architecture with dilated congested sinusoids (red arrow), central vein congestion (yellow arrow)). (D) The liver section from a rat administered Lac-B with HgCl2 shows moderate healing of the necrosis (red arrow) and a slight decrease of the dilated sinusoids (yellow arrow). (E) The liver section from a rat administered Vit-E and Lac-B with HgCl2 shows a marked improvement in hepatocyte degeneration (red arrow), with normal sinusoids (yellow arrow).
Discussion
Human may be exposed to HgCl2 poisoning by multiple routs including oral, inhalation or skin exposures, since it is extensively distributed in the environment. The environmental levels of mercury are rising because of the discharge from hydroelectric, mining and paper industries. It can be found in some skin lightening products and the filling of dental amalgam.21 Therefore, the aims of the current study are to examine the potential hepatoprotective effects of Vit-E and/or Lac-B against HgCl2-induced liver damage in rats, and to further investigate the mechanisms underlying those effects.
In this study, we examined the effect of HgCl2 intoxication on the activity of liver enzymes, and we found that HgCl2 cause significant elevation of serum ALT and AST activities. These enzymes are considered as critical and initial markers in the diagnosis of liver injury, as these enzymes could be released into the circulating blood directly due to hepatic cell damage.22 Our results were parallel to the outcomes of previous studies which revealed the increasing of the liver enzymes activities post excessive exposure of HgCl2.22,23
Our study revealed the administration of HgCl2 caused significant increase in the levels of oxidative stress (MDA), inflammatory (IL-6, TNF-α, and NF-κB), and apoptotic (caspase-3) markers, while GSH level and SOD activity were lowered post HgCl2 injection. It has been approved that when HgCl2 is accumulated within the hepatic cells, this provokes oxidative stress and subsequent liver injury, and it is believed that the principal mechanism of hepatotoxic effect of HgCl2 is the liberation of free radical and production of reactive oxygen species (ROS).24 In addition, the inflammation is usually associated with the production of ROS,25 and the inflammatory cytokines such as IL-6, TNF-α and NF-κB are important transcription factors known to be sensitive to oxidative stress,26,27 see graphical abstract figure.
Additionally, our study revealed the upregulation of the protein’s expressions of CHOP, GPR87, and mTOR in the liver tissues from HgCl2 intoxicated rats. It is documented that in normal healthy conditions, CHOP is expressed at very low levels28; while in diseased states the expression of CHOP is markedly increase and the apoptosis is triggered.28,29 Furthermore, Arfelt and colleagues documented the great relationship between the activation of NF-κB and GPR87-mediated cancer progression.30 Because mTOR is involved in the liver metabolism process of a drug,31 the balanced level of mTOR expression is vital for retaining hepatic cell homeostasis and prevention of liver damage and inflammation. Thus, the dysregulation of mTOR activity may result in liver injury, inflammation and even carcinogenesis.32
The administration of Vit-E and/or Lac-B could reduce the HgCl2-oxidative stress and prevent the overexpression of apoptotic and inflammatory biomarkers in liver tissue; moreover, using of those antioxidants improved the liver morphology. It has been demonstrated that Vit-E and Lac-B alleviated HgCl2-induced testicular atrophy by reducing the testicular MDA, and serum inflammatory cytokines.5 It has been shown that Lac-B decreased the inflammation of rat’s paw edema induced by carrageenan (a food additive which can initiates inflammation), diminished the expression of inflammatory cytokines, and improved its morphological architecture.33 Moreover, in clinical studies, Lac-B exhibited antioxidant and anti-inflammatory activities in healthy individuals and diabetic patients.34,35 Likewise, Vit-E has shown the antioxidant properties against HgCl2-induced reproductive toxicity.36 Additionally, Vit-E possessed a protective action in human lung cell exposed to HgCl2, through inhibition of oxidative stress and apoptosis.37
To sum up, this study suggested that administration of Vit-E and/or Lac-B has the potential to protect the liver from the toxicity induced by HgCl2 and also has the ability to attenuate and reverse HgCl2-induced oxidative stress, apoptosis, and inflammation in liver tissue. Additionally, the modulation of CHOP, GPR87, and mTOR may explain further mechanisms of liver injury and protection.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Undergraduate Student’s Research Support Program, Project no. (URSP-5-20-19).
Author’s Note: AA and IH conceived of the study, participated in its design and coordination; IH, SA and WA carried out the animal experimentation and biochemical analyses; AA and IH conducted the gene and protein analyses, IHH analyzed the data; AA drafted the manuscript and all authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Undergraduate Student’s Research Support Program, Project no. (URSP-5-20-19).
ORCID iD: Ahlam Alhusaini  https://orcid.org/0000-0003-2905-8882
https://orcid.org/0000-0003-2905-8882
References
- 1. Eagles-Smith CA, Silbergeld EK, Basu N, et al. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio. 2018;47(2):170–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nyland JF, Fairweather D, Shirley DL, Davis SE, Rose NR, Silbergeld EK. Low-dose inorganic mercury increases severity and frequency of chronic coxsackievirus-induced autoimmune myocarditis in mice. Toxicol Sci. 2012;125(1):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15(1):351–369. [DOI] [PubMed] [Google Scholar]
- 5. Fadda LM, Alhusaini AM, Al-Qahtani QH, Ali HM, Hasan IH. Role of α-tocopherol and Lactobacillus plantarum in the alleviation of mercuric chloride-induced testicular atrophy in rat’s model: implication of molecular mechanisms. J Biochem Mol Toxicol. 2020;34(6):e22481. [DOI] [PubMed] [Google Scholar]
- 6. Vimy MJ, Lorscheider FL, Sandborgh-Englund G, Ekstrand J, Elinder C-G. Renal function and amalgam mercury. Am J Physiol Regul Integr Comp Physiol. 1997;42(3):R1199–R1200. [DOI] [PubMed] [Google Scholar]
- 7. Ahlbom A, Norell S, Rodvall Y, Nylander M. Dentists, dental nurses, and brain tumours. Br Med J (Clin Res Ed). 1986;292(6521):662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr. 1999;129(12):2170–2176. [DOI] [PubMed] [Google Scholar]
- 9. Riedl CR, Plas E, Hübner WA, Zimmerl H, Ulrich W, Pflüger H. Bacterial colonization of ureteral stents. Eur Urol. 1999;36(1):53–59. [DOI] [PubMed] [Google Scholar]
- 10. Cianciola ME, Echeverria D, Martin MD, Vasken Aposian H, Woods JS. Epidemiologic assessment of measures used to indicate low-level exposure to mercury vapor (Hg). J Toxicol Environ Health. 1997;52(1):19–33. [DOI] [PubMed] [Google Scholar]
- 11. Lohren H, Blagojevic L, Fitkau R, et al. Toxicity of organic and inorganic mercury species in differentiated human neurons and human astrocytes. J Trace Elem Med Biol. 2015;32:200–208. [DOI] [PubMed] [Google Scholar]
- 12. Gökçe HS, Öztürk BC, Çam NF, Andiç-Çakır Ö. Gamma-ray attenuation coefficients and transmission thickness of high consistency heavyweight concrete containing mineral admixture. Cem Concr Compos. 2018;92:56–69. [Google Scholar]
- 13. Böhm V. Vitamin E. Antioxidants (Basel). 2018;7(3):44. doi:10.3390/antiox7030044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y-W, Liong M-T, Tsai Y-C. New perspectives of Lactobacillus plantarum as a probiotic: the gut-heart-brain axis. J Microbiol. 2018;56(9):601–613. [DOI] [PubMed] [Google Scholar]
- 15. Peixoto NC, Roza T, Flores ÉMM, Pereira ME. Effects of zinc and cadmium on HgCl2-δ-ALA-D inhibition and Hg levels in tissues of suckling rats. Toxicol Lett. 2003;146(1):17–25. [DOI] [PubMed] [Google Scholar]
- 16. Ibrahim MA, Bakhaat GA, Tammam HG, Mohamed RM, El-Naggar SA. Cardioprotective effect of green tea extract and vitamin E on Cisplatin-induced cardiotoxicity in mice: toxicological, histological and immunohistochemical studies. Biomed Pharmacother. 2019;113:108731. [DOI] [PubMed] [Google Scholar]
- 17. Liu Q, Ni X, Wang Q, et al. Lactobacillus plantarum BSGP201683 isolated from giant panda feces attenuated inflammation and improved gut microflora in mice challenged with Enterotoxigenic Escherichia coli . Front Microbiol. 2017;8:1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. [DOI] [PubMed] [Google Scholar]
- 19. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. [DOI] [PubMed] [Google Scholar]
- 20. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–474. doi:10.1111/j.1432-1033.1974.tb03714.x [DOI] [PubMed] [Google Scholar]
- 21. Park J-D, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med public Heal. 2012;45(6):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joshi D, Mittal DK, Shukla S, Srivastav AK, Srivastav SK. N-acetyl cysteine and selenium protects mercuric chloride-induced oxidative stress and antioxidant defense system in liver and kidney of rats: a histopathological approach. J Trace Elem Med Biol. 2014;28(2):218–226. [DOI] [PubMed] [Google Scholar]
- 23. Caglayan C, Kandemir FM, Darendelioğlu E, Yıldırım S, Kucukler S, Dortbudak MB. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol. 2019;56:60–68. [DOI] [PubMed] [Google Scholar]
- 24. Yang D, Tan X, Lv Z, et al. Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci Rep. 2016;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang H, Tan X, Yang D, et al. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget. 2017;8(25):40982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benzer F, Kandemir FM, Kucukler S, Comaklı S, Caglayan C. Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in Wistar rats: by modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch Physiol Biochem. 2018;124(5):448–457. [DOI] [PubMed] [Google Scholar]
- 27. Caglayan C, Kandemir FM, Yıldırım S, Kucukler S, Kılınc MA, Saglam YS. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female Wistar rats. Biomed Pharmacother. 2018;102:517–530. [DOI] [PubMed] [Google Scholar]
- 28. Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6(3):439–453. [DOI] [PubMed] [Google Scholar]
- 29. Yao Y, Lu Q, Hu Z, Yu Y, Chen Q, Wang QK. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat Commun. 2017;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arfelt KN, Fares S, Sparre-Ulrich AH, et al. Signaling via G proteins mediates tumorigenic effects of GPR87. Cell Signal. 2017;30:9–18. [DOI] [PubMed] [Google Scholar]
- 31. Domitrović R, Potočnjak I. A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch Toxicol. 2016;90(1):39–79. [DOI] [PubMed] [Google Scholar]
- 32. Cho C-S, Kowalsky AH, Lee JH. Pathological consequences of hepatic mTORC1 dysregulation. Genes (Basel). 2020;11(8):896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayyanna R, Ankaiah D, Arul V. Anti-inflammatory and antioxidant properties of probiotic bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar albino rats. Front Microbiol. 2018;9:3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr. 2003;90(2):449–456. [DOI] [PubMed] [Google Scholar]
- 35. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–543. [DOI] [PubMed] [Google Scholar]
- 36. Muthu K, Krishnamoorthy P. Effect of vitamin C and vitamin E on mercuric chloride-induced reproductive toxicity in male rats. Biochem Pharmacol. 2012;1(102):501–2167. [Google Scholar]
- 37. Ali HM. Mitigative role of garlic and vitamin E against cytotoxic, genotoxic, and apoptotic effects of lead acetate and mercury chloride on WI-38 cells. Pharmacol Reports. 2018;70(4):804–811. [DOI] [PubMed] [Google Scholar]